Abstract
The difficulty of measuring gross N2O production and consumption in soil impedes our ability to predict N2O dynamics across the soil-atmosphere interface. Our study aimed to disentangle these processes by comparing measurements from gas-flow soil core (GFSC) and 15N2O pool dilution (15N2OPD) methods. GFSC directly measures soil N2O and N2 fluxes, with their sum as the gross N2O production, whereas 15N2OPD involves addition of 15N2O into a chamber headspace and measuring its isotopic dilution over time. Measurements were conducted on intact soil cores from grassland, cropland, beech and pine forests. Across sites, gross N2O production and consumption measured by 15N2OPD were only 10% and 6%, respectively, of those measured by GFSC. However, 15N2OPD remains the only method that can be used under field conditions to measure atmospheric N2O uptake in soil. We propose to use different terminologies for the gross N2O fluxes that these two methods quantified. For 15N2OPD, we suggest using ‘gross N2O emission and uptake’, which encompass gas exchange within the 15N2O-labelled, soil air-filled pores. For GFSC, ‘gross N2O production and consumption’ can be used, which includes both N2O emitted into the soil air-filled pores and N2O directly consumed, forming N2, in soil anaerobic microsites.
N2O is one of the most important long-lived greenhouse gases and is expected to be the single most important ozone-depleting substance throughout the 21st century1. Soils account, globally, for about 60% of the total N2O flux to the atmosphere, with 6.6 Tg N yr−1 from natural ecosystems and 4.1 Tg N yr−1 from agricultural systems2. Although it is generally known that microbial nitrification and denitrification in soils are the major sources of atmospheric N2O, it remains a struggle to disentangle and quantify gross rates of microbial N2O production and consumption in soil which, in turn, determine the net N2O flux across the soil-atmosphere interface.
Under anaerobic conditions, incomplete denitrification produces N2O whereas the terminal step of denitrification (i.e. the reduction of N2O to N2) consumes N2O. Hence, microbial N2O production and consumption can occur simultaneously in soil via the activities of different microorganisms or even by a single denitrifying cell3. In addition, within the soil profile and in the soil air-filled pores, N2O can be further reduced to N2 during its transport to the soil surface4,5,6. Soil physical (e.g. water or oxygen content, temperature, porosity) and biochemical factors (e.g. pH, concentrations of electron donors and acceptors) influence the balance between soil N2O production and consumption7, and consequently the net N2O flux to the atmosphere. Soil net N2O uptake has been compiled in a review8, which specifically refers to the net flux of N2O from the atmosphere to the soil and can be detected only if soil N2O consumption exceeds production. Soil N2O consumption, however, is often ignored because it is prone to be masked by the much larger N2O production4 and is difficult to measure directly (e.g. as soil N2 flux) against a very high (78%) atmospheric background9.
The static chamber method, commonly used to measure net N2O flux on the soil surface, cannot quantify the simultaneously occurring gross N2O production and consumption within the soil. One possibility to measure gross N2O production and consumption in soil is the 15N2O pool dilution (15N2OPD) technique, which entails adding 15N2O to the chamber headspace and subsequently measuring the changes in 14N2O and 15N2O over time10. So far, this 15N2OPD technique has been used in managed grassland and cropland soils and in salt marsh landscape, all located in northern California, by the same authors who first evaluated this method under field conditions10,11,12.
In 2013, when the first 15N2OPD measurements were reported10, a debate emerged as to what extent this technique is able to quantify gross N2O production and consumption in soil. Well & Butterbach-Bahl13 questioned the key assumptions of the 15N2OPD technique: the exchange and mixing of soil-derived N2O and 15N2O label between aerobic and anaerobic soil microsites. They argued that gross N2O production and consumption in soil would be underestimated if produced N2O was immediately reduced to N2 without first mixing with the 15N2O-labelled air in interconnected soil pore spaces. This may occur within denitrifier cells and between different microorganisms3 in anaerobic microsites, which here we infer to include not only microsites saturated with water but also isolated pores filled with or enclosed by water and water-entrapped N2O14. Yang et al.15 replied that such constraints could only occur when the soil has a high proportion of anaerobic microsites, and argued that the 15N2O label and soil-derived N2O are likely distributed homogeneously in the chamber headspace from which the calculation of gross N2O fluxes is derived. In summary, the efficacy of the 15N2OPD technique to estimate gross N2O production and consumption is still not settled, and so far this technique has only been compared with results from acetylene inhibition and 15N tracing methods. These latter methods, however, have their own limitations for determining gross N2O production and consumption in soil since they either modify the entire denitrification process as well as its single steps (acetylene inhibition method) or require the addition of 15N-labelled substrate (15N tracing method) with the need to label the soil homogeneously including its anaerobic microsites9,16.
