Abstract
Objectives:
To describe the amyotrophic lateral sclerosis (ALS) patients who sought medication under the Washington State Death with Dignity (DWD) Act since its inception in 2009.
Methods:
Chart review at 3 tertiary medical centers in the Seattle/Puget Sound region and comparison to publicly available data of ALS and all-cause DWD cohorts from Washington and Oregon.
Results:
In Washington State, 39 patients with ALS requested DWD from the University of Washington, Virginia Mason, and Swedish Medical Centers beginning in 2009. The median age at death was 65 years (range 46–86). Seventy-seven percent of the patients used the prescriptions. All of the patients who used the medications passed away without complications. The major reasons for patients to request DWD as reported by participating physicians were loss of autonomy and dignity and decrease in enjoyable activities. Inadequate pain control, financial cost, and loss of bodily control were less commonly indicated. These findings were similar to those of the 92 patients who sought DWD in Oregon. In Washington and Oregon, the percentage of patients with ALS seeking DWD is higher compared to the cancer DWD cohort. Furthermore, compared to the all-cause DWD cohort, patients with ALS are more likely to be non-Hispanic white, married, educated, enrolled in hospice, and to have died at home.
Conclusions:
Although a small number, ALS represents the disease with the highest proportion of patients seeking to participate in DWD. Patients with ALS who choose DWD are well-educated and have access to palliative or life-prolonging care. The use of the medications appears to be able to achieve the patients' goals without complications.
The topic of physician-assisted suicide remains controversial and continues to engender passionate discussion. In the United States, Oregon was the first state to enact the Death with Dignity (DWD) Act in 1997.1 Eleven years later, the state of Washington passed its own DWD Act by referendum,2 modeled on the Oregon law. Between March 2009, when the law went into effect, and December 31, 2014, 725 Washington patients have sought prescriptions under the DWD Act3–9 In Oregon, as of February 2015, 1,327 patients have received prescriptions.10 Since the enactment of the 2 state laws, multiple states, nations, and professional organizations, such as the American Academy of Neurology, have taken up the debate of patients’ “right-to-die” and a physician's role in hastening death in terminally ill patients.
As physicians at the University of Washington Medical Center (UWMC), Virginia Mason Medical Center (VMMC), and Swedish Medical Center, 3 tertiary care institutes in Seattle, Washington, who regularly care for patients with amyotrophic lateral sclerosis (ALS), we have prescribed medications under the Washington DWD Act to 39 of our patients. Herein, we present our experience and describe our cohort in comparison to the 92 patients with ALS who received prescriptions under the DWD Act in Oregon as well as the all-cause DWD cohort in Oregon and Washington.
METHODS
Data collection at the 3 tertiary care centers.
We collected data from 39 patients at the UWMC ALS clinic, VMMC ALS clinic, and Swedish Medical Center general neurology clinic. Patients were already being seen in one of these clinics or were referred by End of Life Washington, a patient support volunteer organization. The state-mandated process for DWD involves the following: eligible patients older than 18 are first required to have a face-to-face visit with the prescribing physician, followed by a consulting physician, usually a neurologist, pulmonologist, or physiatrist, to document that the patient has an expected survival of less than 6 months and has been presented with other end-of-life options, including hospice, comfort care, and pain control. The 2 physicians (prescribing and consulting) determine capacity, that the patient is acting voluntarily, and whether a mental health disorder, such as active depression, may be impairing judgment, necessitating a consultation by a psychiatrist or psychologist. After the patient sees both physicians, the patient must also complete a state-prescribed written request with 2 witnesses present. A second oral request, separated by a minimum of 15 days, must be made by the patient in person or over the phone to the prescribing physician who then prescribes the medication. For this study, patients were identified through the central DWD patient repository at UWMC or the prescribing neurologists at VMMC and Swedish Medical Center.
Chart review at VMMC, Swedish Medical Center, and UWMC.
We reviewed patient charts and the Attending Physician's After Death Reporting Form that each attending/prescribing physician is legally required to fill out for every patient prescribed medication under the DWD Act. The After Death Reporting Form documents the date when the patient was diagnosed as having 6 months to live and the date of death, possible motivating factors contributing to the patient's request, if and when the medication was ingested, time between ingestion and unconsciousness or death, and level of education. Patient charts were reviewed for the date of symptom onset, usually rounded to the beginning of the month, forced vital capacity (FVC), ALS Functional Rating Scale–Revised score,11 disease symptoms, percutaneous endoscopic gastrostomy tube placement, noninvasive ventilation usage, identified religion, and classification by revised El Escorial criteria.12
Standard protocol approvals, registrations, and patient consents.
