Abstract
Objective:
To examine the relationship between gait speed and prior 10 years interleukin-6 (IL-6) burden in older adults. We then assessed whether white matter characteristics influence this relationship.
Methods:
In 179 community-dwelling older adults, gait speed was assessed on an automated walkway and serum IL-6 was assayed on ELISA. Concurrently, white matter characteristics were assessed on MRI by quantifying volume of white matter hyperintensities (WMH), a marker of small vessel disease, and normal-appearing white matter on fractional anisotropy (NAWM-FA), a marker of axonal integrity. IL-6 was assayed at regular intervals at gait assessment and over the prior 10 years and estimates of sustained 10-year IL-6 exposure and the rate of change in IL-6 over 10 years were obtained. Multivariate linear regressions were used to examine the relationships among sustained IL-6 exposure, rate of change in IL-6, gait speed, and white matter characteristics.
Results:
In this sample (age 83 years, 58% female, 41% black, gait speed 0.9 m/s), higher sustained IL-6 levels, but not the rate of change in IL-6 or IL-6 at gait assessment, was significantly related to slower gait (β = −0.27, p < 0.001) and to higher WMH (β = 0.23, p = 0.002), but not NAWM-FA, withstanding covariate adjustments. WMH accounted for 30% attenuation in the relationship between higher sustained IL-6 levels and slower gait speed (p = 0.043) in the mediation analyses.
Conclusions:
Sustained exposure to high IL-6 over 10 years rather than the rate of change in IL-6 or an isolated high IL-6 level may adversely affect gait speed by influencing cerebral WMH.
Increased levels of inflammatory cytokines such as interleukin-6 (IL-6) are cross-sectionally and longitudinally associated with poor physical function in older adults.1–5 IL-6 assayed at regular intervals over a long-term period may better capture sustained IL-6 exposure and rate of change rather than random assays spread apart over time. It is not known whether the sustained long-term exposure to IL-6 or rate of change in IL-6 contributes to the relationship between IL-6 and gait speed beyond a single concurrent indicator of inflammation.
Higher IL-6 is linked to greater volume of white matter hyperintensities (WMH) on MRI in most studies6–8 but not in all.9 WMH are related to slow gait in older adults.10,11 In regions that are free of WMH, which we refer to here as normal-appearing white matter (NAWM), subtle changes are detected on diffusion tensor imaging (DTI) as a reduction in fractional anisotropy of NAWM (NAWM-FA), denoting loss of myelin and astrogliosis at the microstructural level.12,13 Lower NAWM-FA is related to slow gait in elders14,15 as well as chronic inflammation in aging.16 The potential central mechanisms underlying long-term effects of IL-6 on gait slowing remain unknown.
We examined the independent relationships among the longitudinal measures of IL-6 burden, gait speed, and cerebral white matter (WM) characteristics and assessed whether WM characteristics influence these relationships in community-dwelling older adults. We hypothesized that sustained elevation of IL-6 over 10 years is associated with slow gait and this relationship is influenced by cerebral WM disease burden in the brain.
METHODS
Study population.
A total of 1,501 participants were enrolled at the Pittsburgh site of the Health Aging and Body Composition (Health ABC) study, a longitudinal study of physical measures in 3,075 independent community-dwelling older adults aged 70–79 years.17 In the 10th year of the Health ABC study, 819 of the 1,501 alive were screened for an imaging ancillary study. We analyzed data on 179 who had an IL-6 assayed at every timepoint over the 10-year period that included an IL-6 level assayed concurrent with gait speed assessment and brain imaging (flow chart, figure e-1 at Neurology.org).
Standard protocol approvals, registrations, and patient consents.
All participants provided written informed consent for participation. This study was approved by the institutional review boards of the University of Pittsburgh, Pennsylvania, and the University of Tennessee Health Science Center, Memphis.
Inflammatory markers.
Fasting morning blood samples from participants included in this analysis were collected in year 1 (baseline) and years 2, 4, 6, 8, and 10. IL-6 was assayed from serum, citrated plasma, or EDTA plasma.18 At baseline, IL-6 was assayed at the University of Vermont using ELISA (R&D Systems, Minneapolis, MN), and at subsequent timepoints, at Wake Forest University using high-sensitivity Quantikine calorimetric immunoassay (R&D Systems).18,19 The detectable limit for IL-6 (by HS600 Quantikine kit) was 0.10 pg/mL. The interassay coefficients of variation for this assay were 14%, 11%, and 13% for low, medium, and high ranges, respectively. To enable longitudinal analysis of varying IL-6 samples and assays at 2 independent laboratories, IL-6 values were harmonized through a calibration developed at Wake Forest University laboratory as reported previously.19 IL-6 measures were not log-transformed to enable comparisons with other studies.
