Abstract
The family of vertebrate Crumbs proteins, homologous to Drosophila Crumbs (Crb), share large extracellular domains with epidermal growth factor-like repeats and laminin-globular domains, a single transmembrane domain, and a short intracellular C-terminus containing a single membrane proximal 4.1/ezrin/radixin/moesin-binding domain and PSD-95/Discs large/ZO-1-binding motifs. There are 3 Crb genes in humans - Crumbs homolog-1 (CRB1), Crumbs homolog-2 (CRB2), and Crumbs homolog-3 (CRB3). Bilallelic loss-of-function mutations in CRB1 cause visual impairment, with Leber's congenital amaurosis and retinitis pigmentosa, whereas CRB2 mutations are associated with raised maternal serum and amniotic fluid alpha feto-protein levels, ventriculomegaly/hydrocephalus, and renal disease, ranging from focal segmental glomerulosclerosis to congenital Finnish nephrosis. CRB3 has not yet been associated with human disease. In this review, we summarize the phenotypic findings associated with deleterious sequence variants in CRB1 and CRB2. We discuss the mutational spectrum, animal models of loss of function for both genes and speculate on the likely mechanisms of disease.
Key Words: Crumbs proteins, CRB2, CRB1
The Crumbs family of transmembrane cell-cell adhesion proteins share a highly conserved structure with a large extracellular region containing epidermal growth factor (EGF)-like domains and laminin G-like motifs that is linked to a short intracellular tail containing a 4.1/ezrin/radixin/moesin (FERM)-binding domain and a PSD-95/Discs large/ZO-1 (PDZ)-binding domain containing a carboxy-terminal, 4 amino-acid ERLI motif [Omori and Malicki, 2006; Thompson et al., 2013; Zou et al., 2013]. In Drosophila melanogaster, the PDZ-binding motif from the Crb proteins can recruit a plasma membrane-associated protein scaffold comprising Stardust, DPatJ and DLin7 or Par6, and an atypical protein kinase C to form the Crumbs complex [Pocha and Knust, 2013; Thompson et al., 2013]. Crb is expressed in ectoderm and on the subapical membranes of epithelial cells, and aberrant Crb function disrupts the organization of epithelia derived from ectoderm during organogenesis and the maintenance of epithelial cell polarity. Studies in D. melanogaster and Danio rerio showed that the Crumbs complex can also regulate cellular signaling pathways, such as Notch1, mechanistic target of rapamycin complex 1, and the Hippo pathway. In Drosophila, the intracellular FERM-binding domain also binds an upstream regulator of the Hippo pathway, and the loss of Crb inhibits apical Hippo-Warts signaling, preventing inhibition of Yorkie, a transcriptional activator that promotes growth [Thompson et al., 2013]. Humans have 3 Crumbs genes - CRB1, CRB2, and CRB3. Biallelic deleterious sequence variants in CRB2 inherited in an autosomal recessive (AR) pattern have recently been associated with a pleiotrophic, but variable condition, termed CRB2-related syndrome, comprising a phenotypic triad of elevated alpha feto-protein levels, cerebral ventriculomegaly, and renal findings consistent with congenital Finnish nephrosis. In contrast, bilallelic mutations in CRB1 that are inherited in an AR pattern have long been associated with Leber's congenital amaurosis (LCA) and retinitis pigmentosa (RP), whereas as yet there is no known human disease associated with deleterious variants in CRB3. In this review, we summarize the clinical findings, variants, and mechanisms associated with pathogenic variants in CRB1 and CRB2.
CRB1
Clinical Phenotype
The clinical findings associated with retinal dystrophies caused by deleterious sequence variants in CRB1 range from congenital blindness, with LCA, to early-onset rod-cone dystrophy and AR RP [Pellissier et al., 2015]. Mutations in CRB1 account for 10-15% of all patients with LCA [den Hollander et al., 2004, 2008; Bujakowska et al., 2012] and as many as 6.5% of all patients with AR RP [Bernal et al., 2003; den Hollander et al., 2004; Vallespin et al., 2007; Bujakowska et al., 2012]. Visual acuities have ranged from an ability to perceive light, to 20/25 vision. Investigations have revealed undetectable rod electroretinograms (ERGs), in contrast to reduced cone signals. Optical coherence tomography has shown small foveal islands of thinned outer nuclear layer surrounded by thick delaminated retinae with intraretinal hyperreflective lesions [Aleman et al., 2011]. Magnetic resonance imaging scanning of the orbits has been noteworthy for structurally normal optic nerves and subtle changes to occipital lobe white and gray matter [Aleman et al., 2011].
