Abstract
Objective:
We hypothesized that florbetapir, a Food and Drug Administration–approved PET tracer, could distinguish cerebral amyloid angiopathy (CAA)–related intracerebral hemorrhage (ICH) from hypertensive ICH (HTN-ICH).
Methods:
We prospectively enrolled survivors of primary ICH related to probable CAA (per Boston Criteria, n = 10) and HTN-ICH (n = 9) without dementia. All patients underwent florbetapir-PET and multimodal MRI, and patients with CAA had additional Pittsburgh compound B (PiB) PET. Amyloid burden was assessed quantitatively (standard uptake value ratio [SUVR]) and visually classified as positive or negative.
Results:
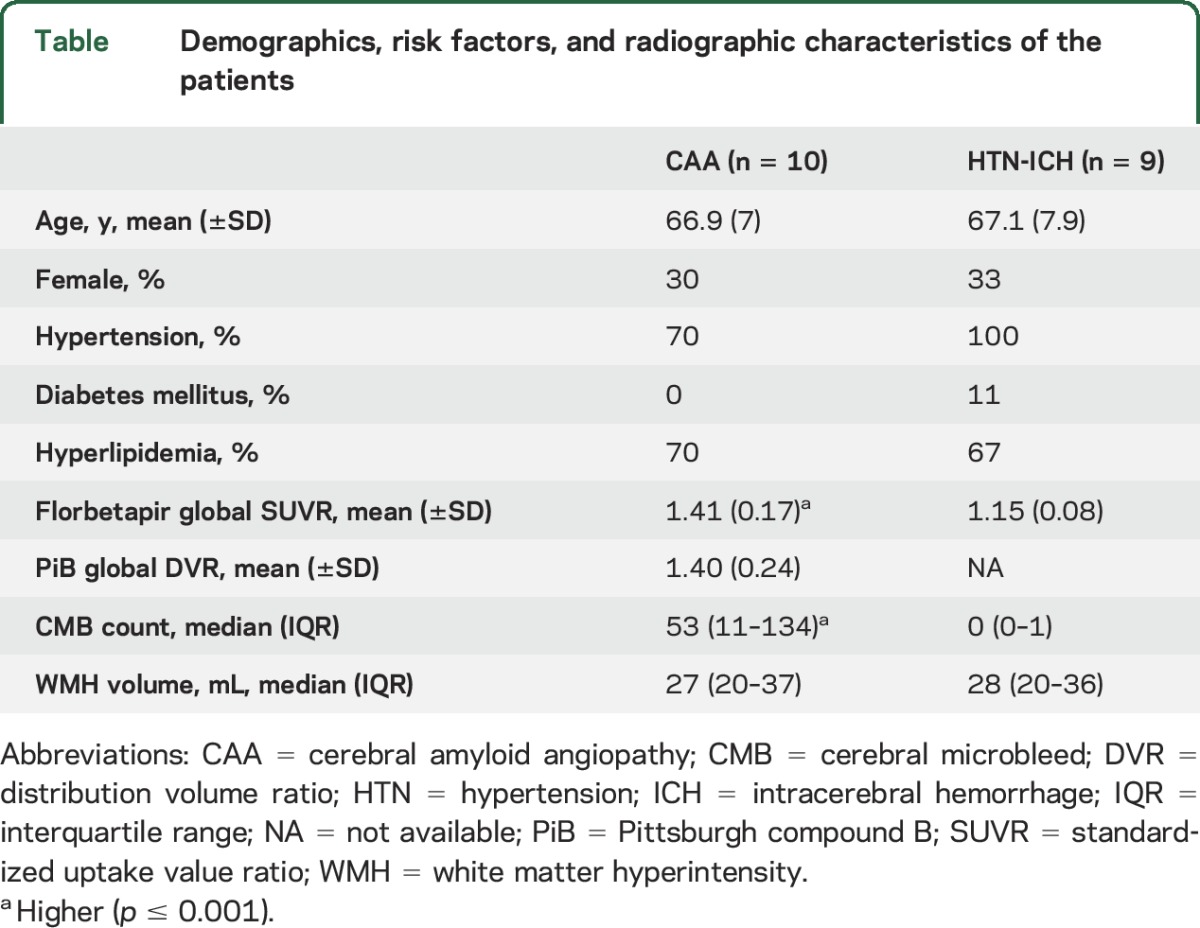
The CAA and HTN-ICH groups had similar age (66.9 vs 67.1), sex, and leukoaraiosis volumes (31 vs 30 mL, all p > 0.8). Florbetapir uptake and PiB retention strongly correlated in patients with CAA both globally within cerebral cortex (r = 0.96, p < 0.001) and regionally in lobar cortices (all r > 0.8, all p ≤ 0.01). Mean global cortical florbetapir uptake was substantially higher in CAA than HTN-ICH (SUVR: 1.41 ± 0.17 vs 1.15 ± 0.08, p = 0.001), as was mean occipital SUVR (1.44 ± 0.12 vs 1.17 ± 0.08, p < 0.001), even after correcting for global SUVR (p = 0.03). Visual rating for positive/negative florbetapir demonstrated perfect interrater agreement (k = 1) and was positive for all 10 patients with CAA vs 1 of 9 HTN-ICH patients (sensitivity 100%, specificity 89%).
