Abstract
Yersinia pestis, one of history’s deadliest pathogens, has killed millions over the course of human history. It has attributes that make it an ideal choice to produce mass casualties and is a prime candidate for use as a biological weapon. When aerosolized, Y. pestis causes pneumonic plague, a pneumonia that is 100% lethal if not promptly treated with effective antibiotics. Currently, there is no FDA approved plague vaccine. The current lead vaccine candidate, a parenterally administered protein subunit vaccine comprised of the Y. pestis virulence factors, F1 and LcrV, demonstrated variable levels of protection in primate pneumonic plague model. As the most likely mode of exposure in biological attack with Y. pestis is by aerosol, this raises a question of whether this parenteral vaccine will adequately protect humans against pneumonic plague. In the present study we evaluated two distinct mucosal delivery platforms for the intranasal (IN) administration of LcrV and F1 vaccine proteins, a live bacterial vector, Lactobacillus plantarum, and a tobacco mosaic virus (TMV) based delivery platform. IN administration of L. plantarum expressing LcrV, or TMV-conjugated to LcrV and F1 (TMV-LcrV+TMV-F1) resulted in the similar induction of high titers of IgG antibodies and evidence of proinflammatory cytokine secretion. However, only the TMV-conjugate delivery platform protected against subsequent lethal challenge with Y. pestis. TMV-LcrV+TMV-F1 co-vaccinated mice had no discernable morbidity and no mortality, while mice vaccinated with L. plantarum expressing LcrV or rLcrV+rF1 without TMV succumbed to infection or were only partially protected. Thus, TMV is a suitable mucosal delivery platform for an F1-LcrV subunit vaccine that induces complete protection against pneumonic infection with a lethal dose of Y. pestis in mice.
Keywords: Yersinia pestis, pneumonic plague, tobacco mosaic virus, mucosal vaccination
Background
Yersinia pestis, the causative agent of Plague, is responsible for the deaths of millions over the course of human history[1,2]. Advancements in public health and the advent of antibiotics have greatly reduced Plague outbreaks in recent years. However, the bacterium is endemic in several areas of the world including Asia, the western United States, South America and Africa, and outbreaks continue to infect thousands of people worldwide each year[3]. Significantly, multidrug antibiotic resistant strains of Y. pestis, have been identified; these strains are resistant to all antibiotics currently recommended for treatment and prophylaxis. These strains represent a potentially significant public health threat [4,5]. The existence of naturally occurring multidrug-resistant Y. pestis and the potential use of Plague as an agent of bioterrorism [1,6] has led to a renewed interest in the development of a safe and effective vaccine.
Y. pestis was independently identified by Alexander Yersin and Shibasaburo Kitasato during a Plague outbreak in Hong Kong in 1894[1,2]. In the more-than-a-century since that discovery, several different Plague vaccines have been developed. Initial attempts included attenuated live (E76) and whole cell killed vaccines; however, adverse side effects and the inability to protect against pneumonic plague, respectively, have limited enthusiasm for these vaccine preparations[7]. More recently, vaccine development efforts have shifted to protein subunit vaccines and live bacterial vaccine vectors. Multiple virulence factors from plague have been investigated as components of a subunit vaccine; with the F1 capsular protein (capsular antigen fraction 1, caf1) and the LcrV protein demonstrating the greatest efficacy against both pneumonic and bubonic plague in animal models (reviewed in [8]). LcrV is both the tip of the Type III secretion system (T3SS) and a secreted immunomodulatory factor in all pathogenic Yersinia spp.; it is an essential virulence factor[9,10]. F1 is the primary structural element of the antiphagocytic capsule expressed only in Y. pestis, but is not required for virulence, as F1(−) strains of Y. pestis remain lethal[11]. When administered parenterally with an alum adjuvant, various compositions of F1-LcrV are protective in rodent models of disease, inducing high titers of protective antibody, as well as CD4 and CD8 T cell mediated protection [8,12–14]. However, in non-human primate models, variable levels of protection against pneumonic disease have been observed, irrespective of high vaccine-specific antibody titers [7,14–17]. These failures have generated doubt as to the ability of these parenteral subunit vaccines to effectively protect humans against pneumonic plague, in the event of a bioterrorist attack. More recently, live bacterial vaccine vectors expressing Y. pestis virulence factors have been investigated for use as mucosal vaccines, which do not require injection and should produce higher levels of protection at mucosal surfaces than parenteral injection. Examples include the lactic acid commensal bacteria, Lactobacillus plantarum and an attenuated form of Salmonella enterica[18–20]. However, these live vector vaccines have yet to be fully evaluated. As a result, there is continued interest in the development of advanced vaccines for Y. pestis.
In the present study we further evaluated the protective efficacy of a mucosally delivered L. plantarum live bacterial vaccine vector[18] expressing LcrV against lethal challenge with Y. pestis, comparing the response to a mucosally delivered LcrV-F1 subunit vaccine covalently linked to a tobacco mosaic virus (TMV)3 delivery platform. TMV is a plant virus that cannot infect animals cells, but has been shown to interact with and stimulate mammalian dendritic cells [21]. TMV-conjugate vaccines have demonstrated protection against challenge with Francisella tularensis [22], and Influenza H1N1 [23] and H5N1 [24]. Here we demonstrate that TMV-conjugated to LcrV and F1 conferred protection against lethal pneumonic challenge with Y. pestis.
Methods and Materials
Animals
Age and gender matched 5–8 week old C57BL/6 mice were obtained from Jackson laboratories (Bar Harbor, ME), and maintained by the husbandry staff of the department of Comparative Medicine at New York Medical College. All experiments were conducted with the approval of the New York Medical College Animal Care and Use Committee.