CRB1 variants have also been found in an estimated 74.1% of individuals with preservation of the para-arteriolar retinal pigment epithelium and an estimated 53.3% of individuals with retinal telangiectasia with exudation (also referred to as Coats-like vasculopathy) [Bujakowska et al., 2012]. Other ocular findings associated with pathogenic CRB1 variants include macular atrophy, pigmented paravenous chorioretinal atrophy, and a predisposition to keratoconus and nanophthalmos [den Hollander et al., 2004; McMahon et al., 2009; Henderson et al., 2010; Zenteno et al., 2011; Bujakowska et al., 2012; Pellissier et al., 2015]. CRB1 mutations may also be a rare cause of cystoid macular edema, a condition that has responded favorably to topical carbonic anhydrase inhibitors [Tsang et al., 2014; Wolfson et al., 2015].
Therapy for the retinal disease caused by CRB1 variants has been attempted in the mouse [Pellissier et al., 2015]. Unexpectedly, targeting a single cell type or combination of cell types using a CRB1 gene therapy vector in a Crb-1 RP mouse model at mid-stage disease reduced, rather than improved, retinal function. In ciliary epithelium with loss of Crb1 function, ectopic expression of human CRB1 transmembrane protein also caused tissue degeneration and invasion of immune cells [Pellissier et al., 2014]. However, targeting both Müller glial cells and photoreceptors with CRB2 ameliorated retinal function and structure in the Crb1 RP mouse model [Pellissier et al., 2015]. In human retinopathy caused by CRB1 mutations, targeting photoreceptors with low levels of endogenous CRB2 with recombinant CRB2 to elevate endogenous levels may prove to be effective in preventing retinal degeneration [Pellissier et al., 2015].
Molecular Genetics
CRB1 comprises 12 exons and exhibits alternative splicing at the 3′ end to yield 2 proteins of 1,376 and 1,406 amino acids containing 19 EGF-like domains, 3 laminin A globular-like domains, and a signal peptide sequence. The longer isoform contains a transmembrane domain and a cytoplasmic domain that includes a conserved membrane proximal FERM-binding domain and a PDZ-binding motif, which enable CRB1 to participate in the formation of adherens junction and link to the actin cytoskeleton [Gosens et al., 2008; Pocha and Knust, 2013].
More than 150 disease-associated variants in CRB1 have been described in 240 patients [Bujakowska et al., 2012; Wolfson et al., 2015]. However, despite the wealth of data, no clear phenotype-genotype correlation has emerged [Pellissier et al., 2015]. The most frequently occurring mutation is p.Cys948Tyr in exon 9, which has been found in 96 reported alleles and accounts for 24% of CRB1 mutations [Bujakowska et al., 2012]. As the majority of mutations are in exons 7 (27%) and 9 (41%) that encode the second and third laminin A globular-like domains, respectively, these domains are considered particularly relevant to CRB1 function, and it has been suggested that these exons should be the first to be screened for variants [Bujakowska et al., 2012]. Although the mutational spectrum is broad, with missense, frameshift, nonsense, and splice site variants, single amino acid substitutions account for 66% of reported variants [Bujakowska et al., 2012]. An estimated 30% of the cases have only 1 detected CRB1 variant, so that intronic sequence variants, digenic or triallelic inheritance, and/or genetic modifiers have been hypothesized to be important in these cases [Bujakowska et al., 2012]. However, cosegregation analysis performed to date does not support digenic inheritance [Vallespin et al., 2007; Bujakowska et al., 2012].