Conclusions:
Florbetapir appears to label vascular amyloid in patients with CAA-related ICH. The approved florbetapir binary visual reading method can have diagnostic value in appropriate clinical settings.
Classification of evidence:
This study provides Class II evidence that florbetapir-PET provides a sensitivity of 100% (95% confidence interval [CI] 66%–100%) and specificity of 89% (95% CI 51%–99%) for determination of probable CAA among cognitively normal patients.
Cerebral amyloid angiopathy (CAA), characterized by accumulation of β-amyloid (Aβ) proteins in the walls of cortical/leptomeningeal vessels, is a common cause of vessel wall breakdown and vascular dysfunction in older adults, making it a major contributor to fatal or disabling intracerebral hemorrhages (ICH) as well as ischemic injury and dementia.1,2 There currently is no specific treatment for CAA, in part because of our inability to identify this condition at early phases prior to appearance of multiple hemorrhagic brain lesions.3 Accurate early diagnosis of CAA also has direct implications for stroke prevention, as this pathology is an important cause of anticoagulant-associated ICH and might dictate use of alternative nonpharmaceutical approaches such as left atrial appendage closure for patients with nonvalvular atrial fibrillation.4,5 The diagnosis of CAA cannot currently be made safely or ruled out by clinically available imaging methods in more than one-third of primary ICHs.6
One of the exciting advances in CAA research has been the demonstration of the ability of Pittsburgh compound B (PiB), originally designed to detect plaque amyloid in Alzheimer disease (AD), to label vascular amyloid as well.7–10 This PET tracer was shown to bind vascular amyloid by radiologic–pathologic correlation in both the common sporadic form of CAA and Iowa-type hereditary CAA, a form of the disorder with little or no plaque deposits of fibrillar Aβ.11,12 Amyloid imaging using PiB-PET has already made important contributions to our understanding of CAA-related hemorrhagic and ischemic disease mechanisms,2,7,8,13,14 such as prediction of future hemorrhages.7
Despite these promising results obtained with PiB, its clinical application and regulatory approval have been limited by its short half-life, requiring onsite synthesis. A more promising approach for clinical use is the recently introduced PET tracer florbetapir (18F-AV-45).15,16 Florbetapir has a long enough half-life for clinical synthesis and use and has been Food and Drug Administration–approved for detection of parenchymal amyloid in suspected AD. Florbetapir has not yet been tested for the diagnosis of CAA, however. We therefore aimed to compare the strength and retention patterns of florbetapir and PiB in patients with CAA without dementia and florbetapir's sensitivity/specificity for CAA relative to similar aged patients with deep ICH caused by chronic hypertension rather than CAA.
METHODS
Study participants.
We prospectively enrolled 10 survivors of CAA-related lobar ICH and 9 patients who had deep hypertensive ICH (HTN-ICH). Both CAA and HTN-ICH patients were recruited from an ongoing single-center prospective longitudinal cohort study of the natural history of ICH (Massachusetts General Hospital, Boston).8 Detailed information including demographics, clinical status, vascular risk factors, and characteristics of the presenting event were prospectively recorded at the time of cohort entry.8,17 None of the patients had dementia according to the DSM-IV-TR18 and all had Mini-Mental State Examination (MMSE) scores of 29 or 30. All patients were free of symptoms suggestive of new stroke for 6 months prior to imaging studies. All 10 patients with CAA were diagnosed with probable CAA according to Boston criteria after presenting with symptomatic ICH.19,20 Radiologic analyses were performed by separate study personnel (J.A.B., P.F.) and the results were recorded without knowledge of participants' clinical information. The study was performed in compliance with Standards for Reporting Diagnostic Accuracy Studies statement guidelines (http://www.stard-statement.org) and the related flow diagram is presented in figure 1.
Figure 1. Standards for Reporting Diagnostic Accuracy Studies flow diagram showing study enrollment and results.
