SECTION 1
A 30-year-old man who recently immigrated from Liberia was admitted to the neurology service for diffuse weakness. He reported 7 years of painless progressive weakness and atrophy. He noted that his legs began to get weak first, which manifested as difficulty keeping up with his peers while playing soccer. This progressively worsened until he had great difficulty walking and arising from a seated position. A few years after the onset of leg weakness, he developed arm weakness. He denied sensory loss. He had some back pain but no limb pain. He denied fasciculations, cramping, or constitutional symptoms. He was not on medications and denied any toxic exposures. His parents were physically normal but were first cousins. He had 2 children, ages 2 and 4 years, who were physically normal.
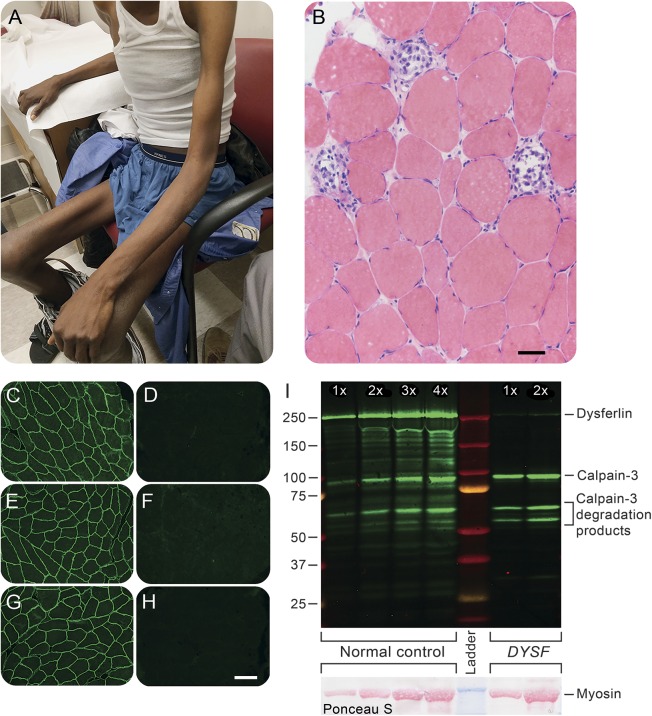
On examination, there was severe muscle atrophy (figure, A), most pronounced in the thighs and humeral region. The middle deltoid and the intrinsic hand muscles were spared. Medical Research Council grade strength was as follows: 4/5 in the proximal arms, elbow, and forearm muscles, 5/5 in the hand intrinsics, and 2/5 in the hip flexors, extensors, adductors and abductors, quadriceps, foot dorsiflexors, and plantar flexion. There was no scapular winging, facial weakness, or neck flexors or extensors weakness. He had no contractures. His reflexes were normal in the arms and absent in the legs. He was unable to stand up without assistance. He walked with a cane with a Trendelenburg gait with circumduction of both legs and bilateral foot drop. His sensory examination was normal.
Figure. Clinical image and muscle biopsy.
Severe atrophy of the humeral and thigh regions with relative sparing of the hands and middle deltoid muscles (A). Hematoxylin & eosin–stained cryosection demonstrates wide variation in muscle fiber diameters and active myonecrosis (B). Dysferlin immunofluorescence staining compares normal control muscle (C, E, G) to the patient (D, F, H); the antibodies used were Hamlet (C, D), Hamlet 2 (E, F), and Romeo (G, H). Dysferlin and calpain-3 Western blotting (I) contrasts a normal control muscle and our patient. Adjacent lanes of control or patient muscle homogenates contain increasing amounts of total protein. The Ponceau S–stained membrane (lower right corner) shows the relative loading of each lane. In our patient, dysferlin is virtually absent while calpain-3 appears normal. The antibodies used for Western blotting were Hamlet (anti-dysferlin) and 12A2 (anti–calpain-3). The size bar in panel A is 50 μm; the size bar in panel G is 100 μm and applies to all of the immunofluorescence images.
His serum creatine kinase (CK) level was elevated between 4,000s and 6,000s IU/L. HIV testing was negative. PPD (purified protein derivative) testing was positive, but further testing revealed only latent TB infection. He underwent nerve conduction studies and needle EMG. Nerve conduction studies were normal. The needle examination revealed fibrillation potentials in multiple proximal and distal muscles of the right arm and leg. Motor unit potentials had small amplitudes, short duration, and polyphasic morphology with increased recruitment.
Questions for consideration:
Based on these findings, what is your differential diagnosis?
What testing would you perform to clarify the diagnosis?