Bacteria
Y. pestis CO92pgm− was a generous gift of Dr. James Bliska (Stonybrook University, NY), and was cultivated using heart infusion agar supplemented with 0.2% Xylose at 28°C. E. coli TOP10 (Invitrogen, Grand Island, NY) was used for cloning of recombinant proteins, and E. coli BL21(DE3) (Invitrogen) was used for recombinant protein expression. LcrV and F1 expressing E. coli strains were grown and maintained in LB media (BD Diagnostics) with kanamycin at 37°C. Growth and cultivation of L. plantarum expressing LipLcrV was previously described[18].
Recombinant Protein
Cloning, expression, and purification of rLcrV were described previously[18]. F1 cloning and expression was performed as follows: total DNA from Y. pestis CO92pgm− was isolated using Wizard SVGenomic DNA purification system according to manufacturer’s instructions (Promega). The caf1 gene was amplified using the primers: caf1 F 5'- CAC CAT GGC AGA TTT AAC TGC AAG CAC CAC TG - 3' and caf1 R 5’- GGA TTA TTG GTT AGA TAC GGT TAC GGT TAC AGC A – 3’. PCR reactions were performed using AccuPrime™ Pfx SuperMix DNA polymerase mix (Invitrogen). Parameters for PCR reactions were as follows: denaturation at 95°C for 5 min, 35 cycles of 95°C for 15 sec–55°C for 30 sec– 68°C for 40 sec, and final extension at 68°C for 5 min. PCR products were cloned into the pET200 vector in E.coli TOP10 using Gateway cloning technology (Invitrogen) according to manufacturer’s instructions. Plasmid DNA was isolated using PureYield™ Plasmid Midiprep System according to manufacturer’s instructions (Promega, Madison, WI, USA). The digested plasmid DNA or DNA fragments from were purified from agarose gels using the Wizard SV Gel and PCR clean-Up system according to manufacturer’s instructions (Promega). All constructs were confirmed by restriction enzyme analysis, PCR and DNA sequencing using standard procedures.
For Recombinant F1 expression, plasmids were transformed into E. coli BL21(DE3). Expression was induced by adding 0.75 mM IPTG to cultures of E. coli BL21(DE3) at an OD600 =0.5 and grown at 37°C for 4 additional hours. Cells were pelleted at 5,000 rpm for 10 min and then store at −20C. Protein was purified using nickel-NTA column chromatography (Sigma, St Louis, MO) under denaturing conditions according to manufacturer’s instructions with the following modification: all buffers contained 8M urea, 150 mM sodium phosphate, and 300mM NaCl. Purified proteins were analyzed by SDS-PAGE, followed by coomassie stain and western blotting. The concentration of proteins was determined by RC DC™ Protein Assay (Bio-Rad), and stored at −20°C.
TMV
TMV-F1 and TMV-LcrV conjugate vaccines were prepared as described previously [23], with minor modifications. Lysine modified TMV [25] was mixed at a 1:1 molar ratio with each antigen in 100 mM 2-(N-morpholino) ethanesulfonic acid and 500 mM NaCl solution at pH 6. 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) was added followed by the addition of 5mM N-hydroxysulfosuccinimide (NHS). EDC concentrations were optimized based in the time and concentration of EDC to generate no “free” protein antigen, which varied by protein. TMV-F1 conjugates required addition of 8 mM EDC in a 2 hour reaction, and TMV-LcrV conjugates required addition of 4mM EDC in a 1 hour reaction. Conjugation was stopped with 1 mM methylamine, and then conjugate vaccines were dialyzed against PBS overnight (Slidalyzer, 10kD MCO). An 8–16%SDS-PAGE gel (BioRad) was used to visualize TMV-Ag conjugates. The final dialyzed vaccine concentration was determined via BCA assay (BioRad), and vaccine aliquots were frozen at −20°C until administered.
Vaccination: Oral vaccination
Mice received 1×10^9 empty vector-L. plantarum, or 1×10^9 lipLcrV-L. plantarum in 250µl PBS by oral gavage twice a day, 6h apart for eight days (4 days followed by two days rest, followed by another four days). Mice received booster vaccinations for 4 consecutive days, twice a day, 6h apart beginning on days 28 and 49. As a positive control for survival, a separate group of mice received 50µg of rLcrV in alum on day 21, and was boosted with 50µg of rLcrV in alum on day 49. Mice were challenged on day 63. Blood was collected prior to each boost and challenge. Serum was obtained by centrifuging blood at 6000RPM for 20 min, and stored at −20°C.
Intranasal L. plantarum vaccination
Mice were lightly anesthetized and vaccinated on days 0 and 1 with 1×10^9 CFU of L. plantarum-empty vector, or 1×10^9 lipLcrV-L. plantarum in 10µl of PBS by IN droplet (5µl per nostril). Mice received single booster doses on days 28, 56, and were challenged on Day 70. As a positive control for survival, a separate group of mice received 50µg of rLcrV in alum on 35 days before challenge. Serum was obtained from blood collected before each boost, as well as challenge, and stored at −20°C. For vaccine specific cytokine analysis, separate groups of mice were boosted a 3rd time on d70. On day 84, they received 100µg of rLcrV emulsified in Freund’s Incomplete Adjuvant subcutaneously in the nape of the neck to activate vaccine specific cells in secondary lymphoid organs. 4 days later, the mice were sacrificed, blood and BAL washes were obtained, and inguinal LNs and SPLs were isolated. Single cell suspensions were generated by pressing the LNs and spleens through a 40µM strainer with the plunger of a syringe. Cells (2×10^5 cells/well) were restimulated in 96-well plates with media, 5µg/ml concanavalin A (mitogen control), or 100µg/ml rLcrV for 72h at 37°C.