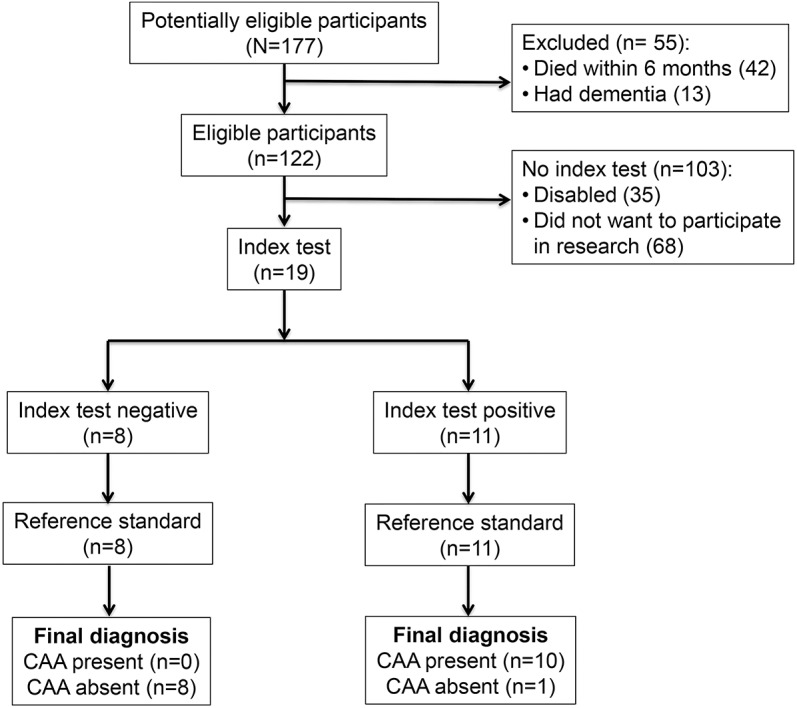
CAA = cerebral amyloid angiopathy.
Standard protocol approvals, registrations, and patient consents.
This study was performed with the approval of and in accordance with the guidelines of the institutional review board of Massachusetts General Hospital. Written informed consent was obtained from all patients (or guardians of patients) participating in the study.
Amyloid imaging acquisition and analysis.
All PET data were acquired using ECAT HR + PET scanner (Siemens, Knoxville, TN). All 19 participants underwent a florbetapir-PET scan using procedures described previously.21 All participants received a single IV bolus of 370 MBq (10 mCi) of florbetapir F18. After transmission data were acquired, a 20-minute PET acquisition for florbetapir was acquired in 4 × 5–minute frames beginning 50 minutes postinjection. The reconstruction method was iterative (4 iteration 16 subsets) with a postreconstruction Gaussian filter of 5 mm. Images were reconstructed, corrected for scatter and attenuation using commercial software packages, and inspected for adequacy of count statistics and absence of head motion. Florbetapir scans were nonlinearly warped using SPM8 to the MNI152 FDG template in statistical parametric mapping, and average PET activities calculated over regions defined by the Harvard/Oxford and UCL Cerebellum atlas in standardized Montreal Neurological Institute space (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). Florbetapir retention standardized uptake value ratios (SUVR) with cerebellar cortex reference region were calculated from average activities in regions of interest (ROIs) for the following target brain regions in the hemisphere not affected by the ICH: frontal cortex, temporal cortex, parietal cortex, and occipital cortex. A composite ROI that represents the whole cerebral cortex was used to calculate the mean cortical SUVR.8 The 10 patients with CAA also had PiB-PET scan using previously described protocols.7,8 The distribution volume ratio (DVR) was computed to express specific PiB retention using cerebellar cortex as the reference tissue. Mean global DVR and the mean DVR from frontal, temporal, parietal, and occipital cortices were calculated using the same ROIs that were applied for florbetapir-based analyses.
In addition to the quantitative measures of amyloid deposition described above, a binary classification of each florbetapir-PET scan was performed, as either visually positive for Aβ (vAβ+) or visually negative for Aβ (vAβ−).15 Two investigators (M.E.G., S.M.G.) who completed the online training for florbetapir reading (https://amyvid.myregistrationp.com/amyvid/login.do) independently interpreted all 19 scans without knowledge of clinical and quantitative PET results.
MRI acquisition and analysis.
Each patient underwent detailed structural MRI scans within a week of PiB-PET imaging, including fluid-attenuated inversion recovery for quantification of white matter hyperintensity (WMH) volume, susceptibility-weighted imaging (SWI) for detection of cerebral hemorrhages, and magnetization-prepared rapid gradient echo for cortical segmentation as described previously.8 Cerebral microbleeds (CMB) were identified per published guidelines.22 WMH volume was measured by validated computer-assisted techniques, calculated based on the hemisphere without ICH in order to omit perilesional signal changes from the WMH measurement.8
Statistical analysis.
