Abstract
BACKGROUND
Previously, the patient-reported Total Illness Burden Index for Prostate Cancer (TIBI-CaP) questionnaire and/or the physician-reported Charlson Comorbidity Index (CCI) have provided assessments of competing comorbidity during treatment decisions for patients with prostate cancer. In the current study, the authors used these assessments to determine comorbidity and prognosis before prostate biopsy and the subsequent diagnosis of prostate cancer to identify those patients least likely to benefit from treatment.
METHODS
A prospective observational cohort study was performed of 104 participants aged 64.0 years ± 6.5 years from 3 institutions representing different health care delivery systems. Patients were identified before undergoing transrectal ultrasound-guided prostate biopsy and followed for a median of 28 months. Associations between the comorbidity scores and nonelective hospital admissions were investigated using logistic regression and Cox proportional hazards models.
RESULTS
Among the 104 patients who underwent prostate biopsy, 2 died during the follow-up period. The overall hospital admission rate was 20% (21 of 104 patients). Higher scores on both the TIBI-CaP (≥ 9) and CCI (≥ 3) were found to be significantly associated with an increased odds for hospital admission (odds ratio, 11.3 [95% confidence interval (95% CI), 2.4–53.6] and OR, 5.7 [95% CI, 1.4–22.4]) and hazards ratios (HRs) for time to hospital admission (HR, 3.8 [95% CI, 1.3–11.2] and HR, 3.2 [95% CI, 1.1–9.1]), respectively.
CONCLUSIONS
TIBI-CaP and CCI scores were found to successfully predict which patients were at high risk for nonelective hospital admission. These patients are likely to have poorer health and a potentially shortened lifespan. Therefore, comorbidity analysis using these tools may help to identify those patients who are least likely to benefit from prostate cancer therapy and should avoid prostate biopsy.
Keywords: prostate cancer, comorbidity, competing risk, health services research
INTRODUCTION
The usually long course and relatively late age of onset of the majority of cases of prostate cancer result in the maxim that most patients die with but not of prostate cancer. This fact engenders increased concern about the likelihood of overtreatment, especially in patients with comorbidities.1,2 Multiple studies have suggested that comorbidity assessment is an especially important factor in stratifying the risk of dying of prostate cancer.3,4 Risk stratification would be useful before proceeding with prostate-specific antigen (PSA) testing and subsequent biopsy to identify those patients who may not benefit from treatment. In addition, avoiding biopsy in patients with high levels of comorbidity could minimize the consequences of treatment-related harms, particularly those that compromise quality of life and impose unnecessary costs to the health care system.1 Therefore, distinguishing those patients who are more likely to benefit from prostate biopsy and subsequent therapy is a priority.
Although the American Urological Association guidelines for the management of clinically localized prostate cancer encourage the use of population-based life tables, these tables may poorly estimate survival in men with multiple comorbidities.5 However, prognostic indices that assess and account for comorbidities may supplement the resources needed to assist physicians in determining a patient’s potential lifespan within the context of clinical decision-making.6
Prior studies have shown the Total Illness Burden Index for Prostate Cancer (TIBI-CaP) to be useful in deciding on a particular therapy after prostate biopsy was performed.7,8 However, to the best of our knowledge, the current study represents the first use of the TIBI-CaP patient-reported questionnaire in a prospective patient population without a previously established diagnosis of prostate cancer.
Therefore, we examined the ability of the TIBI-CaP patient-reported comorbidity questionnaire and the physician-reported Charlson Comorbidity Index (CCI) to predict subsequent morbidity (acute hospitalizations) and mortality in men undergoing prostate biopsy. Acute hospital admission was used as a surrogate metric for the patient’s overall health status.9,10 In particular, we believe comorbidity assessment before prostate biopsy may be helpful in deciding whether to perform a prostate biopsy.
MATERIALS AND METHODS
Setting/Sample
We obtained Institutional Review Board approval from 3 participating medical centers: the University of California at Irvine, the Long Beach Veterans Affairs Medical Center, and the Kaiser Permanente Orange County. These 3 centers represent different health care delivery systems and were used to broaden the characteristics of the study population. Participants had been previously selected to undergo a prostate biopsy after discussion with their treating urologist. The urologist performing the prostate biopsy obtained informed consent from the patient for study enrollment.
