Abstract
Objective:
To describe the ocular motor abnormalities in 9 patients with a lesion involving the nucleus prepositus hypoglossi (NPH), a key constituent of a vestibular-cerebellar-brainstem neural network that ensures that the eyes are held steady in all positions of gaze.
Methods:
We recorded eye movements, including the vestibulo-ocular reflex during head impulses, in patients with vertigo and a lesion involving the NPH.
Results:
Our patients showed an ipsilesional-beating spontaneous nystagmus, horizontal gaze-evoked nystagmus more intense on looking toward the ipsilesional side, impaired pursuit more to the ipsilesional side, central patterns of head-shaking nystagmus, contralateral eye deviation, and decreased vestibulo-ocular reflex gain during contralesionally directed head impulses.
Conclusions:
We attribute these findings to an imbalance in the NPH–inferior olive–flocculus–vestibular nucleus loop, and the ocular motor abnormalities provide a new brainstem localization for patients with acute vertigo.
The nucleus prepositus hypoglossi (NPH) plays a role in the integration of velocity to position signals for horizontal eye movements so that the eyes can be held steady in eccentric positions in the orbit.1,2 The NPH has extensive connections with the vestibulocerebellum and vestibular nuclei (VNs) and participates in the vestibulo-cerebello-ocular motor circuit.3,4 Despite the central location of the NPH in the vestibulo-ocular reflex (VOR) circuitry, neither its role in the VOR, especially during high acceleration stimuli as used in the head impulse test (HIT), nor a detailed analysis of associated eye movement abnormalities has been presented in humans with an NPH lesion. Experimental damage of the NPH in monkeys causes defects of maintaining steady gaze and an asymmetric vestibular nystagmus induced by horizontal rotation, while saccades, smooth pursuit (SP), and sinusoidal VOR are minimally affected.5,6 In cats, unilateral NPH injury produces a failure of horizontal gaze holding bilaterally and an asymmetric VOR induced by rotatory stimuli.7 In humans with NPH lesions, the few existing studies described gaze-evoked nystagmus (GEN), ipsilesional- or contralesional-beating horizontal nystagmus, and postural imbalance with contralateral or bilateral falling.8–10 Here, we report the VOR during head impulses and other ocular motor functions in patients with a focal brainstem lesion involving the NPH.
METHODS
Subjects and evaluation.
We recruited 9 patients with a lesion involving the NPH who had undergone an HIT at the Dizziness Clinic of Seoul National University Bundang Hospital (5 women, 4 men, mean ± SD age = 66 ± 5.4 years, range = 45–74 years); 8 patients had a unilateral NPH lesion, and one (P9) had bilateral involvement. Eight of the 9 patients had an acute infarction involving the unilateral or bilateral NPHs, while one (P3) had a low-grade glioma involving the right NPH. Involvement of the NPH was based on the location of the lesion on axially oriented, high-resolution MRIs, including diffusion-weighted imaging. The lesions on diffusion-weighted imaging were inspected to select the appropriate templates from an anatomic atlas.11 Neurologic and neuro-otologic evaluations were performed during the acute period (average interval 3.3 days, range = 1–7 days in the 8 patients with an infarction) but 30 days after onset of symptoms in the patient with a glioma.
Oculographic study.
Video-oculography (SMI, Teltow, Germany) with a sampling rate of 60 Hz recorded eye movements, including spontaneous nystagmus (SN) in the light and in darkness, GEN with horizontal target displacements of ±30°, horizontal SP and saccades, and head-shaking nystagmus (HSN). Normative data were obtained from 50 age-matched volunteers. Ocular torsion, quantified with fundus photographs, subjective visual vertical (SVV), and caloric testing were obtained in all patients. Detailed methods of each test have been described previously.12–14
Head impulse tests.
Bedside HITs were performed with a rapid rotation of the head of ≈20° amplitude in the planes of the horizontal and the vertical canals. HITs were quantified with the magnetic search coil technique in a 70-cm cubic search coil frame (Skalar, Delft, the Netherlands) for 2 patients with unilateral NPH lesion and one with bilateral infarction involving the NPH. A reduced HIT gain was determined when the mean VOR gains were less than the mean − 2 SD of the control data.14 In the other patients, HITs were manually performed and determined by the senior author (J.-S. K).
Standard protocol approval, registrations, and patient consents.
All experiments followed the tenets of the Declaration of Helsinki. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-1109/135-106). Written informed consents were obtained from the participants.
Representative case.
Patient 1.
A 53-year-old man with hypertension and diabetes mellitus developed acute vertigo and weakness of his left arm and leg. Clinical examination 4 days after the onset of symptoms found a rightward head tilt but no skew deviation. He had SN that beat rightward during visual fixation, increased in darkness and on rightward gaze, and changed into left-beating nystagmus on leftward gaze (video 1 at Neurology.org). In darkness, the right-beating nystagmus had clockwise (relative to the patient view) torsional (upper poles of the eyes beat toward the patient's right shoulder) and up-beating components. A leftward static deviation of the eyes under closed lids was inferred on the basis of rightward correction on eye opening. SP movement to the right was impaired but was normal to the left and vertically. Horizontal and vertical saccades were normal. Bedside HITs showed corrective catch-up saccades during head impulses to the left (video 2). The patient also had left hemiparesis, left hypesthesia, and left central-pattern facial palsy.