We obtained 3 main estimates of IL-6 burden: (1) sustained exposure to IL-6 over prior 10 years obtained by averaging IL-6 levels (pg/mL) at all timepoints for each individual; (2) rate of change in IL-6 over prior 10 years using slope of IL-6 change (pg·mL−1·y−1) estimated for each participant using least absolute deviation regression; and (3) IL-6 level (pg/mL) at brain MRI, which was at a concurrent timepoint of gait assessment. In the 179 individuals, the mean (SD) of the percentage of total absolute variation (pseudo-R2) explained by the L1-norm person-specific lines was 18.2% (17.5%) and across the sample the average mean absolute difference from predicted value was 0.81 pg/mL. IL-6 at year 1 of study and at year 10 of study averaged 1.9 and 3.1 pg/mL, respectively. To account for the variation and intermittent spikes across the 10-year period, we used the least absolute deviation regression to estimate slope trends over time rather than least squares, which have a tendency to be strongly influenced by outliers.
Neuroimaging.
Brain imaging was performed on a 3T Siemens (Munich, Germany) Tim Trio magnetic resonance scanner with a Siemens 12-channel head coil and WMH and FA was measured using previously published methods,20,21 summarized here. WMH were classified on fluid-attenuated inversion recovery (FLAIR) using an automated threshold-based algorithm,21 and normalized to total brain volume (sum of voxels classified as gray matter, WM, and CSF obtained from skull-stripped T1 image).22 Diffusion-weighted images were preprocessed using the FMRIB's Diffusion Toolbox23 and the tensor applied was diagonalized and eigenvalues were determined to compute the FA maps,24 which were registered to the FMRIB58_FA template23 as described previously.25 Then, NAWM-FA was obtained utilizing the cerebral WM and WMH segmentation obtained from the magnetization-prepared rapid gradient echo and T2-weighted FLAIR image.26 The Johns Hopkins University White Matter Atlas was overlaid on to the segmented WMH images to obtain regional WMH in the corpus callosum and bilateral anterior thalamic radiation, corticospinal tracts, inferior fronto-occipital fasciculi, inferior longitudinal fasciculi, superior longitudinal fasciculi, and uncinate fasciculi.23,27
Gait measures.
Gait speed (assessed the same day of the brain imaging) was measured on a 4-meter-long GaitMatII (EQ Inc., Chalfont, PA) that contains pressure-sensitive sensors that capture various gait parameters. Two practice walks across the walkway preceded the steady-state gait speed measures, which were averaged over 2 consecutive traverses. Changes in gait speed at initiation and termination of gait were not included in the gait speed estimation.
Covariates.
These included demographic variables and health behavior variables (self-reported smoking, alcohol use, and moderate intensity exercise in the last 12 months), body mass index (BMI, kg/m2), comorbidities (self-reported or hospital-record identified diagnoses of hypertension, diabetes mellitus, angina, myocardial infarction, congestive heart failure, intermittent claudication, stroke, or TIA), modified Mini-Mental State Examination score (3 MS),28 isometric grip strength (assessed using a hand-held dynamometer) (JAMAR Technologies, Inc., Hatfield, PA), age-related parkinsonism (quantified on the Unified Parkinson's Disease Rating Scale), intracranial volume on MRI, microstructural properties of total gray matter assessed on DTI, and APOE ε4 carrier status.
Statistical analysis.
The associations of the 3 IL-6 measures (sustained 10-year IL-6, rate of IL-6 change, IL-6 concurrent with brain MRI) with gait speed and WM measures were assessed using Pearson correlations adjusting for multiple comparisons (Bonferroni correction) and separate linear regression models. First, unadjusted regressions were calculated with each of the IL-6 measures as the primary independent predictor variable and gait speed as dependent variable. We adjusted for sociodemographic variables (model A), BMI, smoking, alcohol, high-intensity exercise hypertension, coronary heart disease, diabetes mellitus II, stroke, grip strength, 3 MS score, APOE ε4 carrier status, intracranial volume, microstructure total gray matter volume (model B, selecting covariates using backward selection criteria [p < 0.01]), and NAWM-FA and WMH volume (models C and D, respectively), which were added to the fully adjusted model and statistical tests for mediation29 of the associations between gait speed and IL-6 measures. We also examined whether baseline IL-6 level or variance in IL-6 levels over 10 years influenced the relationship between our IL-6 measures and gait speed. We finally explored the association between IL-6 and WMH of tracts linked to mobility using Pearson correlation with Bonferroni correction for multiple comparisons. Statistical analyses were performed on SAS 9.3 and SPSS 22.0 statistical software packages.