The primary research question was whether florbetapir-PET can help diagnose CAA among cognitively normal patients (Class II evidence). The sample size was determined based on available data from previous amyloid imaging and CAA diagnostic studies. Bivariate comparisons were performed using Fisher exact test, t test, or Wilcoxon rank-sum test as appropriate. Pearson r was used to measure bivariate correlation as appropriate. Multivariate regression analysis was used to test the independent association between variables of interest after adjustment for covariates. Models developed using a forward selection method to reduce the number of variables did not differ substantially from those that included all covariates. Appropriate arithmetic transformations were applied to the continuous variables that were non-normally distributed for use in multivariate models. Sensitivity and specificity were calculated by standard formulas. The kappa statistic was used to test interrater reliability of amyloid positivity ratings. All statistical analyses were performed using SPSS software. A threshold for significance of p < 0.05 was used. All tests of significance were 2-tailed.
RESULTS
Ten survivors of probable CAA-related ICH and 9 survivors of HTN-ICH were prospectively enrolled between March 2013 and January 2015. The participants had no cognitive deficits by history or objective testing (MMSE ≥29). Patients with CAA-ICH and HTN-ICH had similar mean ages (66.9 vs 67.1; table) and proportion of female patients (30% vs 33%, both p > 0.2). All patients with HTN-ICH and 70% of patients with CAA were hypertensive (p = 0.21). The distribution of other vascular risk factors (hyperlipidemia and diabetes) was not different between the groups (both p > 0.2). WMH volume was similar between CAA and HTN-ICH (median 27 vs 28 mL, p = 0.9). Patients with CAA had higher CMB counts, as in previous studies (p < 0.001).6,23 No adverse event from any of the study procedures was observed in any participant.
Table.
Demographics, risk factors, and radiographic characteristics of the patients
Within the CAA cohort, we tested our hypothesis that the pattern of cortical florbetapir retention would parallel PiB uptake, a demonstrated marker of vascular amyloid. Florbetapir uptake and PiB retention strongly correlated in patients with CAA both globally within cerebral cortex (r = 0.96, p < 0.001, figure 2) and regionally in occipital, frontal, temporal, and parietal cortices (all r > 0.8, all p ≤ 0.01). These associations remained significant after adjustment for age and sex. Both PiB retention and florbetapir uptake correlated with age (p < 0.05 for both correlations), but not sex. Presence or absence of vascular risk factors (hypertension, diabetes mellitus, hyperlipidemia) was not associated with any global or regional PiB or florbetapir measure (all p > 0.2).
Figure 2. Scatterplot of mean global florbetapir and Pittsburgh compound B (PiB) values in cerebral amyloid angiopathy (CAA).
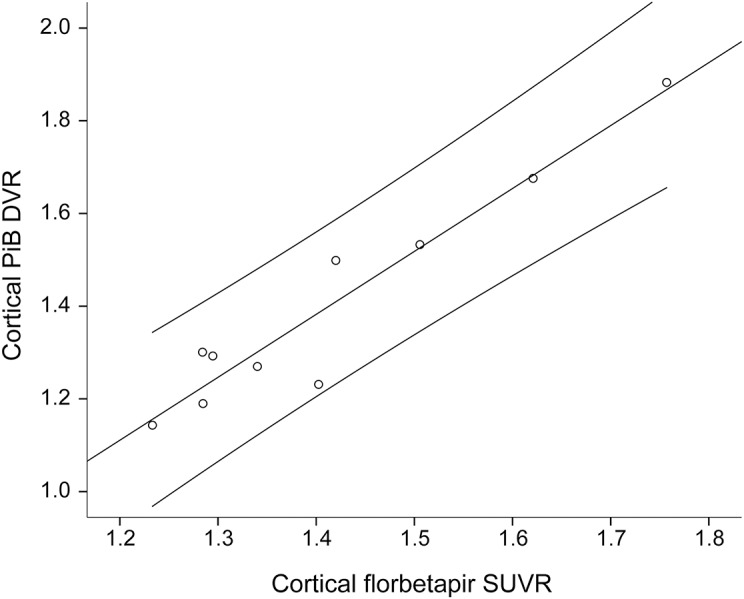
The linear association between mean global cortical florbetapir retention and PiB uptake values in the CAA cohort (n = 10, r = 0.96, p < 0.001). Lines represent the best fit (middle) and the 95% confidence interval. DVR = distribution volume ratio; SUVR = standardized uptake value ratio.