Design
A prospective observational cohort of 107 participants who were being evaluated for clinically localized prostate cancer were enrolled between January 2009 and March 2010 and reviewed after 3 years from the time the last patient was enrolled for the occurrence of nonelective hospital admissions. A total of 3 subjects who had < 6 months of follow-up were excluded, leaving 104 patients without a previous diagnosis of prostate cancer at the time of the biopsy for the analysis.
Study Measures
Participants were asked to complete the TIBI-CaP questionnaire before undergoing a transrectal ultrasound-guided prostate biopsy. The TIBI is a previously validated patient-reported comorbidity measure of 12 health domains including severity that was later modified for patients with prostate cancer (TIBI-P or TIBI-CaP).7,11 TIBI scores measured at baseline have been used to empirically identify subgroups of patients with a differential likelihood of experiencing subsequent outcomes including mortality and cardiovascular events over follow-up periods of ≥ 5 years.12,13 The CCI was completed for each patient by the physician before the prostate biopsy was performed.14
Data Collection and Outcomes
In addition to the TIBI-CaP and CCI, demographic information (ie, age, race, body mass index [BMI] in kg/m2, and PSA) was collected. The follow-up end date used was the date of the last documented note in the medical record as of December 30, 2011 (3 years from the time of initiation of the study). Outcomes consisted of nonelective hospital admissions and mortality. Hospital admission was defined as admittance through the emergency department, a direct admission, an inpatient transfer from an outside hospital, documentation of hospitalization at an outside facility, or required observation within the emergency department for a period of time. Patients admitted for prostate biopsy complications, planned outpatient/ inpatient surgical procedures, or surgical complications were excluded. The Veterans Affairs Medical Center and Kaiser Permanente are nearly fully captured health systems, which minimized missed events. The biopsy results and the subsequent therapy chosen were documented.
Statistical Analysis
Hospitalized patients were compared with nonhospitalized patients with respect to age, BMI, PSA, race, TIBI-CaP, and CCI using 2-group Student t tests. A Spearman rank correlation was used to assess agreement between TIBI-CaP and CCI scores. Associations between patient characteristics including age, BMI, TIBI-CaP, and CCI and hospital admissions were investigated using univariate and multivariate logistic regression. Time to hospital admission was investigated using Kaplan-Meier analysis and Cox proportional hazards regression. Because of the small numbers of nonelective admissions, comorbidity scores were dichotomized using cutpoints (TIBI-CaP ≥ 9 and CCI ≥ 3) that represented the upper 10% for each of the TIBI-CaP and CCI distributions. These cutpoint values are consistent with values reported in previous studies.8,15 A P value < .05 was considered to be statistically significant.
RESULTS
The characteristics of the participants are shown in Table 1. The mean age of the patients at the time of study enrollment was 64.0 years (standard deviation, 6.5 years). The majority of patients were white/non-Hispanic (72%) and the remaining patients were 13% black, 8% Hispanic, and 8% Asian. The median follow-up time was 28 months (range, 6 months–35 months). Patients who underwent biopsy were found to have either a prostate nodule or an elevated PSA level (mean, 8.1 ng/mL [range, 0.5 ng/mL–80 ng/mL]), with 88% of patients (91 of 104 patients) having a PSA level ≥ 4 ng/mL. The median TIBI-CaP score was 3.0 (range, 0–19); 10% of patients (10 of 98 patients) had a score of ≥ 9. The median CCI score was 0 (range, 0–12) and 12% of patients (12 of 104 patients) had a score of ≥ 3.0.
TABLE 1.
Patient Characteristics
| Characteristic | All
|
Not Hospitalized During Follow-Up
|
Hospitalized During Follow-Up
|
P
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | Student t Test | |
| Age | 104 | 64 | 6.5 | 83 | 63.4 | 6.3 | 21 | 66.1 | 7.1 | .09 |
| BMI | 104 | 28.7 | 4.3 | 83 | 28.1 | 3.7 | 21 | 30.9 | 5.8 | .05 |
| PSA | 104 | 8.1 | 8.2 | 83 | 8.2 | 8.8 | 21 | 7.5 | 5.4 | .64 |
| No. | Median | Range | No. | Median | Range | No. | Median | Range | Mann-Whitney U Test | |
|
| ||||||||||
| Charlson Comorbidity Index | 103 | 0.0 | 0–12 | 82 | 0.0 | 0–6 | 21 | 2.0 | 0–12 | .002 |
| TIBI-CaP | 98 | 3.0 | 0–19 | 79 | 3.0 | 0–11 | 19 | 6.0 | 1–19 | <.001 |
| No. | % | No. | % | No. | % | Chi-Square Test | ||||
|
| ||||||||||
| Race | ||||||||||
| White | 75 | 72 | 62 | 75 | 13 | 62 | .68 | |||
| Hispanic | 8 | 8 | 6 | 7 | 2 | 9 | ||||
| Black | 13 | 13 | 9 | 11 | 4 | 19 | ||||
| Asian | 8 | 8 | 6 | 7 | 2 | 9 | ||||
Abbreviations: BMI, body mass index; PSA, prostate-specific antigen; SD, standard deviation TIBI-CaP, Total Illness Burden Index for Prostate Cancer.
