Abstract
Objective:
To assess the efficacy of a progressive aerobic exercise training program on cognitive and everyday function among adults with mild subcortical ischemic vascular cognitive impairment (SIVCI).
Methods:
This was a proof-of-concept single-blind randomized controlled trial comparing a 6-month, thrice-weekly, progressive aerobic exercise training program (AT) with usual care plus education on cognitive and everyday function with a follow-up assessment 6 months after the formal cessation of aerobic exercise training. Primary outcomes assessed were general cognitive function (Alzheimer's Disease Assessment Scale–Cognitive subscale [ADAS-Cog]), executive functions (Executive Interview [EXIT-25]), and activities of daily living (Alzheimer's Disease Cooperative Study–Activities of Daily Living [ADCS-ADL]).
Results:
Seventy adults randomized to aerobic exercise training or usual care were included in intention-to-treat analyses (mean age 74 years, 51% female, n = 35 per group). At the end of the intervention, the aerobic exercise training group had significantly improved ADAS-Cog performance compared with the usual care plus education group (−1.71 point difference, 95% confidence interval [CI] −3.15 to −0.26, p = 0.02); however, this difference was not significant at the 6-month follow-up (−0.63 point difference, 95% CI −2.34 to 1.07, p = 0.46). There were no significant between-group differences at intervention completion and at the 6-month follow-up in EXIT-25 or ADCS-ADL performance. Examination of secondary measures showed between-group differences at intervention completion favoring the AT group in 6-minute walk distance (30.35 meter difference, 95% CI 5.82 to 54.86, p = 0.02) and in diastolic blood pressure (−6.89 mm Hg difference, 95% CI −12.52 to −1.26, p = 0.02).
Conclusions:
This study provides preliminary evidence for the efficacy of 6 months of thrice-weekly progressive aerobic training in community-dwelling adults with mild SIVCI, relative to usual care plus education.
ClinicalTrials.gov identifier:
Classification of evidence:
This study provides Class II evidence that for adults with mild SIVCI, an aerobic exercise program for 6 months results in a small, significant improvement in ADAS-Cog performance.
Vascular cognitive impairment (VCI) is the second most common cause of dementia after Alzheimer disease (AD).1 Cerebral small vessel disease plays a critical role in covert ischemia and the development of sub-cortical ischemic vascular cognitive impairment (SIVCI),2 the most common form of VCI. SIVCI is defined by the presence of white matter lesions (WMLs) and lacunar infarcts, and has the clinical consequence of increased dementia risk.3,4 Aerobic exercise might delay the progression of SIVCI by modifying cardiovascular and metabolic risk factors.5–7 A previous observational study8 demonstrated an association between physical activity and a lower risk of mild SIVCI. However, no clinical trial has utilized aerobic exercise to augment cognitive function in individuals with mild SIVCI. Thus, we conducted a proof-of-concept single-blind randomized controlled trial designed to provide preliminary evidence of efficacy of a 6-month, thrice-weekly, progressive aerobic exercise training program on cognitive and everyday function among older adults with mild SIVCI. In addition, we assessed the feasibility of delivering the exercise training program in this population by examining rates of recruitment, intervention compliance, and observed withdrawal rates over the 6-month intervention period.
METHODS
Design and setting.
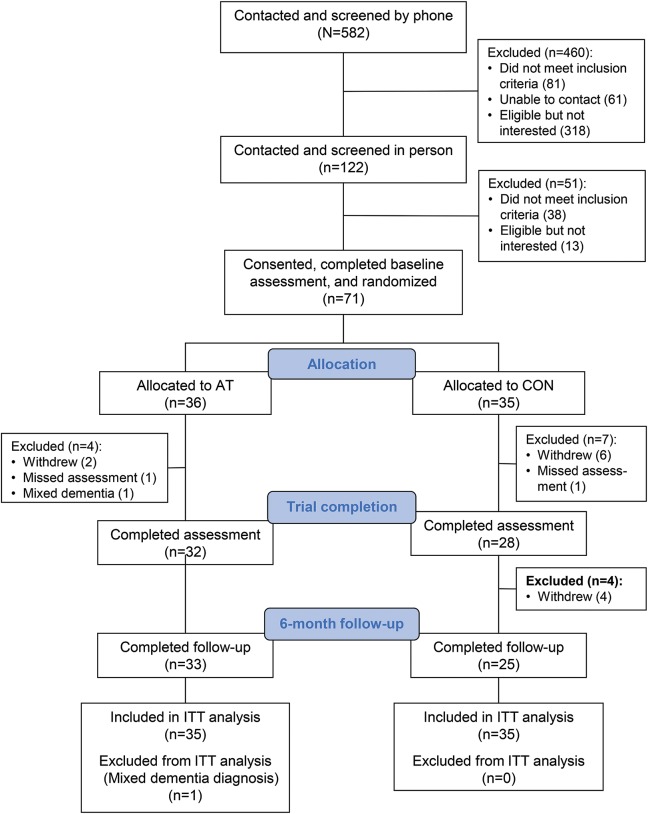
The study involved 2 phases: a 6-month intervention phase followed by a 6-month follow-up phase. Measurements were made at 3 times: baseline, end of the intervention period (6 months postrandomization), and 6-month follow-up (12 months postrandomization; figure). The assessors were blinded to the participants' assignments. Data were collected at a research laboratory on the Vancouver General Hospital campus.