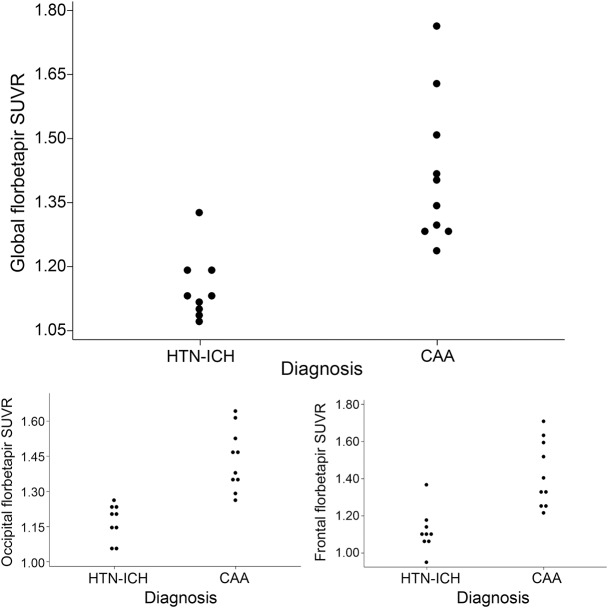
We then performed both quantitative and qualitative analyses to determine the ability of florbetapir-PET to diagnose CAA. Mean global cortical florbetapir uptake was higher in CAA than HTN-ICH (SUVR 1.41 ± 0.17 vs 1.15 ± 0.08, p = 0.001). The mean occipital SUVR was also higher in CAA (1.44 ± 0.12 vs 1.17 ± 0.08, p < 0.001) (figure 3). This association remained significant even after correcting for global SUVR (p = 0.03), whereas the difference in regional SUVR for the other cortical lobes was not independent of global SUVR. Addition of age to the regression model did not change the significance of this relationship.
Figure 3. Comparison of mean cortical florbetapir standardized uptake value ratio (SUVR) between hypertensive intracerebral hemorrhage and cerebral amyloid angiopathy (CAA).
Dot plots show mean global cortical as well as occipital and frontal cortical florbetapir SUVR values in patients with hypertensive intracerebral hemorrhage (HTN-ICH) and CAA. Based on visual assessment of the scans blinded to quantitative analyses, patients with mean global cortical SUVR less than 1.21 were identified as having negative scans, the rest as positive scans.
Classification of the florbetapir scans as positive or negative demonstrated perfect interrater agreement (κ = 1) between 2 trained neurologists blinded to all other information. Visual assessment of florbetapir-PET was positive for all 10 patients with CAA vs only 1 of 9 HTN-ICH patients (figures 3 and 4), yielding a sensitivity of 100% (95% confidence interval [CI] 66%–100%), specificity 89% (95% CI 51%–99%). The mean global SUVR of the 8 negative scans ranged between 1.08 and 1.19 whereas the SUVR range for the pooled group of 11 positive scans was 1.23–1.76 (figure 3), showing no overlap in mean global SUVR between negative and positive scans. The one HTN-ICH patient who was amyloid-positive had a global SUVR of 1.33. This patient's age was 72 at the time of presentation with a thalamocapsular hemorrhage. She had HTN and hyperlipidemia, but no unusual characteristics for HTN-ICH and MMSE score of 29.
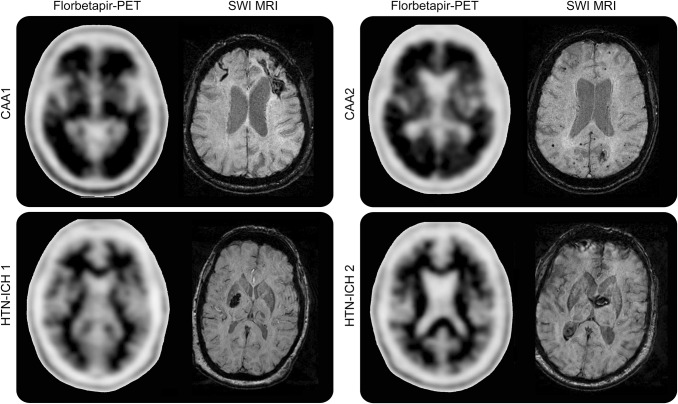
Figure 4. Examples of florbetapir-positive and -negative scans from patients with cerebral amyloid angiopathy (CAA) and hypertensive intracerebral hemorrhage (HTN-ICH).
The upper panel shows florbetapir-positive PET scans from 2 patients with CAA. On susceptibility-weighted imaging (SWI) MRI of the first patient (CAA 1), a left frontal lobar ICH and right frontal superficial siderosis are seen, whereas the SWI MRI of the second patient (CAA 2) shows a left occipital ICH and multiple lobar microbleeds. The florbetapir scans for these patients with CAA show 2 or more brain areas (each larger than a single cortical gyrus) in which there is reduced or absent gray-white contrast, corresponding to intense gray matter radioactivity. The lower panel shows SWI MRIs displaying deep hypertensive ICHs (HTN-ICH 1 and 2). The corresponding negative florbetapir scans show more radioactivity in white matter than in gray matter, creating clear gray-white contrast.