Video-oculography documented right-beating SN with a mean slow-phase velocity (SPV) of 2.9 ± 0.4°/s during visual fixation. When visual fixation was removed, the right-beating nystagmus had a decelerating waveform with an initial SPV of 12.3 ± 2.6°/s (figure 1A). The patient showed asymmetric horizontal GEN (figure 1B) and impaired SP to the right (figure 1C). The amplitude of horizontal saccades was normal but with drift (time constant [Tc] = 2.2 ± 0.3 seconds) after rightward (30°) saccades but little drift (Tc = 25.9 ± 3.3 seconds) after leftward (30°) saccades (figure 1D). Caloric testing was normal. Search coil recordings of HITs documented decreased gains and corrective catchup saccades for stimulation of the left horizontal semicircular canal (HC) and increased gain for stimulation of each anterior canal (AC), more for the right than for the left AC (figure 1E). During AC stimulation, there was a premature deceleration resulting in corrective catchup saccades (figure 1E). The patient also showed clockwise (relative to the patient’s view) ocular torsion and rightward tilt of the SVV (11.7°, binocular viewing, normal range = −2.4 to 2.6° [positive value indicates rightward tilt]). Diffusion-weighted MRIs showed a linear infarction in the right medial medulla, mainly in the tegmentum (figure 2, P1). The vertigo improved over several days, and 50 days later, he only had a small ipsilesionally beating SN (SPV < 1°/s) in darkness and no GEN. However, the pattern of abnormalities on HITs was unchanged.
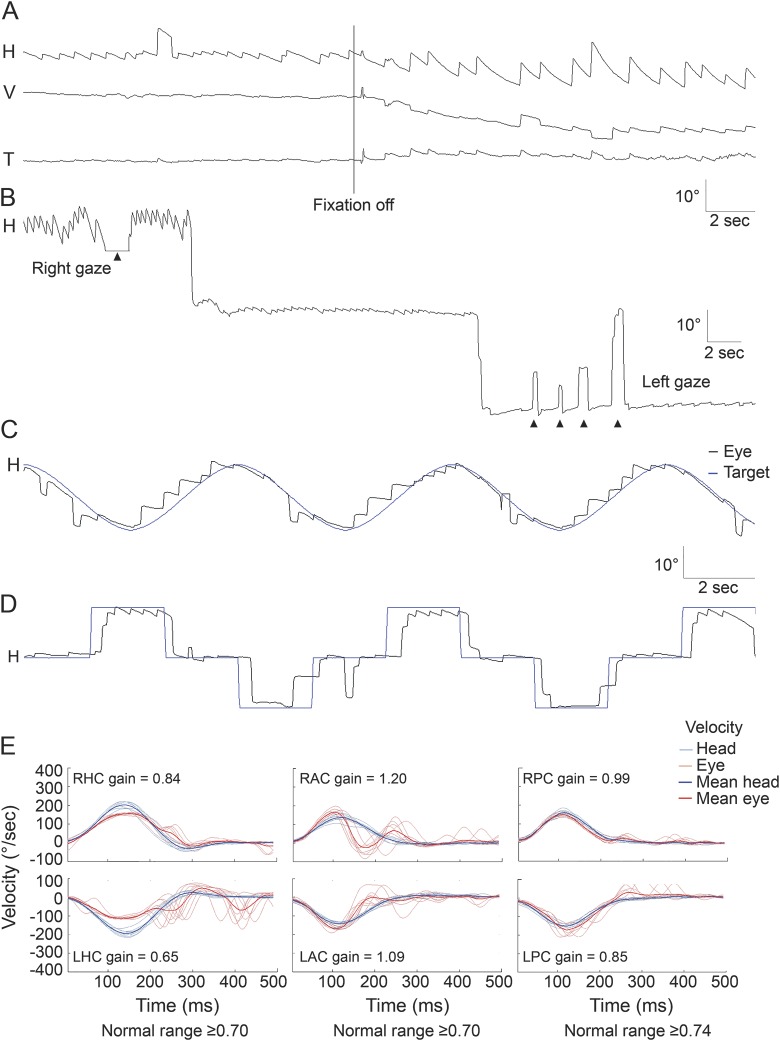
Figure 1. Abnormal eye movements in patient 1 with a lesion involving the right nucleus prepositus hypoglossi.
(A) Patient 1 shows spontaneous right-beating nystagmus with a mean slow-phase velocity (SPV) of 2.9 ± 0.4°/s, which increases when fixation is eliminated, showing a decelerating waveform and an initial SPV of 12.3 ± 2.6°/s. (B) Lateral gaze produces gaze-evoked nystagmus, more intense when looking to the ipsilesional side. (C) Smooth pursuit is impaired ipsilesionally (gain ≈0.2 in response to a sinusoidal target motion at a peak velocity of 10°/s). (D) Horizontal saccades are normal. (E) Recordings of head impulse tests with the magnetic search coil technique show decreased gain of the vestibulo-ocular reflex for the left horizontal canal (HC). The gains for both anterior canals (ACs) are increased, but premature deceleration results in corrective catchup saccades. In contrast, the gains for both posterior canals (PCs) are within the normal range. The gains were measured as the ratio of mean eye velocity to mean head velocity during a 40-millisecond window centered at peak head acceleration. H = horizontal; T = torsional; V = vertical.