To date, the enigmatic lack of measurements of gross N2O production and consumption in soil impedes our ability to predict N2O dynamics across the soil-atmosphere interface. Our study aimed to disentangle gross N2O production and gross N2O consumption in soil by comparing measurements from 15N2OPD technique and gas-flow soil core (GFSC) method. The latter is an established method that directly measures gross N2O production and consumption in soil by simultaneously quantifying N2O and N2 fluxes17 without the use of an inhibitor or 15N labelling of substrate9,16. We hypothesized that if the assumption of the 15N2OPD method (i.e. exchange and mixing of soil-derived N2O and 15N2O label between aerobic and anaerobic soil microsites) is attained, then the 15N2OPD and GFSC methods should yield comparable estimates of gross N2O production and consumption in soil. We tested this hypothesis using different soils from four ecosystems: grassland, cropland, beech and pine forests (Table 1), covering a range of soil biochemical characteristics as well as soil aeration status (e.g. water content and soil texture) and N availability.
Table 1. Site characteristics.
| Site characteristics | Grassland | Cropland | Beech forest | Pine forest |
|---|---|---|---|---|
| Location | 47.57°N, 11.03°E | 48.19°N, 11.96°E | 51.76°N, 9.58°E | 43.72°N, 10.28°E |
| Mean annual temperature (°C) | 6.7 | 8.5 | 7.3 | 14.1 |
| Mean annual precipitation (mm) | 1373 | 1029 | 1100 | 918 |
| Elevation (m above sea level) | 870 | 510 | 510 | 10 |
| Vegetation/Crop | Poaceae; Taraxacum | Zea mays | Fagus sylvatica | Pinus pinaster |
| Soil type | Haplic Cambisol | Calcaric Cambisol | Dystric Cambisol | Calcareous Regosol |
| Soil texture (% sand/silt/clay) | 10/68/23 | 30/52/18 | 12 / 54/34 | 93/3/4 |
| Soil bulk density (g cm−3) | 0.59 | 1.17 | 0.64 | 1.30 |
| Soil pH | 7.1 | 6.7 | 3.8 | 5.7 |
| Soil total organic carbon (g C kg−1) | 135 | 20 | 127 | 10 |
| Soil total nitrogen (g N kg−1) | 8.0 | 1.7 | 6.6 | 0.7 |
| Soil C:N ratio | 16.9 | 11.8 | 18.9 | 13.5 |
Soil characteristics in the grassland, cropland and pine forest sites were measured in the top 10 cm of mineral soil19,21; in the beech forest site, these were measured in the top 5 cm of mineral soil.
Results
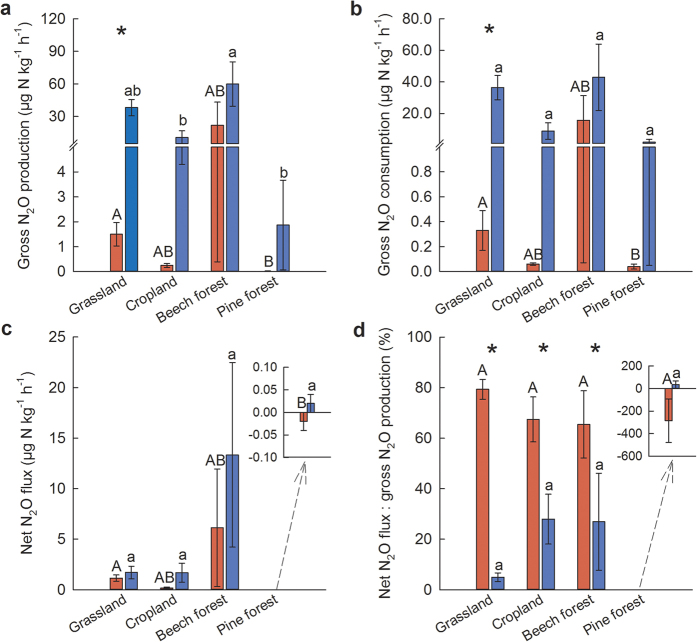
From the 15N2OPD measurements, gross N2O production and consumption rates and net N2O flux (Fig. 1a–c) were higher (p = 0.01–0.03) in the silty loam Cambisol soil in manured grassland than in the sandy Regosol soil in unmanaged pine forests, and neither differed from the sandy loam Cambisol soil in cropland or the silty loam Cambisol soil in unmanaged beech forest. For the grassland, cropland and beech forest, net N2O emissions accounted for 66–79% of gross N2O production (Fig. 1d). For the pine forest, net N2O uptake (Fig. 1c) was paralleled by larger gross N2O consumption (Fig. 1b) than gross N2O production (Fig. 1a); these fluxes were very small but still above our detection limit.
Figure 1. Soil gross and net N2O fluxes.
Gross N2O production (a), gross N2O consumption (b), net N2O flux (c), and the ratio of net N2O flux to gross N2O production (d), measured by 15N2O pool dilution (15N2OPD; red bars) and gas-flow soil core (GFSC; blue bars). For each method, means (± s.e., n = 4 replicate sampling points) with different capital (for 15N2OPD) and small letters (for GFSC) indicate significant differences among sites (one-way ANOVA with Fisher’s LSD test at p ≤ 0.05 or Kruskal-Wallis ANOVA with multiple comparisons of mean ranks at p ≤ 0.05). For each site, asterisks above the bars indicate significant differences between the two methods (paired t test at p ≤ 0.05).