DISCUSSION
We found that florbetapir detects CAA and differentiates patients with CAA-related ICH from HTN-ICH by both quantitative and visually qualitative scoring methods. Cortical florbetapir retention in CAA was observed to match the pattern and severity of PiB uptake. We also found that the binary visual determination of florbetapir positivity fully correlated with the quantitative analyses, a mean global SUVR of 1.21 accurately categorizing scans as amyloid positive/negative. The binary visual scoring system thus appears to be a robust marker for diagnosing advanced CAA in the appropriate clinical context.
The main focus of our analysis was assessing florbetapir as a clinically available diagnostic marker for distinguishing CAA from non-CAA causes of ICH, particularly hypertensive small vessel disease. HTN and CAA are the most common etiologies of deep hemispheric and lobar ICH, respectively, in older adults,24 as well as causing ischemic injuries such as leukoaraiosis or lacunar infarcts.23 Distinguishing CAA from non-CAA can play a major role in clinical decision-making, as CAA confers a particularly high risk of future ICH and dementia.25 Patients who present with a CAA-related lobar ICH have a 9% annual recurrence risk and even those diagnosed solely with microbleeds have 5% annual risk of first-time ICH.26 Such risk can increase by 2- to 4-fold with use of anticoagulants, so making the CAA diagnosis early can be essential for selecting optimal stroke prevention approaches in patients with possible indications for anticoagulation such as atrial fibrillation.27–29 Several alternatives to warfarin anticoagulation with lower associated ICH risks have recently emerged,4,5,30 underlining the potential importance of identifying CAA in practice. Compared to CAA, the recurrence risk of deep HTN-ICH is relatively low (∼2% per year) and does not necessarily preclude future warfarin anticoagulation.29 The diagnosis of CAA might similarly affect other clinical decisions suggested to increase ICH risk such as statins for secondary stroke prevention.31–33
The diagnosis of probable CAA by the Boston criteria has high specificity, but currently available methods are insensitive to the presence of CAA in situations such as occurrence of an isolated lobar ICH without microbleeds, hemorrhagic lesions in both deep and lobar locations, or cerebellar hemorrhages.19,20 In our largest analyzed series of consecutive primary ICH patients who had MRI (n = 526), patients with uncertain diagnoses were common (isolated lobar ICH, n = 122, 23.2%; mixed location or cerebellar ICH, n = 76, 14.5%) relative to the more definite diagnostic categories (probable CAA, n = 191, 36.3%; HTN-ICH, n = 137, 26%).6 Older patients with multiple lobar microbleeds without ICH (lobar microbleed-only patients) show clinical, radiologic, and risk factor profiles of CAA,26 but low microbleed counts on MRI are associated with a decreased probability of confirming CAA on autopsy.34 These data suggest that for more than one third of primary ICH patients and for lobar microbleed-only patients with low microbleed burden, adjunctive diagnostic methods are needed for establishing the diagnosis of CAA. The only such adjunct method currently available is CSF analysis for Aβ and tau levels,35 but lumbar puncture is an invasive method that additionally provides no spatial information on disease location.
The increased florbetapir uptake observed in these CAA-ICH patients, like previous demonstrations of increased PiB retention, likely reflects binding of the ligands to vascular amyloid. This interpretation is consistent with extensive previous reports of associations between PiB retention and the hemorrhagic and ischemic markers of CAA.7–14,36 The observation of increased relative florbetapir retention in occipital cortex (even after adjustment for global SUVR) further supports this interpretation, as this pattern parallels the posterior predominance of CAA pathology.9,10,37 Some of the observed florbetapir retention likely also reflects accompanying parenchymal AD pathology, which often co-occurs with CAA (despite our restriction to cognitively normal patients with MMSE 29–30). We note, however, that confounding by concomitant AD pathology would tend to bias away from the demonstrated occipital predominance.9
Our results suggest that the relatively long-lived and widely available florbetapir can be applied to both clinical practice and single or multicenter trials related to CAA. Findings supporting its use include the perfect interrater agreement obtained in binary classifications of amyloid, the perfect sensitivity, and the very high specificity of the positive/negative categorizations for identifying presence/absence of CAA. For an amyloid-negative florbetapir scan, the probability that it was a true negative (negative predictive value) was 100%, a finding in line with results of previous radiologic-pathologic validation of florbetapir for parenchymal AD pathology.15,16 Our finding of a false-positive (1 HTN-ICH patient who was florbetapir-positive) likely reflects the background rate of approximately 14% of otherwise healthy older adults who have an amyloid-positive scan.21 One previous study found that 4 out of 9 cognitively healthy older controls had high PiB retention, the highest reported rate of false positivity in amyloid imaging field but the sensitivity to detect CAA was still excellent (91%) in this particular study.38 It is plausible that the high specificity that we found might suffer in patients 80 and older due to increased incidence of asymptomatic amyloid positivity. Our data nevertheless suggest that a negative florbetapir scan might be used to exclude a diagnosis of severe CAA, providing valuable guidance to the management of patients with isolated lobar ICH, mixed location ICH, and cerebellar ICH, and patients who cannot undergo MRI.
