Abstract
Objective:
To characterize comorbid chronic conditions, describe health services use, and estimate health care costs among community-dwelling older adults with prior stroke.
Methods:
This is a retrospective cohort study using administrative data from Ontario, Canada. We identified all community-dwelling individuals aged 66 and over on April 1, 2008 (baseline), who had experienced a stroke at least 6 months prior. We estimated the prevalence of 14 comorbid conditions at baseline; we captured all physician visits, emergency department visits, hospital admissions, home care contacts, and associated costs over 5 years stratifying by number of comorbid conditions. Where possible, we distinguished between health services use for stroke- and non-stroke-related reasons.
Results:
A total of 29,673 individuals met our criteria. Only 1% had no comorbid conditions, while 74.9% had 3 or more. The most common conditions were hypertension (89.8%) and arthritis (65.8%); 5 other conditions had a prevalence of 20% or more (ischemic heart disease, diabetes, chronic obstructive pulmonary disease, inflammatory bowel disease, and dementia). Use of all health services doubled with increasing comorbidity and was largely attributed to non-stroke-related reasons. Total and per-patient costs increased with comorbidity. Main cost drivers shifted from physician and home care visits to hospital admissions with greater comorbidity.
Conclusions:
Our findings demonstrate the importance of community-based patient-centered care strategies for stroke survivors that address their range of health needs and prevent more costly acute care use.
Stroke is a leading cause of death and disability among older adults.1 Due to population aging and greater poststroke survival,2 an increasing number of older people are living with the effects of stroke.
The frequency of most chronic conditions (CCs), including the co-occurrence of multiple CCs, also increases with age.3 A recent study found that older adults with prior stroke were more likely to have other CCs than those without prior stroke.4 Comorbid CCs are associated with greater short- and long-term stroke mortality,5,6 worse rehabilitation outcomes,7,8 and reduced use of secondary prevention.9
Most research has focused on concordant comorbid conditions (those related to stroke) and outcomes such as stroke mortality, recurrence, rehabilitation, or stroke-specific health care costs.10–13 Little research has fully characterized the frequency of common comorbid CCs or the effects on overall health services use or costs. A more complete understanding of comorbidity among older adults with stroke is necessary for the implementation of interventions that meet the full range of needs in older adults with stroke, including the accommodation of discordant CCs. The overall objective of this study is to examine the relationship between the number of comorbid conditions and health services use among a cohort of community-dwelling older adults living with stroke. The specific objectives are to (1) characterize comorbid CCs, (2) describe their health services use for stroke-related and non-stroke-related reasons over a 5-year period, and (3) estimate the associated health care costs over this period.
METHODS
Study design and setting.
This is a retrospective cohort study using linked health administrative databases from Ontario, Canada. The vast majority of provincial residents are covered under the public health insurance plan (Ontario Health Insurance Plan [OHIP]), which includes physician visits, acute care hospital use, and home care.
This study was conducted with support from the Aging, Community, and Health Research Unit (ACHRU) at McMaster University. ACHRU was established to design, evaluate, and translate interprofessional community-based programs to improve health care access, health-related quality of life, and health outcomes while reducing costs for older adults with multiple CCs. This study was developed to address ACHRU's information needs for an intervention for older stroke survivors with comorbid CCs.
Data.
The databases included the Registered Persons Database (RPDB) for demographic data, OHIP claims for physician visits, the Discharge Abstract Database (DAD) for inpatient hospitalization records, the National Ambulatory Care Reporting System (NACRS) for emergency department and ambulatory care records, and the Home Care Database for home care service records. Additional databases were accessed for specific diagnostic information, including the Ontario Mental Health Reporting System, the Ontario Cancer Registry, and the Ontario Drug Benefit claims database. All databases, except RPDB, are collected for administrative purposes and submission is mandatory for the relevant health service contact. They have been well-studied for accuracy. Chart re-abstraction studies of NACRS have found 84% interrater reliability on diagnostic elements14 while those of DAD have found over 80% completeness of diagnostic reporting and 88% positive predictive value of the reported diagnosis.15 Nonclinical elements, such as service dates, have near perfect agreement with medical charts.15 A stroke-focused DAD evaluation found 97% complete diagnostic reporting with 90% positive predictive value for diagnosis.16–26
The data were linked using unique, encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences (ICES) in Toronto, Canada.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Research Ethics Board at each Sunnybrook Health Sciences Centre and McMaster University (certificate 13-394-C).