Expression and Animal Models
CRB1 expression is strongest in the human retina and brain and has been demonstrated in Müller glia cells and cone and rod photoreceptors [Pellissier et al., 2015]. In the developing murine eye, Crb1 expression is detectable from E11.5 in the retinal anlage and labels the developing iris and proliferative retinoblasts up to E16.5 [den Hollander et al., 2002]. In early postnatal stages and in the adult eye, Crb1 is present in the apical membranes of the retinal epithelial cells, in Müller cells, and in the outer limiting membrane of the inner segments of photoreceptors [den Hollander et al., 2002]. Crb1 transcripts are also detected in the central nervous system from E10.5, with expression predominantly in the ventral part of the neural tube, including the ventral spinal cord, the ventral part of the mesencephalon (tegmentum), the mammillary and the hypothalamic regions [den Hollander et al., 2002]. In the adult murine brain, Crb1 expression is confined to areas of neuronal production and migration [den Hollander et al., 2002].
In mice, Crb1 maintains adherens junctions between photoreceptors and Müller glia cells [Pellissier et al., 2014]. Knockout Crb1-/-, knockin Crb1C249W/-, and naturally occurring Crb1rd8/rd8 mice show mild retinal disorganization that is particularly marked in the inferior retinal quadrant [van de Pavert et al., 2007; Pellissier et al., 2014]. Mouse models of Crb1 loss of function together with models of Crb2 and Pals1 loss of function also display some of the phenotypic features associated with human CRB1 variants, including focal laminar disorganization, pseudorosette formation, photoreceptor degeneration, and functional impairment in the electroretinogram [Alves et al., 2013, 2014; Kim et al., 2015]. These findings are in accordance with clinical features of the patients carrying CRB1 mutations, whose retinae demonstrate thickening with an altered laminar organization, coarse outer and inner zones, and a thick surface layer around the optic nerve that, in combination, resembles a normal retina at a less mature stage of development [Jacobson et al., 2003].
Mechanism
Crb1 shares the same structure as other Crb proteins, with a large extracellular region composed of EGF-like and laminin A/G-like domains and a short, but essential intracellular domain that contains a FERM-binding motif for interaction with EPB4.1L5 and an extreme C-terminal PDZ-binding motif responsible for its critical interaction with the PDZ domain of protein associated with lin-seven 1 (Pals1) [Kim et al., 2015]. Crb complexes are formed from Crb and the cytoplasmic proteins, PALS1 (also known as membrane protein palmitoylated 5, or MPP5), and PALS1-associated tight junction protein, or multi-PDZ domain protein 1 [Alves et al, 2014]. The Crb complex proteins localize at the apical side of the epithelium that forms the adhesive belt formed between photoreceptor cells and Müller glia located in the outer limiting membrane of the retina [Kim et al., 2015]. At this membrane, retinal cells are connected by adherens junctions that separate the apical portion of the plasma membrane from the basolateral domain [Alves et al., 2014]. The association of eye defects with loss of CRB1 function is supported by studies that show that ablation of Cbr1 and Crb2 in the murine retina leads to defects in lamination and proliferation of retinal progenitor cells that is similar to LCA, with dysregulation of the Notch1 and YAP/Hippo signaling pathways involved in cell proliferation [Pocha and Knust, 2013]. The Crb and partitioning-defective complexes act as cell polarity regulators and reside apically to the adherens junctions [Alves et al., 2014]. Mutations or changes in the expression of the components of this complex alter polarity and adhesion in the retinal epithelium, such that dividing cells detach from the apical lamina [Alves et al., 2014]. Disruption to the spatiotemporal aspects of retinogenesis subsequently results in retinal thinning and degeneration, causing mild to severe impairment of retinal function and vision [Alves et al., 2014].
CRB2
Clinical Phenotype
Biallelic mutations in CRB2 cause CRB2-related syndrome that is characterized by the phenotypic triad of greatly elevated maternal serum alpha-fetoprotein (MSAFP) and amniotic fluid alpha-fetoprotein levels, cerebral ventriculomegaly and cystic renal disease with histopathological findings resembling congenital Finnish nephrosis [Slavotinek et al., 2015; Jaron et al., 2016; Lamont et al., 2016]. Thirteen individuals have been reported with this phenotype to date (table 1) [Slavotinek et al., 2015; Jaron et al, 2016; Lamont et al., 2016]. Although initially ascertained due to raised MSAFP and renal disease, hydrocephalus or ventriculomegaly has been present in 11/13 (85%) individuals. Aqueductal stenosis was noted in 5/13 (38%), gray matter heterotopias in 3/13 (23%), and abnormalities of the corpus callosum, including thinning of this tract, in 2/13 (15%) (table 1). Seizures were manifest in 2 individuals, although 1 of these patients had only 1 seizure by report, type unknown [Jaron et al., 2106].