Intranasal TMV-conjugate vaccination
Mice were vaccinated with 25µg of TMV-LcrV (consisting of 12.5µg of TMV and 12.5 µg of vaccine protein), 25µg TMV-F1, or 25µg of TMV-LcrV+25µg of TMV-F1, 12.5µg of rLcrV, 12.5µg of rF1, or 12.5µg rLcrV+12.5µg rF1 in 40µl of normal saline on day 0. In 2 experiments, mice were boosted in the same manner on days 14, 35, and 56. In subsequent experiments the mice were boosted only on days 21 and 35. All mice were challenged on day 70.
Challenge
Y. pestis CO92pgm− was grown to an OD620 of 1.1 in heart infusion broth supplemented with 2.5mM CaCl2 and 0.2% Xylose at 37°C in a shaking incubator (175 RPM). Culture medium was removed by centrifugation and washing (3×) with normal saline at 5000 RPM for 10 min at room temp with a soft brake. The LD50 of Y. pestis CO92pgm− in C57BL/6 mice was determined to be 2.5 × 10^4 CFU (data not shown). Bacteria were resuspended in normal saline to an OD620 of 1.1, assuming a concentration of 1 × 10^9 CFU. Bacteria were diluted to a concentration of 5 × 10^6/ml which corresponds to 2.5 × 10^5 bacteria in 50µl (10 LD50). 50µl of bacteria was administered to lightly anesthetized mice in biosafety cabinet. Dilutions of bacteria were streaked on heart infusion agar supplemented with 0.2% Xylose to confirm the number of bacterial CFU administered. Mice were weighed inspected daily for signs of morbidity (ruffled fur, motheaten appearance, hunched posture, loss of activity). Mice that lost more than 20% of their pre-infection body weight, or were severely moribund, were euthanized. Experiments were continued until all mice succumbed to infection, recovered and regained their pre-infection weight, or until day 20.
ELISA
Plates were coated with rLcrV and rF1 (3 µg/ml) in 0.1M Carbonate Buffer, pH 9.4 overnight at 4°C. Plates were blocked with 1% BSA in PBS for 1h at room temperature, and washed with 0.05% Tween in PBS. Sera were diluted 1:2 starting at the indicated concentration in blocking buffer, and were incubated for 1h at room temperature. Plates were washed again, and HRP-labeled goat anti-mouse antibody (Ig heavy and light chain, IgG1, IgG2a, IgG2b, IgG3, or IgA-specific;Southern Biotech, Birmingham, AL) diluted 1:5000 in blocking buffer was added to each well for 1h at room temperature. The plates were washed and developed with TMB substrate (KPL, Gaithersburg, Maryland) for 30 min at room temperature. The reaction was stopped by addition of 2N sulfuric acid, and absorbance was read at 450nm and 570nm (Molecular Devices).
Cytokine
Cytokine and chemokine (IL1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-17, MCP1, IFNγ, TNFα, MIP-1α, GMCSF, and RANTES) levels were measured in culture supernatants (pg/ml) using a multiplex ELISA according to the manufacturer’s instructions (Quansys Biosciences, Logan, UT) using a Quansys dedicated luminescence plate reader and proprietary software. IFNγ production was also measured in culture supernatants using a cytokine ELISA (BD biosciences, San Jose, CA) according to the manufacturer’s instructions. Supernatants were analyzed in duplicate.
Statistical Analysis
Statistical analyses were performed using Prism 6.0 (Graphpad Software, La Jolla, CA). Survival curves were compared using a Log-rank (Mantel-Cox) test. Differences in the mean absorbance values of antibody binding for each vaccine group were evaluated at each serum dilution by two-way ANOVA followed by a Dunnett’s post-hoc test for multiple comparisons. Cytokine and BAL antibody levels, and serum antibody titers were compared using a one-way ANOVA followed by a Holm-Sidak’s pot-hoc test for multiple comparisons. p values of <0.05 were considered statistically significant.
Results
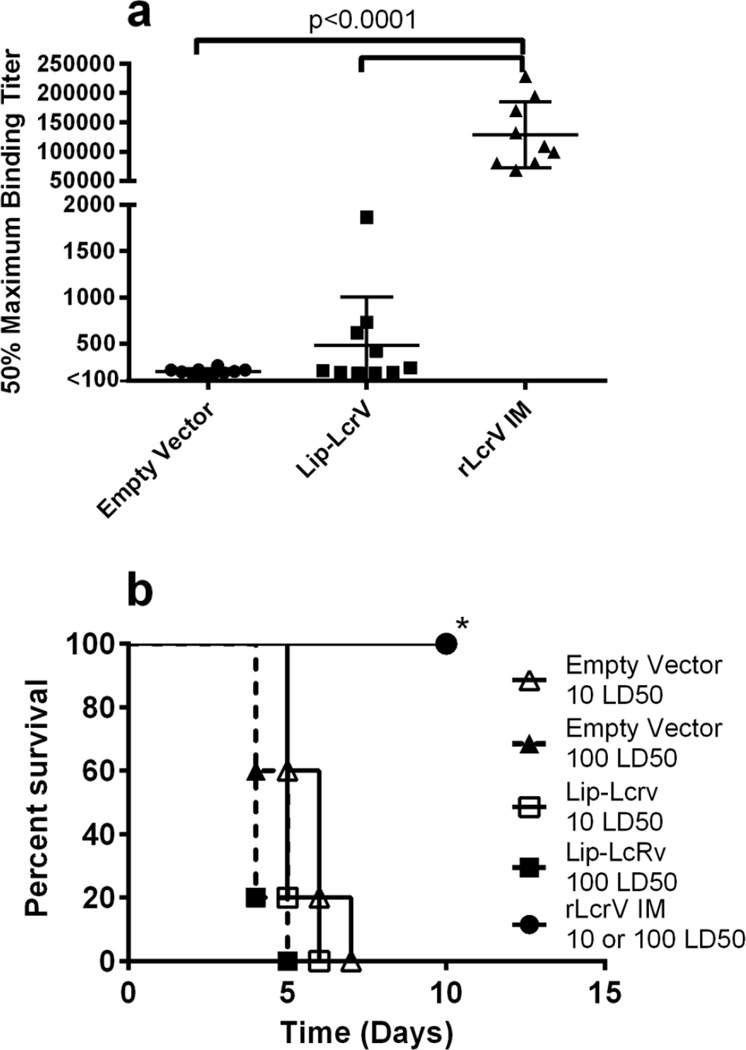
We previously described an oral vaccine platform consisting of a recombinant strain of the commensal organism, L. plantarum, expressing the LcrV protein of Y. pestis CO92[18]. The protein was expressed with a leader sequence from the OspA protein of Borrelia burgdorferi containing a lipidation motif that targets membrane localization of the protein (lipLcrV-L. plantarum)[26]. Oral vaccination with lipLcrV-L. plantarum followed by two boosts resulted in production of serum IgG specific for LcrV (Fig. 1); however, levels were significantly lower than those in mice vaccinated and boosted intramuscularly with rLcrV in alum (p < 0.0001). rLcrV in alum is a control for survival, as the IM administration of LcrV and F1 subunit proteins in alum has previously been demonstrated to induce protective immunity in rodent models of Plague [8,12–14]. Production of Th1 cytokines was also previously observed when L. plantarum expressing LcrV was incubated with either mouse bone marrow derived DCs or human peripheral blood DCs, compared to vector alone[18]. Despite this, mice were not protected against intranasal (IN) challenge with 10 or 100 LD50 of Y. pestis CO92 pgm−; all vaccinated mice succumbed to infection (Fig. 1).