From the GFSC measurements, gross N2O production (Fig. 1a) was higher (p = 0.02) in the beech forest than in the cropland and pine forest and intermediate in the grassland. Gross N2O consumption (p = 0.37; Fig. 1b) and net N2O fluxes (p = 0.06; Fig. 1c) did not differ among sites. Net N2O fluxes accounted, on average, for only 24% of gross N2O production (Fig. 1d), and hence most (76%) of the produced N2O was further reduced to N2.
Although significant differences in gross N2O production and consumption between the 15N2OPD technique and GFSC method were only found in the grassland site (p = 0.02 for both; Fig. 1a,b), the fluxes measured by the GFSC method were up to two orders of magnitude larger than those measured by the 15N2OPD technique (Fig. 1a,b). The large spatial variation within each site (indicated by the large standard errors) resulted in non-statistically detectable differences between these two methods. However, for gross N2O production, rates measured by the 15N2OPD technique were on average 10% of those measured by the GFSC method (Fig. 1a). For gross N2O consumption, rates measured by the 15N2OPD technique were on average 6% of those measured by the GFSC method (Fig. 1b). Net N2O fluxes from the soil cores used for the 15N2OPD measurement were on average 94% of those measured by the GFSC method, which did not differ in any of the sites (p = 0.11–0.61; Fig. 1c). In three sites, except in the pine forest that had very low fluxes, the ratios of net N2O flux to gross N2O production measured by the 15N2OPD technique were higher (p < 0.01–0.05) than those measured by the GFSC method (Fig. 1d).
Soil water-filled pore space (WFPS), microbial C and N, and denitrification enzyme activity (DEA) were generally higher (p ≤ 0.02) in the grassland than in the pine forest (Table 2). Soil NH4+ concentrations were higher (p < 0.01) in the grassland and beech forest compared to the cropland, whereas soil NO3− concentrations were higher (p = 0.02) in the cropland than in the grassland and pine forest (Table 2). Gross N2O production and consumption, measured by either the 15N2OPD technique or the GFSC method, showed positive correlations with WFPS, NH4+, microbial C and N, and DEA (R = 0.56–0.93, p < 0.05; Supplementary Table S1). Net N2O fluxes from the soil cores used for the 15N2OPD measurements correlated positively with the same soil properties (R = 0.64–0.92, p < 0.01; Supplementary Table S1), whereas no correlation was found with net N2O flux measured by the GFSC method.
Table 2. Soil physical and biochemical characteristics in the top 5 cm, determined from the soil cores immediately after the measurement of gross N2O fluxes.
| Soil characteristics | Grassland | Cropland | Beech forest | Pine forest |
|---|---|---|---|---|
| Water-filled pore space (%) | 79 ± 1 a | 57 ± 2 ab | 70 ± 14 ab | 25 ± 1 b |
| NH4+ (mg N kg−1) | 4.34 ± 0.97 a | 0.66 ± 0.12 b | 2.35 ± 0.37 a | 1.30 ± 0.18 ab |
| NO3− (mg N kg−1) | 1.00 ± 0.14 b | 5.42 ± 0.60 a | 4.17 ± 2.14 ab | 0.71 ± 0.38 b |
| Microbial C (g C kg−1) | 3.26 ± 0.13 a | 0.76 ± 0.03 c | 2.68 ± 0.24 ab | 1.72 ± 0.10 bc |
| Microbial N (mg N kg−1) | 211.02 ± 4.84 a | 69.22 ± 0.90 c | 160.90 ± 11.35 ab | 98.70 ± 5.37 bc |
| Denitrification enzyme activity (g N kg−1 h−1) | 5.16 ± 0.64 a | 0.21 ± 0.07 bc | 0.83 ± 0.17 ab | 0.00 ± 0.00 c |
Means ± s.e. (n = 4) within each row followed by different letter indicate significant differences among sites (one-way ANOVA with Fisher’s LSD test at p ≤ 0.05 or Kruskal-Wallis ANOVA with multiple comparisons of mean ranks at p ≤ 0.05.
Discussion
Both the 15N2OPD and GFSC methods have been proposed to be able to measure gross N2O production and consumption in soils9,10. The comparable net N2O fluxes determined by these methods (Fig. 1c) suggest that both methods can yield similar results in terms of the net effect of concurrently occurring production and consumption of N2O. However, the measured gross N2O production and consumption rates (Fig. 1a,b), and thus the ratios of net N2O flux to gross N2O production (Fig. 1d), differed between the two methods. Hence, we reject our hypothesis that 15N2OPD technique and GFSC method yield comparable estimates of gross N2O fluxes.