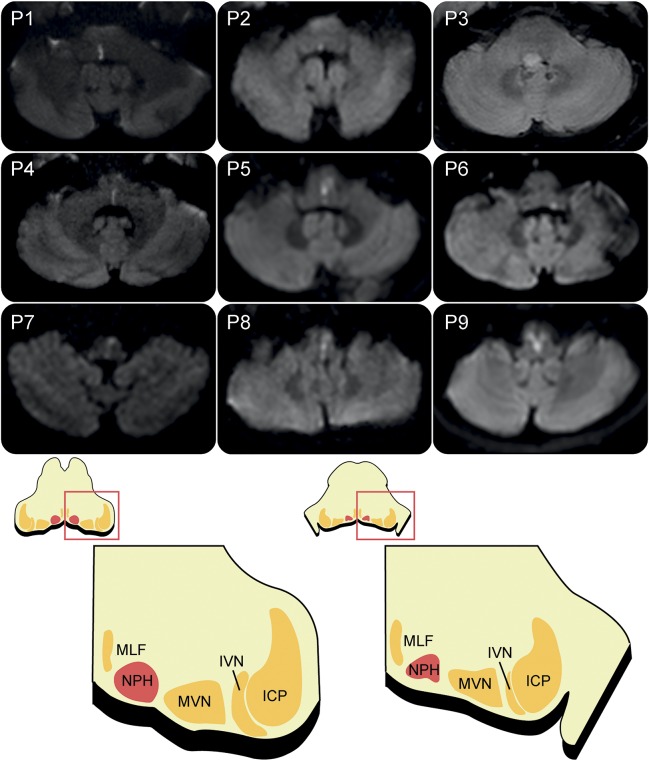
Figure 2. Brain imaging of the patients and illustration of the nucleus prepositus hypoglossi (NPH).
Patients 1 through 8 have a unilateral NPH lesion, and patient 9 has an infarction involving both NPHs. Patient 3 has a small glioma located in the right-sided dorsal pons, and the other 8 patients have an acute infarction involving the NPH. ICP = inferior cerebellar peduncle; IVN = inferior vestibular nucleus; MLF = medial longitudinal fasciculus; MVN = medial vestibular nucleus.
RESULTS
The extent of the lesion on axial MRIs of each patient is presented in figure 2. The lesions invariably involved the dorsal brainstem extending from the lower pons to the medulla near to the midline. Acute vertigo was the main presentation in 8 patients with unilateral or bilateral NPH infarction, and one patient with unilateral pontomedullary glioma reported progressively increasing unsteadiness over the course of a month.
Spontaneous nystagmus.
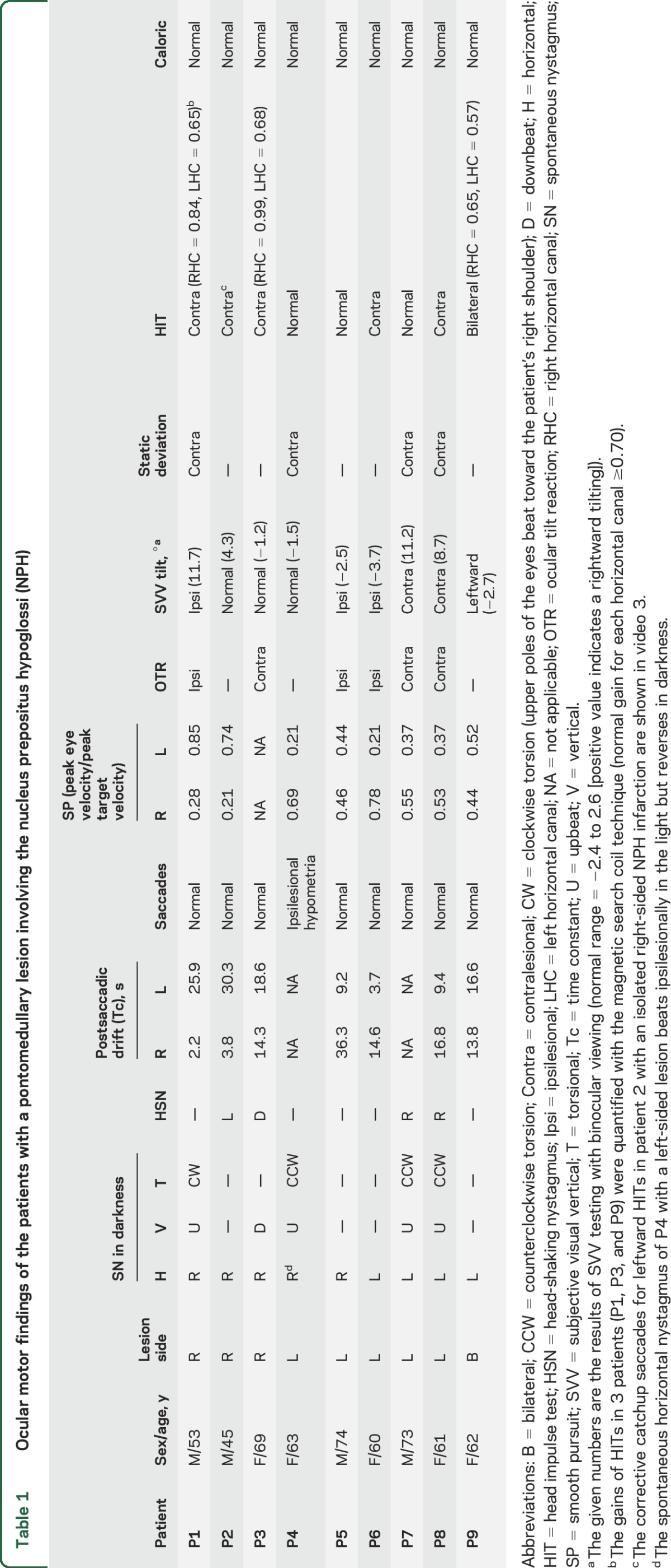
During fixation, 4 of the 8 patients with a unilateral NPH lesion showed horizontal SN that beat toward the ipsilesional side. The SN markedly increased in darkness in 3 patients, while one patient (P4) showed a reversal of nystagmus. In darkness, 7 of the 8 patients with a unilateral NPH lesion showed horizontal SN beating toward the ipsilesional side with a decelerating slow-phase waveform.