RESULTS
In this sample of 179 older adults (mean age 83 years, 58% female, 37% black), the mean gait speed was 0.92 m/s and average 3 MS score was 93 (table 1).
Table 1.
Sample characteristics (n = 179)
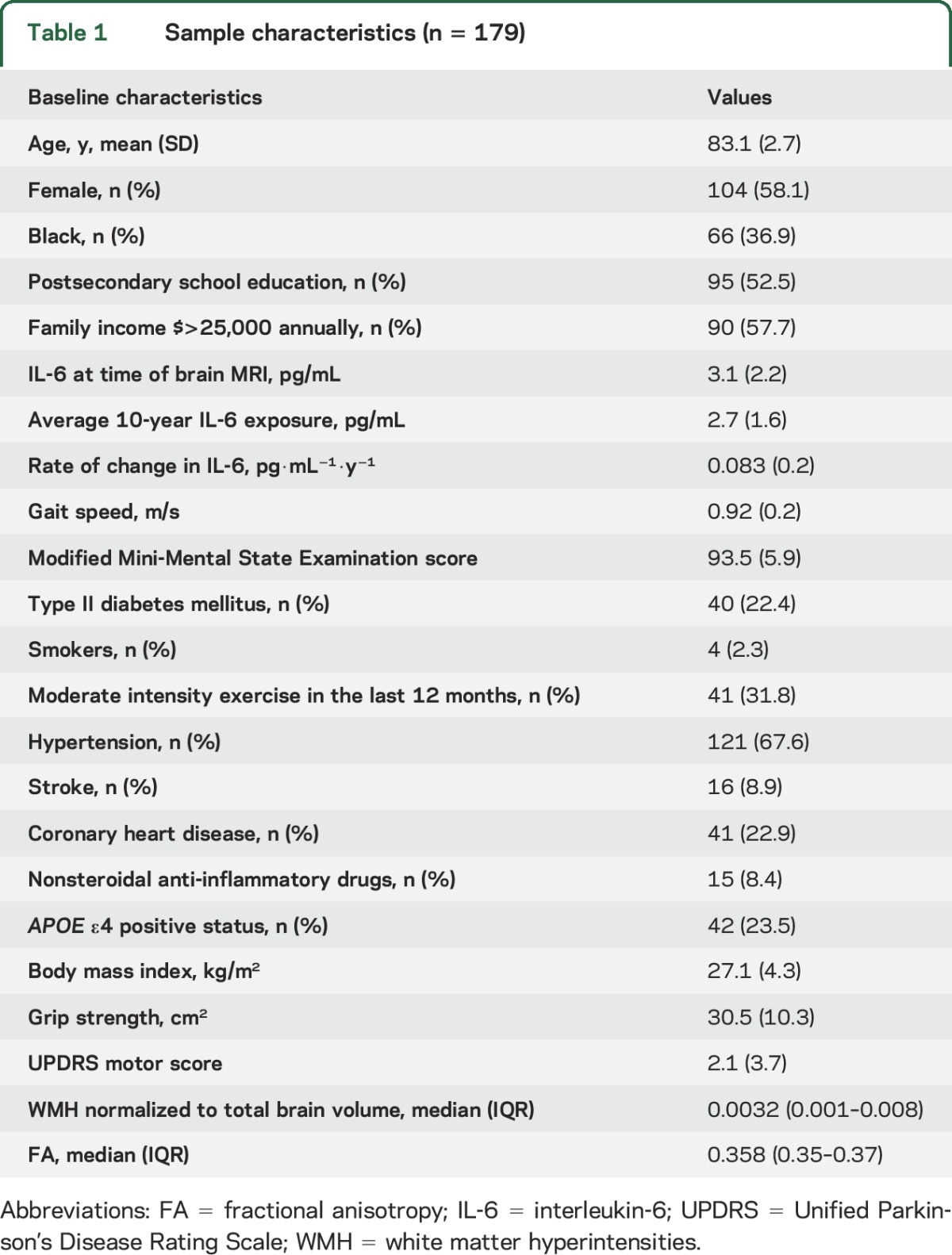
Table 2 shows the unadjusted correlations among IL-6 measures, gait speed, and WM characteristics. Higher 10-year sustained IL-6 exposure and higher IL-6 at brain MRI correlated significantly with slower gait speed and with greater WMH but not with lower NAWM-FA. Additionally, slower gait speed correlated significantly with worse WM characteristics (lower NAWM-FA and greater WMH burden).