When using the 15N2OPD technique, gross N2O production is determined from the dilution of 15N2O label by 14N2O produced in the soil10. An implicit assumption of this approach is that the headspace-labelled 15N2O that diffuses into the soil results in a homogeneous mixture of 15N2O with soil-derived N2O in the soil air-filled pores, which also imply that these pores must be interconnected to the soil surface for homogenous mixing to occur. Our conservative calculations of diffusive transport of 15N2O into interconnected soil air-filled pores suggest that 15N2O must have diffused into these pores and back to the headspace within 0.5 h. However, there may be two situations when gross N2O production and consumption will be underestimated by this method: 1) produced N2O is immediately consumed within denitrifier cells3, and 2) produced N2O diffuses out of denitrifier cells and is consumed by other microorganisms, which may have N2O reductase but cannot act on the preceding substrates of the denitrification pathway18, without being mixed first with the 15N2O label during the 3-hour measurement period. Both situations can occur in anaerobic microsites, which here we infer to microsites saturated with water, isolated pores filled with or enclosed by water forming a diffusion barrier, and water-entrapped N2O as expounded by Clough et al.14. If these situations happen, disparity between 15N2OPD and GFSC measurements would be large in a fine-textured soil with high water content whereas they would be comparable in a coarse-textured soil with low water content. The fact that our results showed the large differences between the 15N2OPD and GFSC measurements in the silty loam soil of grassland with high WFPS and they were particularly comparable in the sandy soil of pine forest with low WFPS (Fig. 1a,b; Table 2) suggest that the 15N2OPD technique was not able to quantify gross N2O production in these above-mentioned two situations. With the GFSC method, gross N2O production is measured as the sum of emitted N2O and N2, and thus those immediately consumed N2O to N2 within denitrifier cells and between different microorganims in anaerobic microsites are included in this measurement.
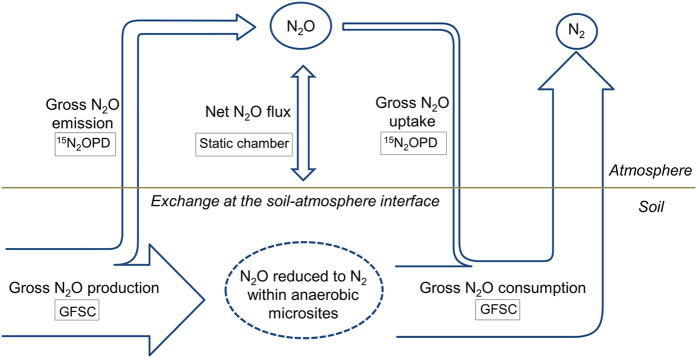
We summarize our results into a conceptual model in order to illustrate two decoupled pathways of N2O production and consumption in soil (Fig. 2). In the first pathway, N2O is produced in anaerobic microsites and reduced immediately to N2 without first mixing with the 15N2O label. Based on our results, only the GFSC method but not the 15N2OPD technique was able to quantify this pathway. The second pathway covers the soil-derived N2O that diffuses into the interconnected soil air-filled pores and mixes with the 15N2O label, which was captured by the 15N2OPD technique. Even if the N2O that has moved into the soil air-filled pores is being consumed during its diffusion towards the soil-atmosphere interface4, as long as the produced N2O mixes with the 15N2O label this can be included in the 15N2OPD calculations of gross N2O production. It is clear that both 15N2OPD and GFSC methods yield complementary important information, and thus a differentiation in the use of terminologies is needed. Since the 15N2OPD technique reflects the N2O dynamics in the gas phase of the soils and its exchange with the atmosphere, we propose to use the terms ‘gross N2O emission’ and ‘gross N2O uptake’ to denote the gross N2O fluxes in interconnected soil air-filled pores measured by this method. Since the GFSC method measures gross N2O fluxes not only in interconnected soil air-filled pores but also in anaerobic microsites, we propose that the terms ‘gross N2O production’ and ‘gross N2O consumption’ be used (Fig. 2). Below we will use these proposed terminologies to distinguish between the processes measured by these two methods.
Figure 2. Conceptual diagram of gross N2O fluxes.
Gross N2O emission and gross N2O uptake, measured by 15N2O pool dilution (15N2OPD), which largely includes gas exchange in interconnected air-filled pores in the soil; gross N2O uptake = gross N2O emission – net N2O flux. Gross N2O production and gross N2O consumption, measured by gas-flow soil core (GFSC), which encompasses the soil air-filled pores as well as anaerobic microsites (e.g. soil micro spots saturated with water, isolated pores filled with or enclosed by water, and water-entrapped N2O); gross N2O consumption = N2 emission, and gross N2O production = gross N2O consumption + net N2O flux.