Of the 104 men in the screening population, 30 (29%) were diagnosed with cancer based on the enrollment prostate biopsy (data not shown). The most common treatments were radical prostatectomy (47%), external beam radiotherapy (27%), or active surveillance (13%). The majority of men (20 of 30 patients [67%]) were diagnosed with Gleason score 6 prostate cancer. The numbers were too small to detect a demonstrable difference among admission rates between the cancer treatment groups.
There was an overall mortality rate of 2% (2 of 104 patients). One 63-year-old patient died of a metastatic squamous cell carcinoma of the mastoid and a 76-year-old patient died of a stroke. The overall hospital admission rate during follow-up was 20% (21 of 104 patients), which included both of the patients who died. Patients admitted to the hospital were older (aged 66.1 years vs 63.4 years; P = .09) and had a significantly higher BMI (30.9 kg/m2 vs 28.1 kg/m2; P = .046) and more comorbidities (6.9 vs 3.1 [P = .002] for TIBI-CaP and 2.5 vs 0.7 [P = .02] for CCI) compared with patients who were not admitted to the hospital during follow-up (Table 1).
The most common reasons for hospital admission were problems with the cardiovascular, gastrointestinal, or respiratory systems (Table 2). In particular, the cardiovascular system was responsible for the majority of hospital admissions (14 of 21 admissions [67%]).
TABLE 2.
Cause of Hospital Admissiona
| Reason for Admission (n=21) | No. (%) |
|---|---|
| Cardiovascular | 14 (67) |
| Chest pain/CHF | 8 (38) |
| Stroke | 4 (19) |
| DVT | 2 (10) |
| Gastrointestinal | 2 (10) |
| Pulmonary | 2 (10) |
| Renal | 1 (5) |
| Other | 2 (10) |
Abbreviations: CHF, congestive heart failure; DVT, deep venous thrombosis.
Cause of hospitalization is shown by organ system.
Because TIBI-CaP and CCI scores showed a significant Spearman correlation of 0.5 (P < .001), separate multivariate models were performed using each comorbidity measure (Table 3). After adjusting for age and BMI using multivariate logistic regression, higher comorbidity scores as measured by both the TIBI-CaP and CCI were associated with an increased odds ratio (OR) for hospital admission during the follow-up period. TIBI-CaP scores of ≥ 9 were found to be significantly associated with an increased adjusted OR of 11.3 (95% confidence interval [95% CI], 2.4–53.6; P = .002). The CCI scores of ≥ 3 were found to be significantly associated with an increased adjusted OR of 5.7 for hospitalization during follow-up (95% CI, 1.4–22.4; P = .01) (Table 3).
TABLE 3.
Association Between Comorbidity and Hospitalization During Follow-Up (OR) and Time to Hospitalization in a Cox Proportional Hazards Model (HR)a
| Comorbidity Instrument | Not Hospitalized | Hospitalized | Adjusted ORb | 95% CI | P |
|---|---|---|---|---|---|
| TIBI-CaP | |||||
| 0–8 | 76 | 12 | |||
| 9–19 | 3 | 7 | 11.3 | 2.4–53.6 | .002 |
| Charlson Comorbidity Index | |||||
| 0–2 | 77 | 14 | |||
| 3–12 | 5 | 7 | 5.7 | 1.4–22.4 | .013 |
| Adjusted HRb | 95% CI | P | |
|---|---|---|---|
| TIBI-CaP (0–8 vs 9–19) | 3.8 | 1.3–11.2 | .017 |
| Charlson Comorbidity Index (0–2 vs 3–12) | 3.2 | 1.1–9.1 | .033 |
95% CI, 95% confidence interval; HR, hazards ratio; OR, odds ratio; TIBI-CaP, Total Illness Burden Index for Prostate Cancer.