Table 2.
Unadjusted correlations (Pearson r and p value) between main variables of interest corrected for multiple comparisons
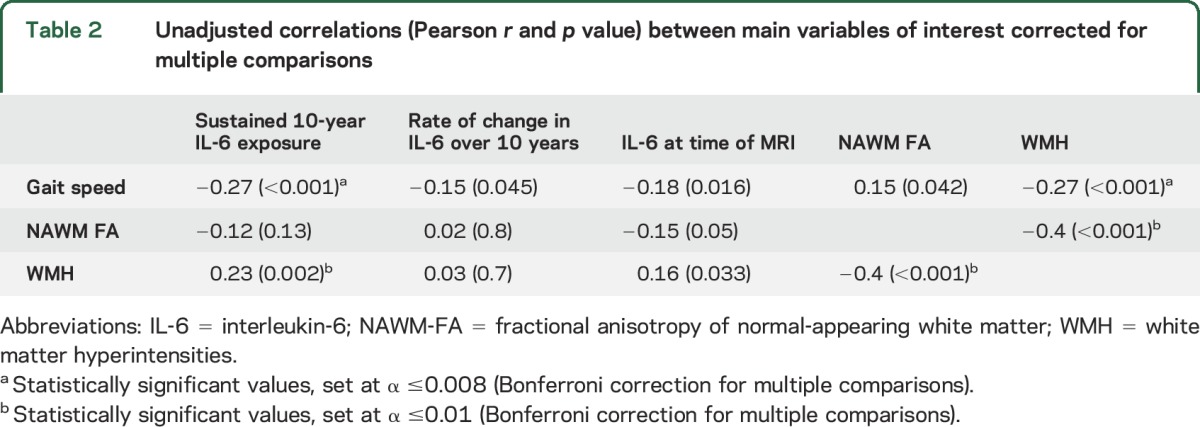
Table 3 shows the unadjusted and adjusted β coefficients of the association between sustained IL-6 exposure over 10 years, gait speed, and WM characteristics. A higher sustained 10-year exposure to IL-6 was significantly associated with slower gait speed (β = −0.266, p < 0.001). This association, though attenuated by approximately 47%, remained statistically significant after accounting for covariates (model B, β= −0.141, p = 0.03). The strength of association between higher sustained 10-year IL-6 exposure and slow gait speed diminished by approximately 63% and was no longer significant with WMH in the model (model C, β = −0.099, p = 0.133). Tests of mediation effects revealed that WMH accounted for approximately 30% of the attenuation in the strength of the relationship between sustained IL-6 and slow gait (p = 0.043; table 3). The association between sustained IL-6 levels and slow gait speed remained significant with NAWM-FA in the fully adjusted model. NAWM-FA did not significantly attenuate the relationship between sustained 10-year IL-6 levels and slow gait speed in the mediation model (p = 0.9). Furthermore, the association between higher 10-year sustained IL-6 exposure and slow gait speed remained statistically significant after accounting for IL-6 level at MRI (β = −0.15, p = 0.04) and the rate of change in IL-6 over 10 years (β = −0.17, p = 0.01) in the fully adjusted model. Finally, adjusting for the IL-6 level at Health ABC study entry or the variance in IL-6 levels over the prior 10 years did not substantially influence the unadjusted association between sustained IL-6 exposure and slow gait speed (β = −0.27 for both, p = 0.002 and 0.006, respectively).
Table 3.