Permission was obtained from the institutional review boards at the 3 sites.
Washington State data.
By law, the Washington State Department of Health collects information on all patients who are prescribed medication, regardless of whether the medication was used, under the DWD law through the After Death Reporting Form, as well as their death certificate, and publishes an annual report.3–9 The total number of patients with ALS who died in Washington between 2009 and 2014 was calculated from the Mortality Table published online by the Washington State Department of Health.13
Oregon State data.
In distinction to the Washington State law, the Oregon law does not explicitly require that the patient “self-administer” but implies that the medication must be ingested.1 Furthermore, the Oregon Public Health Division publishes a yearly and cumulative summary characterizing only patients who took the prescribed DWD medication. Oregon reports data specifically on patients with ALS, as opposed to the more general “neurodegenerative disease” category used by Washington State.
Deidentified group data on patients with ALS who requested prescriptions under the DWD Act were obtained by applying to the Oregon Health Authority. Data were acquired for those who died using the medication as well as those who did not use the medication. Grouped data of the patients' sex, age group, median age at death, race, marital status, and education were also obtained. The total number of patients with ALS who died between 1998 and 2013 was calculated from the Oregon Vital Statistics Annual Report14 and from Hedberg et al.15
Statistical analysis.
The Fisher exact test via program R (2015) was used for comparisons of descriptive categories between the ALS and all-cause cohorts. Similar analyses were performed for comparison of Washington State patients who used medication vs those who did not. The p values <0.05 were considered significant.