Table 1.
Clinical findings in patients with CRB2-related syndrome
| Slavotinek et al. [2015] (n = 6) | Lamont et al. [2016] (n = 5) | Jaron et al. [2016] (n = 2) | Total (%) (n = 13) | |
|---|---|---|---|---|
| Central nervous system | ||||
| Ventriculomegaly | 4/6 | 5/5 | 2/2 | 11/13 (85) |
| Aqueductal stenosis | 3/5 | 2/2 | 5/13 (38) | |
| Gray matter heterotopias | 1/6 | 2/5 | 3/13 (23) | |
| Abnormal corpus callosum | 2/2 | 2/13 (15) | ||
| Cerebellar hypoplasia | 1/6 | 1/5 | 1/13 (8) | |
| Quadrigeminal cyst | 1/5 | 1/13 (8) | ||
| Seizures | 1/5 | 1/2 | 2/13 (15) | |
| Cardiac | ||||
| Ventricular septal defect | 1/6 | 1/2 | 2/13 (15) | |
| Atrial septal defect | 1/5 | 1/13 (8) | ||
| Patent ductus arteriosus | 1/2 | 1/13 (8) | ||
| Pericardial effusion | 1/6 | 1/5 | 2/13 (15) | |
| Scimitar syndrome | 1/5 | 1/13 (8) | ||
| Renal | ||||
| Renal echogenicity | 2/6 | 4/5 | + | 6/13 (46) |
| Micro- or macrocysts | 3/6 | 1/5 | 4/13 (31) | |
| Ureterohydronephrosis | 2/2 | 2/13 (15) | ||
| ‘Finnish nephrosis’ | 1/6 | 2/5 | 3/13 (23) | |
| Eye | ||||
| Abnormal retinae | 1/5 | 1/13 (8) | ||
| Abnormal optic nerve/disc | 2/5 | 1/2 | 3/13 (23) | |
| Other | ||||
| Uterus didelphys | 1/5 | 1/13 (8) | ||
| B-cell lymphoma | 1/5 | 1/13 (8) | ||
| Right lung hypoplasia | 1/2 | 1/13 (8) |
Involvement of the kidneys was demonstrated by renal echogenicity in 6/13 (46%), macro- or microcysts in 4/13 (31%), histopathological changes consistent with Finnish nephrosis in 3/13 (23%), and hydro-ureteronephrosis in 2/13 (15%) patients. Cardiac manifestations have been less common, with single occurrences of ventricular septal defect, an atrial septal defect in a child with Scimitar syndrome and a patent ductus arteriosus that required surgical repair (table 1). Other findings have included the development of B-cell lymphoma at 3 years of age and right lung hypoplasia (table 1) [Jaron et al., 2016; Lamont et al., 2016].
In view of the ocular findings associated with CRB1 mutations, retinal changes have been actively sought in patients with CRB2-related syndrome. To date, only one individual has demonstrated retinal abnormalities and a defect in the outer retinal laminae at the foveal site; reduced visual acuity, nystagmus, and irregular retinal pigmentation were observed in a female at 7 months of life [Lamont et al., 2016]. Several others with biallelic CRB2 mutations have had optic atrophy or a ‘faded’ optic nerve, although only the mother's history, rather than the medical records, was available for one of these children [Lamont et al., 2016].
Dysmorphic features have not frequently been described, but one individual had a broad forehead, low-set ears, retrognathia, widely spaced nipples, a bilateral single transverse crease and broad thumbs at birth, and frontal bossing, a high palate and broad first toes at 3 years and 8 months of age [Jaron et al., 2016]. His sib had right occipital plagiocephaly, a low-set left ear, depressed nasal bridge, a high and narrow palate, a mildly shield-shaped chest with widely spaced nipples, and a broad hallux at 2 years and 4 months of age [Jaron et al., 2016].