Figure. CONSORT flow diagram for the randomized controlled trial.
AT = aerobic training; CON = usual care plus education; ITT = intent-to-treat analysis.
This study was designed to address whether, among individuals with SIVCI, 6 months of thrice-weekly, progressive aerobic training (AT) can improve (1) cognitive performance as indexed by the Alzheimer's Disease Assessment Scale–Cognitive subscale (ADAS-Cog); (2) global executive function as indexed by the Executive Interview (EXIT-25); and (3) performance of activities of daily living as indexed by the Alzheimer's Disease Cooperative Study–Activities of Daily Living (ADCS-ADL) scale.
This study provides Class II evidence that AT has short-term benefits on ADAS-Cog performance because more than 2 primary outcomes were specified a priori.
Standard protocol approvals, registrations, and patient consents.
Ethical approval was provided by the University of British Columbia's Clinical Research Ethics Board (H07-01160). The trial was registered at ClinicalTrials.gov (NCT01027858). The trial protocol has been published previously.9 Participants provided written informed consent.
Participants.
The sample consisted of adults with a clinical diagnosis of mild SIVCI. We recruited from the University of British Columbia Hospital Clinic for Alzheimer’s Disease and Related Disorders, the Vancouver General Hospital Stroke Prevention Clinic, and specialized geriatric clinics in metro Vancouver, Canada. Clinical diagnosis of VCI was made based on the presence of both small vessel ischemic disease and cognitive syndrome.10 Small vessel ischemic disease was defined as evidence of relevant cerebrovascular disease by brain CT or MRI defined as the presence of both (1) periventricular and deep WMLs and (2) absence of cortical and or cortico-subcortical nonlacunar territorial infarcts and watershed infarcts, hemorrhages indicating large vessel disease, signs of normal-pressure hydrocephalus, or other specific causes of WMLs (i.e., multiple sclerosis, leukodystrophies, sarcoidosis, brain irradiation). In addition to the neuroimaging evidence, the presence or a history of neurologic signs such as Babinski sign, sensory deficit, gait disorder, or extrapyramidal signs consistent with subcortical brain lesions was required and confirmed by study physicians (G.-Y.R.H. and P.E.L.). Cognitive syndrome was defined as a baseline Montreal Cognitive Assessment (MoCA)11 score <26/30 and a Mini-Mental State Examination (MMSE)12 score of ≥20 at screening. Progressive cognitive decline was confirmed through medical records or caregiver/family member interviews. For additional details on inclusion and exclusion criteria, see the e-Methods at Neurology.org.
Sample size.
No previous randomized trial has examined the effect of aerobic exercise on cognitive function in mild SIVCI. The required sample size for this study was calculated based on predictions of 6-month changes on the ADAS-Cog. An exercise trial among community-dwelling adults with ADAS-Cog as the primary outcome measure demonstrated an effect size of 0.60.13 Thus, assuming a one-sided α = 0.05, 30 participants per group would provide 75% power to detect this effect size. We recruited 35 participants per group to accommodate a conservative 15% dropout rate.
Descriptors.
At baseline, participants underwent a clinical assessment with study physicians (G.-Y.R.H. and P.E.L.) to confirm current health status and eligibility for the study. Age in years and education level were assessed by self-report. Standing height was measured as stretch stature to the 0.1 centimeter per standard protocol. Weight was measured twice to the 0.1 kilogram on a calibrated digital scale. Waist-to-hip ratio was determined by measuring the widest part of the hip circumference and the waist just above the navel in centimeters. We used the Functional Comorbidity Index14 to assess the number of comorbid conditions related to physical functioning. General mobility was assessed by the Short Physical Performance Battery15 and by the Timed Up-and-Go Test.16 Total WML volume was quantified for a subset (n = 38) who underwent structural 3T MRI at baseline.
Primary outcome measures.
Although the study contained 3 prespecified primary outcomes, the study was powered based on expected changes in ADAS-Cog. We determined a priori that observing a statistically significant difference (2-tailed p < 0.05) on any of the 3 outcomes at the end of the intervention would be considered preliminary evidence of efficacy of AT in this population.9 The ADAS-Cog is an 11-item assessment of memory, language, and praxis; scores range from 0 to 70, with higher scores indicating greater cognitive impairment.17 The ADAS-Cog has acceptable reliability and validity and responsiveness to treatment effects.18 The EXIT-25 assesses global executive function over 25 items; scores range from 0 to 50, with higher scores indicating greater executive dysfunction. Scores ≥15 are indicative of clinically significant executive dysfunction.19 The ADCS-ADL is a 23-item informant-rated questionnaire that measures an individual's performance of activities of daily living.20 Scores range from 0 to 78, with higher scores indicating better performance.