Study cohort.
The cohort included all individuals 66 years and over who resided in the community and had a diagnosis of stroke as of April 1, 2008 (baseline). We set our lower age limit at 66 years since prescription drug coverage starts at age 65 and a 1-year look-back for relevant prescriptions was required for certain diagnostic definitions. We excluded individuals residing in nursing homes.
We looked for evidence of ischemic or hemorrhagic stroke within the 5 years prior to baseline by searching all DAD diagnostic fields for relevant ICD-10-CA codes, derived from a recent stroke care evaluation in Ontario27 (appendix e-1 at Neurology.org). We excluded individuals with a new stroke within the 6 months prior to baseline to avoid capturing the postacute period. We further excluded individuals who were 105 years or older, who were receiving palliative care, or who had no health system contact in the 5 years prior to baseline.
From baseline, each individual was followed until the first of nursing home admission, a move out of province, death, or the end of the 5-year follow-up (March 31, 2012). At the start of each follow-up year, we adjusted the cohort denominator to remove those no longer eligible for inclusion (due to death, nursing home admission, or an out-of-province move).
We described the cohort at baseline by age, sex, neighborhood income, and the presence of comorbid CCs. The comorbid CCs identified were anxiety or depression, arthritis, cancer, cerebral vascular disease (excluding stroke), chronic obstructive pulmonary disease (COPD), dementia, diabetes, upper gastrointestinal bleed, hypertension, ischemic heart disease (IHD), liver disease, osteoporosis/osteopenia, inflammatory bowel disease (IBD), and renal disease (with and without chronic dialysis). These conditions were selected because of their prevalence and relevance to the ACHRU intervention studies. Each condition, except cancer, was defined using either of the following methods: (1) a search for diagnostic codes in the OHIP, DAD, or NACRS databases within the 5 years prior to baseline or specific prescription drug claims within the 1 year prior to baseline; or (2) entry into a diagnosis-specific database created at ICES. Cancer diagnoses were identified using the provincial registry. Details for each diagnosis are listed in appendix e-1; several have been previously validated or use a similar standard approach.16–26 The extent of comorbidity was estimated by summing across the total number of comorbid conditions at baseline. We grouped the cohort by number of comorbid conditions (0, 1, 2, 3, 4, 5, 6, 7, 8 or more).
Health services utilization variables.
We identified all health services utilization from baseline to the end of the 5-year follow-up, including physician visits (to primary and specialist care providers), unplanned emergency department visits, hospitalizations, and home care contacts. We created hospitalization episodes to avoid double-counting interhospital transfers. For each hospitalization episode, we estimated the total length of stay (admission to final discharge) and time in either the intensive care unit (ICU) or as alternate level of care (ALC). ALC refers to when a patient no longer requires hospital care but cannot be discharged due to a lack of alternative services elsewhere. For physician visits, emergency department visits, and hospitalizations, we distinguished between those related to stroke and those for all other reasons (appendix e-2). For home care services, we counted the total number of nursing visits, but the reason for the visit could not be determined.
Statistical analyses and costing.
We used descriptive statistics to characterize the cohort including the prevalence of each condition at baseline. For each year of follow-up, we estimated the total amount of each type of health service used by number of comorbid conditions; corresponding estimates of per year and average annual usage were adjusted to the year-specific denominator. Costs, in Canadian dollars, for each type of health service were calculated by multiplying the service volume (either total number of visits or hours of service, depending on type) by the published unit cost (cost per visit or hour, depending on type). Of note, we used all home care visit types (not just nursing visits) to estimate total home care costs. Details on the source of published costs can be found in appendix e-3. Total service costs for each year of follow-up were calculated by adding the total costs per type. Average annual per-patient costs were estimated by dividing total service costs by the number of individuals in the cohort per year. We further estimated the proportion of total health services costs attributed to each of physician visits, emergency department visits, hospitalizations, and home care. Where applicable, costs were standardized to 2012 dollars to facilitate comparisons across years (although the inflation rate in the period was close to zero).