Hospital admission (yes/no) is shown with regard to the range of comorbidities counted by 2 variations in comorbidity tools: the TIBI-CaP and the Charlson Comorbidity Index. Patients at high risk for hospital admission were compared with those who were at low risk for hospital admission, which included TIBI-CaP (high risk >8) and the Charlson Comorbidity Index (≥3). Each score was compared using the ORs (percentage) and HRs (time to event) with the P values listed.
HRs were adjusted for age and body mass index.
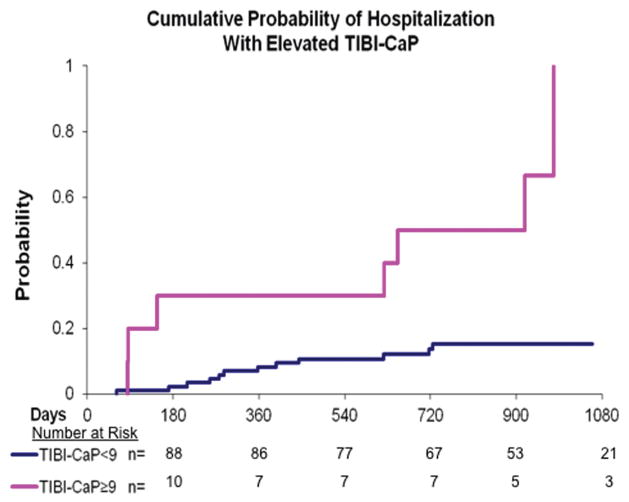
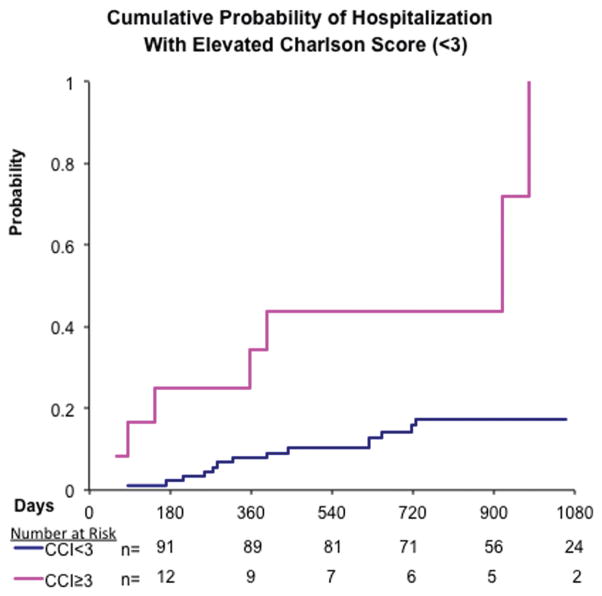
Higher comorbidity scores were also associated with a greater likelihood of hospitalization during the follow-up period as evidenced by an increased hazards ratio (HR) after adjusting for age and BMI (Table 3). Those patients with TIBI-CaP scores ≥ 9 had an HR of 3.8 (95% CI, 1.3–11.2) compared with those with scores of 0 to 8 (Fig. 1). CCI scores of ≥ 3 were also found to be associated with an increased HR of 3.2 (95% CI, 1.1–9.1) compared with a CCI score of < 3 after adjusting for age and BMI (Fig. 2).
Figure 1.
A Kaplan-Meier curve for hospital admission by Total Illness Burden Index for Prostate Cancer (TIBI-CaP) score is shown that demonstrates the time to hospitalization comparing patients split between 2 comorbidity groups (those with a TIBI-CaP score of ≥ 9 and those with a TIBI-CaP score of < 9). The group with a score of ≥ 9 previously was described in the validation of the TIBI-CaP score.
Figure 2.
A Kaplan-Meier curve for hospital admission by Charlson Comorbidity Index (CCI) is shown that demonstrates the time to hospitalization comparing patients by CCI score group (≥ 3 or < 3). Previous studies have confirmed that a CCI score of ≥ 3 is considered to be significant.