It is important to point out that the 15N2OPD technique is able to yield information on gross N2O uptake from the atmosphere to the soil. For years there has been a discussion on the importance of N2O uptake in the soil from the atmosphere and substantial progress has been hampered because until now only the net N2O fluxes on the soil surface can be routinely measured with inexpensive static chamber method. With the 15N2OPD technique, we now have an operational approach that can be used for field measurements and can separate the net N2O fluxes across the soil-atmosphere interface into gross N2O emission and gross N2O uptake. It is a significant advancement since this technique will allow us to investigate the factors that control N2O uptake by soils under actual field conditions, which is a commonly unquantified sink of ecosystem N budgets.
Moreover, our results contrast to the notion that substantial N2O uptake only happens in soils with net negative N2O flux. This was shown by the larger gross N2O uptake (measured by 15N2OPD technique) in the grassland that had larger net N2O emissions than in the pine forest that had a net negative N2O flux (Fig. 1b,c). The positive correlations of gross N2O uptake with soil biochemical characteristics (Supplementary Table S1) suggest that high gross N2O uptake occurs in soils with high microbial activity and high substrate availability (Table 2). The ratios of net to gross N2O emissions (66–79% in grassland, cropland and beech forest; Fig. 1d) were similar to the values reported by Yang et al.10 and Yang and Silver12 from managed grassland and cropland in California (net to gross N2O emission ratio of 68–70%). These generally comparable ratios may open the possibility of making estimates of gross N2O emissions and uptake based on measured net N2O emissions.
The large fraction of gross N2O production that was consumed to N2 (measured by GFSC method) suggests that gross N2O production and consumption were closely coupled, which is in line with our aforementioned deduction (i.e. most N2O was immediately reduced to N2 in anaerobic microsites). Hence, the similar correlations found for gross N2O production and consumption with soil biochemical characteristics (Supplementary Table S1) as those found for gross N2O emission and uptake (measured by 15N2OPD technique) suggests that these gross N2O fluxes were regulated by the same process, denitrification4.
Our findings show that whereas the 15N2OPD technique is a valuable tool to separate net N2O flux across the soil-atmosphere interface into gross N2O emission and uptake, it did not allow measuring a large part of gross N2O production and consumption in anaerobic microsites. In order to avoid misinterpretations of terminologies, we propose that the terms ‘gross N2O emission and uptake’ should be used for gross N2O fluxes measured with the 15N2OPD technique and ‘gross N2O production and consumption’ should be used for gross N2O fluxes measured with the GFSC method.
Methods
Study sites and soil sampling
Soil samples were collected from four ecosystems: grassland, cropland, beech and pine forests, covering different vegetation, soil types and climatic conditions (Table 1). The montane grassland is manured 2–3 times a year and cut for hay three times a year19. The cropland is a conventional corn-winter wheat rotation. The unmanaged beech forest (Fagus sylvatica) is 163 years old20, and the unmanaged Mediterranean pine forest (Pinus pinaster) is 52 years old21.
At each site, we selected four sampling points as replicates with a minimum distance of 25 m from each other. At each replicate, eight intact soil cores (250 cm3 each) were taken using stainless-steel cores (8 cm diameter, 5 cm height): four of which were used for the 15N2OPD measurement and the other four for the GFSC measurement. The 15N2OPD measurement was conducted concurrently with the GFSC measurement, such that the soil cores for these two methods were handled similarly in all aspects. Neither soil moisture nor substrate level was adjusted.
15N2O pool dilution
Four intact soil cores were placed in an incubation glass (6.6 L volume), equipped with Luer-lock stopcock for gas sampling. Upon closure of the incubation vessel, we injected into the chamber headspace 7 mL of 15N2O label gas, containing 100 ppmv of 98% single labelled 15N-N2O, 275 ppbv sulfurhexafluoride (SF6, as a tracer for physical loss of N2O) and the rest as synthetic air. This injected amount increased the N2O concentration in the headspace by approx. 106 ppbv N2O with 12.5 atom% 15N enrichment and SF6 concentration of 292 pptv. At 0.5, 1, 2, and 3 h following label gas injection, 100 mL and 12 mL gas samples were taken out and stored in pre-evacuated 100 mL glass bottles and 12 mL glass tubes (Exetainer; Labco Limited, Lampeter, UK), respectively, with rubber septa. The sampled air volume was then replaced with 112 mL of a gas mixture (80% helium and 20% oxygen) to maintain the headspace at atmospheric pressure and oxygen concentration, without altering the isotopic composition of the headspace N2O. The dilution that this replacement caused was accounted for in the calculations. The 100 mL gas samples were used to analyze isotopic composition using an isotope ratio mass spectrometer (IRMS) (Finnigan Deltaplus XP, Thermo Electron Corporation, Bremen, Germany). The 12 mL gas samples were used to measure N2O and SF6 concentrations using a gas chromatograph equipped with an electron capture detector (GC 6000 Vega Series 2, Carlo Erba Instruments, Milan, Italy). The detection limit of the entire measurement set-up and instrument precision was <0.9 ppbv N2O h−1.