RESULTS
We identified 29,673 community-dwelling older adults with a prior stroke. At baseline (April 1, 2008), 31.9% were 66–74 years, 46.0% 75–84 years, and 22.1% 85 years and older; 50.1% were male. The majority (74.9%) had 3 or more comorbid CCs and only 1% had no CCs. The average number of comorbid conditions was 3.5 with little variation by age. The most common comorbid conditions were hypertension (89.8%), arthritis (65.8%), and IHD (38.1%) (table). One-fifth (21.2%) of the cohort had dementia and 5.9% experienced a mood or anxiety disorder. A total of 60.7% (n = 18,012) of the cohort was followed for 5 years (until March 31, 2012); 78.5% of censoring was due to death and the remainder due to nursing home admission.
Table.
Baseline characteristics of community-dwelling older adults who experienced a stroke, Ontario, Canada, April 1, 2008
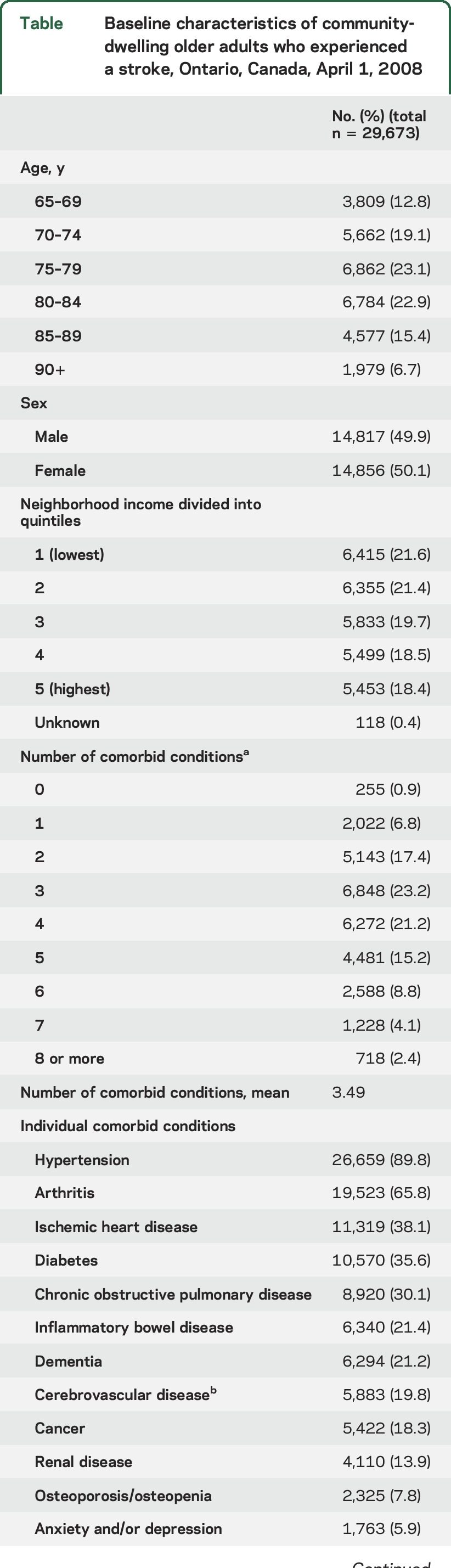
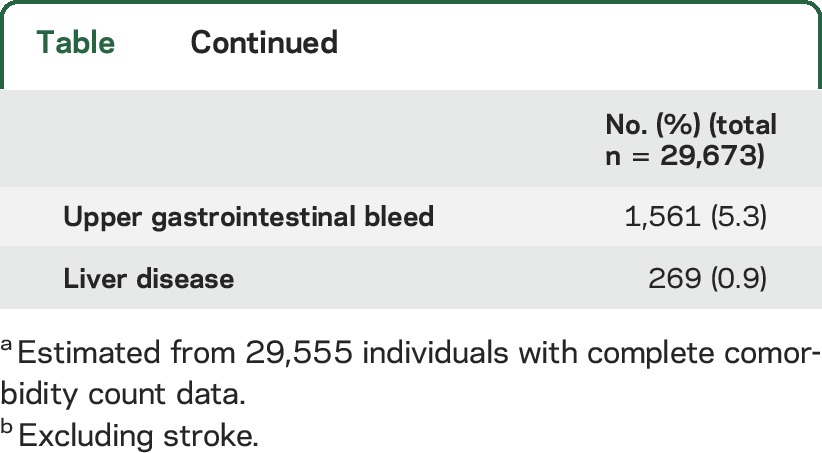
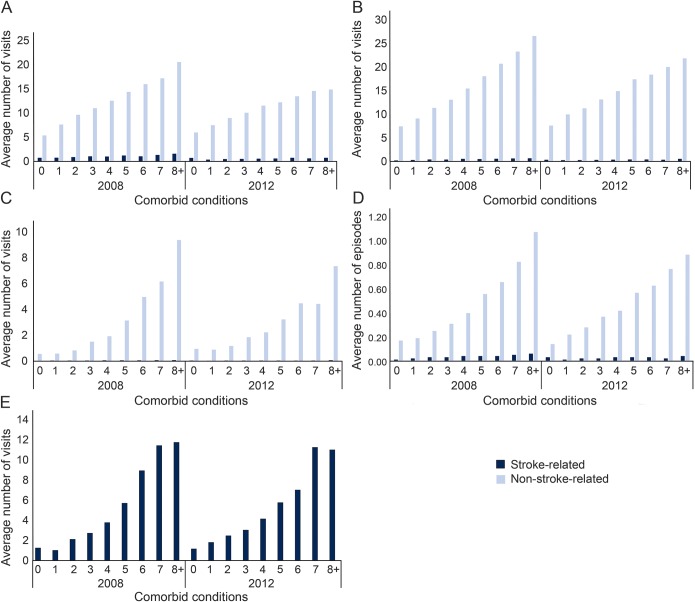
Figure 1 displays the average annual number of primary care visits, specialist visits, emergency department visits, acute care hospitalizations, and home care nursing visits stratified by number of comorbid conditions in 2008 and 2012. In each year, the average annual use of primary care visits, specialist visits, emergency department visits, and acute care hospitalizations increased with the number of comorbid conditions. In all cases, use at least tripled among those with 8 or more comorbid conditions relative to those with no comorbid conditions with the most striking increases observed for emergency department visits and acute care hospitalizations. Overall, use