RESULTS
We identified 39 patients with ALS in the 3 Seattle medical centers who requested medication under DWD. Between 2009 and 2014, 1,146 Washington State residents died of ALS. It is difficult to estimate a true percentage of patients with ALS who sought DWD, as our cohort represents only patients known to our 3 institutions. However, the percentage of patients with ALS who sought DWD is probably between 3.4% (39/1,146) and 6.7% (77/1,146), with the latter estimate based on the assumption that all DWD patients reported in Washington State with a neurodegenerative disease had ALS. Similarly, 5% (92/1,795) of Oregon patients with ALS who died sought medication under DWD between 1998 and 2014. The percentage of patients with ALS who sought DWD is significantly higher than the 0.6% (494/73,319) of patients with cancer who did so in Washington State and 0.2% (725/298,178) of all participating patients who died. ALS is the second most frequently reported disease after cancer (77.3%, 1,162/1,502 Oregon and Washington DWD patients).
Demographics and characteristics of patients with ALS at 3 tertiary Washington referral centers.
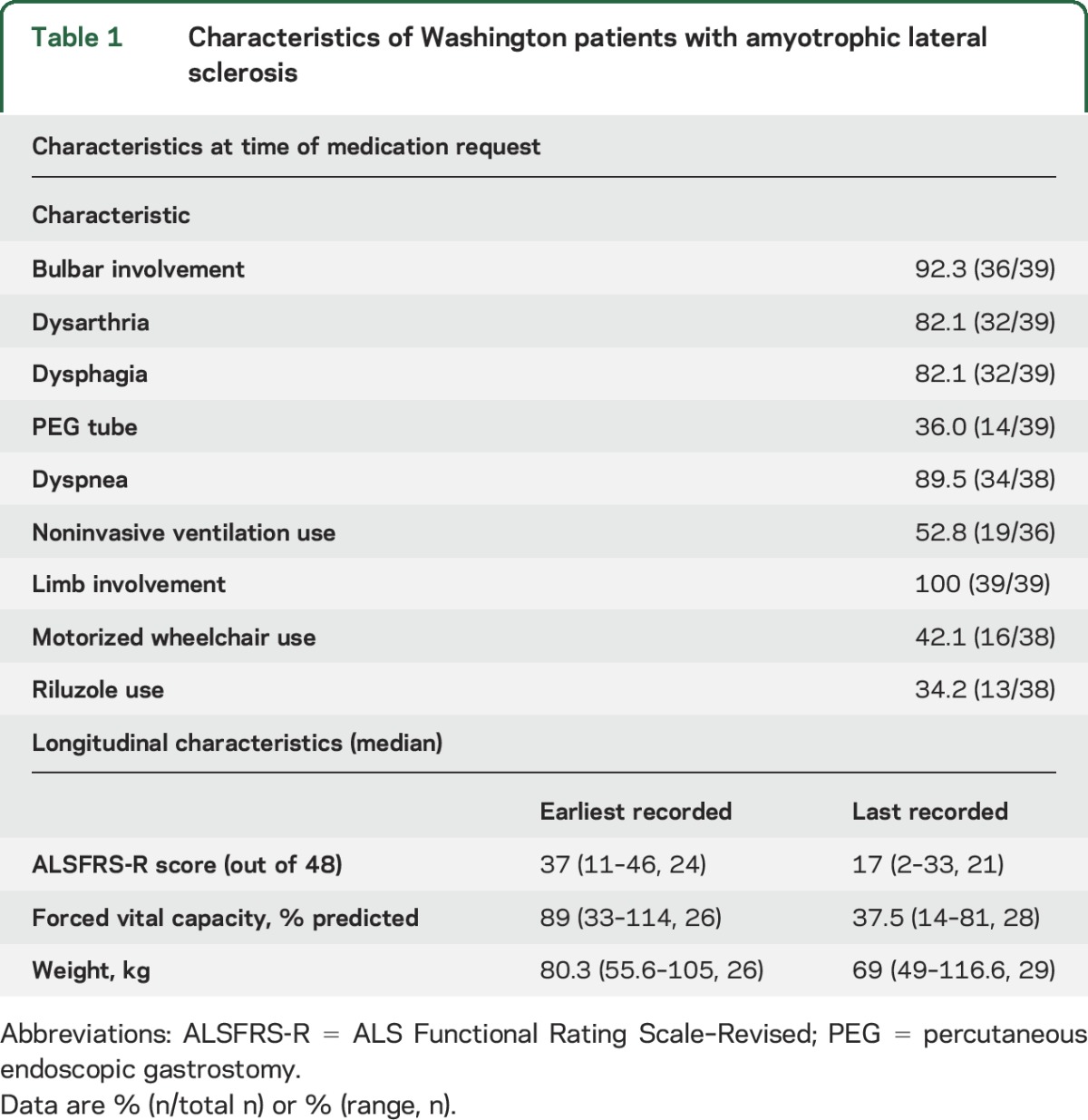
The median age at death was 65 years (range 46–86). Bulbar onset occurred in 30.7% (12/39). By revised El Escorial criteria, 79.5% had definite ALS on presentation.12 The median duration of disease before the request for lethal medication was 712 days (range 207–2,407).
By the time patients requested DWD medications (table 1), 92.3% had developed bulbar involvement: dysarthria (in 32/39) and/or dysphagia (in 32/39). However, only 36.0% of patients had undergone gastrostomy tube placement. Most of the patients (89.5%) had dyspnea and about half (52.8%) used noninvasive ventilation. At the time of the DWD