DISCUSSION
Concerns regarding the overtreatment of prostate cancer have contributed to the U.S. Preventive Services Task Force delivering a grade D recommendation regarding PSA screening.16 Although controversial, this recommendation will compel physicians to scrutinize the selection of candidates for prostate cancer screening and diagnosis. It is interesting to note that the PIVOT (Prostate Cancer Intervention Versus Observation Trial) study demonstrated little benefit for the surgical treatment of patients with prostate cancer and the potential for a profound impact on quality of life related to incontinence and impotence; however, patients in the analysis with a PSA level ≥ 10 ng/mL and classified as being of intermediate or high risk according to the D’Amico risk group classification17 experienced a benefit in all-cause and prostate cancer-specific mortality, respectively. The results suggest benefits for select patients and indicate the need for improved screening criteria. Although the PIVOT study did not demonstrate a significant association between comorbidity and mortality at a median of 10 years, the results of the current study suggest that comorbidity does predict subsequent morbidity as reflected by nonelective hospital admissions.9,10 Therefore, we suggest that incorporating an accurate comorbidity assessment into candidate selection may not only be useful at the time of treatment but should be considered even earlier in the decision-making process for PSA screening and prostate biopsy.
One of the most widely used tools to assess 10-year survival using information about a patient’s comorbid conditions is the CCI, also referred to as the Charlson score.14 The CCI is physician-reported and usually retrospective, but does not assess the severity of disease. A CCI ≥ 3 is considered to be an indication of high comorbidity and has previously been shown to predict higher mortality for causes other than prostate cancer in patients already diagnosed with localized prostate cancer.15,18–20 In the current study, patients with a CCI ≥ 3 were found to have an increased risk of hospital admissions (OR, 5.7).
Conversely, a point-of-care patient-reported measure of comorbidity could provide more timely and possibly more accurate information regarding the presence and severity of comorbid conditions to be used in discussions regarding medical decisions.21 In the initial validation study by Litwin et al assessing mortality after 3.5 years, the authors found that patients with TIBI-CaP scores ≥ 9 were significantly more likely to die of other causes compared with those with TIBI-CaP scores of 0 to 2.8 We found that a TIBI-CaP score of ≥ 9 was associated with an 11-times higher risk of hospital admission after adjusting for BMI compared with a TIBI-CaP score of < 9.
The most common causes of admission to an acute care hospital identified in the current study were cardiovascular-related (67% of non–prostate-related hospital admissions), which is consistent with national trends.22 Cardiovascular disease remains the leading cause of mortality.23 Specifically, those patients who are admitted to the hospital with congestive heart failure in particular tend to have more comorbidities (≥ 5 in approximately 40% of men with congestive heart failure), as well as higher mortality rates.24
To the best of our knowledge, the current study represents the first use of the TIBI-CaP patient-reported questionnaire in a prospective patient population without a previously established diagnosis of prostate cancer. Additional studies are needed to investigate the use of comorbidity assessment along with prostate cancer risk stratification to determine which patients should receive a prostate biopsy and ensuing therapy. Although the study was limited by a small sample size, patient diversity was strengthened by enrolling patients from 3 different institutions comprised of different patient populations (University of California at Irvine, Long Beach Veterans Affairs Medical Center, and Kaiser Permanente Orange County), in addition to its prospective design.
Several other limitations should be considered when interpreting the results of the current study. First, because follow-up consisted of chart review and documentation of the last note in the chart, this study may underrepresent the number of admissions to the hospital because some patients may have presented to hospitals outside of this study for subsequent medical care. In addition, the current study population technically represented a pre-screened cohort because the physicians and patients already decided to pursue prostate biopsy. Thus, patients with even higher comorbidity levels may have already been excluded.
Comorbidity assessment is useful, but must be done in a way that minimizes the burden to the patient and the physician. We developed an electronic version of the TIBI-CaP that patients may complete outside of the clinic setting before their appointment with their physician to provide an objective measure of the patient’s health status to enrich the discussion between the patient and physician when making health care decisions.
Conclusions
Prostate cancer screening and use of the PSA test continues to be controversial given the longevity of the disease and the side effects of screening and therapy. Both physician-reported (CCI) and patient-reported (TIBI-CaP) measures of comorbidity have identified patients at high risk for nonelective hospital admission and may aid medical decision-making, specifically among patients considering prostate biopsy. Before prostate biopsy, providers should assess the number and severity of the patient’s comorbid conditions to discuss whether proceeding with biopsy and/or therapy is likely to be beneficial.