Gaze-evoked nystagmus.
All 8 patients with a unilateral NPH lesion had asymmetric horizontal GEN, greater during ipsilesional gaze (table 1). Six patients with GEN in the light showed a smaller Tc (mean = 7.1 ± 4.3 seconds, range = 2.2–14.3 seconds) after saccades to the ipsilesional side than the Tc (mean = 23.7 ± 7.2 seconds, range = 14.6–36.3 seconds) after contralesional saccades (mean asymmetry of Tc = 53.9 ± 27.7%, range = 13%–84%). Of note, 2 patients without SN during straight-ahead fixation also showed asymmetric GEN, which was stronger on looking toward the ipsilesional side. In contrast, one patient with bilateral NPH infarction showed relatively symmetric GEN (asymmetry of Tc = 9.2%).
Table 1.
Ocular motor findings of the patients with a pontomedullary lesion involving the nucleus prepositus hypoglossi (NPH)
Head-shaking nystagmus.
Four patients showed HSN with central patterns,13 horizontal HSN in the direction opposite the SN in 3, and a perverted downbeat nystagmus after horizontal head shaking in one.
Smooth pursuit.
Horizontal SP in response to sinusoidal target motion was impaired toward the ipsilesional side in 8 patients with a unilateral NPH lesion. One patient with bilateral NPH lesion showed bilaterally impaired SP. Five of the 8 patients with a unilateral NPH lesion also showed bilaterally impaired SP, but worse toward the ipsilesional side.
Saccades and static ocular deviation.
Horizontal saccades were normal in 7 patients with a unilateral NPH lesion, while only one showed ipsilesional hypometria. Four patients with a unilateral lesion showed static ocular deviation to the contralesional side under closed lids.
Ocular tilt reaction and SVV.
Seven of the 8 patients with a unilateral NPH lesion showed head tilt, skew deviation, or ocular torsion, 4 with a contraversive ocular tilt reaction (OTR), and 3 patients with an ipsiversive OTR. Five of the 8 patients with a unilateral lesion showed abnormal SVV tilt in the direction of the OTR, and one patient without an OTR showed abnormal SVV tilt toward the ipsilesional side.
Caloric tests and HITs.
None of the patients showed caloric paresis. Bedside HITs revealed corrected catchup saccades when stimulating the contralesional HC in 5 of the 8 patients with a unilateral NPH lesion. Search coil recordings of HITs in a patient (P1, representative case) with a right-sided NPH infarction documented decreased VOR gains for the left HC (gain at 0.65 for the contralesional HC and 0.84 for the ipsilesional HC [normal ≥0.70]) and increased gain for both ACs (1.20 for the ipsilesional AC and 1.09 for the contralesional AC [normal ≤1.02]). One patient (P3) with a right-sided glioma also showed decreased head impulse VOR gain for the contralesional HC (0.68 for the contralesional HC and 0.99 for the ipsilesional HC) and increased gain for both ACs (1.06 for the ipsilesional AC and 1.08 for the contralesional AC). A patient (P9) with an infarction involving both NPHs showed decreased gains for both HCs (0.65 for the right HC and 0.57 for the left HC) but normal gains for each vertical canal.
DISCUSSION
Our patients with NPH lesions showed a distinct pattern of eye movement abnormalities: ipsilesional-beating SN; horizontal GEN, more intense on looking toward the ipsilesional side; central patterns of HSN; impaired SP, greater ipsilesionally; and static contralateral ocular deviation. Furthermore, patients with NPH lesions showed decreased head impulse VOR gains for the contralesional HC and increased gains for both ACs.