We modeled the vertical diffusive transport of 15N2O label through the 5 cm long soil cores, using the diffusion equation 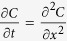 in which C, t and x denote concentration, time and path length, respectively22. The free-air N2O diffusion coefficient at 15 °C, 0.1582 cm s−1, was used and adjusted for soil tortuosity based on the air-filled porosity23, which was calculated using the measured bulk density and gravimetric moisture contents. Our most conservative calculations, using the lowest air-filled porosity and assuming an impervious boundary condition at bottom of the soil cores, showed that the 15N2O label had diffused into the 5 cm long soil cores and back to the headspace within 0.5 h. Thus, our sampling interval during the 3-hour measurement period was sufficient to allow mixing of the label gas with the soil-derived N2O in interconnected air-filled pores and to quantify the changes in N2O concentrations and 15N2O enrichments in the headspace.
in which C, t and x denote concentration, time and path length, respectively22. The free-air N2O diffusion coefficient at 15 °C, 0.1582 cm s−1, was used and adjusted for soil tortuosity based on the air-filled porosity23, which was calculated using the measured bulk density and gravimetric moisture contents. Our most conservative calculations, using the lowest air-filled porosity and assuming an impervious boundary condition at bottom of the soil cores, showed that the 15N2O label had diffused into the 5 cm long soil cores and back to the headspace within 0.5 h. Thus, our sampling interval during the 3-hour measurement period was sufficient to allow mixing of the label gas with the soil-derived N2O in interconnected air-filled pores and to quantify the changes in N2O concentrations and 15N2O enrichments in the headspace.
Gross N2O emission rate was calculated using the following equations modified from Yang et al.10:
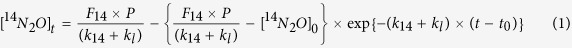 |
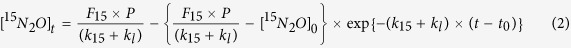 |
where [14N2O]t is the concentration of 14N2O at time t, calculated as the product of N2O concentration and 14N-N2O atom%; [15N2O]t is the concentration of 15N2O, calculated as the product of N2O concentration and 15N-N2O atom% excess, assuming that the 15N isotopic composition of background N2O is 0.3688 atom%10; t represents the time of gas sampling from the headspace; F14 represents the 14N2O mole fraction (0.997) and F15 represents the 15N2O mole fraction (0.003) of emitted N2O; k14 and k15 represent the first-order rate constants of 14N2O and 15N2O reduction to N2, respectively, calculated based on the fractionation factor (α = k15/k14) that has an average value of 0.9924 ± 0.0036 in literature10; kl represents the first-order rate constant for loss of inert transport tracer, SF6; P is gross N2O emission rate. The k14 and k15 represent the biological loss, and kl represents the physical loss. Since the changes of their concentrations in the headspace are simultaneously affected by biological consumption and physical loss, we used the sum of these constants (k14 + kl or k15 + kl) in the above equations.
We estimated the parameters for P and k15 by simultaneously fitting the measured [14N2O]t and [15N2O]t with equation (1) and (2). The best fit of [14N2O]t and [15N2O]t was found using the least square approach and minimizing the following error function:
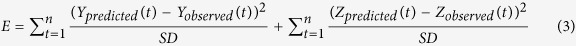 |
where E is minimal weighted error (E); Y, Z and n indicate 14N2O, 15N2O concentrations, and the number of measurements, respectively; SD refers to the standard deviation of the observed concentrations over the course of measurements24,25. Equation (3) was minimized using the ‘fminsearchbnd’ function in MATLAB (MathWorks, Version R2011b, USA). Gross N2O uptake was calculated as the difference between gross N2O emission and net N2O flux10.
Gas-flow soil core
The GFSC method is a fully automated, direct and sensitive quantification of the change of N2O and N2 concentrations in the headspace above the soil cores. The soil air of the four soil cores and the headspace of the incubation vessel were completely replaced by a gas mixture consisting of 20% O2 (purity grade of 5.5), 80% He (purity grade of 5.0), N2O (400 ppbv) and N2 (25 ppmv). This complete exchange was done by automated repeated cycles of evacuation and gas purging, achieved through a built-in purging system in an extremely air-tight chamber that is connected directly to a gas chromatograph (Shimadzu GC-17A, Shimadzu, Munich, Germany)17,26,27,28. Eighteen hours of evacuation-purging cycles ensure a complete removal of the background atmospheric air27, after which the headspace and tubing connections to the gas chromatograph were further purged for three hours. Subsequently, the system switched to a static chamber mode, and the headspace air of the incubation vessel was analyzed hourly over four hours through a directly connected gas chromatograph with an electron capture detector for N2O analysis and a pulse discharge He ionization detector (Vici AG, Schenkon, Switzerland) for N2 analysis26. To sample the headspace, a slight overpressure was created by injecting 40 mL of the He-based gas mixture to the headspace, directing headspace air to the sampling loops26. The dilution of this non-intrusive overpressure sampling technique was accounted for in the calculation of N2O and N2 concentrations26. In order to achieve the best possible tightness of the incubation system against intrusion of atmospheric N2, all tubing connections, valves as well as the entire incubation vessel were placed under water. Before starting the N2O and N2 measurements, the air-tightness of the system was always checked with an empty incubation vessel, which was connected in parallel with the measuring vessel. Based on the sensitivity and repeatability of the gas chromatograph measurements, the detection limits were <0.03 ppmv h−1 for N2 and <0.45 ppbv h−1 for N2O. The measured N2 flux from the soil equals to gross N2O consumption whereas the sum of N2 and N2O fluxes equals to gross N2O production17,26,27,28.