of service was predominantly driven by non-stroke-related reasons, even among those with no or only one comorbid condition. Both stroke and non-stroke-related reasons for use increased with the number of comorbid conditions. Average hospital length of stay, ICU stays, and ALC days showed similar increases with greater comorbid conditions (data not shown). The average annual number of home care nursing visits increased from 1.3 visits among those with 0 conditions to 11.8 visits among those with 3 or more conditions (figure 1E).
Figure 1. Average annual number of health service contacts by community-dwelling older adults who experienced a stroke stratified by reason for contact, 2008 and 2012.
(A) Primary care physician visits. (B) Specialist physician visits. (C) Emergency department visits. (D) Acute care hospital episodes. (E) Nursing home care visits (unable to distinguish between stroke- and non-stroke-related).
Between 2008 and 2012, the average annual number of primary care and specialist visits, regardless of comorbidity, declined from 13.2 to 11.0 and 15.6 to 14.3, respectively. This appeared to be driven by changes in those with the highest level of comorbidity since those with no or one comorbidity showed either consistent or greater average use over time. There was little change in average annual number of hospitalizations or home care nursing visits over time for the full cohort or by comorbidity; while the average annual number of emergency department visits overall was unchanged, there were slight differences by comorbidity with decreased use among those with the highest number of comorbid conditions.