While a prior study described SN that could beat ipsilesionally or contralesionally in unilateral pontomedullary lesions involving NPH,9 most of our patients with a unilateral NPH lesion showed ipsilesional-beating SN with slow phases having a decelerating waveform, the hallmark of impaired neural integration. The NPH and adjacent medial VN (MVN) serve as the horizontal neural integrator, and experimental lesions there cause GEN,15 just as our patients had, although additional damage to the nearby paramedian tracts/nuclei may also contribute to gaze-holding defects.16 The NPH lies next to the midline of the lower pons and enlarges with an egg-shaped outline, the longer axis directed horizontally, on cross sections of the medulla oblongata.11 The NPH occupies the medial part of the dorsal medulla beneath an elevation of the floor of the fourth ventricle, reaching the lesion in P6 that is located more laterally than the other patients. Because the most lateral part of the NPH is next to the medial MVN in the medulla,11 discriminating the involved structure(s) on the basis of imaging is difficult. Meanwhile, patients with isolated unilateral MVN infarction usually show horizontal-torsional SN beating contralesionally and horizontal GEN that is more intense when looking toward the healthy side.14 This is the opposite pattern shown by most of our patients with NPH lesions. The characteristics of the SN and GEN in our patients point to NPH involvement rather than the VN (table 2).
Table 2.
Comparison of ocular motor abnormalities between unilateral lesions involving the vestibular nucleus (VN) and nucleus prepositus hypoglossi (NPH)
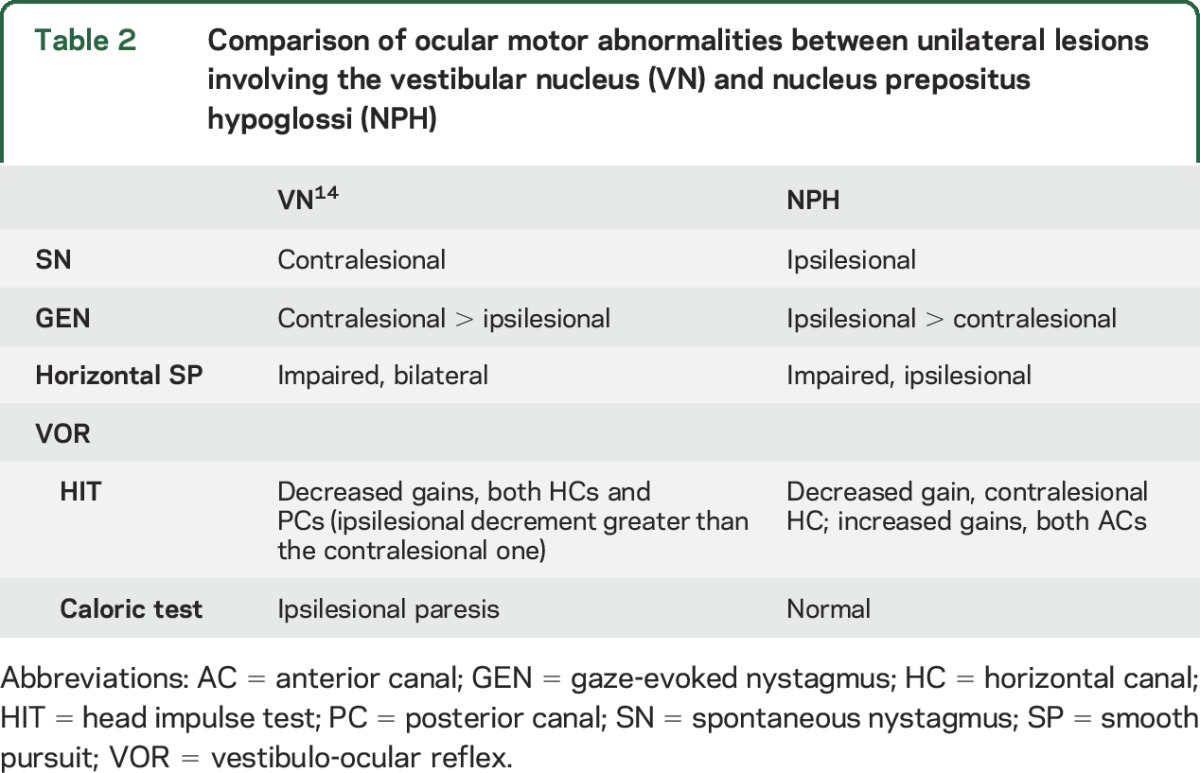
All patients with a unilateral NPH lesion had asymmetric horizontal GEN, greater during ipsilesional gaze. This asymmetric GEN in NPH lesions need not be due to a simple additive effect of SN on symmetrical GEN. The lower Tc of postsaccadic drift after eccentric gaze to the ipsilesional side in our patients indicates that integration was more severely impaired when the eyes lie ipsilesionally in the orbit.1 Unilateral NPH lesions in monkeys reduce the Tc to 1/10 of normal, especially in the ipsilesional hemifields.5 The dependence of nystagmus on eye position in peripheral vestibular lesions (Alexander law) has been attributed to adaptive changes in the brainstem velocity–to–position neural integrator,17 although it has been recently hypothesized that the cause is nonlinear processing of information in the VN.18
Five of the 8 patients with a unilateral NPH lesion showed corrective catchup saccades for the contralesional VOR, and search coil recordings of HITs documented a decreased VOR gain for the contralesional HC and increased gain for both ACs. The NPH has extensive and reciprocal connections with the VN both directly and indirectly via the vestibulocerebellum.19 The NPH has inhibitory projections to both inferior olivary nuclei (IONs), but crossed projections are 3 times denser than uncrossed projections.20 The NPHs also inhibit each other via GABAergic commissural projections (figure 3A).20 Thus, the inhibitory projections from the damaged NPH to the contralesional NPH and to both IONs, but more to the contralesional ION, are diminished in a unilateral NPH lesion. This would produce a relative inhibition of the ipsilesional ION and activation of the contralesional ION (figure 3B). Because the climbing fibers from the IONs cross to contact the Purkinje cells of the contralateral flocculus, which in turn inhibit the VN on the same side as the flocculus, reduced firing of the inhibitory climbing fibers from the ipsilesional ION would lead to disinhibition (i.e., activation) of the contralesional flocculus and increased suppression of the contralesional VN (figure 3B).9,21 In summary, unilateral NPH injury induces a relative hypofunction of the ipsilesional ION, ipsilesional flocculus, and contralesional VN (figure 3B).