Secondary outcome measures.
Executive functions.
Three aspects of executive functions (EF) were assessed. Response inhibition was assessed with the Stroop Test (incongruent minus congruent trials).21 Set shifting was assessed with the Trail-Making Tests (part B minus part A).22 Working memory was assessed with verbal digit span tests (digits forward minus digits backwards).23 Details on these tests are shown in the e-Methods.
General cardiovascular capacity, physical activity level, and physiologic markers.
We used the 6-Minute Walk Test (6MWT)24 to assess general cardiovascular capacity. Meters walked in 6 minutes was recorded. Current level of physical activity was determined by the Physical Activities Scale for the Elderly questionnaire.25 Body mass index was calculated using the formula mass (kg)/height (m2). Resting systolic and diastolic blood pressure and heart rate were taken using an automated device (OMRON M2 HEM-7121-E, Kyoto, Japan). The mean of 2 recordings, taken 5 minutes apart, is reported.
Randomization.
The randomization sequence was generated using the web application randomization.com with a ratio of 1:1 to AT or usual care plus education. A research team member not involved with the study held this sequence at a remote location. After the completion of consent and baseline testing, the research coordinator contacted the team member holding the list to determine the next allocation.
Experimental groups.
AT group.
All AT group classes were led by instructors certified to instruct seniors. Class attendance was recorded for each participant by the instructors throughout the 6-month intervention. Each 60-minute class included a 10-minute warm-up, a 40-minute walk, and a 10-minute cool down. Walking occurred outdoors and followed predetermined routes around local areas. The intensity of the AT program was monitored and progressed using 3 approaches: (1) heart rate monitoring with an initial intensity of 40% of age-specific target heart rate (i.e., heart rate reserve [HRR]). HRR was calculated by subtracting resting heart rate from maximum heart rate (using the formula 206.9 − 0.67 × age26) and was recalculated each month. Participants progressed over the first 12 weeks to the range of 60%–70% of HRR. Once the target HRR of 65% was achieved, it was sustained for the remainder of the intervention period; (2) subjective monitoring using the Borg Rating of Perceived Exertion (RPE)27 with a target RPE of 14–15; and (3) the “talk” test,28 starting at a walking pace allowing comfortable conversation and progressing to a walking pace where conversation was difficult. For participants taking β-blocker medication (7 in each group; table 1), subjective measures of intensity were prioritized.
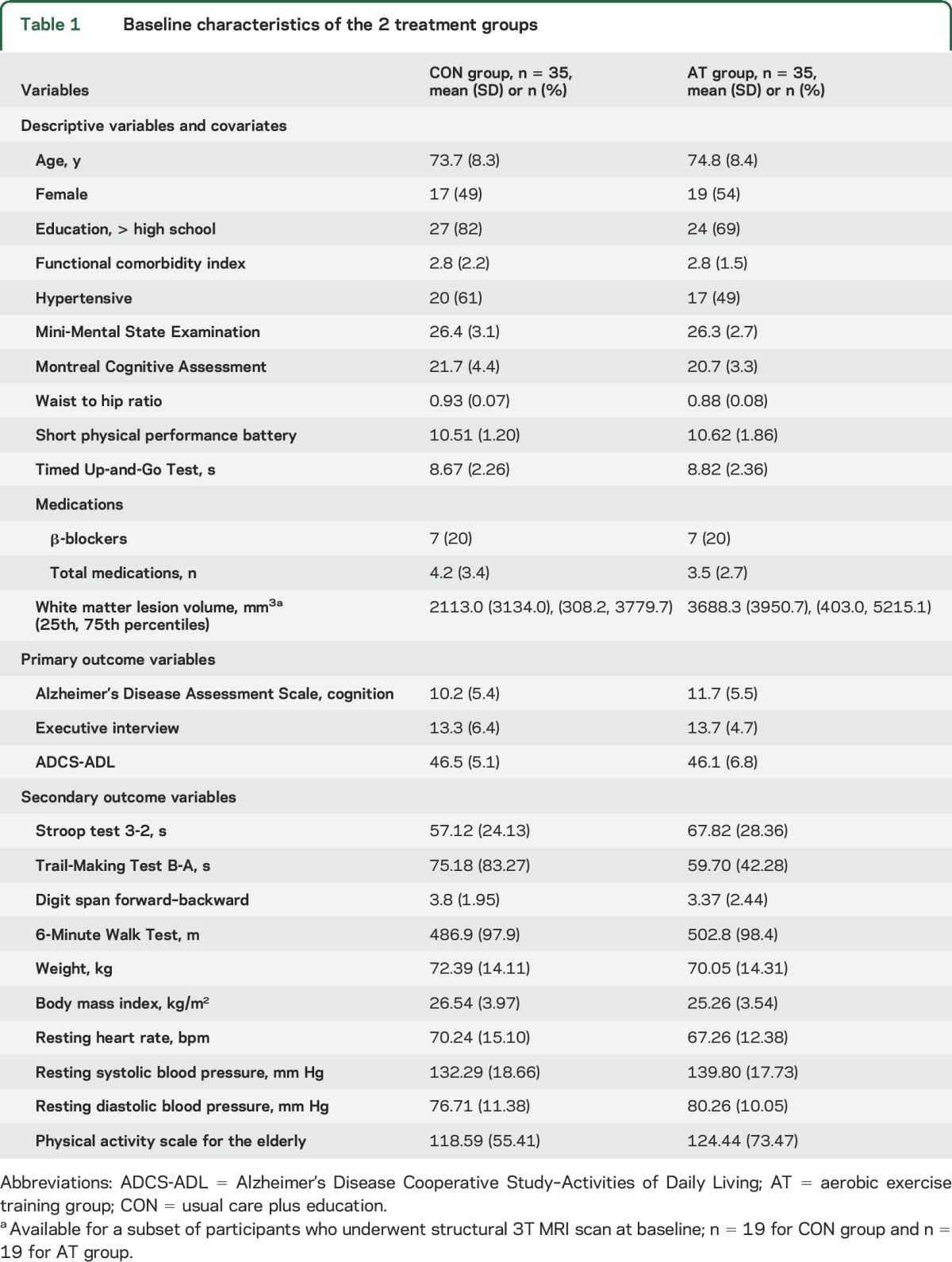
Table 1.