Figure 1.
Oral administration of Lip-LcrV L. plantarum is not protective. a, 50% maximum binding titers were calculated for total LcrV specific Ig levels in mice vaccinated and boosted with Lip-LcrV L. plantarum(n=10), L. plantarum transfected with plasmid lacking LcrV (empty vector) (n=10), or IM with rLcrV in alum(n=5). b, survival of mice orally vaccinated and boosted with Lip-LcrV L. plantarum, empty vector or vaccinated and boosted IM with rLcrV in alum after challenge with 10× LD50 or 100× LD50 of Y. pestis CO92pgm−. These data are representative of two experiments. *-p<0.01 rLcrV IM 10 or 100 LD50 vs all other groups.
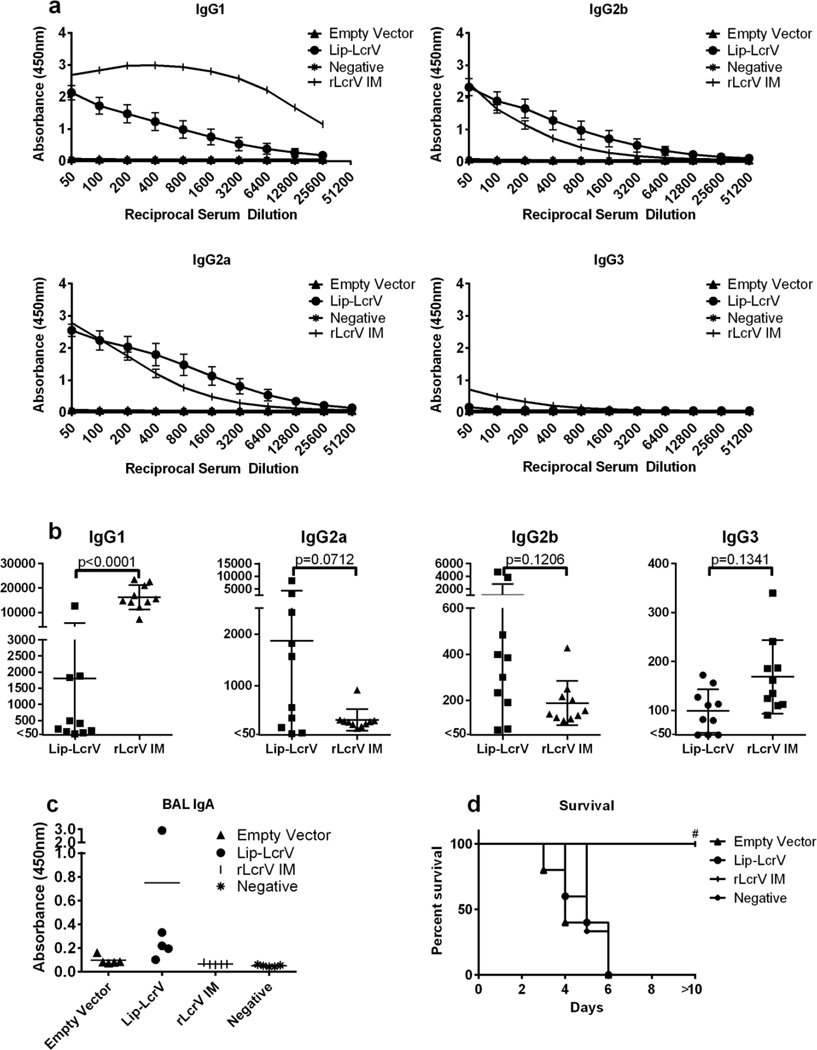
As a bioweapon, Y. pestis most likely would be delivered as an aerosol, causing pneumonic plague; thus, we altered our approach, testing the efficacy of lipLcrV-L. plantarum administered via IN droplet. IN administration constituted a single immunization followed by 2 or 3 individual boosts. Far fewer doses of intranasal vaccine were required to establish high antibody titers compared to oral vaccine administration. IN vaccination generated levels of serum anti-LcrV IgG subtypes similar to those induced by IM vaccination with rLcrV in alum, with the exception of IgG1, which was produced at higher levels in IM vaccinated mice (Fig. 2a, b). LcrV-specific SIgA was only detected in BAL fluid from mice vaccinated with lipLcrV-L. plantarum (Fig 2c). However, antibody titers in lipLcrV-L. plantarum vaccinated mice did demonstrate significant variability. Vaccine-specific cytokine production was measured by multiplex ELISA in culture supernatants following 72h ex vivo stimulation of NALT, head and neck LNs, and splenocytes with rLcrV. With the exception of a single mouse, cytokine production was undetectable (data not shown). To boost vaccine-specific cytokine production, mice received a subcutaneous injection of rLcrV in Freund’s incomplete adjuvant 2 weeks after the final boost, and cytokine production was then measured in splenocyte culture after ex-vivo stimulation with rLcrV for 72h. Production of IFNγ, IL-2, and MCP1 was observed, but was not significantly greater than mice that received vector alone (Fig. S1). IL-17 production was significantly higher in in lipLcrV-L. plantarum vaccinated mice compared to rLcrV IM vaccinated mice (Fig. S1). No other vaccine specific cytokines were observed. However, despite generating comparable immune responses to those in IM vaccinated mice, all lipLcrV-L. plantarum IN vaccinated mice succumbed to IN challenge with 10 LD50 of Y. pestis CO92 pgm−, whereas mice vaccinated IM with rLcrV in alum survived (Fig. 2d). IN vaccinated mice succumbed to infection at a rate similar to that of vector alone vaccinated mice, no correlation between antibody titer, or IL-17 production was observed.