Association of gait speed and NAWM-FA and WMH on brain MRI (dependent variables) with sustained exposure to IL-6 over prior 10 years (independent variable) in the sample of older adults who had IL-6 assayed at all 6 timepoints over the 10-year period (n = 179)
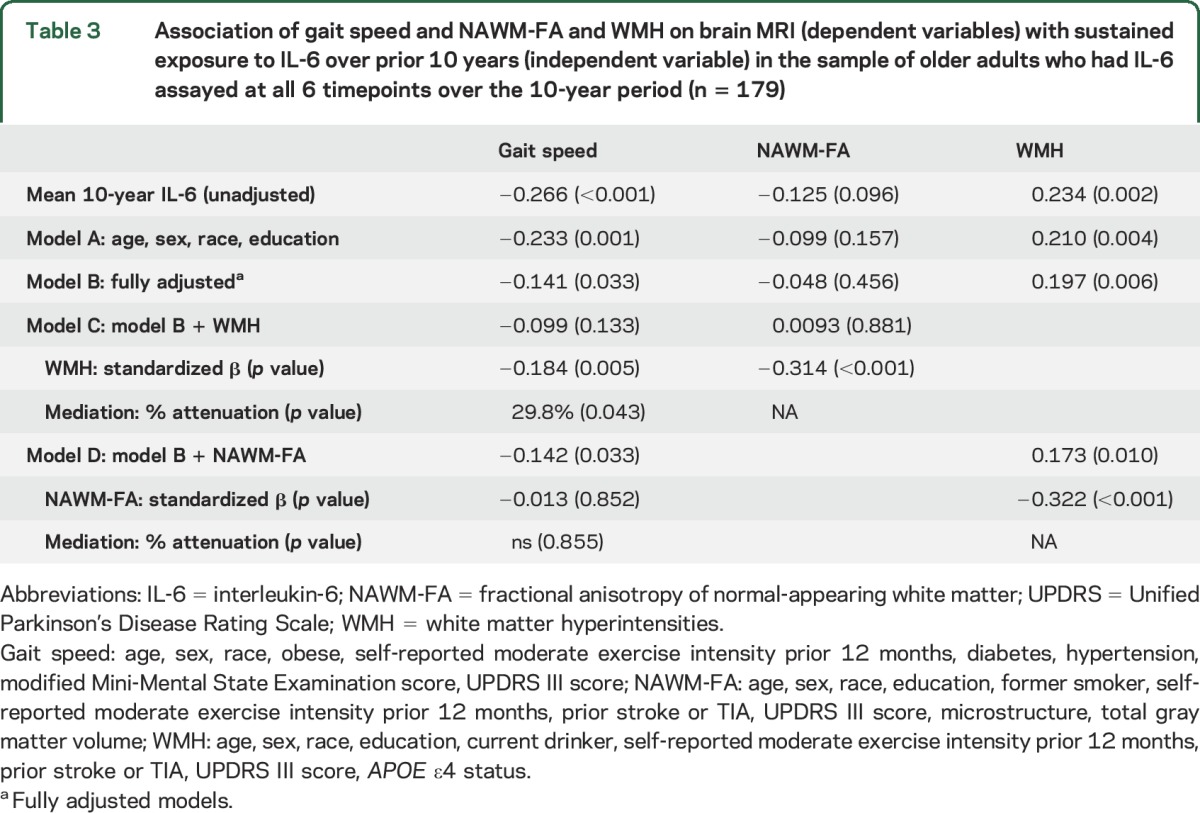
Higher sustained 10-year IL-6 exposure was associated with greater WMH volume (β = 0.234, p = 0.002) and remained statistically significant after accounting for covariates that included APOE ε4 status (table 3, model B, β = 0.197, p = 0.006). With NAWM-FA in the model, there was a modest diminution in strength of association between higher sustained 10-year IL-6 exposure and greater WMH by approximately 26% but the statistical significance was still retained (model D: β = 0.173, p = 0.01).
The association between rate of change in IL-6 over prior 10 years and gait speed was not significant in the fully adjusted model (β = −0.1, p = 0.061). There was no significant association between rate of change in IL-6 and NAWM-FA or WMH volume.
Higher IL-6 level at brain MRI was not significantly associated with slow gait speed after adjustment for sustained IL-6 levels over the prior 10 years (β = 0.004, p = 0.5). Higher IL-6 at MRI was also associated with greater WMH volume (β = 0.159, p = 0.03), and withstood adjustments for demographic covariates, but this relationship was not significant in the fully adjusted model. The association between higher IL-6 level and lower NAWM-FA was not significant when adjusted for WMH (β = −0.09, p = 0.12). IL-6 at time of MRI was not significantly associated with slow gait speed in the fully adjusted model (β = −0.052, p = 0.4) and therefore formal mediation effects of WMH or NAWM-FA in this relationship were not conducted.
Our exploratory analysis of the regional WMH relationships revealed that sustained exposure to high IL-6 over 10 years was significantly correlated with greater WMH in the corpus callosum (r = 0.21, p = 0.005), bilateral anterior thalamic radiation (r = 0.15, p = 0.04), corticospinal tracts (left: r = 0.23, p = 0.002), inferior fronto-occipital fasciculi (r = 0.21, p = 0.006), inferior longitudinal fasciculi (r = 0.16, p = 0.04), and superior longitudinal fasciculi (r = 0.20, p = 0.007), but not the uncinate fasciculi (r = 0.13, p = 0.2); the regional associations in the corpus callosum, corticospinal tracts, inferior fronto-occipital fasciculi, and superior longitudinal fasciculi withstood correction for multiple comparisons (α set at ≤0.0071, Bonferroni correction for 7 regional tracts).