Baseline characteristics of the 2 treatment groups
The AT group was also given a pedometer to serve as both an incentive and monitoring tool. Participants recorded the number of steps each day taken outside the AT classes on standard logs provided by the research team. Table e-1 provides descriptive information on the number of steps recorded across the intervention period.
Usual care plus education group.
Participants in the usual care plus education (CON) group received usual care as well as monthly educational materials about VCI and healthy diet. However, no specific information regarding physical activity was provided. In addition, research staff phoned the CON participants on a monthly basis to maintain contact and to acquire research data.
Feasibility.
To assess feasibility, we calculated the recruitment rate, intervention compliance (determined by percentage of the total AT classes attended), and the withdrawal rate (determined from active requests to withdraw from the study) over the 6-month intervention period. The AT intervention was deemed feasible if the following 3 conditions were achieved: (1) a recruitment rate >15%; (2) a withdrawal rate <15%; and (3) an average compliance of 60% for the AT classes.
Adverse events monitoring.
All participants were instructed to report any adverse effects due to the AT exercises to our research coordinator, such as falls or musculoskeletal pain persisting longer than 48 hours. Participants were also questioned about the presence of any adverse effects, such as musculoskeletal pain or discomfort, at each exercise session. All instructors also monitored participants for symptoms of angina and shortness of breath during the exercise classes. External experts from our safety monitoring committee reviewed all adverse events reported on a monthly basis.
Statistical analysis.
Primary analyses were conducted in the statistical package SPSS 22.0 (IBM Corporation, Armonk, NY) and evaluated between-group differences (AT vs CON) on the outcomes of interest using mixed models. Time was considered as a repeated, categorical variable and was included as a fixed effect in addition to group, group-by-time interaction, and baseline MoCA score. The intercept was specified as a random effect. The covariance matrix was specified as first-order autoregressive to account for correlations among within-subject residuals across time points. By including group and baseline MoCA score in all models, it was assumed that data were missing at random (See table e-2 for information on missing data). Analyses were based on intention-to-treat, restricted maximum likelihood estimation using all available data from the randomized participants (n = 70). For the primary outcome measures, complete case analyses were also conducted, including only those participants who completed the 6-month follow-up assessment (n = 58). Furthermore, per protocol analyses were conducted, in which only AT participants with ≥60% class attendance were included (27 out of the 35 randomized). These analyses replicated the intention-to-treat analyses (table e-3).
A final set of analyses determined whether changes in ADAS-Cog performance correlated with changes in other outcomes, independent of treatment group and baseline MoCA score. These correlation analyses were limited to variables that showed a significant between-group difference over time.
RESULTS
Seventy-one individuals were recruited between December 2009 and April 2014. The final follow-up measurements were made in April 2015. After randomization, one AT group participant was deemed ineligible due to the diagnosis of mixed dementia and was excluded from all analyses (figure). Table 1 provides the baseline descriptive characteristics of the 70 participants.
Primary outcome measures.
Table 2 shows the covariate-adjusted change from baseline to 6 months (end of intervention) and from baseline to 12 months (end of 6-month follow-up) for the 3 primary outcome variables, as well as the adjusted between-group difference scores obtained from the analyses. We focus on the intention-to-treat results and note any differences when using complete case analysis.
Table 2.