SECTION 2
The history, neurologic examination, elevated CKs, and EMG are consistent with a myopathy. This myopathy could be either acquired or inherited. Exposure to toxins and infectious diseases were essentially excluded by the medical history and laboratory testing and will not be discussed in detail here. The differential is hence narrowed to autoimmune vs inherited myopathies.
In general, patients with inflammatory myopathies have proximal more than distal arm and leg weakness; an exception in older individuals is inclusion body myositis, which is typically asymmetric and affects the quadriceps and flexors of the fingers and wrists. Our patient has predominantly lower extremities weakness, which is symmetric and affects both distal and proximal muscles. This pattern, combined with the severity of his atrophy and the duration of his symptoms, reduces the likelihood of an inflammatory myopathy.
Inherited myopathies include congenital myopathies, metabolic myopathies, myotonic disorders, and muscular dystrophies. The onset of symptoms in his 20s along with the severity of his atrophy and the absence of myotonia or exercise intolerance makes a muscular dystrophy most likely. The consanguinity of his parents raises suspicion for an autosomal recessive muscular dystrophy but does not exclude the possibility of a de novo autosomal dominant dystrophy.
The degree of CK elevation may also be helpful. Certain muscular dystrophies are associated with more profound CK elevations including dystrophinopathies (Duchenne and Becker muscular dystrophies), limb-girdle muscular dystrophy (LGMD) 1C (caveolin-3), LGMD 2A (calpain-3), LGMD 2B (dysferlin), LGMD 2I (FKRP), and LGMD 2L (anoctamin 5).1
Specific patterns of weakness, particularly in the earlier stages of disease, can be helpful in distinguishing different muscular dystrophies. Examples of helpful patterns are listed in table e-1 at Neurology.org.1 Certain muscular dystrophies may share a common genetic basis but present with very different patterns of weakness. Specific examples include dysferlin (DYSF) and anoctamin 5 (ANO5) gene mutations, which may present with either an LGMD pattern (LGMD 2B or 2L, respectively) or distal leg weakness (Miyoshi myopathy type 1 or 3, respectively).
This patient's pattern of weakness appears to best fit distal leg, proximal arm pattern, though admittedly his weakness extends proximally in the legs and spares his scapular region. The differential is broad and would include facioscapulohumeral dystrophy (FSHD), scapuloperoneal muscular dystrophy, Emery-Dreifuss muscular dystrophy, Pompe disease, LGMD 1B (lamin A/C), LGMD 2A (calpain-3), LGMD 2B (dysferlin), LGMD 2C–F (sarcoglycans), LGMD 2I (FKRP-dystroglycanopathy) LGMD 2L (ANO5), and Becker muscular dystrophy. FSHD is unlikely given the absence of facial weakness. The absence of scapular winging makes FSHD, scapuloperoneal and calpainopathy muscular dystrophies less likely. Patients with Emery-Dreifuss muscular dystrophy (X-linked and LMNA dominant forms) often present with early contractures not present in this patient. Pompe disease often involves some level of respiratory muscle disease, which does not appear here. There is no history or evidence of calf hypertrophy, which is often seen in dystrophinopathies (Becker muscular dystrophy), dystroglycanopathies (e.g., LGMD 2I), and sarcoglycanopathies (LGMD 2C–F).
Based on the patient's history, CK, and clinical examination, he most likely has LGMD 2B or LGMD 2L, though many of the possible diagnoses discussed cannot be completely excluded without additional testing.
A muscle biopsy of the left deltoid was performed (figure, B). This revealed wide variation in size and shape of muscle fibers with extensive myonecrosis and regeneration. Many fibers had internalized nuclei. There was a mild degree of lymphocytic endomysial and perimysial, perivascular inflammation (not shown). Immunoperoxidase staining revealed a reduction in dysferlin and sarcolemmal upregulation of utrophin in many fibers. Dystrophin staining (carboxy terminus, rod domain, and amino terminus) was normal. These findings are suggestive of a muscular dystrophy and the immunohistochemical findings suggested a dysferlinopathy; however, dysferlin staining can be difficult to interpret and reduced dysferlin can be seen in other muscular dystrophies such as caveolinopathies or calpainopathies.2
A next-generation sequencing (NGS) panel was performed (Medical Neurogenetics, Atlanta, GA). This revealed a heterozygous pathogenic mutation in the SCGA gene (c.574C>T, p.Arg192Ter, reference GRCH37; dbSNP rs387907298) along with a homozygous variant of undetermined significance (VUS) in the DYSF gene (c.3167G>A, pArg1056Gln, reference GRCH37; dbSNP rs150877497). The VUS in the DYSF gene has not been described in disease populations, but is present with very low frequency (0.0001) in the ExAC database (Exome Aggregation Consortium, Cambridge, MA).3
SECTION 3
Questions for consideration:
Based on prior testing and NGS findings, what is the most likely cause of the patient's muscular dystrophy?