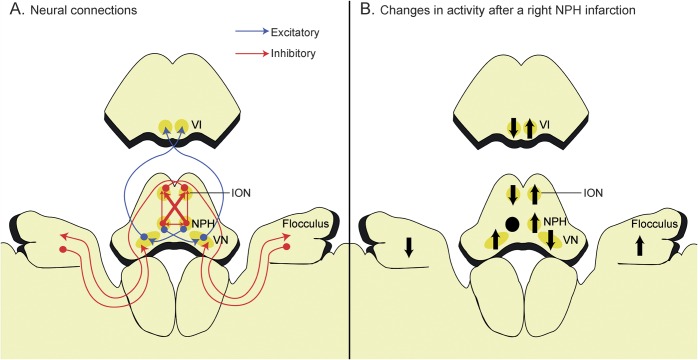
Figure 3. Schematic illustration of the connections among the nucleus prepositus hypoglossi (NPH), inferior olivary nucleus (ION), flocculus, and vestibular nucleus (VN).
(A) The NPH inhibits its contralateral partner through the commissural connections and suppresses the contralateral ION more than the ipsilateral one. The ION sends climbing fibers that cross in the brainstem to reach the Purkinje cells in the contralateral flocculus, which in turn inhibit the VN on the same side. (B) A unilateral NPH injury would result in increased inhibition of the ipsilesional ION, disinhibition of the contralesional flocculus, and suppression of the contralesional VN, which would in turn decrease the gain of the vestibulo-ocular reflex during head impulses in the contralesional direction. The NPH is displaced anteriorly for the clarity of presentation. Black arrows indicate changes in activity after the NPH lesion. VI = abducens nucleus.
Consistent with this mechanism, the HIT gain was decreased in a patient with unilateral floccular infarction, especially during contralesional head impulses.12 Unilateral pharmacologic inactivation of the NPH in cats produced a normal VOR during rotation toward the ipsilesional side but diminished responses during rotation toward the healthy side.7 Because the cerebellar Purkinje cells provide a frequency-dependent signal,12,22 the functional deficit of the ipsilesional flocculus in unilateral NPH injury could decrease the VOR gain selectively during contralesional HITs (a high-frequency stimulus) while leaving the caloric response (a low-frequency stimulus) intact, as observed in our patients. Alterations in the NPH-ION-flocculus-VN loop can also account for the increased VOR gains for the ACs because the flocculus preferentially inhibits the ipsilateral AC pathway.23 Previous studies showed that deficient inhibitory projections from floccular Purkinje cells to the VN induce a higher gain during AC than during posterior semicircular canal (PC) stimulation.24 The impaired VOR only during contralesional HITs observed with NPH lesions contrasts to findings in patients with unilateral MVN lesions, in whom the head impulse VOR gains were decreased for HCs and PCs on both sides, but more ipsilesionally (table 2).14 Although positive HITs are usually a sign of a peripheral vestibular lesion, decreased HIT VOR gains do not exclude central vestibular damage but are localizing only when combined with other ocular motor abnormalities.
The SN of one patient (P4) with an NPH lesion next to the brainstem midline beat ipsilesionally in the light but reversed in darkness. Another patient (P5) with a lesion adjacent to the medullary midline also showed contralesionally beating SN only in darkness. The HITs in those 2 patients were normal. Midline lesions of the inhibitory commissural connections between the 2 NPHs symmetrically reduce bilateral vestibular responses.9 Alternatively, a central vestibular imbalance with a unilateral NPH lesion might be undetectable with the HITs, especially when crossed inhibitory projections to the ION from the intact NPH are also damaged at the brainstem midline.