Soil controlling factors
Soil water content (one-day oven-drying at 105 °C and expressed as WFPS using 2.65 g cm−3 as particle density and the measured bulk density; Table 1), NH4+ and NO3− concentrations (0.5 M K2SO4 extraction), and microbial biomass C and N (CHCl3 fumigation-extraction) were determined from the soil cores immediately after the gas measurements. NH4+ and NO3− concentrations in the soil extract were determined using continuous flow autoanalyzer (Skalar Scan plus system, Skalar Analytical B.V., Breda, Netherlands). Microbial biomass C and N were determined as the difference in 0.5 M K2SO4-extractable organic C and N (analyzed using persulfate oxidation with an infrared detector; Multi N/C 3100 TOC/TNb-Analysator, Analytik Jena, Jena, Germany) between the fumigated and unfumigated soils divided by kEC = 0.45 and kEN = 0.6829. DEA was determined from the N2O produced during an anaerobic incubation with glucose and NO3− added in excess and acetylene inhibited N2O reduction of to N230.
Statistical analysis
The above soil properties, determined separately from the soil cores used for 15N2OPD and GFSC measurements, did not differ between these two measurements (p > 0.05; paired t test); thus, the values from the two measurements were averaged to represent a replicate sampling point. Data sets were first tested for normal distribution (Shapiro-Wilk’s test) and equality of variance (Levene’s test). We used log-transformation for variables with non-normal distributions or unequal variances and assessed the differences in gross N2O fluxes and soil properties among sites using one-way analysis of variance (ANOVA) with Fisher’s least significant difference test. When none of the data transformations were able to attain normal distribution and equality of variance, differences among sites were tested using the Kruskal-Wallis ANOVA with multiple comparisons test. The differences in gross and net N2O fluxes between the 15N2OPD and GFSC methods for each site were assessed using the paired t test. Relationships of gross N2O fluxes with soil properties were assessed using spearman rank correlation test. Statistical significance was set at p ≤ 0.05. Statistical analyses were conducted using SPSS (SPSS, Chicago, Illinois, USA).
Additional Information
How to cite this article: Wen, Y. et al. Disentangling gross N2O production and consumption in soil. Sci. Rep. 6, 36517; doi: 10.1038/srep36517 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, Co 749/1-1). Yuan Wen was supported by China Scholarship Council. Zhe Chen was supported by Alexander von Humboldt Foundation and National Natural Science Foundation of China (21107109). Further funding was provided by the Helmholtz/BMBF TERENO initiative, BMBF SUSALPS project, DFG BU 1173/17-1, DFG SFB 990/2 (project A05), DFG VE 219/14-1, and BMBF SIGNAL project.
Footnotes
Author Contributions M.D.C., E.V., M.D., B.W. and K.B.-B. designed the study; Y.W. and Z.C. carried out the measurements and analyzed data; B.W. modeled the diffusive transport of 15N2O label in soil; A.C. and Y.W. solved the 15N2OPD equations in MATLAB and experimentally tested them; G.W., Z.C., B.W., R.K., M.D. and K.B.-B. established the GFSC method; M.D. and Z.C. conceptualized Fig. 2; M.D.C., Y.W. and E.V. wrote most parts of the manuscript; all authors reviewed and rewrote parts of the manuscript.