request, the median percentage of the FVC was 37.5% with 26 of 28 patients demonstrating an FVC ≤50% (table 1). All patients had limb involvement, with a significant percentage (42.1%) requiring the use of a motorized wheelchair and showing significant functional impairment from disease progression as indicated by the decline in their score on the ALS Functional Rating Scale–Revised. A significant percentage of patients did not specify a religious affiliation (56%, 14/25 patients who divulged this information on a clinic intake form.) Two of the 39 patients moved to Washington before making the DWD request.
Table 1.
Characteristics of Washington patients with amyotrophic lateral sclerosis
Comparison to all-cause Oregon and Washington State DWD participants.
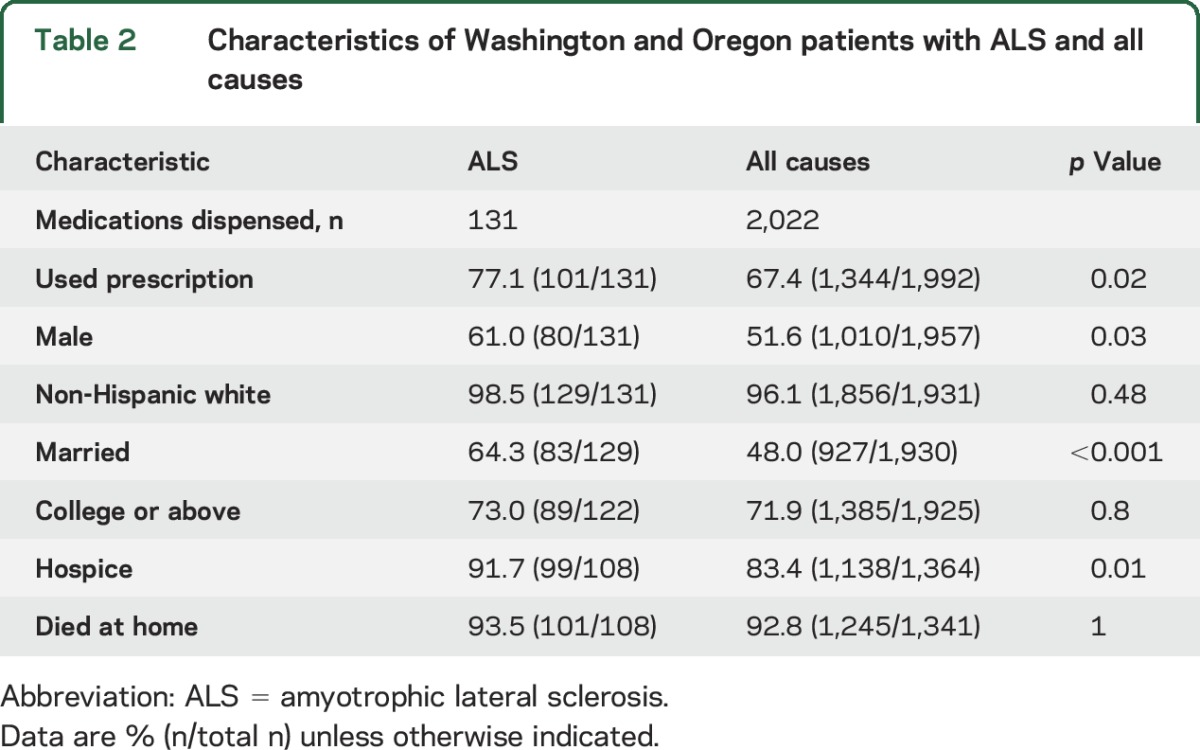
In the 92 Oregon patients with ALS who sought DWD, the median age at death was 67 years (range 34–86). The median age and range of ages at death of the combined ALS cohort was younger compared to the all-cause DWD participants (figure). Compared to the all-cause DWD cohort, there was a statistically significant increase in the proportion of patients with ALS who were male, married, and enrolled in hospice care (table 2). Almost all the ALS and all-cause DWD cohorts were non-Hispanic white and college educated who died at home. Only one patient with ALS (2.6%) in our cohort and 2 (2.7%) in the Oregon series required psychiatric consultation. This is slightly less than the 4.9% reported for the all-cause cohort.
Figure. Age distribution of patients with ALS and all-cause Death with Dignity patients in Oregon and Washington.
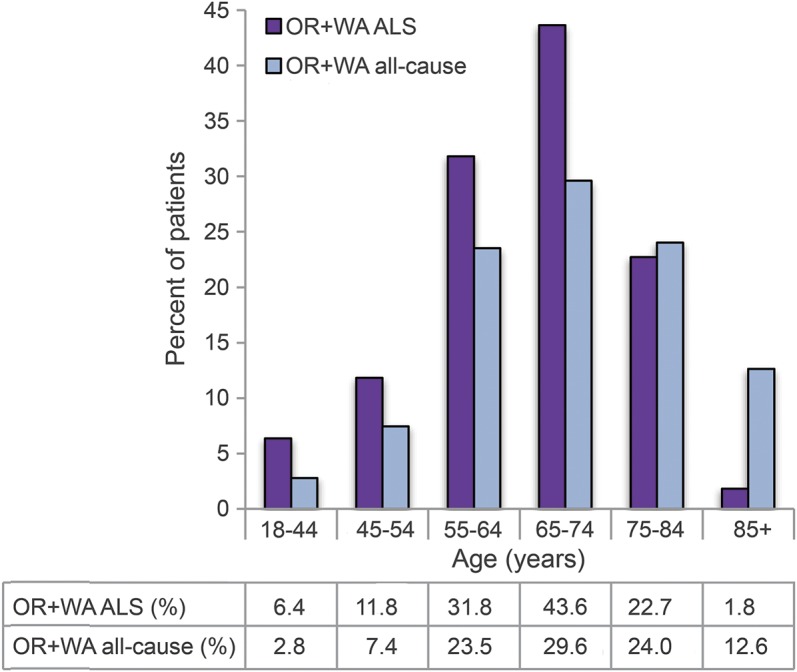
ALS = amyotrophic lateral sclerosis; OR = Oregon; WA = Washington.
Table 2.
Characteristics of Washington and Oregon patients with ALS and all causes
Motivators for participation in DWD.