Figure 2.
IN administration of Lip-LcrV L. plantarum is not protective. a, LcrV specific IgG1, IgG2a, IgG2b, and IgG3 levels following vaccination and boost with Lip-LcrV L. plantarum (Lip-LcrV) (n=5), empty vector (n=5), rLcrV in alum (rLcrV IM) (n=5), or unvaccinated (negative) (n=5). b, 50% maximal binding titers were calculated for the indicated LcrV specific isotypes in Lip-LcrV and rLcrV IM vaccinated mice. Titers could not be generated empty vector or negative mice. c, LcrV specific IgA in BAL washes of vaccinated and control mice. d, survival of mice after challenge with 10× LD50 of Y. pestis CO92pgm−. #-p<0.01 rLcrV IM vs. Empty Vector, Lip-LcrV, rLcrV IM survival. These data are representative of five experiments.
IM vaccination of mice with rLcrV in alum resulted in complete protection of mice, supporting previous data that LcrV specific responses induced to protein subunits are sufficiently capable of protecting mice from lethal challenge [8,12–14]. However, parenteral administration of subunit vaccines induces variable protection in non-human primates, implying that they may not protect humans against pneumonic plague [7,14–17]. We have previously utilized TMV as a delivery platform to amplify cellular and humoral immune responses to subunit vaccines, inducing protection against mucosally administered pathogens, F. tularensis and Influenza A [21–24]. Therefore, we altered our protocol incorporating the use of a TMV platform for the IN delivery of a subunit vaccine consisting of rLcrV and rF1 proteins. Recombinant TMV expressing a surface exposed lysine in the coat protein was previously generated in order to improve amine reactivity [25]. rLcrV or rF1 was covalently linked to the surface of the virus, such that each virus was decorated with the protein. 25µg of vaccine (containing 12.5µg of TMV + 12.5µg of recombinant vaccine protein) was administered IN in 40–50 µl of normal saline. In initial experiments 3 boosts were performed on day 14, 28, and 56. However, it was subsequently determined that the 3rd boost did not enhance serum anti-vaccine antibody to any significant degree, and only two boosts (d21 and d35) were performed in later experiments. In all cases, the mice were challenged on d70 with 10 LD50 of Y. pestis CO92 pgm−. IN administration of TMV-LcrV, TMV-F1, or both TMV-LcrV and TMV-F1 (TMV-LcrV+TMV-F1) produced higher titers of vaccine-protein specific IgG1, IgG2a, and IgG2b antibodies than those observed in mice vaccinate IM with rLcrV+rF1 in alum (Fig 3a, b, c, respectively). Interestingly, IN co-vaccination with both TMV-LcrV and TMV-F1 resulted in increased anti-LcrV and anti-F1 antibody levels compared to mice vaccinated IN with either TMV-LcrV or TMV-F1 alone (Fig 3 a–c). Mice vaccinated with TMV-LcrV alone demonstrated ~60% protection against subsequent challenge with Y. pestis CO92 pgm− (Fig. 4). These mice demonstrated outward signs of illness, including weight loss, reduced activity, and moth-eaten appearance, however, outward signs of illness were visually far less severe than those observed in unvaccinated control mice regardless of whether mice succumbed to infection or recovered (reduced morbidity). Mice vaccinated IN with TMV-F1 or TMV-LcrV+TMV-F1, or vaccinated IM with rLcrV+rF1 in alum, were completely protected from lethal challenge (Fig 5). These mice showed no outward signs of illness, did not lose weight and showed no reduced activity. By contrast, mice that received rLcrV, rF1, or rLcrV+rF1 IN had reduced antibody levels compared to those observed in mice that received vaccine proteins conjugated to TMV, and reduced survival rates. All mice that received rLcrV IN succumbed to infection, while only a single mouse, of five, that received rF1 IN survived. By contrast, mice that received TMV-LcrV and TMV-F1 IN demonstrated significantly greater protection from lethal challenge (p<0.05). The mice that received rLcrV, rF1, or rLcrV+rF1 IN demonstrated severe morbidity compared to TMV-treated mice. Mice vaccinated with rF1+rLcrV IN demonstrated ~50% survival, higher than that observed with vaccination using each protein independently, but significantly less than that observed following IN vaccination with TMV-LcrV+TMV-F1 (p<0.05). Mice vaccinated with rF1+rLcrV IN were also visibly less moribund than mice vaccinated with each protein individually, but did demonstrate outward signs of illness, whereas mice vaccinated with TMV-LcrV+TMV-F1 appeared healthy after infection. These data indicate that TMV has adjuvant activity, and can work to overcome barriers to intranasal vaccine administration [27] and improve immunity to linked subunit protein vaccines. Remarkably, we were able to demonstrate full protection from lethal pneumonic challenge with Y. pestis via IN vaccination with a protein subunit vaccine linked to a TMV delivery system.