Estimated mean change in primary outcome variables from baseline
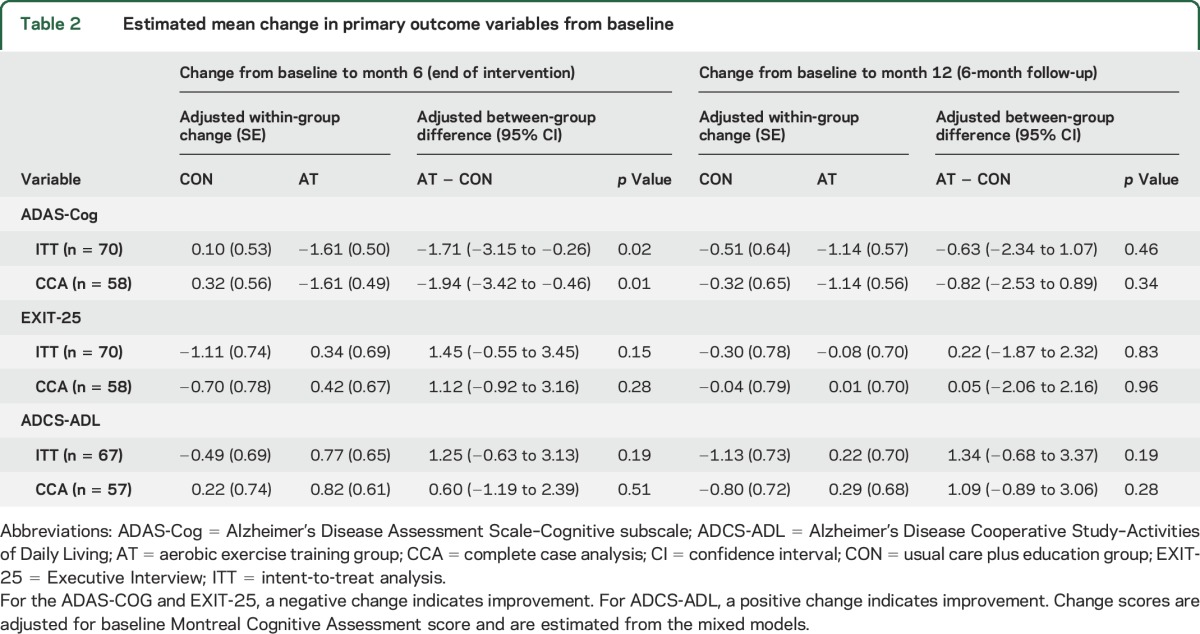
There was a small, statistically significant difference in change in ADAS-Cog performance at 6 months; this difference decreased by 12 months and was no longer statistically significant. Small between-group differences were also observed for EXIT-25 and ADCS-ADL at 6 months and 12 months but none were statistically significant (table 2). Complete case analysis produced similar results to those described above.
Secondary outcome measures.
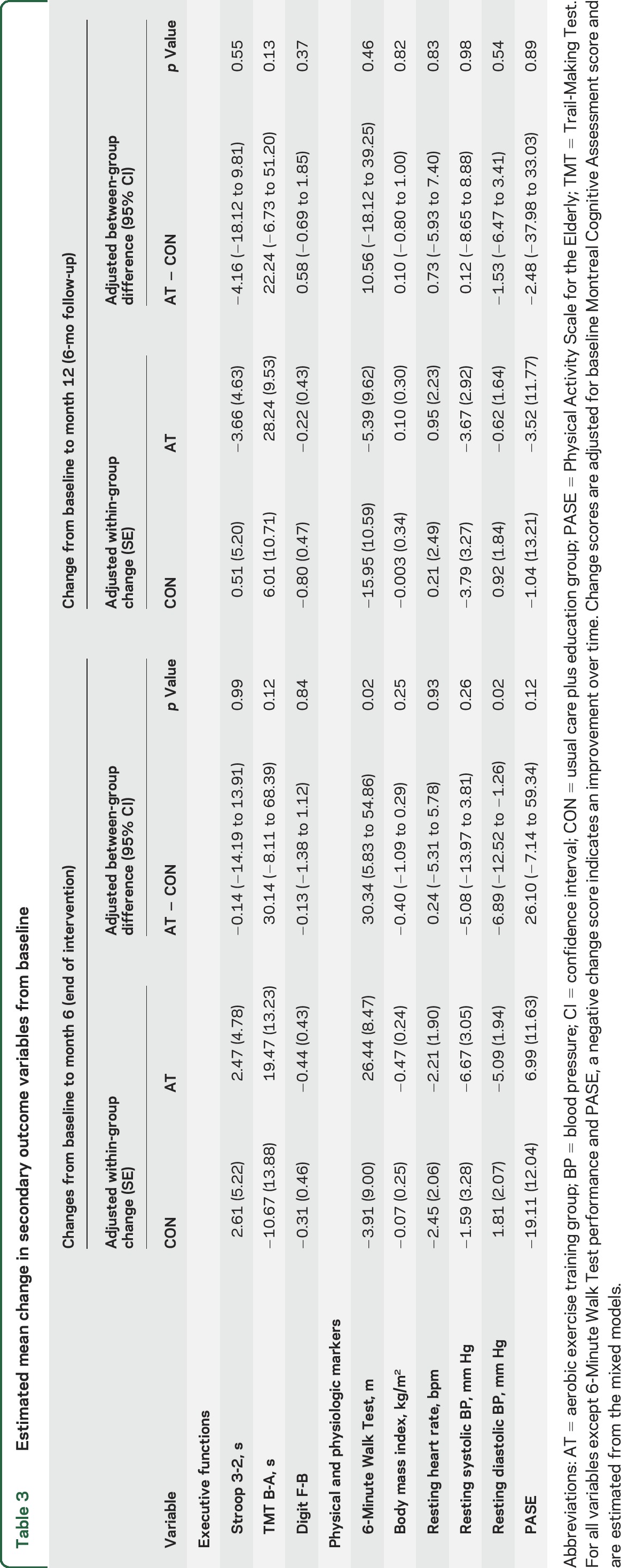
Table 3 provides the estimated between-group and within-group differences over time in the secondary outcomes. There were significant between-group differences in 6MWT and in diastolic blood pressure favoring the AT group. Both effects did not persist at the 6-month follow-up. There were no between-group differences in the 3 specific EF measures or in other secondary measures. During the 6-month intervention period, improvements in ADAS-Cog correlated with reduced resting diastolic blood pressure (partial r = 0.32, p = 0.01) but not with improvements in 6WMT distance (partial r = −0.17, p = 0.22).