How could the presence of a VUS be more clearly determined to be pathogenic in this patient?
The patient is a carrier of a pathologic α-sarcoglycan mutation. The sarcoglycanopathies are autosomal recessive and therefore the presence of a single mutation is insufficient to cause disease. However, a deletion in the other SCGA allele was not excluded. Dysferlinopathies are also autosomal recessive but the patient has the same VUS on both alleles of the gene producing dysferlin. This makes a dysferlinopathy more likely; however, as this variant has not been described in patients with muscular dystrophy, a causal link is not established.
The patient’s muscle biopsy was further evaluated by immunofluorescence, demonstrating normal sarcoglycan staining (not shown) and an absence of dysferlin staining with 3 different anti-dysferlin antibodies (figure, C–H). Hamlet, Hamlet 2, and Romeo antibodies bind to different epitopes of the dysferlin protein. A Western blot using Hamlet confirmed the nearly complete absence of dysferlin in the patient's muscle (figure, I).
DISCUSSION
This patient has a dysferlinopathy causing severe, diffuse muscle weakness and atrophy.
Dysferlinopathies are autosomal recessive myopathies caused by mutations in the DYSF gene.4 The clinical presentation is notably varied including an LGMD 2B presentation, early involvement of the medial calf muscle (Miyoshi myopathy), or a combination of proximal and distal weakness. The phenotype may also evolve with progression of disease. The age at onset is also variable, with most patients presenting in the second or third decade. Congenital muscular dystrophy due to dysferlin mutations has also been described.5,6 CK levels are typically quite elevated. A study of patients in France revealed a mean elevation approximately 20 times normal CK levels.7
NGS is a useful tool in the diagnosis of muscular dystrophy as many LGMDs have overlapping clinical presentations. When considering NGS, one should keep in mind that 2 of the most common muscular dystrophies (FSHD1 and myotonic dystrophy [DM1]) are not amenable to this diagnostic technology. In addition, when sequencing a large number of genes, it is common to identify VUS. In autosomal recessive diseases, variants are often more easily dismissed as they are frequently heterozygous. However, in both autosomal dominant and recessive diseases, the presence of a variant should be considered clinically interesting but not definitive without further studies such as familial segregation studies or bioassays in the patient or an animal model.
In this patient, a homozygous VUS was seen in the dysferlin gene. Of note, his clinical phenotype fits a dysferlinopathy. His muscle biopsy revealed the absence of dysferlin in muscle cells. These findings confirm the pathogenicity of his novel, homozygous DYSF mutation.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge Terese Nelson for performing the muscle biopsy immunostaining and Mary Cox for performing the Western blotting.
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Colin Quinn: conceptualization, design, and drafting the manuscript. Tanya M. Bardakjian: conceptualization and revising the manuscript for intellectual content. Dr. Steven Moore: conceptualization and revising the manuscript for intellectual content. Dr. Chafic Karam: conceptualization and revising the manuscript for intellectual content.
STUDY FUNDING
S.A. Moore is supported by NIH grant U54-NS053672, Iowa Wellstone Muscular Dystrophy Cooperative Research Center.
DISCLOSURE
C. Quinn, S. Moore, and T. Bardakjian report no disclosures relevant to the manuscript. C. Karam served on the editorial board of the Neurology® Resident & Fellow Section. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Barohn RJ, Dimachkie MM, Jackson CE. A pattern recognition approach to patients with a suspected myopathy. Neurol Clin 2014;32:569–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubowtiz VR, Sewry CA, Oldfors A. Muscle Biopsy: A Practical Approach, 4th ed. Philadelphia: Saunders; 2013. [Google Scholar]
- 3.ExAC Browser. Variant: 2:71797810 G/A. Available at: http://exac.broadinstitute.org/variant/2-71797810-G-A. Accessed May 4, 2016.
- 4.Liu J, Aoki M, Illa I, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet 1998;20:31–36. [DOI] [PubMed] [Google Scholar]
- 5.Paradas C, González-Quereda L, De Luna N, et al. A new phenotype of dysferlinopathy with congenital onset. Neuromuscul Disord 2009;19:21–25. [DOI] [PubMed] [Google Scholar]
- 6.Ceyhan-Birsoy O, Talim B, Swanson LC, et al. Whole exome sequencing reveals DYSF, FKTN, and ISPD mutations in congenital muscular dystrophy without brain or eye involvement. J Neuromuscul Dis 2015;2:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen K, Bassez G, Krahn M, et al. Phenotypic study in 40 patients with dysferlin gene mutations: high frequency of atypical phenotypes. Arch Neurol 2007;64:1176–1182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.