Figure 3.
IN vaccination and boost with TMV-LcrV+TMV-F1 induces higher anti-LcrV and anti-F1 antibody production compared to IM vaccination with rLcrV+rF1. Anti-LcrV and anti-F1 antibody binding curves (left column) and 50% maximal binding titers (right column) for IgG1 (a), IgG2a (b), and IgG2b (c) in mice vaccinated with TMV-LcrV (n=5), TMV-F1(n=8), TMV-LcrV+TMV-F1(n=7), rLcrV IN(n=5), rF1 IN(n=5), rLcrV+rF1 IN(n=5), rLcrV+rF1 IM(n=5), or unvaccinated mice(n=5). Titers could not be calculated for anti-F1 antibodies in TMV-LcrV or rLcrV IN vaccinated mice or unvaccinated mice, and anti-LcrV antibodies in TMV-F1 or rF1 IN vaccinated mice or unvaccinated mice; these groups are not therefore not represented in the 50% binding titer graphs. These data are representative of 5 experiments for TMV-LcrV and 3 experiments for TMV-F1 and TMV-LcrV+TMV-F1.
Figure 4.
TMV-LcrV + TMV-F1 completely protects against morbidity and mortality associated with pneumonic infection with 10× LD50 Y. pestis CO92pgm−. Mice were challenged on day 0 and then weighed daily until completion of the experiment. These data are representative of 5 experiments for TMV-LcrV and 3 experiments for TMV-F1 and TMV-LcrV+TMV-F1. *-p<0.05, TMV-LcrV IN vs. rLcrV IN, TMV-F1 IN vs. rF1 IN, TMV-LcrV+TMV-F1 IN vs. rLcrV+rF1 IN, rLcrV+rF1 IM vs. rLcrV+rF1 IN.
Discussion
In the present study we demonstrated that a protein subunit vaccine consisting of the LcrV and F1 virulence factors from Y. pestis CO92 bound to TMV, induced complete protection from subsequent lethal challenge with the bacteria when delivered mucosally. Mice vaccinated with both proteins demonstrated 100% survival and no morbidity. On the other hand, mice vaccinated with a live bacterial vaccine vector, L. plantarum, were not protected against lethal challenge. The failure of the lipLcrV-L. plantarum vaccine to demonstrate any level of protection against lethal challenge is curious given the robust humoral immune response generated by the vaccine. The lack of survival did not appear to be the result of improper folding and/or presentation of LcrV in the recombinant Lactobaccilus, as antibodies generated against the vaccine were capable of binding to the rLcrV used for vaccination and could detect LcrV in whole lysates of Y. pestis CO92 pgm− (data not shown). The similarities between the measured immune responses generated by IM vaccination with rLcrV and IN vaccination with lipLcrV-L. plantarum, implies that the differences in the protective effect between the two vaccines does not lie in either the antibodies or cytokines tested here, but in some as of yet unidentified factor(s) that contributed to protection from pneumonic plague. TMV has been previously demonstrated to enhance humoral and cell mediated immunity to subunit vaccines, enhancing antigen uptake and presentation by professional antigen presenting cells [21]. We hypothesize that direct uptake of the viral particle, albeit a recombinantly expressed plant pathogen, enhances the response to the associated subunit protein by simulate antiviral signaling. This hypothesis is supported by studies demonstrating enhanced protection from respiratory challenge with both F. tularensis and Influenza A [22–24]. L. plantarum is a commensal organism, and may not induce the same level of activation as TMV, though further study is needed to confirm this. This could provide an explanation for the protective effects of the TMV conjugated vaccine over the live bacterial vector, and is the focus of continuing study.
Previous reports have suggested a correlation between combined LcrV and F1 specific IgG1 antibody titer and protection[28,29]. IgG1 is often associated with Th2 immune responses in mice; however, in practice we and others often observe elevated IgG1 in response to exogenous antigen, even in the presence of Th1 responses ([30] and P. Arnaboldi personal observations). IN vaccination with lipLcrV-L. plantarum induced somewhat lower titers of LcrV-specific IgG1 compared to the mice vaccinated IM with rLcrV protein. However, titers in lipLcrV-L. plantarum were still present. Production of IgG2a and IgG2b and cytokine levels between the IN and IM vaccines groups were similar. We find it unlikely that the difference in IgG1 titers alone is responsible for complete lack of protection in lipLcrV-L. plantarum vaccinated mice compared to rLcrV vaccinated mice. In support of this, mice vaccinated with TMV-LcrV alone also demonstrated higher titers of IgG1 compared with mice vaccinated IM with rLcrV+rF1; however, in we observe ~60% survival in TMV-LcrV vaccinated mice compared to 100% survival in IM vaccinated mice (Fig 3a and 4). Furthermore, in one experiment we observed that mice vaccinated IM with rLcrV+rF1 in alum produced very low levels of IgG1. It is unclear why this occurred in this experiment; however, despite a lack of IgG1, the mice were protected from lethal challenge. When combined, these results imply that IgG1 titers do not necessarily correlate well with survival, and that other immune parameters, including cell mediated immunity, are involved and must be investigated. SIgA is not required for protection from pneumonic plague. LcrV specific SIgA was observed in BAL washes of lipLcrV-L. plantarum vaccinated mice but not in rLcrV IM vaccinated mice, yet only the latter were protected from lethal pneumonic challenge.