Table 3.
Estimated mean change in secondary outcome variables from baseline
Feasibility.
Based on the 440 participants who could be contacted and screened by telephone and deemed eligible, our observed recruitment rate was 16% (i.e., 71/440). Based on the 122 individuals who were screened in person, 84 were deemed eligible; our observed recruitment rate was 85% (i.e., 71/84; figure). Of the 70 participants who were randomized and included in our analyses, the withdrawal rate during the 6-month intervention period was 17% (i.e., 6/35) in the CON group, 3% (i.e., 1/35) in the AT intervention group, and 10% (i.e., 7/70) in the overall sample. One participant from each experimental group missed their trial completion assessment (figure). The average exercise compliance observed in the AT group was 68%.
Adverse events.
Two study-related adverse events were reported in the AT group and one in the CON group. All 3 were nonsyncopal falls. One of the falls in the AT group resulted in a broken tooth and required assessment in the Hospital Emergency Department; the remaining 2 did not result in injury.
DISCUSSION
This proof-of-concept randomized trial suggests engaging in AT may benefit cognitive function and reduce cardiovascular risk in community-dwelling adults with mild SIVCI, a population at risk for dementia and functional decline. We found a small difference in change in cognitive function, as measured by the ADAS-Cog at the end of the intervention, relative to usual care with education. We also found that AT significantly improved general cardiovascular capacity and reduced resting diastolic blood pressure. Further, our data suggest identification and recruitment of this population is feasible and a thrice-weekly AT can be delivered to this target population with an acceptable level of compliance.
The observed effects on ADAS-Cog aligns with our previous study,29 which demonstrated that 6 months of progressive aerobic exercise significantly improved memory (both verbal and spatial), but not EF, in women with probable mild cognitive impairment aged 70–80 years. Memory is a key cognitive domain assessed by ADAS-Cog and has been shown to be particularly impaired in persons with small subcortical strokes.30 In the current study, the difference of 1.71 points observed on the ADAS-Cog between AT and control groups falls within the range observed in previous pharmaceutical trials among individuals with vascular dementia or mild SIVCI (i.e., 1–2 points)31–33; however, the difference was less than that deemed to be the minimal clinically important difference (3 points).34
In contrast, AT did not produce improvements in EF. A previous study demonstrated that 6 months of AT significantly improved EF in older women with amnestic MCI35 and in older adults with glucose intolerance.36 There are differences in both the frequency and intensity of AT between the 2 studies (i.e., 3×/week and 60%–70% of HRR in our study vs 4×/week and 75%–85% HRR in the previous studies). In addition, on average, our study participants were older and had lower baseline MMSE scores. Finally, based on the average EXIT-25 scores at baseline, the study participants did not have substantial executive dysfunction (i.e., scores <15/50),19 which might have reduced sensitivity to improvement.
We found that improved ADAS-Cog performance was associated with reduced diastolic blood pressure. Hypertension is regarded as a key and modifiable risk factor for SIVCI37 and it has been suggested that the association between diastolic blood pressure and stroke risk is J-shaped among older adults, such that as diastolic blood pressure either increases or decreases from 71 mm Hg, stroke risk increases.38 Notably, we observed that AT reduced diastolic blood pressure from approximately 80 to 75 mm Hg, which might confer protection against future stroke. Our correlational finding also suggests that reduced blood pressure may be a pathway by which aerobic exercise promotes cognitive health among those with mild SIVICI. Nevertheless, the relationship between late-life blood pressure and cognitive function is still poorly understood.