Acknowledgments
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Lin K, Lipsitz R, Miller T, Janakiraman S U.S. Preventive Services Task Force. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:192–199. doi: 10.7326/0003-4819-149-3-200808050-00009. [DOI] [PubMed] [Google Scholar]
- 2.Daskivich TJ, Chamie K, Kwan L, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117:2058–2066. doi: 10.1002/cncr.25751. [DOI] [PubMed] [Google Scholar]
- 3.Schroder FH. Stratifying risk-the U.S. Preventive Services Task Force and prostate-cancer screening. N Engl J Med. 2011;365:1953–1955. doi: 10.1056/NEJMp1112140. [DOI] [PubMed] [Google Scholar]
- 4.Daskivich TJ, Chamie K, Kwan L, et al. Comorbidity and competing risks for mortality in men with prostate cancer. Cancer. 2011;117:4642–4650. doi: 10.1002/cncr.26104. [DOI] [PubMed] [Google Scholar]
- 5.Walz J, Suardi N, Shariat SF, et al. Accuracy of life tables in predicting overall survival in patients after radical prostatectomy. BJU Int. 2008;102:33–38. doi: 10.1111/j.1464-410X.2008.07614.x. [DOI] [PubMed] [Google Scholar]
- 6.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307:182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stier DM, Greenfield S, Lubeck DP, et al. Quantifying comorbidity in a disease-specific cohort: adaptation of the total illness burden index to prostate cancer. Urology. 1999;54:424–429. doi: 10.1016/s0090-4295(99)00203-4. [DOI] [PubMed] [Google Scholar]
- 8.Litwin MS, Greenfield S, Elkin EP, Lubeck DP, Broering JM, Kaplan SH. Assessment of prognosis with the total illness burden index for prostate cancer: aiding clinicians in treatment choice. Cancer. 2007;109:1777–1783. doi: 10.1002/cncr.22615. [DOI] [PubMed] [Google Scholar]
- 9.Campbell SE, Seymour DG, Primrose WR. ACMEPLUS Project. A systematic literature review of factors affecting outcome in older medical patients admitted to hospital. Age Ageing. 2004;33:110–115. doi: 10.1093/ageing/afh036. [DOI] [PubMed] [Google Scholar]
- 10.Bottle A, Aylin P, Majeed A. Identifying patients at high risk of emergency hospital admissions: a logistic regression analysis. J R Soc Med. 2006;99:406–414. doi: 10.1258/jrsm.99.8.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenfield S, Sullivan L, Dukes KA, Silliman R, D’Agostino R, Kaplan SH. Development and testing of a new measure of case mix for use in office practice. Med Care. 1995;33(suppl 4):AS47–AS55. [PubMed] [Google Scholar]
- 12.Daskivich T, Sadetsky N, Kaplan SH, Greenfield S, Litwin MS. Severity of comorbidity and non-prostate cancer mortality in men with early-stage prostate cancer. Arch Intern Med. 2010;170:1396–1397. doi: 10.1001/archinternmed.2010.251. [DOI] [PubMed] [Google Scholar]
- 13.Greenfield S, Billimek J, Pellegrini F, et al. Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: a cohort study. Ann Intern Med. 2009;151:854–860. doi: 10.7326/0003-4819-151-12-200912150-00005. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Abdollah F, Sun M, Schmitges J, et al. Cancer-specific and other-cause mortality after radical prostatectomy versus observation in patients with prostate cancer: competing-risks analysis of a large North American population-based cohort. Eur Urol. 2011;60:920–930. doi: 10.1016/j.eururo.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 16.Moyer VA U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 17.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 18.Albertsen PC, Fryback DG, Storer BE, Kolon TF, Fine J. Long-term survival among men with conservatively treated localized prostate cancer. JAMA. 1995;274:626–631. [PubMed] [Google Scholar]
- 19.Fowler JE, Jr, Terrell FL, Renfroe DL. Comorbidities and survival of men with localized prostate cancer treated with surgery or radiation therapy. J Urol. 1996;156:1714–1718. [PubMed] [Google Scholar]
- 20.Tewari A, Johnson CC, Divine G, et al. Long-term survival probability in men with clinically localized prostate cancer: a case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol. 2004;171:1513–1519. doi: 10.1097/01.ju.0000117975.40782.95. [DOI] [PubMed] [Google Scholar]
- 21.Bayliss EA, Ellis JL, Steiner JF. Subjective assessments of comorbidity correlate with quality of life health outcomes: initial validation of a comorbidity assessment instrument. Health Qual Life Outcomes. 2005;3:51. doi: 10.1186/1477-7525-3-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 23.Minino AM. NCHS data brief, no 64. Hyattsville, MD: National Center for Health Statistics; 2011. Death in the United States, 2009. [Google Scholar]
- 24.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]