The prognosis in CRB2-related syndrome was initially thought to be poor, with no survival reported beyond 7 months of age from the first report of 6 affected individuals [Slavotinek et al., 2015]. However, the outcome has since been recognized as more variable and normal cognition and health have subsequently been reported in 2 individuals of 6 and 7 years of age, with both having ventriculo-peritoneal shunts inserted for treatment of a hydrocephalus at an early age [Jaron et al., 2016; Lamont et al., 2016]. It is unclear if there will prove to be a phenotype-genotype correlation associated with the prognosis.
Missense variants in CRB2 have also been associated with steroid resistant nephrotic syndrome with an underlying renal pathology of focal segmental glomerulosclerosis (FSGS) in 4 individuals who were not known to have any cerebral manifestations or other extrarenal findings [Ebarasi et al., 2015]. Furthermore, 3 out of 4 patients with isolated FSGS had missense substitutions in the 10th EGF domain [Jaron et al., 2016], and one patient with FSGS was a compound heterozygote for a missense mutation in an EGF-like domain and for a frameshift mutation previously observed in CRB2-related syndrome [Lamont et al., 2016]. CRB2 has also been resequenced for mutations in 85 patients with RP and 79 patients with LCA, and 11 missense substitutions were detected, but no patient had 2 mutations compatible with AR inheritance of CRB2 variants as a cause for their disease [van den Hurk et al., 2005].
CRB2-related syndrome should be suspected on finding significantly raised MSAFP and amniotic fluid alpha-fetoprotein levels in the second trimester of pregnancy, together with cerebral ventriculomegaly and renal findings suggestive of nephrosis. Management has been supportive and investigations for features of ciliopathy have been recommended [Jaron et al., 2016], although the one individual in whom a cilia biopsy was examined proved to have normal cilial function.
Mutations and Molecular Genetics
In all cases of CRB2-related syndrome, compound heterozygosity or homozygosity for sequence variants predicted to affect the function of CRB2 were demonstrated (table 2) [Slavotinek et al., 2015; Jaron et al., 2016; Lamont et al., 2016]. The sequence variants were hypothesized to act by loss of function based on nonsense and frameshift mutations and biallelic inheritance. CRB2 is located at chromosome 9q33.3 and contains 13 exons that encode a 1,285 amino acid transmembrane protein. Alternative splicing of the gene results in 2 isoforms, with isoform 1, a putative type I transmembrane protein of 1,285 amino acids, and isoform 2, a secreted protein of 1,176 amino acids; most interest has so far been devoted to the isoform encoding the transmembrane protein, which shares 24.4% sequence identity with human CRB1.
Table 2.
Reported variants in CRB2
| Genomic position | Nucleotide alteration | Amino acid alteration | ExAC browsera | Recurrent variant | Phenotype | Functional study |
|---|---|---|---|---|---|---|
| chr9: g. 126132826G>T | c.[1494G>T] | p.(Trp498Cys) | 90/120,792 | no | CRB2-related syndrome | no |
| chr9: g. 126133191G>C | c.[1859G>C] | p.(Cys620Ser) | not present | no | SRNS | yes |
| chr9: g. 126133214C>T | c.[1882C>T] | p.(Arg628Cys) | 2/119,188 | no | SRNS | no |
| chr9: g. 126133218G>C | c.[1886G>C] | p.(Cys629Ser) | not present | no | SRNS | yes |
| chr9: g. 126133229C>T | c.[1897C>T] | p.(Arg633Trp) | not present | no | CRB2-related syndrome | no |
| chr9: g. 126133349A>C | c.[1928A>C] | p.(Glu643Ala) | 5/117,750 | no | CRB2-related syndrome | no |
| chr9: g. 126133697G>A | c.[2277G>A] | p.(Trp759*) | 2/118,572 | no | CRB2-related syndrome | no |
| chr9: g. 126133821C>G | c.[2400C>G] | p.(Asn800Lys) | 17/118,106 | yes | CRB2-related syndrome | no |
| chr9: g. 126135914_126135915 | c.[3108_3109ins | p.(Gly1036Ala/s*42) | not present | yes | CRB2-related syndrome/ | no |
| insCCGGCGCGGCCCCGGC | CCGGCGCGGCCCCGGC] | SRNS | ||||
| chr9: g. 126136037A>C | c.[3227A>C] | p.(Asp1076Ala) | not present | no | CRB2-related syndrome | no |
| chr9: g. 126136101_126136102delCT | c.[3291_3292delCT] | p.(Cys1098Ser/s*53) | not present | no | CRB2-related syndrome | no |
| chr9: g. 126136153C>T | c.[3343C>T] | p.(Arg1115Cys) | not present | no | CRB2-related syndrome | no |
| chr9: g. 126136196G>C | c.[3386G>C] | p.(Cys1129Ser) | not present | no | CRB2-related syndrome | no |
| chr9: g. 126139229G>A | c.[3746G>A] | p.(Arg1249Gln) | 111/82,212 | no | SRNS | no |
All variant nomenclature used hg19 as the reference sequence. Reported variants after Slavotinek et al. [2015].