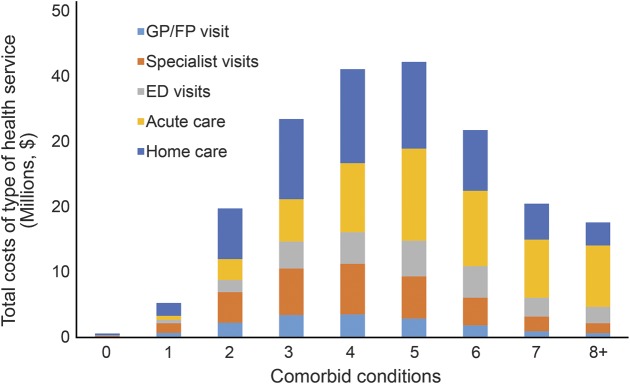
Figure 2 displays total health services utilization costs by number of comorbid conditions for 2008. The proportion attributed to each type of health services utilization is shown within the overall cost bars. Total cohort cost was $213.5 million. Total cost increased from $615,000 among those with no comorbid conditions to $42 million among those with 5 comorbid conditions and then declined to $17.6 million among those with 8 or more comorbid conditions. These cost differences reflect both the group size based on number of comorbid conditions and differences in health services utilization. Most notably, the share of costs attributed to acute care (emergency department visits and hospitalizations) increased with comorbidity while the share of costs attributed to physician visits and home care use declined. Data from 2012 showed a similar pattern but lower overall costs, likely due to the smaller cohort size at 5 years (data not shown).
Figure 2. Total annual health services utilization costs for community-dwelling older adults who experienced a stroke in 2008 by type of health service and number of chronic conditions.
ED = emergency department; FP = family practitioner; GP = general practitioner.
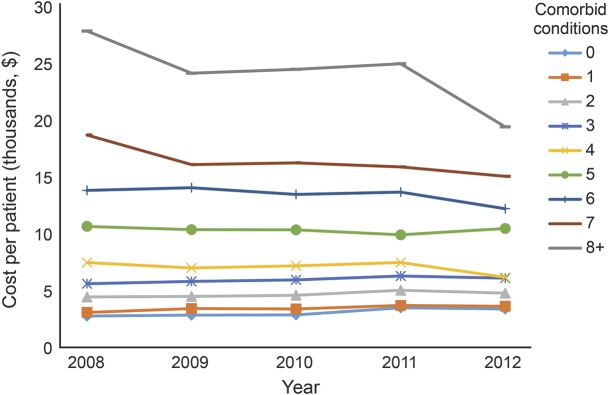
Figure 3 displays average annual per-patient costs, adjusted to 2012 dollars, for each study year by number of comorbid CCs. Average annual per-patient costs increased with the number of comorbid conditions but differences widened with increasing comorbidity. For example, in 2008, average per-patient costs were $2,775 for those without comorbid conditions, $3,090 for those with 1, $5,623 for those with 3, $10,671 for those with 5, and $27,898 for those 8 or more comorbid conditions. Overall average per-patient costs in 2008 were $8,223, which is skewed by the greater number of individuals with multiple comorbid conditions. Over the 5 years, the overall average per-patient cost showed a slight decline but this was inconsistent across levels of comorbidity with the greatest change observed among those with the highest level of comorbidity.
Figure 3. Average annual per-patient health services costs for community-dwelling older adults who experienced a stroke between 2008 and 2012, adjusted to 2012 dollars.
DISCUSSION
We found that in a cohort of older adults with prior stroke, the vast majority were living with multiple comorbid CCs. While concordant conditions, such as hypertension and diabetes, were common, so were discordant conditions, such as arthritis and IBD. We also found that greater comorbidity was associated with higher stroke- and non-stroke-related health services utilization. Not surprisingly, health care costs increased with the number of comorbid CCs, but the main cost drivers shifted from community-based to acute care services.
A striking finding is the sheer volume of health services utilization. For example, in 2008, the average older adult with prior stroke visited a primary care physician over 13 times—more than 1 visit per month—not including specialist visits. It is also striking that the majority of health services use captured here was not directly attributed to stroke, even among those individuals who did not have 1 of the 14 other CCs. While many of these conditions share risk factors or treatment plans with stroke, others do not and may require special consideration in the design or implementation of stroke recovery and secondary prevention programs. For example, arthritis, identified in two-thirds of our cohort, has been associated with poorer functional recovery following stroke.28 This appears to be attributed to stroke rehabilitation activities that do not accommodate for common arthritis-related limitations and changes to arthritis medications due to cardiovascular risks.7 Other common conditions, such as COPD and dementia, likely also have implications for rehabilitation and secondary prevention activities.