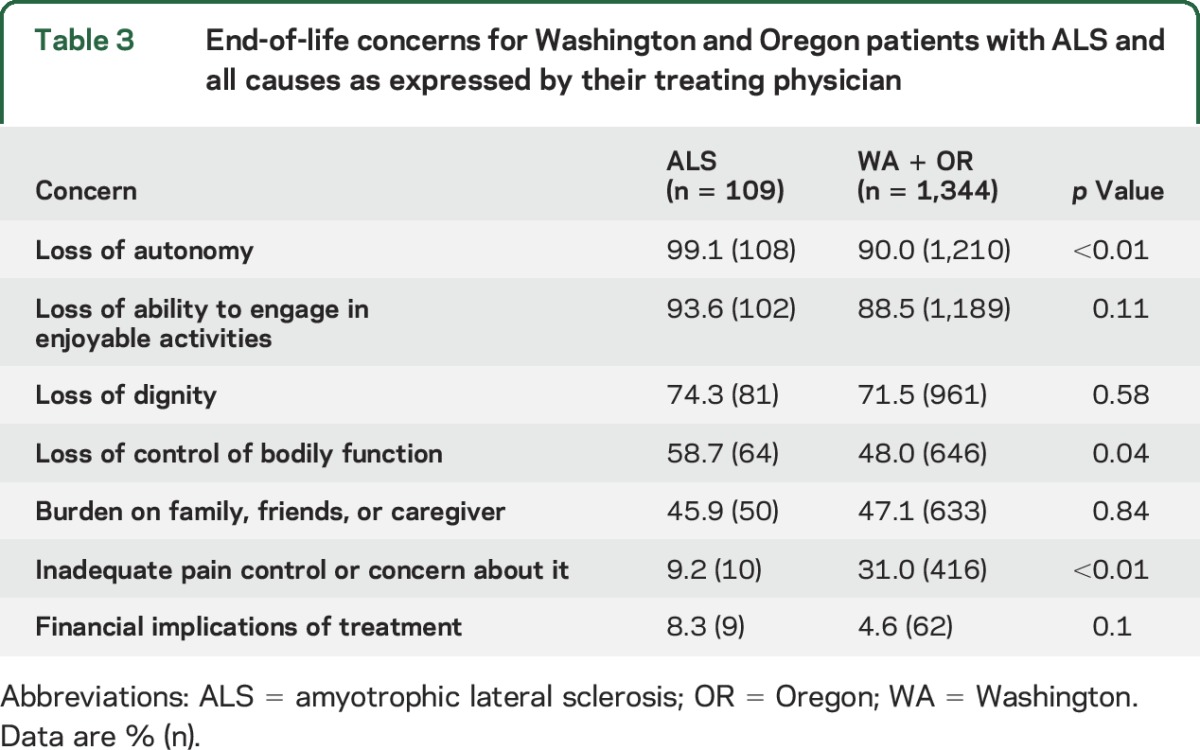
Based on the reporting forms in the states of Oregon and Washington, the physicians involved with the DWD process for their patients with ALS believed the primary motivation for participation to be loss of autonomy, ability to participate in enjoyable activities, and dignity (table 3). Physicians who prescribed DWD medications thought that their patients with ALS were more concerned about the loss of autonomy compared to the all-cause cohort, which was statistically significant (p < 0.01). Physicians believed that inadequate pain control was more of a concern for the all-cause DWD cohort, which mainly comprised patients with cancer.
Table 3.
End-of-life concerns for Washington and Oregon patients with ALS and all causes as expressed by their treating physician
Patient experience with the DWD medication.
The median number of days between first oral request and death was 77 in Oregon and 88 in Washington (range 0–615 days); 92.6% died at home. The patients were prescribed pentobarbital (50.0%, 55/110 patients), secobarbital (46.4%, 51/110 patients), or a combination of chloral hydrate, morphine sulfate, and phenobarbital (4.6%, 4/110 patients). The median time between ingestion and unconsciousness was 4.5 minutes for Washington State and 4 minutes for Oregon (range 1–60 minutes) and median time between ingestion and death was 28 minutes for Washington State and 30 minutes for Oregon (range 4 minutes to 26 hours). All patients who ingested the medication died without regaining consciousness.