One strength of our study was use of HTN-ICH as a comparison group, an entity that shares many characteristics with CAA, such as propensity for ischemic and hemorrhagic damage.6,23 The CAA and HTN-ICH cohorts were well-matched in terms of age, sex, and WMH volumes. Our study also has notable limitations. Our sample was small, but nonetheless yielded clinically meaningful differences because of the robust separation between amyloid-positive and amyloid-negative patients. Our sample was also restricted to participants able to come to the hospital for research imaging >6 months after their ICH; such survival bias might favor the null hypothesis, as these patients could be more likely to have less severe CAA. Finally, although we used previously validated diagnostic criteria,19,20 we did not have pathologic evidence of underlying small vessel disease type. Again, any misclassification would tend to bias towards the null hypothesis rather than towards intergroup differences.
Our results support the ability of amyloid imaging with florbetapir-PET to diagnose CAA in the appropriate clinical context, providing actionable information for a range of potential clinical decisions related to bleeding risk. Although the current study was restricted to ICH patients, the results also raise the possibility that florbetapir-PET might detect advanced CAA pathology prior to ICH for the purposes of therapeutic trials or future primary prevention treatments.
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- CAA
cerebral amyloid angiopathy
- CI
confidence interval
- CMB
cerebral microbleed
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision
- DVR
distribution volume ratio
- HTN
hypertension
- ICH
intracerebral hemorrhage
- MMSE
Mini-Mental State Examination
- PiB
Pittsburgh compound B
- ROI
region of interest
- SUVR
standardized uptake value ratio
- SWI
susceptibility-weighted imaging
- WMH
white matter hyperintensity
AUTHOR CONTRIBUTIONS
M. Edip Gurol: study concept and design, analysis and interpretation, drafting and revising the manuscript for intellectual content. J. Alex Becker: acquisition of data, analysis and interpretation. Panagiotis Fotiadis: acquisition of data, critical revision of manuscript for intellectual content. Grace Riley: acquisition of data, critical revision of manuscript for intellectual content. Kristin Schwab: acquisition of data, critical revision of manuscript for intellectual content. Keith A. Johnson: study concept and design, critical revision of manuscript for intellectual content. Steven M. Greenberg: study concept and design, critical revision of manuscript for intellectual content, study supervision.
STUDY FUNDING
M. Edip Gurol: NINDS K23 NS083711. Steven M. Greenberg: NINDS R01 NS070834. Avid Radiopharmaceuticals, Inc. provided the florbetapir tracer used for the respective PET scans. Avid did not contribute any funds for Pittsburgh compound B PET scans, MRI scans, staff salaries, or any other funds. No funding source had any role in the planning of this study, analyses, interpretation of results, or manuscript preparation.