As with other services, the average annual number of home care nursing visits increased with comorbidity; however, even among the group with the greatest number of comorbid conditions, the average annual number of visits was low, with an average of only 5 visits per year. Of note, this estimate includes individuals who did not receive home care and may be skewed. At the same, though, it has been well-reported that older adults who experience a stroke often live with long-term functional impairments.29,30 Although we cannot speculate based on our data, others have demonstrated ongoing unmet needs for supportive home care services for stroke survivors.31
We found that the drivers of health care costs varied by the number of comorbid conditions. Physicians, in particular specialists, and home care were the biggest cost contributors in those with few comorbid conditions but acute care was the largest single contributor in those with multiple comorbid conditions. This likely reflects the general increase of acute care use reported elsewhere.32,33 Overall costs declined over the 5-year follow-up, likely reflecting the diminishing cohort, but there was little change in the relative contribution of each health service type to costs. Average per-patient costs declined slightly for the group with the greatest number of comorbidities but increased for those with no or few comorbidities. This reflects underlying changes in service utilization and not changing costs since these were standardized to 2012 dollars. Increased costs among those with no or few comorbid conditions may in part be explained by the addition of new diagnoses over the 5 years that we were unable to capture. Regardless, there is a clear increase in average per-patient costs with increasing comorbidity. Future research should also consider the relative effect of specific comorbid conditions on costs, in particular those conditions or groups of conditions associated with highest costs. This work would also benefit from the inclusion of prescription medication costs, given their role in CC management.
This study has several strengths, including our population-based cohort of older adults with prior stroke and the use of administrative data to track health service utilization. We did not restrict the study to stroke-concordant conditions, allowing for a more thorough description of multimorbidity than in many prior studies. This study also has limitations. First, we could only include health services that were covered by the public health insurance system, which means that privately covered home care services were excluded. Second, we were unable to add new diagnoses over follow-up, since it is difficult to capture specific onset dates for most CCs using administrative data. Third, we used a simple count of CCs to estimate the burden of comorbidity since there is little guidance on conceptualizing comorbidity in people with stroke. Future research is needed to better understand the effect of other definitions of comorbidity including concordant/discordant dichotomies, symptom burden, and high counts. Further, we considered only 14 comorbid CCs, so likely underestimate comorbidity in this population; however, our list does include the most prevalent conditions as reported elsewhere.3 Finally, we used a simple strategy to distinguish stroke- and non-stroke-related health service use, which may underestimate use that could be indirectly attributed to stroke (for example, a fall-related injury that resulted from an impairment due to stroke). Regardless, our findings on the predominance of non-stroke-related use raise an important note of consideration for future evaluations of stroke care programs since it is possible that they would have an effect on overall service use, and not just stroke-specific outcomes.
This study highlights a number of implications for future research, practice, and policy. We found a very high burden of multiple CCs, notably the frequent cooccurrence of discordant CCs. Research is needed to better characterize the interplay between stroke and common discordant conditions, such as arthritis, and the implications for stroke rehabilitation, secondary prevention, and outcomes, as well as non-stroke-related care and outcomes. This is central to the design and implementation of care pathways and programs that accommodate patient preferences and priorities. The increasing use of acute care services with greater comorbidity reinforces the need for investment in community-based care to prevent complications of existing conditions and reduce the need for the most expensive services.