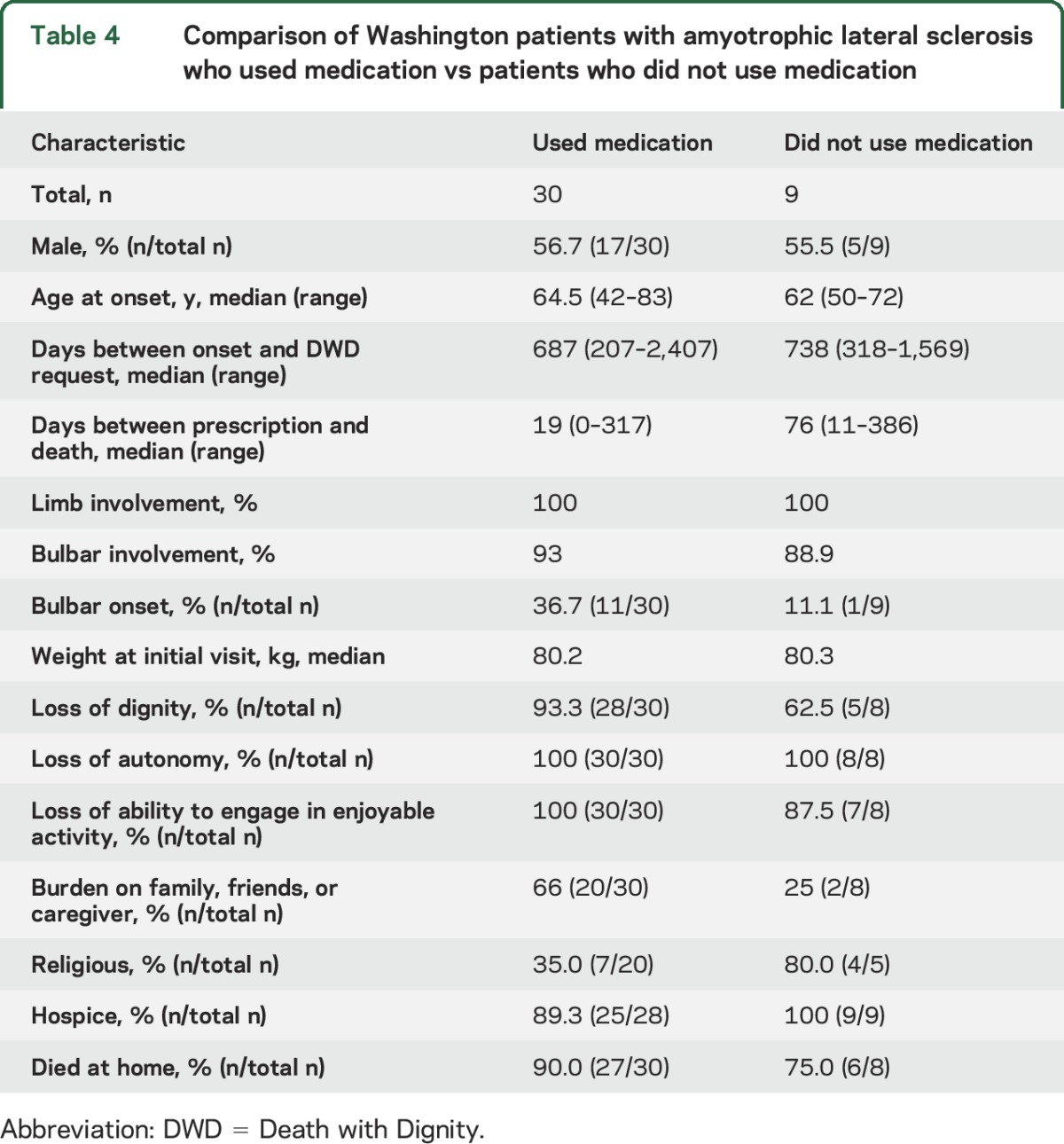
Comparison of Washington patients who used the medication vs those who did not.
In the combined Washington State and Oregon cohorts, 77.1% of patients used the medication. This percentage is statistically significantly higher than the all-cause Washington and Oregon cohort (66.0%). In Washington, all patients died, with 77.0% (30/39) using the DWD medication. The 9 patients who did not use the medication were similar to patients who used the medication in sex, age at onset, days between onset and terminal diagnosis, days between prescription and death, and limb or bulbar involvement (table 4). The cohort that used medication was more concerned about being a burden on family, friends, or caregiver (p = 0.0498). More patients in the cohort that used medication had bulbar-onset ALS and did not identify with a religious affiliation; however, these differences were not statistically significant.
Table 4.
Comparison of Washington patients with amyotrophic lateral sclerosis who used medication vs patients who did not use medication
DISCUSSION
Our cohort of 131 patients with ALS represents a small subset of DWD patients in the 2 states. We estimate the frequency of patients with ALS who sought DWD to be 3.4% to 6.7%. This estimate may not be accurate because it is based on death certificates filed with the states. The estimated frequency may be slightly increased compared to the first decade following enactment of the Oregon law (1998–2007) when 2.7% of patients with ALS sought DWD. While the total number of patients remains small, ALS is the disease with the highest percentage of patients seeking DWD. This is similar to the findings in the Netherlands, which also found that the rate of euthanasia and physician-assisted suicide was higher in patients with ALS relative to cancer patients.16,17
Similar to the all-cause DWD population and a recent study at the Seattle Cancer Care Alliance (SCCA) of patients with terminal cancer,18 patients with ALS also tended to be non-Hispanic white and well educated. However, subtle differences reflective of the distinct characteristics of ALS were evident. The ALS DWD cohort was younger and more likely to be male. More patients were married compared to DWD patients in the SCCA (55%) and in the all-cause DWD cohort (48.1%). This may reflect the younger ALS cohort that is presumably less likely to be widowed. Although higher than is generally reported in ALS, the proportion of patients with bulbar onset was also similar to the percentage in the Netherlands cohorts.16 We speculate that the greater number of patients with bulbar onset in our cohort may have been more motivated to participate in DWD than other patients because of their loss of verbal communication and ability to articulate ideas, especially in light of the greater number of well-educated patients who took part.