The activity carried on primary vestibular afferents during head rotations made up of low frequencies is perseverated centrally by a velocity-storage mechanism that sustains nystagmus, even after the firing of the peripheral vestibular system has ceased.1,13 Our patients with an NPH lesion have a central vestibular imbalance with lesser tone on the contralesional side, leading to an ipsilesional-beating SN. The contralesional HSN seen in 3 patients, however, suggests that the central vestibular imbalance is reversed transiently after horizontal head shaking. The dissociation between SN and HSN direction in our patients with a focal unilateral brainstem lesion adjacent to the midline suggests that vestibular activity did not accumulate during head shaking on the ipsilesional side but was stored normally on the intact side. A previous study showed that midline lesions at the pontomedullary junction reduce the Tc of per-rotary and postrotatory nystagmus, implying an impaired velocity-storage mechanism.25 The perverted downbeat HSN in one patient may be explained by the amplified AC pathway signals resulting from the disruption of cerebellar inhibitory projections or from asymmetric involvement of the brainstem otolith pathways that leads to a tilt in the gravity-dependent vector of the canal reflex.26
Our patients with unilateral NPH infarction typically showed impaired SP, greater toward the ipsilesional side. This finding is unlikely due to the weak ipsilesional-beating SN. The NPH receives afferents from the nucleus of the optic tract that contribute to motion perception and SP.27 The NPH may influence SP by relaying this information to the flocculus and paraflocculus.19 The NPH also receives a projection from the contralateral fastigial oculomotor region that helps accelerate contralateral and decelerate ipsilateral saccades and impairs contralateral SP when damaged.27,28
Horizontal saccades were symmetric in most of our patients with a unilateral NPH lesion, except one with hypometric ipsilesional saccades. Experimental lesions of the NPH in monkeys and cats minimally affect the metrics of horizontal saccades.5,7 The NPH, in contrast to its essential role for the VOR and neural integration, may not be crucial for saccade generation. In contrast, the ipsilesional ocular deviation under closed lids was observed in half of the patients with a unilateral NPH lesion. Given the symmetric saccades in most of these patients, the deviation might be a direct effect of the unilateral NPH lesion on activity of the VN on both sides: relative disinhibition of the ipsilesional VN and suppression of the contralesional VN. These activity changes between the VN on both sides would lead to hyperactivity of the contralesional abducens nucleus and resultant ocular deviation (figure 3B).
The diagnosis of stroke in patients with acute sustained vertigo requires a careful and focused clinical examination. This study defines a unique pattern of vestibular and ocular motor abnormalities in acutely dizzy patients with brainstem infarction involving the NPH, which differs from the ocular motor syndrome of the adjacent VN. We suggest a pathophysiology based on abnormalities in the NPH–inferior olive–flocculus–VN loop.
Supplementary Material
GLOSSARY
- AC
anterior semicircular canal
- GEN
gaze-evoked nystagmus
- HC
horizontal semicircular canal
- HIT
head impulse test
- HSN
head-shaking nystagmus
- ION
inferior olivary nucleus
- MVN
medial vestibular nucleus
- NPH
nucleus prepositus hypoglossi
- OTR
ocular tilt reaction
- PC
posterior semicircular canal
- SN
spontaneous nystagmus
- SP
smooth pursuit
- SPV
slow-phase velocity
- SVV
subjective visual vertical
- Tc
time constant
- VN
vestibular nucleus
- VOR
vestibulo-ocular reflex
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
S.-H.K., as the first author, contributed to analysis and interpretation of the data and drafting of the manuscript. J.-S.K., as the corresponding author, contributed to design of the study, interpretation of the data, and critical revision of the manuscript. D.S.Z. and S.d.L. contributed to interpretation of the data and critical revision of the manuscript. H.J.K. contributed to technical and material support.
STUDY FUNDING
This study was supported by grants from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A080750).
DISCLOSURE
S. Kim reports no disclosures relevant to the manuscript. D. Zee serves as an associate editor of Frontiers in Neuro-otology and a member of the editorial board of The Cerebellum. He received speaker’s honoraria from Abbott Pharmaceuticals and Micromedical Technologies and royalties from Oxford University Press. S. du Lac and H. Kim report no disclosures relevant to the manuscript. J. Kim serves as an associate editor of Frontiers in Neuro-otology, serves on the editorial boards of the Journal of Korean Society of Clinical Neurophysiology, Research in Vestibular Science, Journal of Clinical Neurology, Frontiers in Neuro-ophthalmology, Journal of Neuro-ophthalmology, and Journal of Vestibular Research, and has received research support from SK Chemicals, Co. Ltd. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Leigh RJ, Zee DS. The Neurology of Eye Movements, 5th ed. New York, NY: Oxford University Press; 2015. [Google Scholar]