Cellular immune responses to Plague have been shown to be important for protection [7,13,16,31,32]. Cytokine production in response to ex vivo restimulation with rLcrV was not observed following vaccination alone. Cytokine production was only detectable after in vivo stimulation followed by ex vivo restimulation. This implies that vaccination alone does not induce a strong inflammatory cytokine response, a key factor in side effects associated with vaccination. Despite the clear difference in protection in lipLcrV-L. plantarum vaccinated mice and rLcrV IM vaccinated mice, observed vaccine-specific cytokine secretion between the two groups was minimal and virtually identical, possibly implying that the cytokines and chemokines included in our multiplex panel (IL1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-17, MCP1, IFNγ, TNFα, MIP-1α, GMCSF, and RANTES) did not play a significant role in vaccine induced protection. Differences in protection may be attributable to a cytokine/chemokine profile that was not included in our multiplex panel, or that the observed response involved different subsets of immune cells (e.g., CD4 vs. CD8), which were not assessed in our studies. Studies are being carried out to thoroughly investigate the individual contributions of CD4 and CD8 T cells in the vaccine response, as well as a more expansive determination of cytokine secretion.
Also of interest is the apparent adjuvanting effect of co-vaccination with both TMV-LcrV and TMV-F1. Anti-LcrV and anti-F1 is elevated in co-vaccinated mice compared to mice that receive each vaccine component individually. This suggests that there is not only an additive effect of both vaccines on survival, but an additive effect on the resultant immune response. It will be of interest to see if the enhanced production of anti-LcrV responses in covaccinated mice is able to provide complete protection against F1(−) strains of Y. pestis, where the contribution of F1-specific immunity will be nullified.
The results presented here represent a study of the potential of an intranasally delivered subunit vaccine for pneumonic plague. Preliminary evidence suggests that this vaccine is also protective against bubonic plague (data not shown). Though we were unable to identify specific mechanisms that directly correlate with a protective vaccine response in our experiments, these data suggest that the immune response required for protection is atypical, and not associated with previously described humoral and Th1 cytokine responses. Further studies are currently being conducted to determine if this is, in fact, the case, and to identify specific immune mechanisms that correlate with protection against Plague. Further studies will also be conducted to evaluate the protective capabilities of the TMV-vaccine in the presence of different fully virulent strains of Y. pestis and ultimately to determine if mucosal delivery with a TMV platform will enhance vaccine efficacy in primate models. These two vaccine platforms, the non-protective L. plantarum live vector vaccine and the strongly protective TMV subunit vaccine, offer us a unique ability to evaluate differential immune responses to the same vaccine proteins. Regardless we have demonstrated that TMV is a suitable mucosal delivery platform for an F1-LcrV subunit vaccine that induces complete protection against pneumonic infection with a lethal dose of Y. pestis in mice.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. James Bliska, PhD who generously supplied the Yersinia pestis strain used in this study.
Funding: This work was supported by the National Institutes of Health [grant number R01 AI084952]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: TMV-Tobacco Mosaic Virus, IN-intranasal, IM-intramuscular
Conflict of Interest Statement: PMA, MS, CD, LAP, AM, and RD have no conflicts associated with this study.
References
- 1.Prentice MB, Rahalison L. Plague. Lancet. 2007;369(9568):1196–1207. doi: 10.1016/S0140-6736(07)60566-2. [DOI] [PubMed] [Google Scholar]
- 2.Williamson ED. Plague. Vaccine. 2009;27(Suppl 4):D56–D60. doi: 10.1016/j.vaccine.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 3.Butler T. Plague gives surprises in the first decade of the 21st century in the United States and worldwide. Am J Trop Med Hyg. 2013;89(4):788–793. doi: 10.4269/ajtmh.13-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galimand M, Carniel E, Courvalin P. Resistance of Yersinia pestis to Antimicrobial Agents. Antimicrobial Agents and Chemotherapy. 2006;50(10):3233–3236. doi: 10.1128/AAC.00306-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch TJ, Fricke WF, McDermott PF, White DG, Rosso ML, Rasko DA, et al. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One. 2007;2(3):e309. doi: 10.1371/journal.pone.0000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alibek K, Handelman S. Biohazard: The Chilling True Story of the Largest Covert Biological Weapons Program in the World--Told from Inside by the Man Who Ran it. New York: Random House; 1999. [Google Scholar]