There are limitations. The Vascular Dementia Assessment Scale cognitive subscale (VADAS-Cog) may be more sensitive to modest changes in cognition in comparison to the ADAS-Cog.18 Yet data supporting the use of the VADAS-Cog data were limited when the current study was begun. The number of participants who withdrew during the 6-month intervention was different between the 2 groups (2 vs 10); future studies will need to address this problem to maximize retention. Moreover, compared with those who completed the 6-month intervention, individuals who withdrew had lower baseline cognitive function, as measured by the MMSE, MoCA, and ADAS-Cog. Thus, our current results may not apply to those who were at a more severe stage of SIVCI. While this study used well-defined clinical criteria for SIVCI diagnosis that include neuroimaging,10 the clinical presentation may include mixed pathology such as concomitant AD.2 Significant between-group differences were only observed in 1 of the 3 primary outcomes (ADAS-Cog). Although this met our prespecified criteria for preliminary efficacy,9 future studies with larger sample sizes and application of biomarkers are needed to rigorously evaluate the role of aerobic exercise on cognitive function among older adults with mild SIVCI. Further, all positive effects of 6 months of AT dissipated after a 6-month follow-up period, which suggests that strategies to increase the sustainability of these effects are needed. This might entail a longer intervention period (e.g., 12 months) or the inclusion of behavioral components (e.g., self-monitoring or incentive schemes) to facilitate maintenance of this frequency and intensity of walking upon intervention cessation. Finally, the 16% recruitment rate observed in this study emphasizes potential feasibility challenges that may exist among future trials that include older adults with SIVCI.
Despite these limitations, our proof-of-concept study provides preliminary evidence that a 6-month program of thrice-weekly progressive AT promotes cognitive function and reduces cardiovascular risk in older adults with mild SIVCI.
Supplementary Material
GLOSSARY
- 6MWT
6-Minute Walk Test
- AD
Alzheimer disease
- ADCS-ADL
Alzheimer's Disease Cooperative Study–Activities of Daily Living
- ADAS-Cog
Alzheimer's Disease Assessment Scale–Cognitive subscale
- AT
aerobic training
- CON
usual care plus education
- EF
executive functions
- EXIT-25
Executive Interview
- HRR
heart rate reserve
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- RPE
Rating of Perceived Exertion
- SIVCI
subcortical ischemic vascular cognitive impairment
- VCI
vascular cognitive impairment
- VADAS-Cog
Vascular Dementia Assessment Scale cognitive subscale
- WML
white matter lesion
Footnotes
Supplemental data at Neurology.org
Editorial, page 2072
AUTHOR AFFILIATION
From the Department of Physical Therapy (T.L.-A., J.R.B., J.C.D., J.J.E., L.A.B.), Division of Geriatric Medicine (P.E.L.), and Division of Neurology (C.J., G.-Y.R.H.), University of British Columbia; Djavad Mowafaghian Centre for Brain Health (T.L.-A., J.R.B., J.C.D., L.A.B., G.-Y.R.H.), Centre for Hip Health and Mobility (T.L.-A., J.R.B., J.C.D., M.M., W.C.), and Centre for Clinical Epidemiology and Evaluation (P.M.B.), Vancouver Coastal Health Research Institute; and University of British Columbia Hospital Clinic for Alzheimer Disease and Related Disorders (P.E.L., C.J., G.-Y.R.H.), Vancouver, Canada.
AUTHOR CONTRIBUTIONS
Dr. Liu-Ambrose had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: T.L.-A., J.R.B., J.C.D., J.J.E., P.E.L., C.J., L.A.B., P.M.B., M.M., W.C., G.-Y.R.H. Acquisition, analysis, or interpretation of data: T.L.-A., J.R.B., J.C.D., J.J.E., P.M.B., M.M., W.C., G.-Y.R.H. Drafting of the manuscript: T.L.-A., J.R.B., J.C.D. Critical revision of the manuscript for important intellectual content: T.L.-A., J.R.B., J.C.D., J.J.E., P.E.L., C.J., L.A.B., P.M.B., M.M., W.C., G.-Y.R.H. Statistical analysis: T.L.-A., J.R.B., J.C.D., P.M.B. Obtained funding: T.L.-A., J.J.E., L.A.B., C.J., P.M.B., G.-Y.R.H. Administrative, technical, or material support: T.L.-A., J.C.D., G.-Y.R.H., P.E.L., M.M., W.C. Study supervision: T.L.-A., G.-Y.R.H.