Supplementary Material
GLOSSARY
- ACHRU
Aging, Community, and Health Research Unit
- ALC
alternate level of care
- CC
chronic condition
- COPD
chronic obstructive pulmonary disease
- DAD
Discharge Abstract Database
- ICD-10-CA
International Classification of Diseases–10, Canada
- IBD
inflammatory bowel disease
- ICES
Institute for Clinical Evaluative Sciences
- ICU
intensive care unit
- IHD
ischemic heart disease
- NACRS
National Ambulatory Care Reporting System
- OHIP
Ontario Health Insurance Plan
- RPDB
Registered Persons Database
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
A. Gruneir: study concept and design, acquisition of data, analysis and interpretation, writing first draft of manuscript, critical revision of the manuscript for important intellectual content. L. Griffith: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content. K. Fisher: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content. D. Panjwani: study concept and design, critical revision of the manuscript for important intellectual content. S. Gandhi: study concept and design, critical revision of the manuscript for important intellectual content. L. Sheng: study analysis and interpretation, critical revision of the manuscript for important intellectual content. C. Patterson: study concept and design, critical revision of the manuscript for important intellectual content, overall supervision. A. Gafni: study concept and design, critical revision of the manuscript for important intellectual content, overall supervision. J. Ploeg: study concept and design, funding, critical revision of the manuscript for important intellectual content, overall supervision. M. Markle-Reid: study concept and design, funding, critical revision of the manuscript for important intellectual content, overall supervision.
STUDY FUNDING
This work is part of a program of research (Aging, Community and Health Research Unit) supported by the Canadian Institutes of Health Research Signature Initiative in Community- Based Primary Healthcare (cihr-irsc.gc.ca/e/43626.html) (funding reference number: TTF 128261) and the Ontario Ministry of Health and Long-Term Care Health (MOHLTC) System Research Fund Program (grant 06669). This study was also supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario MOHLTC. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI). The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES, the Ontario MOHLTC, or CIHI is intended or should be inferred. Dr. Markle-Reid is supported, in part, by funding from the Canada Research Chair program. Dr. Griffith and Dr. Gruneir are supported by CIHR New Investigators Awards.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.World Heart Federation. Heart disease: stroke. In: World Heart Federation [online]. Available at: world-heart-federation.org/cardiovascular-health/stroke/. Accessed July 17, 2015. [Google Scholar]
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet 2014;383:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pefoyo AJ, Bronskill SE, Gruneir A, et al. The increasing burden and complexity of multimorbidity. BMC Public Health 2015;15:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallacher KI, Batty GD, McLean G, et al. Stroke, multimorbidity and polypharmacy in a nationally representative sample of 1,424,378 patients in Scotland: implications for treatment burden. BMC Med 2014;12:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson CS, Carter KN, Brownlee WJ, Hackett ML, Broad JB, Bonita R. Very long-term outcome after stroke in Auckland, New Zealand. Stroke 2004;35:1920–1924. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt M, Jacobsen JB, Johnsen SP, Botker HE, Sorensen HT. Eighteen-year trends in stroke mortality and the prognostic influence of comorbidity. Neurology 2014;82:340–350. [DOI] [PubMed] [Google Scholar]
- 7.Wood JP, Connelly DM, Maly MR. “Holding me back”: living with arthritis while recovering from stroke. Arch Phys Med Rehabil 2009;90:494–500. [DOI] [PubMed] [Google Scholar]
- 8.Turhan N, Atalay A, Muderrisoglu H. Predictors of functional outcome in first-ever ischemic stroke: a special interest to ischemic subtypes, comorbidity and age. NeuroRehabilitation 2009;24:321–326. [DOI] [PubMed] [Google Scholar]
- 9.Moroney JT, Tseng CL, Paik MC, Mohr JP, Desmond DW. Treatment for the secondary prevention of stroke in older patients: the influence of dementia status. J Am Geriatr Soc 1999;47:824–829. [DOI] [PubMed] [Google Scholar]