- 7.Philipovskiy AV, Smiley ST. Vaccination with live Yersinia pestis primes CD4 and CD8 T cells that synergistically protect against lethal pulmonary Y. pestis infection. Infect Immun. 2007;75(2):878–885. doi: 10.1128/IAI.01529-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson ED, Oyston PC. Protecting against plague: towards a next-generation vaccine. Clin Exp Immunol. 2013;172(1):1–8. doi: 10.1111/cei.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewoody RS, Merritt PM, Marketon MM. Regulation of the Yersinia type III secretion system: traffic control. Front Cell Infect Microbiol. 2013;3:4. doi: 10.3389/fcimb.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Depaolo RW, Tang F, Kim I, Han M, Levin N, Ciletti N, et al. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe. 2008;4(4):350–361. doi: 10.1016/j.chom.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quenee LE, Cornelius CA, Ciletti NA, Elli D, Schneewind O. Yersinia pestis caf1 variants and the limits of plague vaccine protection. Infect Immun. 2008;76(5):2025–2036. doi: 10.1128/IAI.00105-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh AK, Kingston JJ, Gupta SK, Batra HV. Recombinant Bivalent Fusion Protein rVE Induces CD4+ and CD8+ T-Cell Mediated Memory Immune Response for Protection Against Yersinia enterocolitica Infection. Front Microbiol. 2015;6:1407. doi: 10.3389/fmicb.2015.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szaba FM, Kummer LW, Duso DK, Koroleva EP, Tumanov AV, Cooper AM, et al. TNFalpha and IFNgamma but not perforin are critical for CD8 T cell-mediated protection against pulmonary Yersinia pestis infection. PLoS Pathog. 2014;10(5):e1004142. doi: 10.1371/journal.ppat.1004142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinc G, Pennington JM, Yolcu ES, Lawrenz MB, Shirwan H. Improving the Th1 cellular efficacy of the lead Yersinia pestis rF1-V subunit vaccine using SA-4-1BBL as a novel adjuvant. Vaccine. 2014;32(39):5035–5040. doi: 10.1016/j.vaccine.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Smiley ST. Current challenges in the development of vaccines for pneumonic plague. Expert Rev Vaccines. 2008;7(2):209–221. doi: 10.1586/14760584.7.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parent MA, Berggren KN, Kummer LW, Wilhelm LB, Szaba FM, Mullarky IK, et al. Cell-mediated protection against pulmonary Yersinia pestis infection. Infect Immun. 2005;73(11):7304–7310. doi: 10.1128/IAI.73.11.7304-7310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smiley ST. Immune defense against pneumonic plague. Immunol Rev. 2008;225:256–271. doi: 10.1111/j.1600-065X.2008.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.del Rio B, Fuente JL, Neves V, Dattwyler R, Seegers JF, Gomes-Solecki M. Platform technology to deliver prophylactic molecules orally: an example using the Class A select agent Yersinia pestis. Vaccine. 2010;28(41):6714–6722. doi: 10.1016/j.vaccine.2010.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres-Escobar A, Juarez-Rodriguez MD, Branger CG, Curtiss R., 3rd Evaluation of the humoral immune response in mice orally vaccinated with live recombinant attenuated Salmonella enterica delivering a secreted form of Yersinia pestis PsaA. Vaccine. 2010;28(36):5810–5816. doi: 10.1016/j.vaccine.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branger CG, Sun W, Torres-Escobar A, Perry R, Roland KL, Fetherston J, et al. Evaluation of Psn, HmuR and a modified LcrV protein delivered to mice by live attenuated Salmonella as a vaccine against bubonic and pneumonic Yersinia pestis challenge. Vaccine. 2010;29(2):274–282. doi: 10.1016/j.vaccine.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemnade JO, Seethammagari M, Collinson-Pautz M, Kaur H, Spencer DM, McCormick AA. Tobacco mosaic virus efficiently targets DC uptake, activation and antigen-specific T cell responses in vivo. Vaccine. 2014;32(33):4228–4233. doi: 10.1016/j.vaccine.2014.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banik S, Mansour AA, Suresh RV, Wykoff-Clary S, Malik M, McCormick AA, et al. Development of a Multivalent Subunit Vaccine against Tularemia Using Tobacco Mosaic Virus (TMV) Based Delivery System. PLoS One. 2015;10(6):e0130858. doi: 10.1371/journal.pone.0130858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallajosyula JK, Hiatt E, Hume S, Johnson A, Jeevan T, Chikwamba R, et al. Single-dose monomeric HA subunit vaccine generates full protection from influenza challenge. Hum Vaccin Immunother. 2014;10(3):586–595. doi: 10.4161/hv.27567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallajosyula JK, Jeevan T, Chikwamba R, Webby RJ, McCormick AA. A Single Dose TMV-HA Vaccine Protects Mice from H5N1 Influenza Challenge. International Journal of Vaccine Research. 2016;1(2):6. [Google Scholar]
- 25.Smith ML, Lindbo JA, Dillard-Telm S, Brosio PM, Lasnik AB, McCormick AA, et al. Modified tobacco mosaic virus particles as scaffolds for display of protein antigens for vaccine applications. Virology. 2006;348(2):475–488. doi: 10.1016/j.virol.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 26.del Rio B, Seegers JF, Gomes-Solecki M. Immune response to Lactobacillus plantarum expressing Borrelia burgdorferi OspA is modulated by the lipid modification of the antigen. PLoS One. 2010;5(6):e11199. doi: 10.1371/journal.pone.0011199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riese P, Sakthivel P, Trittel S, Guzman CA. Intranasal formulations: promising strategy to deliver vaccines. Expert Opin Drug Deliv. 2014;11(10):1619–1634. doi: 10.1517/17425247.2014.931936. [DOI] [PubMed] [Google Scholar]
- 28.Williamson ED, Vesey PM, Gillhespy KJ, Eley SM, Green M, Titball RW. An IgG1 titre to the F1 and V antigens correlates with protection against plague in the mouse model. Clin Exp Immunol. 1999;116(1):107–114. doi: 10.1046/j.1365-2249.1999.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinc G, Pennington JM, Yolcu ES, Lawrenz MB, Shirwan H. Improving the Th1 cellular efficacy of the lead Yersinia pestis rF1-V subunit vaccine using SA-4-1BBL as a novel adjuvant. Vaccine. 2014;32(39):5035–5040. doi: 10.1016/j.vaccine.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166(12):7381–7388. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 31.Smiley ST. Cell-mediated defense against Yersinia pestis infection. Adv Exp Med Biol. 2007;603:376–386. doi: 10.1007/978-0-387-72124-8_35. [DOI] [PubMed] [Google Scholar]
- 32.Lin JS, Park S, Adamovicz JJ, Hill J, Bliska JB, Cote CK, et al. TNFalpha and IFNgamma contribute to F1/LcrV-targeted immune defense in mouse models of fully virulent pneumonic plague. Vaccine. 2010;29(2):357–362. doi: 10.1016/j.vaccine.2010.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.