Finally, we compared the 179 participants with IL-6 assays at all timepoints to those excluded as they lacked 1 to 3 IL-6 assays over the 10-year period (n = 101, table e-1). Those excluded had higher IL-6 measures, worse NAWM-FA, lower cognitive performance, and slower gait, although the differences in gait were not statistically significant (table e-1). As a sensitivity analysis, we reanalyzed the whole sample (n = 280), which included the 101 participants who had at least 3 IL-6 assays over the 10-year with a final IL-6 assay concurrent with MRI (average number of IL-6 assays 5, range 3–6). We again found that sustained exposure to high IL-6 level over prior 10 years, but not the rate of change in IL-6 or IL-6 at time of MRI, was associated with slower gait, and this association was influenced by worse WM characteristics (table e-2).
DISCUSSION
In this cohort of older adults with mean age of 83 years, sustained exposure to high IL-6 levels over the prior 10 years is strongly related to slower gait and this association is explained by higher WMH burden in the brain. Each participant had an IL-6 assay conducted at every timepoint of the study, culminating with a final assay at time of gait assessment and brain MRI. Hence, we were able to comprehensively characterize long-term IL-6 burden by measuring sustained IL-6 exposure over 10 years and by estimating the rate of change in IL-6 over this period. Among the measures we utilized to characterize IL-6 burden, we found that only sustained 10-year IL-6 exposure was robust enough to withstand several statistical adjustments, whereas rate of change in IL-6 over 10 years or IL-6 at gait assessment and MRI timepoint were not significant on their own, did not withstand statistical adjustments, or were not significant when accounted for each other in the model. This is the first report indicating that in community-dwelling older adults, higher sustained IL-6 exposure could be detrimental to gait speed, possibly mediated by higher WMH burden in the brain.
The association between IL-6 and mobility decline is frequent but not universal; a significant association between high IL-6 levels and subsequent mobility decline was observed in the Health ABC study,3,4 the Women's Health and Aging Study,30 and InCHIANTI,2 but not in the MacArthur studies of successful aging.31 The Cardiovascular Health Study All-Stars study (mean age 84.9 years, with a demographic that was similar to our study sample) examined association of IL-6 levels at 2 timepoints 9 years apart concurrent with assessment of physical and cognitive impairment; higher baseline IL-6 levels were more strongly associated with slow gait than change in IL-6 over the 2 timepoints.5 This study supports our findings on the lack of robust association between rate of change in IL-6 over 10 years and slow gait speed.
We found that higher IL-6 at time of MRI was related to worse NAWM-FA but not to WMH, whereas sustained IL-6 levels were associated with WMH but not with NAWM-FA. In aging, WMH represent the aftermath of longstanding WM injury that begins with changes in NAWM.32 IL-6 influences inflammatory signaling leading to microglial sensitization33,34 and neuronal damage,35 which decreases the spatial restrictiveness within fibers, making them less anisotropic,12 leading to a lower NAWM-FA signal on DTI.12 Over time, cytokines increase blood–brain permeability enabling reactive gliosis,36 one of the pathologic hallmarks of WMH. Thus, the relationship between concurrent high IL-6 and low NAWM-FA may become blunted over time and high IL-6 sustained over a long-term period could influence downstream development of WMH. This reasoning, though speculative, may explain the lack of relationship between sustained IL-6 and NAWM-FA and between concurrent IL-6 and WMH.
We also found that sustained exposure to high IL-6 level was associated with greater WMH in the corpus callosum, corticospinal tracts, inferior fronto-occipital fasciculi, and superior longitudinal fasciculi. WMH in these tracts can disrupt neural transmission, leading to gait slowing in older adults.10,11,37 Tracts that traverse large areas of watershed WM are more prone to WM injury and WMH.38 Recent evidence also suggests that inflammation influences corpus callosal changes.39,40 Therefore, we speculate that tracts such as the corpus callosum, corticospinal, and long association tracts are likely to bear the brunt of longstanding effects of sustained high inflammation. The attenuation of the relationship between IL-6 exposure and slow gait by WMH points to the regional vulnerability of WM and a compromised neural connectivity of gait driven by an inflammation-mediated pathophysiologic mechanism.