While many motivators for DWD were similar between the ALS and all-cause cohorts, patients with ALS were more significantly concerned about the loss of autonomy and control of bodily functions. Fewer patients with ALS were concerned about inadequate control of pain compared to the all-cause cohort composed of mainly patients with cancer. A higher percentage of patients with ALS were prescribed the DWD medication compared to the all-cause cohort and the SCCA cohort.18
A significant number of the ALS cohort did not take the medication, possibly using the prescription as a safeguard against continued loss of autonomy. Compared to the 9 patients in the Washington cohort who did not take the medication and died naturally of the disease, more patients who used the medication had bulbar onset, were more concerned about being a burden on family, caregiver, and friends, and were less religious, consistent with previous studies in patients with ALS reporting their wish to participate in either euthanasia or assisted suicide in both Europe19 and the United States.20
Despite the best efforts of experienced ALS clinicians, predicting a 6-month survival for patients with ALS is challenging. The largest number of days between first oral request and death was 615 days. Six of the 39 Washington patients who died survived 6 months after receiving their prescription. However, both state laws only require that 6 months’ prognosis be arrived by “reasonable medical judgment.”1,2
Physicians involved in implementing DWD for their patients with ALS must rule out situational depression or frontotemporal dementia.21,22 In our series, no participants were found by their prescribing or consulting physician to have clinical depression affecting their competency. Competency, as defined by Washington State law, is the ability to make and communicate “an informed decision”2 to “request and obtain a prescription for medication that the qualified patient may self-administer to end his or her life in a humane and dignified manner, that is based on an appreciation of the relevant facts and after being fully informed….”2 The physicians in our study were comfortable making this determination and therefore psychiatric consultation was seldom used. Only one patient with ALS at our 3 institutions was found to have frontotemporal dementia clinically and was denied participation.
In the state of Washington, physicians caring for patients with ALS who seek DWD must also assess whether the patient can “self-administer” the medication. Because a significant portion of our Washington ALS cohort had upper extremity weakness and/or dysphagia, this requirement may appear to be hard to fulfill. However, according to the Washington law, “self-administer” is defined as “a qualified patient's act of ingesting medication to end his or her life in a humane and dignified manner.”2 Sometimes, our patients needed to compress a syringe to “self-administer” the medication into their mouth or gastronomy tube. This method allows for compliance with the law.
Our study was limited by its small size and retrospective nature. We also cannot account for patients with ALS from Washington State who sought care outside of our 3 tertiary care centers or sought the prescription from their primary care practitioner. Our study did not employ neuropsychologists or psychiatrists to assess formally for clinical depression or subtle cognitive changes, though we typically do screen if clinical suspicion is high. Another limitation is the lack of patient input as the motivating factors described are based solely on the impression of the attending physicians participating in the DWD process and may reflect their bias. A more comprehensive prospective longitudinal study, as has been performed in Europe,23 would be more illuminating.
Nonetheless, our study provides data on the largest documented US cohort with ALS seeking DWD. The DWD process appears to be well-regulated with multiple checks and safeguards. In our study, there were no dose failures or complications. The few patients with ALS who chose DWD were generally well-educated, had access to palliative and life-prolonging care, and sought autonomy in deciding the terms and timing of the end of their life. Patients who eventually took the medication, as compared to those who were given the medication but chose not to use it, took less comfort in religion and had greater concerns about being a burden to their families and friends.
ACKNOWLEDGMENT
The authors thank Vivian Siu and the Oregon Center for Health Statistics for help in obtaining statistics of Oregon ALS DWD patients. The authors are deeply appreciative of the help of Tracie Granger and Caryl Tongco in obtaining study approval. The authors appreciate biostatistical advice from Katherine Tan, Brenda Price, and Kevin Cain. Finally, they are grateful to Drs. Claire Creutzfeldt, Elizabeth Loggers, Will Longstreth, Zachary Simmons, Robert Wood, and S. Elizabeth Zauber for reviewing and editing the manuscript.