- 2.McFarland JL, Fuchs AF. Discharge patterns in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movement in behaving macaques. J Neurophysiol 1992;68:319–332. [DOI] [PubMed] [Google Scholar]
- 3.Baker R, Berthoz A. Is the prepositus hypoglossi nucleus the source of another vestibulo-ocular pathway? Brain Res 1975;86:121–127. [DOI] [PubMed] [Google Scholar]
- 4.McCrea R, Baker R. Anatomical connections of the nucleus prepositus of the cat. J Comp Neurol 1985;237:377–407. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko CR. Eye movement deficits after ibotenic acid lesions of the nucleus prepositus hypoglossi in monkeys, I: saccades and fixation. J Neurophysiol 1997;78:1753–1768. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko CR. Eye movement deficits following ibotenic acid lesions of the nucleus prepositus hypoglossi in monkeys II. Pursuit, vestibular, and optokinetic responses. J Neurophysiol 1999;81:668–681. [DOI] [PubMed] [Google Scholar]
- 7.Godaux E, Mettens P, Chéron G. Differential effect of injections of kainic acid into the prepositus and the vestibular nuclei of the cat. J Physiol 1993;472:459–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho HJ, Choi HY, Kim YD, Seo SW, Heo JH. The clinical syndrome and etiological mechanism of infarction involving the nucleus prepositus hypoglossi. Cerebrovasc Dis 2008;26:178–183. [DOI] [PubMed] [Google Scholar]
- 9.Seo SW, Shin HY, Kim SH, et al. Vestibular imbalance associated with a lesion in the nucleus prepositus hypoglossi area. Arch Neurol 2004;61:1440–1443. [DOI] [PubMed] [Google Scholar]
- 10.Kim JS, Choi KD, Oh SY, et al. Medial medullary infarction: abnormal ocular motor findings. Neurology 2005;65:1294–1298. [DOI] [PubMed] [Google Scholar]
- 11.Büttner-Ennever JA, Horn AK. Olszewski, Baxter's Cytoarchitecture of the Human Brainstem, 3th ed. Basel, Switzerland: Karger; 2014. [Google Scholar]
- 12.Park HK, Kim JS, Strupp M, Zee DS. Isolated floccular infarction: impaired vestibular responses to horizontal head impulse. J Neurol 2013;260:1576–1582. [DOI] [PubMed] [Google Scholar]
- 13.Choi KD, Oh SY, Park SH, Kim JH, Koo JW, Kim JS. Head-shaking nystagmus in lateral medullary infarction patterns and possible mechanisms. Neurology 2007;68:1337–1344. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Lee SH, Park JH, Choi JY, Kim JS. Isolated vestibular nuclear infarction: report of two cases and review of the literature. J Neurol 2014;261:121–129. [DOI] [PubMed] [Google Scholar]
- 15.Arnold DB, Robinson DA, Leigh RJ. Nystagmus induced by pharmacological inactivation of the brainstem ocular motor integrator in monkey. Vis Res 1999;39:4286–4295. [DOI] [PubMed] [Google Scholar]
- 16.Nakamagoe K, Iwamoto Y, Yoshida K. Evidence for brainstem structures participating in oculomotor integration. Science 2000;288:857–859. [DOI] [PubMed] [Google Scholar]
- 17.Robinson DA, Zee DS, Hain TC, Holmes A, Rosenberg LF. Alexander's law: its behavior and origin in the human vestibulo-ocular reflex. Ann Neurol 1984;16:714–722. [DOI] [PubMed] [Google Scholar]
- 18.Bockisch CJ, Khojasteh E, Straumann D, Hegemann SC. Eye position dependency of nystagmus during constant vestibular stimulation. Exp Brain Res 2013;226:175–182. [DOI] [PubMed] [Google Scholar]
- 19.McCrea RA, Horn AK. Nucleus prepositus. Prog Brain Res 2006;151:205–230. [DOI] [PubMed] [Google Scholar]
- 20.Arts MP, De Zeeuw CI, Lips J, Rosbak E, Simpson JI. Effects of nucleus prepositus hypoglossi lesions on visual climbing fiber activity in the rabbit flocculus. J Neurophysiol 2000;84:2552–2563. [DOI] [PubMed] [Google Scholar]
- 21.Colin F, Manil J, Desclin JC. The olivocerebellar system, I: delayed and slow inhibitory effects: an overlooked salient feature of cerebellar climbing fibers. Brain Res 1980;187:3–27. [DOI] [PubMed] [Google Scholar]
- 22.Raymond JL, Lisberger SG. Neural learning rules for the vestibulo-ocular reflex. J Neurosci 1998;18:9112–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito M, Nisimaru N, Yamamoto M. Specific patterns of neuronal connexions involved in the control of the rabbit's vestibulo-ocular reflexes by the cerebellar flocculus. J Physiol 1977;265:833–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker MF, Zee DS. Cerebellar disease alters the axis of the high-acceleration vestibuloocular reflex. J Neurophysiol 2005;94:3417–3429. [DOI] [PubMed] [Google Scholar]
- 25.Katz E, de Jong JV, Buettner-Ennever J, Cohen B. Effects of midline medullary lesions on velocity storage and the vestibulo-ocular reflex. Exp Brain Res 1991;87:505–520. [DOI] [PubMed] [Google Scholar]
- 26.Kim HA, Lee H, Sohn SI, Kim JS, Baloh RW. Perverted head shaking nystagmus in focal pontine infarction. J Neurol Sci 2011;301:93–95. [DOI] [PubMed] [Google Scholar]
- 27.Voogd J, Schraa-Tam CK, van der Geest JN, De Zeeuw CI. Visuomotor cerebellum in human and nonhuman primates. Cerebellum 2012;11:392–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson FR, Straube A, Fuchs AF. Participation of caudal fastigial nucleus in smooth pursuit eye movements, II: effects of muscimol inactivation. J Neurophysiol 1997;78:848–859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.