ExAC browser: http://exac.broadinstitute.org/. SRNS = Steroid resistant nephrotic syndrome.
Several recurrent mutations have been described, including p.Asn800Lys (p.N800K), a missense mutation that has been reported with Ashkenazi Jewish ethnicity [Slavotinek et al., 2015; Jaron et al., 2016]. This sequence variation was found in 2/128 Ashkenazi Jewish control genomes from a recent study [Carmi et al., 2014], resulting in a carrier frequency of 1 in 64 [Jaron et al., 2016]. It has been suggested that testing for CRB2 mutations should commence with p.Asn800Lys in this population. Interestingly, as yet there are no described p.Asn800Lys homozygotes, raising speculation that this event may either be lethal or cause a milder phenotype that remains undiagnosed [Jaron et al., 2016]. Other recurrent mutations include the missense variant, p.(Glu643Ala), and a 16-bp insertion causing a duplication of a 16-bp segment, c.3105_2106insGGCCCGGCGCGGCCCC, resulting p.(Gly1036Alafs*42) (table 2). Finally, it is also possible that biallelic nonsense mutations in CRB2 will be lethal in the embryonic period in humans, similar to the early mortality observed in the Crb2 knockout mouse [Xiao et al., 2011].
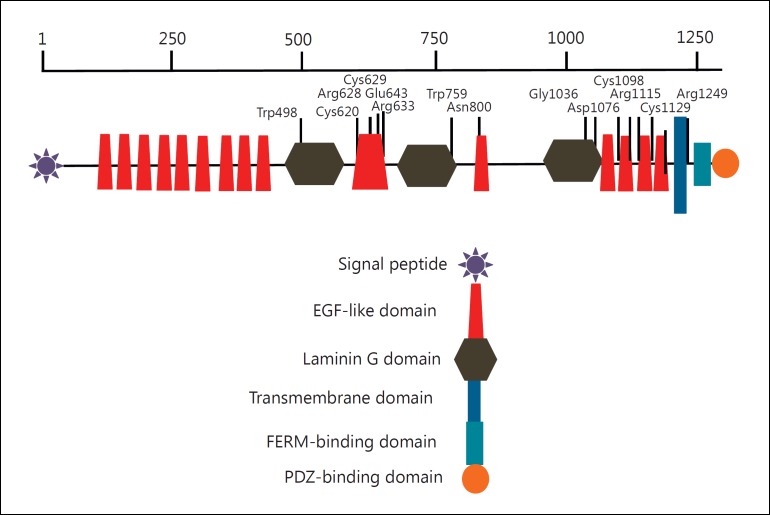
Human CRB2 contains 15 extracellular EGF-like domains and 3 extracellular laminin G-like domains, with a similar domain structure to CRB1 (fig. 1). Modeling of Crb in D. melanogaster has shown that the majority of the protein is extracellular, with a short cytoplasmic segment containing the PDZ-binding domains that interacts with PALS1/MPP5 (fig. 2). To date, almost all of the mutations associated with CRB2-related syndrome have occurred in the extracellular domain of the protein (table 2) and frequently involved cysteine residues that alter disulfide bridge formation or charged amino acids required for protein domain interactions [Lamont et al., 2016]. Individuals with CRB2-related syndrome have also been shown to have heterozygous variants in ciliopathy genes and genes for nephrosis, including NPHS1, BBS7, and BBS12, suggesting that modifying alleles or genetic burden may contribute to clinical variability. Phenotypic variability in CRB2-related syndrome may also result from digenic or triallelic inheritance as described below [Jaron et al., 2016; Lamont et al., 2016], similar to other ciliopathies.