The results of this study have important clinical relevance to age-related mobility decline. Several anti-inflammatory and immune-modulatory therapies, including dietary and behavioral interventions, have potential efficacy in reducing low-grade chronic inflammation in aging. Vascular risk factor modification can potentially mitigate chronically elevated IL-6 levels and potentially affect gait slowing in older adults.4 Our findings suggest that low IL-6 levels sustained over a long-term period may be more meaningful for maintaining WM health and gait speed in older adults.
There are several strengths of this study. We studied participants who had IL-6 levels drawn at every timepoint over the 10-year period. Inclusion into study ascertained that those with mobility disability or impaired mobility were excluded. WM was assessed with both 3T MRI and DTI, which were obtained concurrent with gait speed assessment. The sample was also racially diverse. Finally, our analysis accounted for several confounders known to influence gait in older adults.
Certain limitations need to be considered. Our sample of older adults was likely robust based on MRI and study eligibility and may therefore differ from others who report on similar phenomena in terms of sensitivity of IL-6 assays across timepoints, and sources of bias due to survival, health status, functional status, or cognition. Then again, the finding that sustained high IL-6 levels over 10 years influence gait speed and that WM disease appears to be a key driver of this association in this selective sample is noteworthy. We did not study other inflammatory cytokines such as C-reactive protein that are also linked to mobility decline in aging because C-reactive protein levels were not obtained at the timepoint of brain MRI and gait assessment in the Health ABC study. Brain imaging was not performed at baseline in the study and therefore, we were unable to examine the longitudinal relationships between WMH accrual and the IL-6 measures.
This study suggests that higher sustained exposure to IL-6 over 10 years is associated with slower gait in older adults and this relationship is mediated by cerebral WMH.
Supplementary Material
ACKNOWLEDEGMENT
The authors thank Howard Aizenstein, MD, PhD, and his laboratory for neuroimaging acquisition and processing of imaging data.
GLOSSARY
- 3 MS
modified Mini-Mental State Examination score
- BMI
body mass index
- DTI
diffusion tensor imaging
- FLAIR
fluid-attenuated inversion recovery
- Health ABC
Health Aging and Body Composition
- IL-6
interleukin-6
- NAWM
normal-appearing white matter
- NAWM-FA
fractional anisotropy of normal-appearing white matter
- WM
white matter
- WMH
white matter hyperintensities
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
N.K. Nadkarni contributed to the study design, performed analyses, data interpretation and wrote and edited the manuscript. R.M. Boudreau performed statistical analyses and contributed to the data interpretation and drafting of the manuscript. O.L. Lopez contributed to the data interpretation and contributed to the editing of the manuscript. S.A. Studenski contributed to the study design, interpretation of the data, and editing of the manuscript. G. Liu contributed to the statistical analyses and interpretation of data. S. Kritchevsky contributed to the study design and editing of the manuscript. K. Yaffe supported the study and contributed to the study design and editing of the manuscript. A. Newman supported the study and contributed to the study design, data interpretation, and editing of the manuscript. C. Rosano supported the study and contributed to the study design, interpretation of the data, and editing of the manuscript.
STUDY FUNDING
This research was supported by National Institute on Aging awards K23AG049945, R01AG029232, N01AG62101, N01AG62103, and N01AG 62106; the Intramural Research Program of the NIH; the Pittsburgh Pepper Center (P30 AG024827); and the Pittsburgh Alzheimer's Disease Research Center (P50 AG005133).