GLOSSARY
- ALS
amyotrophic lateral sclerosis
- DWD
Death with Dignity
- FVC
forced vital capacity
- SCCA
Seattle Cancer Care Alliance
- UWMC
University of Washington Medical Center
- VMMC
Virginia Mason Medical Center
AUTHOR CONTRIBUTIONS
Dr. Wang: design and conceptualization, data acquisition and statistical analysis, drafting, revising, and final submission of manuscript. Dr. Elliott: design and conceptualization, data acquisition, and revising of manuscript. Dr. Jung Henson: design and conceptualization, data acquisition, and revising of manuscript. Dr. Gerena-Maldonado: data acquisition, and revising of manuscript. Ms. Strom: data acquisition. Ms. Downing: data acquisition. Ms. Vetrovs: data acquisition. Ms. Kayihan: data acquisition. Ms. Paul: data acquisition. Ms. Kennedy: data acquisition. Dr. Benditt: revising of manuscript. Dr. Weiss: design and conceptualization, data acquisition, drafting and revising of manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Oregon State Legislature. ORS 127.800-995; 1997. [Google Scholar]
- 2.State of Washington. The Washington Death with Dignity Act, Initiative 1000; 2008. [Google Scholar]
- 3.Washington State Department of Health. Washington State Attending Physician's After Death Reporting Form; 2009. [Google Scholar]
- 4.Washington State Department of Health. Washington State Department of Health 2009 Death with Dignity Act Report; 2010. [Google Scholar]
- 5.Washington State Department of Health. Washington State Department of Health 2010 Death with Dignity Act Report; 2011. [Google Scholar]
- 6.Washington State Department of Health. Washington State Department of Health 2011 Death with Dignity Act Report; 2012. [Google Scholar]
- 7.Washington State Department of Health. Washington State Department of Health 2012 Death with Dignity Act Report; 2013. [Google Scholar]
- 8.Washington State Department of Health. Washington State Department of Health 2013 Death with Dignity Act Report; 2014. [Google Scholar]
- 9.Washington State Department of Health. Washington State Department of Health 2014 Death with Dignity Act Report; 2015. [Google Scholar]
- 10.Oregon Public Health Division. Oregon's Death with Dignity Act—2014; 2015. [Google Scholar]
- 11.Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 1999;169:13–21. [DOI] [PubMed] [Google Scholar]
- 12.Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 13.Washington State Department of Health. Table C4: crude rates for selected causes by sex for residents [online]. Available at: http://www.doh.wa.gov/portals/1/Documents/5400/DeathC42014.xls. Accessed September 2015.
- 14.Oregon Health Authority. Oregon vital statistics annual reports: volume 2 [online]. Available at: https://public.health.oregon.gov/BirthDeathCertificates/VitalStatistics/annualreports/Pages/index.aspx. Accessed September 2015.
- 15.Hedberg K, Hopkins D, Leman R, Kohn M. The 10-year experience of Oregon's death with dignity act: 1998–2007. J Clin Ethics 2009;20:124–132. [PubMed] [Google Scholar]
- 16.Maessen M, Veldink JH, Onwuteaka-Philipsen BD, et al. Trends and determinants of end-of-life practices in ALS in the Netherlands. Neurology 2009;73:954–961. [DOI] [PubMed] [Google Scholar]
- 17.Veldink JH, Wokke JH, van der Wal G, Vianney de Jong JM, van den Berg LH. Euthanasia and physician-assisted suicide among patients with amyotrophic lateral sclerosis in the Netherlands. N Engl J Med 2002;346:1638–1644. [DOI] [PubMed] [Google Scholar]
- 18.Loggers ET, Starks H, Shannon-Dudley M, Back AL, Appelbaum FR, Stewart FM. Implementing a Death with Dignity program at a comprehensive cancer center. N Engl J Med 2013;368:1417–1424. [DOI] [PubMed] [Google Scholar]
- 19.Stutzki R, Schneider U, Reiter-Theil S, Weber M. Attitudes toward assisted suicide, life-prolonging measures in Swiss ALS patients and their caregivers. Front Psychol 2012;3:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganzini L, Silveira MJ, Johnston WS. Predictors and correlates of interest in assisted suicide in the final month of life among ALS patients in Oregon and Washington. J Pain Symptom Manage 2002;24:312–317. [DOI] [PubMed] [Google Scholar]
- 21.Lipton AM, White CL III, Bigio EH. Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathol 2004;108:379–385. [DOI] [PubMed] [Google Scholar]
- 22.Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller B. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology 2003;60:1094–1097. [DOI] [PubMed] [Google Scholar]
- 23.Maessen M, Veldink JH, Onwuteaka-Philipsen BD, et al. Euthanasia and physician-assisted suicide in amyotrophic lateral sclerosis: a prospective study. J Neurol 2014;261:1894–1901. [DOI] [PubMed] [Google Scholar]