DISCLOSURE
M. Gurol is funded by NIH grant K23 NS083711. J. Becker, P. Fotiadis, G. Riley, K. Schwab, and K. Johnson report no disclosures relevant to the manuscript. S. Greenberg is funded by NIH grant R01 NS070834. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol 2011;70:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurol ME. Molecular neuroimaging in vascular cognitive impairment. Stroke 2016;47:1146–1152. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg SM, Al-Shahi Salman R, Biessels GJ, et al. . Outcome markers for clinical trials in cerebral amyloid angiopathy. Lancet Neurol 2014;13:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lubitz SA, Bauer KA, Benjamin EJ, et al. . Stroke prevention in atrial fibrillation in older adults: existing knowledge gaps and areas for innovation: a summary of an American Federation for Aging research seminar. J Am Geriatr Soc 2013;61:1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haley KE, Greenberg SM, Gurol ME. Cerebral microbleeds and macrobleeds: should they influence our recommendations for antithrombotic therapies? Curr Cardiol Rep 2013;15:425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charidimou A, Boulouis G, Haley K, et al. . White matter hyperintensity patterns in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2016;86:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurol ME, Dierksen G, Betensky R, et al. . Predicting sites of new hemorrhage with amyloid imaging in cerebral amyloid angiopathy. Neurology 2012;79:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurol ME, Viswanathan A, Gidicsin C, et al. . Cerebral amyloid angiopathy burden associated with leukoaraiosis: a positron emission tomography/magnetic resonance imaging study. Ann Neurol 2013;73:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson KA, Gregas M, Becker JA, et al. . Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol 2007;62:229–234. [DOI] [PubMed] [Google Scholar]
- 10.Ly JV, Donnan GA, Villemagne VL, et al. . 11C-PIB binding is increased in patients with cerebral amyloid angiopathy-related hemorrhage. Neurology 2010;74:487–493. [DOI] [PubMed] [Google Scholar]
- 11.Bacskai BJ, Frosch MP, Freeman SH, et al. . Molecular imaging with Pittsburgh compound B confirmed at autopsy: a case report. Arch Neurol 2007;64:431–434. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg SM, Grabowski T, Gurol ME, et al. . Detection of isolated cerebrovascular beta-amyloid with Pittsburgh compound B. Ann Neurol 2008;64:587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charidimou A, Hong YT, Jager HR, et al. . White matter perivascular spaces on magnetic resonance imaging: marker of cerebrovascular amyloid burden? Stroke 2015;46:1707–1709. [DOI] [PubMed] [Google Scholar]
- 14.Ly JV, Rowe CC, Villemagne VL, et al. . Cerebral β-amyloid detected by Pittsburgh compound B positron emission topography predisposes to recombinant tissue plasminogen activator-related hemorrhage. Ann Neurol 2010;68:959–962. [DOI] [PubMed] [Google Scholar]
- 15.Clark CM, Pontecorvo MJ, Beach TG, et al. . Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol 2012;11:669–678. [DOI] [PubMed] [Google Scholar]
- 16.Clark CM, Schneider JA, Bedell BJ, et al. . Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA 2011;305:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurol ME, Irizarry MC, Smith EE, et al. . Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology 2006;66:23–29. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th Ed, Text Revision). Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 19.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–539. [DOI] [PubMed] [Google Scholar]
- 20.Smith EE, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Curr Atheroscler Rep 2003;5:260–266. [DOI] [PubMed] [Google Scholar]
- 21.Johnson KA, Sperling RA, Gidicsin CM, et al. . Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer's disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement 2013;9:S72–S83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg SM, Vernooij MW, Cordonnier C, et al. . Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith EE, Nandigam KR, Chen YW, et al. . MRI markers of small vessel disease in lobar and deep hemispheric intracerebral hemorrhage. Stroke 2010;41:1933–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep 2012;14:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry 2012;83:124–137. [DOI] [PubMed] [Google Scholar]
- 26.van Etten ES, Auriel E, Haley KE, et al. . Incidence of symptomatic hemorrhage in patients with lobar microbleeds. Stroke 2014;45:2280–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: stroke prevention in atrial fibrillation II study. Lancet 1994;343:687–691. [PubMed] [Google Scholar]
- 28.Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999;131:492–501. [DOI] [PubMed] [Google Scholar]
- 29.Eckman MH, Rosand J, Knudsen KA, et al. . Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2003;34:1710–1716. [DOI] [PubMed] [Google Scholar]
- 30.Ruff CT, Giugliano RP, Braunwald E, et al. . Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 31.Lauer A, Greenberg SM, Gurol ME. Statins in intracerebral hemorrhage. Curr Atheroscler Rep 2015;17:526. [DOI] [PubMed] [Google Scholar]
- 32.Westover MB, Bianchi MT, Eckman MH, Greenberg SM. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol 2011;68:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein LB, Amarenco P, Szarek M, et al. . Hemorrhagic stroke in the stroke prevention by aggressive reduction in cholesterol levels study. Neurology 2008;70:2364–2370. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Ramirez S, Romero JR, Shoamanesh A, et al. . Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimers Dement 2015;11:1480–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verbeek MM, Kremer BP, Rikkert MO, et al. . Cerebrospinal fluid amyloid beta(40) is decreased in cerebral amyloid angiopathy. Ann Neurol 2009;66:245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dierksen GA, Skehan ME, Khan MA, et al. . Spatial relation between microbleeds and amyloid deposits in amyloid angiopathy. Ann Neurol 2010;68:545–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinters HV. Cerebral amyloid angiopathy: a critical review. Stroke 1987;18:311–324. [DOI] [PubMed] [Google Scholar]
- 38.Baron JC, Farid K, Dolan E, et al. . Diagnostic utility of amyloid PET in cerebral amyloid angiopathy-related symptomatic intracerebral hemorrhage. J Cereb Blood Flow Metab 2014;34:753–758. [DOI] [PMC free article] [PubMed] [Google Scholar]