References
- Ravishankara A. R., Daniel J. S. & Portmann R. W. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326, 123–125 (2009). [DOI] [PubMed] [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis . (eds Stocker T. F. et al.) (Cambridge University Press, 2013). [Google Scholar]
- Knowles R. Denitrification. Microbiol. Rev. 46, 43–70 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis-Lardy L., Wrage N., Metay A., Chotte J. & Bernoux M. Soils, a sink for N2O? A review. Glob. Change Biol. 13, 1–17 (2007). [Google Scholar]
- Conen F. & Neftel A. Do increasingly depleted δ15N values of atmospheric N2O indicate a decline in soil N2O reduction? Biogeochemistry 82, 321–326 (2007). [Google Scholar]
- Koehler B. et al. An in-depth look into a tropical lowland forest soil: nitrogen-addition effects on the contents of N2O, CO2 and CH4 and N2O isotopic signatures down to 2-m depth. Biogeochemistry 111, 695–713 (2012). [Google Scholar]
- Saggar S. et al. Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Sci. Total Environ. 465, 173–195 (2013). [DOI] [PubMed] [Google Scholar]
- Schlesinger W. H. An estimate of the global sink for nitrous oxide in soils. Glob. Change Biol. 19, 2929–2931 (2013). [DOI] [PubMed] [Google Scholar]
- Groffman P. M. et al. Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol. Appl. 16, 2091–2122 (2006). [DOI] [PubMed] [Google Scholar]
- Yang W. H., Teh Y. A. & Silver W. L. A test of a field-based 15N-nitrous oxide pool dilution technique to measure gross N2O production in soil. Glob. Change Biol. 17, 3577–3588 (2011). [DOI] [PubMed] [Google Scholar]
- Yang W. H. & Silver W. L. Gross nitrous oxide production drives net nitrous oxide fluxes across a salt marsh landscape. Glob. Change Biol. 22, 2228–2237 (2016). [DOI] [PubMed] [Google Scholar]
- Yang W. H. & Silver W. L. Net soil-atmosphere fluxes mask patterns in gross production and consumption of nitrous oxide and methane in a managed ecosystem. Biogeosciences 13, 1705–1715 (2016). [Google Scholar]
- Well, R. & Butterbach-Bahl K. Comments on “A test of a field-based 15N-nitrous oxide pool dilution technique to measure gross N2O production in soil” by Yang et al. (2011), Global Change Biology, 17, 3577–3588. Glob. Change Biol . 19, 133–135 (2013). [DOI] [PubMed] [Google Scholar]
- Clough T. J., Sherlock R. R. & Rolston D. E. A review of the movement and fate of N2O in the subsoil. Nutr. Cycl. Agroecosys. 72, 3–11 (2005). [Google Scholar]
- Yang W. H., Teh Y. A. & Silver W. L. Measuring gross N2O production in soil: a reply to Well and Butterbach-Bahl. Glob. Change Biol . 19, 985–987 (2013). [DOI] [PubMed] [Google Scholar]
- Butterbach-Bahl K., Baggs E. M., Dannenmann M., Kiese R. & Zechmeister-Boltenstern S. Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Phil. Trans. R. Soc. B . 368, 20130122 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenmann M., Butterbach-Bahl K., Gasche R., Willibald G. & Papen H. Dinitrogen emissions and the N2:N2O emission ratio of a Rendzic Leptosol as influenced by pH and forest thinning. Soil Biol. Biochem. 40, 2317–2323 (2008). [Google Scholar]
- Sanford R. A. et al. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc. Natl Acad. Sci. USA 109, 19709–19714 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unteregelsbacher S. et al. Increased methane uptake but unchanged nitrous oxide flux in montane grasslands under simulated climate change conditions. Eur. J. Soil Sci. 64, 586–596 (2013). [Google Scholar]
- Corre M. D., Beese F. O. & Brumme R. Soil nitrogen cycle in high nitrogen deposition forest: changes under nitrogen saturation and liming. Ecol. Appl. 13, 287–298 (2003). [Google Scholar]
- Rosenkranz P. et al. N2O, NO and CH4 exchange, and microbial N turnover over a Mediterranean pine forest soil. Biogeosciences 3, 121–133 (2006). [Google Scholar]
- Atkins P. W. Physical Chemistry . (Oxford University Press, 1986). [Google Scholar]
- Gliński J. & Stępniewski W. Soil Aeration and Its Role for Plants . (CRC Press, 1985). [Google Scholar]
- Teh Y. A., Rhew R. C., Atwood A. & Abel T. Water, temperature, and vegetation regulation of methyl chloride and methyl bromide fluxes from a shortgrass steppe ecosystem. Glob. Change Biol. 14, 77–91 (2008). [Google Scholar]
- Rhew R. C. Sources and sinks of methyl bromide and methyl chloride in the tallgrass prairie: Applying a stable isotope tracer technique over highly variable gross fluxes. J. Geophys. Res. 116, G03026 (2011). [Google Scholar]
- Butterbach-Bahl K., Willibald G. & Papen H. Soil core method for direct simultaneous determination of N2 and N2O emissions from forest soils. Plant Soil 240, 105–116 (2002). [Google Scholar]
- Wang R. et al. Measurement of N2, N2O, NO, and CO2 emissions from soil with the gas-flow-soil-core technique. Environ. Sci. Technol. 45, 6066–6072 (2011). [DOI] [PubMed] [Google Scholar]
- Chen Z. et al. Relationships between denitrification gene expression, dissimilatory nitrate reduction to ammonium and nitrous oxide and dinitrogen production in montane grassland soils. Soil Biol. Biochem. 87, 67–77 (2015). [Google Scholar]
- Shen S. M., Pruden G. & Jenkinson D. S. Mineralization and immobilization of nitrogen in fumigated soil and the measurement of microbial biomass nitrogen. Soil Biol. Biochem. 16, 437–444 (1984). [Google Scholar]
- Smith M. S. & Tiedje J. M. Phases of denitrification following oxygen depletion in soil. Soil Biol. Biochem. 11, 261–267 (1979). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.