STUDY FUNDING
Canadian Stroke Network and the Heart and Stroke Foundation of Canada. The funding source had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Rockwood K, Wentzel C, Hachinski V, Hogan DB, MacKnight C, McDowell I. Prevalence and outcomes of vascular cognitive impairment: Vascular Cognitive Impairment Investigators of the Canadian Study of Health and Aging. Neurology 2000;54:447–451. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol 2003;2:89–98. [DOI] [PubMed] [Google Scholar]
- 3.Vermeer SE, Longstreth WT Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007;6:611–619. [DOI] [PubMed] [Google Scholar]
- 4.Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol 2002;1:426–436. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JL, Slentz CA, Houmard JA, et al. Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention Through Defined Exercise). Am J Cardiol 2007;100:1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart KJ, Bacher AC, Turner K, et al. Exercise and risk factors associated with metabolic syndrome in older adults. Am J Prev Med 2005;28:9–18. [DOI] [PubMed] [Google Scholar]
- 7.LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation 2005;112:505–512. [DOI] [PubMed] [Google Scholar]
- 8.Middleton L, Kirkland S, Rockwood K. Prevention of CIND by physical activity: different impact on VCI-ND compared with MCI. J Neurol Sci 2008;269:80–84. [DOI] [PubMed] [Google Scholar]
- 9.Liu-Ambrose T, Eng JJ, Boyd LA, et al. Promotion of the mind through exercise (PROMoTE): a proof-of-concept randomized controlled trial of aerobic exercise training in older adults with vascular cognitive impairment. BMC Neurol 2010;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erkinjuntti T, Inzitari D, Pantoni L, et al. Research criteria for subcortical vascular dementia in clinical trials. J Neural Transm 2000;59:23–30. [DOI] [PubMed] [Google Scholar]
- 11.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 13.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008;300:1027–1037. [DOI] [PubMed] [Google Scholar]
- 14.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol 2005;58:595–602. [DOI] [PubMed] [Google Scholar]
- 15.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 17.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356–1364. [DOI] [PubMed] [Google Scholar]
- 18.Ylikoski R, Jokinen H, Andersen P, et al. Comparison of the Alzheimer's Disease Assessment Scale Cognitive Subscale and the Vascular Dementia Assessment Scale in differentiating elderly individuals with different degrees of white matter changes: the LADIS study. Dement Geriatr Cogn Disord 2007;24:73–81. [DOI] [PubMed] [Google Scholar]
- 19.Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. J Am Geriatr Soc 1992;40:1221–1226. [DOI] [PubMed] [Google Scholar]
- 20.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease: The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11(suppl 2):S33–S39. [PubMed] [Google Scholar]
- 21.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol 1935;18:643. [Google Scholar]
- 22.Spreen O, Strauss E. A Compendium of Neurological Tests. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 23.Wechsler D. Wechsler Adult Intelligence Scale–Revised: The Psychological Corporation. San Diego: Harcourt Brace Jovanovich; 1981. [Google Scholar]
- 24.Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest 2003;123:387–398. [DOI] [PubMed] [Google Scholar]
- 25.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The Physical Activity Scale for the Elderly (PASE): evidence for validity. J Clin Epidemiol 1999;52:643–651. [DOI] [PubMed] [Google Scholar]
- 26.Gellish RL, Goslin BR, Olson RE, McDonald A, Russi GD, Moudgil VK. Longitudinal modeling of the relationship between age and maximal heart rate. Med Sci Sports Exerc 2007;39:822–829. [DOI] [PubMed] [Google Scholar]
- 27.Borg G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int J Sports Med 1982;3:153–158. [DOI] [PubMed] [Google Scholar]
- 28.Persinger R, Foster C, Gibson M, Fater DC, Porcari JP. Consistency of the talk test for exercise prescription. Med Sci Sports Exerc 2004;36:1632–1636. [PubMed] [Google Scholar]
- 29.Nagamatsu LS, Chan A, Davis JC, et al. Physical activity improves verbal and spatial memory in older adults with probable mild cognitive impairment: a 6-month randomized controlled trial. J Aging Res 2013:861893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacova C, Pearce LA, Costello R, et al. Cognitive impairment in lacunar strokes: the SPS3 trial. Ann Neurology 2012;72:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia JJ, Wei C, Liang J, et al. The effects of DL-3-n-butylphthalide in patients with vascular cognitive impairment without dementia caused by subcortical ischemic small vessel disease: a multicentre, randomized, double-blind, placebo-controlled trial. Alzheimers Demen 2016;12:89–99. [DOI] [PubMed] [Google Scholar]
- 32.Auchus AP, Brashear HR, Salloway S, Korczyn AD, De Deyn PP, Gassmann-Mayer C. Galantamine treatment of vascular dementia: a randomized trial. Neurology 2007;69:448–458. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson D, Doody R, Helme R, et al. Donepezil in vascular dementia: a randomized, placebo-controlled study. Neurology 2003;61:479–486. [DOI] [PubMed] [Google Scholar]
- 34.Schrag A, Schott JM. Alzheimer's Disease Neuroimaging I: what is the clinically relevant change on the ADAS-Cog? J Neurol Neurosurg Psychiatry 2012;83:171–173. [DOI] [PubMed] [Google Scholar]
- 35.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol 2010;67:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker LD, Frank LL, Foster-Schubert K, et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer's disease. J Alzheimers Dis 2010;22:569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan U, Porteous L, Hassan A, Markus HS. Risk factor profile of cerebral small vessel disease and its subtypes. J Neurol Neurosurg Psychiatry 2007;78:702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vishram JK, Borglykke A, Andreasen AH, et al. Impact of age on the importance of systolic and diastolic blood pressures for stroke risk: the Monica, Risk, Genetics, Archiving, and Monograph (MORGAM) Project. Hypertension 2012;60:1117–1123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.