DISCLOSURE
N. Nadkarni, R. Boudreau, and S. Studenski report no disclosures relevant to the manuscript. O. Lopez reports consultancy for Grafoil and Lundbeck. G. Liu and S. Kritchevsky report no disclosures relevant to the manuscript. K. Yaffe reports board membership on Takeda, Inc. and Alzheimer's Association Medical & Scientific Advisory Board. A. Newman and C. Rosano report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Verghese J, Holtzer R, Oh-Park M, Derby CA, Lipton RB, Wang C. Inflammatory markers and gait speed decline in older adults. J Gerontol A Biol Sci Med Sci 2011;66:1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2004;59:242–248. [DOI] [PubMed] [Google Scholar]
- 3.Cesari M, Kritchevsky SB, Nicklas B, et al. Oxidative damage, platelet activation, and inflammation to predict mobility disability and mortality in older persons: results from the Health Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci 2012;67:671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc 2004;52:1105–1113. [DOI] [PubMed] [Google Scholar]
- 5.Jenny NS, French B, Arnold AM, et al. Long-term assessment of inflammation and healthy aging in late life: the Cardiovascular Health Study all stars. J Gerontol A Biol Sci Med Sci 2012;67:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright CB, Moon Y, Paik MC, et al. Inflammatory biomarkers of vascular risk as correlates of leukoariosis. Stroke 2009;40:3466–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornage M, Chiang YA, O'Meara ES, et al. Biomarkers of inflammation and MRI-defined small vessel disease of the brain: the Cardiovascular Health Study. Stroke 2008;39:1952–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology 2012;78:720–727. [DOI] [PubMed] [Google Scholar]
- 9.Wersching H, Duning T, Lohmann H, et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 2010;74:1022–1029. [DOI] [PubMed] [Google Scholar]
- 10.Benson RR, Guttmann CR, Wei X, et al. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology 2002;58:48–55. [DOI] [PubMed] [Google Scholar]
- 11.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc 2005;53:649–654. [DOI] [PubMed] [Google Scholar]
- 12.Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med 1999;42:515–525. [PubMed] [Google Scholar]
- 13.Gouw AA, Seewann A, Vrenken H, et al. Heterogeneity of white matter hyperintensities in Alzheimer's disease: post-mortem quantitative MRI and neuropathology. Brain 2008;131:3286–3298. [DOI] [PubMed] [Google Scholar]
- 14.Bolandzadeh N, Liu-Ambrose T, Aizenstein H, et al. Pathways linking regional hyperintensities in the brain and slower gait. Neuroimage 2014;99:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Heuvel DM, ten Dam VH, de Craen AJ, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry 2006;77:149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bettcher BM, Yaffe K, Boudreau RM, et al. Declines in inflammation predict greater white matter microstructure in older adults. Neurobiol Aging 2015;36:948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci 2001;56:M644–M649. [DOI] [PubMed] [Google Scholar]
- 18.Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 2003;61:76–80. [DOI] [PubMed] [Google Scholar]
- 19.Metti AL, Aizenstein H, Yaffe K, et al. Trajectories of peripheral interleukin-6, structure of the hippocampus, and cognitive impairment over 14 years in older adults. Neurobiol Aging 2015;36:3038–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkatraman VK, Aizenstein HJ, Newman AB, et al. Lower digit symbol substitution score in the oldest old is related to magnetization transfer and diffusion tensor imaging of the white matter. Front Aging Neurosci 2011;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res 2006;148:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 2001;20:45–57. [DOI] [PubMed] [Google Scholar]
- 23.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 24.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 1996;36:893–906. [DOI] [PubMed] [Google Scholar]
- 25.Andersson JLR, Jenkinson M. Non-linear Registration, aka Spatial Normalisation: FMRIB Technical Report TR07JA2. Available at: fmrib.ox.ac.uk/analysis/techrep. Accessed July 6, 2016. [Google Scholar]
- 26.Barrick TR, Charlton RA, Clark CA, Markus HS. White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage 2010;51:565–577. [DOI] [PubMed] [Google Scholar]
- 27.Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007;36:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng EL, Chui HC. The modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314–318. [PubMed] [Google Scholar]
- 29.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 2008;40:879–891. [DOI] [PubMed] [Google Scholar]
- 30.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc 2002;50:1947–1954. [DOI] [PubMed] [Google Scholar]
- 31.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci 2000;55:M709–M715. [DOI] [PubMed] [Google Scholar]
- 32.de Groot M, Verhaaren BF, de Boer R, et al. Changes in normal-appearing white matter precede development of white matter lesions. Stroke 2013;44:1037–1042. [DOI] [PubMed] [Google Scholar]
- 33.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 2012;8:1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham C, Maclullich AM. At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain Behav Immun 2013;28:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aktas O, Ullrich O, Infante-Duarte C, Nitsch R, Zipp F. Neuronal damage in brain inflammation. Arch Neurol 2007;64:185–189. [DOI] [PubMed] [Google Scholar]
- 36.Hsuchou H, Kastin AJ, Mishra PK, Pan W. C-reactive protein increases BBB permeability: implications for obesity and neuroinflammation. Cell Physiol Biochem 2012;30:1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moscufo N, Guttmann CR, Meier D, et al. Brain regional lesion burden and impaired mobility in the elderly. Neurobiol Aging 2011;32:646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke 1997;28:652–659. [DOI] [PubMed] [Google Scholar]
- 39.Bettcher BM, Watson CL, Walsh CM, et al. Interleukin-6, age, and corpus callosum integrity. PLoS One 2014;9:e106521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arfanakis K, Fleischman DA, Grisot G, et al. Systemic inflammation in non-demented elderly human subjects: brain microstructure and cognition. PLoS One 2013;8:e73107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