- 10.Kolominsky-Rabas PL, Heuschmann PU, Marschall D, et al. Lifetime cost of ischemic stroke in Germany: results and national projections from a population-based stroke registry: the Erlangen Stroke Project. Stroke 2006;37:1179–1183. [DOI] [PubMed] [Google Scholar]
- 11.Johansen HL, Wielgosz AT, Nguyen K, Fry RN. Incidence, comorbidity, case fatality and readmission of hospitalized stroke patients in Canada. Can J Cardiol 2006;22:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karatepe AG, Gunaydin R, Kaya T, Turkmen G. Comorbidity in patients after stroke: impact on functional outcome. J Rehabil Med 2008;40:831–835. [DOI] [PubMed] [Google Scholar]
- 13.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke 1996;27:1459–1466. [DOI] [PubMed] [Google Scholar]
- 14.Canadian Institute for Health Information. CIHI Data Quality Study of Ontario Emergency Department Visits for Fiscal Year 2004–2005: Executive Summary. Ottawa: Canadian Institute for Health Information; 2008. [Google Scholar]
- 15.Canadian Institute for Health Information. CIHI Data Quality Study of 2007–2008 Discharge Abstract Database. Ottawa: Canadian Institute for Health Information; 2010. [Google Scholar]
- 16.Canadian Institute for Health Information. CIHI Data Quality Study of the 2008–2009 Discharge Abstract Database. Ottawa: Canadian Institute for Health Information; 2010. [Google Scholar]
- 17.Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512–516. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J 2002;144:290–296. [DOI] [PubMed] [Google Scholar]
- 19.Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physician diagnosed COPD in health administrative databases. COPD 2009;6:388–394. [DOI] [PubMed] [Google Scholar]
- 20.Tu K, Campbell NR, Chen ZL, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med 2007;1:e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 21.Tu K, Mitiku T, Lee DS, Guo H, Tu JV. Validation of physician billing and hospitalization data to identify patients with ischemic heart disease using data from the Electronic Medical Record Administrative data Linked Database (EMRALD). Can J Cardiol 2010;26:e225–e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopushinsky SR, Covarrubia KA, Rabeneck L, Austin PC, Urbach DR. Accuracy of administrative health data for the diagnosis of upper gastrointestinal diseases. Surg Endosc 2007;21:1733–1737. [DOI] [PubMed] [Google Scholar]
- 23.Molnar AO, van Walraven C, McArthur E, Fergusson D, Garg AX, Knoll G. Validation of administrative database codes for acute kidney injury in kidney transplant recipients. Can J Kidney Health Dis 2016;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson D, Richards H, Chapman A. The national ambulatory care reporting system: factors that affect the quality of its emergency data. Int J Inf Qual 2008;2:97–114. [Google Scholar]
- 25.Widdifield J, Bernatsky S, Paterson JM, et al. Accuracy of Canadian health administrative databases in identifying patients with rheumatoid arthritis: a validation study using the medical records of rheumatologists. Arthritis Care Res 2013;65:1582–1591. [DOI] [PubMed] [Google Scholar]
- 26.Levy AR, O'Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario drug benefit database. Can J Clin Pharmacol 2003;10:67–71. [PubMed] [Google Scholar]
- 27.Hall R, Khan F, O'Callaghan C, et al. Ontario Stroke Evaluation Report 2012: Prescribing System Solutions to Improve Stroke Outcomes. Toronto: Institute for Clinical Evaluative Sciences; 2012. [Google Scholar]
- 28.Nguyen-Oghalai TU, Ottenbacher KJ, Granger CV, Goodwin JS. Impact of osteoarthritis on the rehabilitation of patients following a stroke. Arthritis Rheum 2005;53:383–387. [DOI] [PubMed] [Google Scholar]
- 29.Hartman-Maeir A, Soroker N, Ring H, Avni N, Katz N. Activities, participation and satisfaction one-year post stroke. Disabil Rehabil 2007;29:559–566. [DOI] [PubMed] [Google Scholar]
- 30.Markle-Reid M, Orridge C, Weir R, et al. Interprofessional stroke rehabilitation for stroke survivors using home care. Can J Neurol Sci 2011;38:317–334. [DOI] [PubMed] [Google Scholar]
- 31.Hunter DJ, Grant HJ, Purdue MP, Spasoff RA, Dorland JL, Bains N. An epidemiologically-based needs assessment for stroke services. Chronic Dis Can 2004;25:138–146. [PubMed] [Google Scholar]
- 32.Terner M, Reason B, McKeag AM, Tipper B, Webster G. Chronic conditions more than age drive health system use in Canadian seniors. Healthc Q 2011;14:19–22. [DOI] [PubMed] [Google Scholar]
- 33.Lochner KA, Goodman RA, Posner S, Parekh A. Multiple chronic conditions among Medicare beneficiaries: state-level variations in prevalence, utilization, and cost, 2011. Medicare Medicaid Res Rev 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.