Fig. 1.
Schematic drawing of mutation position in CRB2, showing that the majority of mutations affect the extracellular domain.
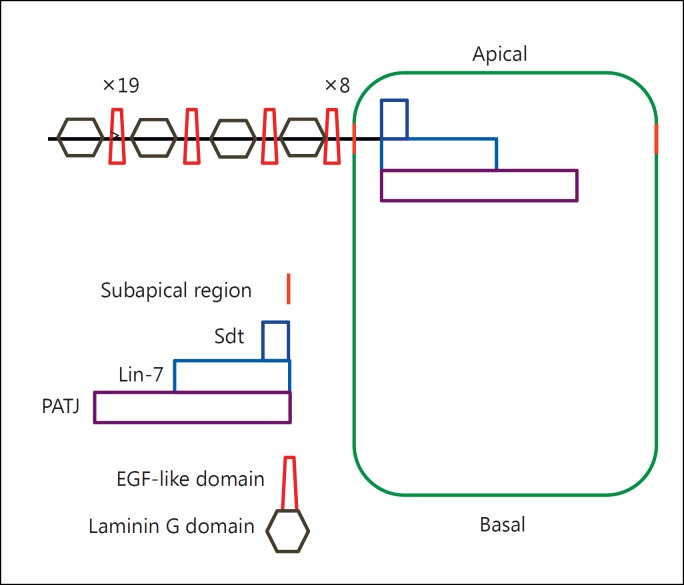
Fig. 2.
Schematic drawing of Crb in D. melanogaster, showing the position of the protein at the subapical region of the plasma membrane (marked in orange). The protein contains a large extracellular portion with numerous EGF-like domains (×19 and ×8 refer to repetitions of this repeat) and laminin G domains and a short intracellular portion that interacts with Sdt, Lin-7 and PATJ to form the Crumbs complex (after figs. 1 and 2B from Bulgakova and Knust, 2009).
Expression and Animal Models
CRB2 is expressed in human fetal retinal pigment epithelium, choroid and human adult brain and kidney, with weaker expression in the heart, placenta, and lung [van den Hurk et al., 2005]. Conditional null mice lacking Crb2 in the developing retina show severe progressive thinning and degeneration of the photoreceptor layer, abnormal lamination of immature rod photoreceptors, and disruption of the adherens junctions between retinal photoreceptors and Müller glia cells [Alves et al., 2013]. Complete loss of Crb2 in mice leads to fatal developmental anomalies that begin at gastrulation, with defective head folding, heart tube looping, foregut invagination, and somite development [Xiao et al., 2011]. In the developing brain, conditional ablation of Crb2 in the murine dorsal telencephalon leads to defects in the maintenance of apical polarity and cortical abnormalities [Dudok et al., 2016].
In zebrafish, there are 2 CRB2 orthologues, crb2a and crb2b. crb2a is transcribed in the optic vesicle and undifferentiated retinal neuroepithelium and has overlapping expression with crb1 expression in the undifferentiated neural tube epithelium, suggesting functional redundancy [Zou et al., 2013]. crb2b expression is restricted to the ventral-most regions of the anterior central nervous system [Zou et al., 2013]. The expression of crb1, crb2a, and crb2b follows a spatial-temporal distribution from anterior to posterior and from ventral to dorsal in the embryo that lags behind the expression of other adherens junction components. crb2b is also expressed in the pronephric glomerulus from 48 until 96 h after fertilization, and zebrafish injected with antisense morpholinos targeting this gene have compromised glomerular permeability with renal cysts and cardiac edema [Ebarasi et al., 2015]. Zebrafish that were homozygous for a stable loss-of-function mutation in crb2b showed abnormal glomerular histology, with an expanded Bowman's space, absence of the foot processes and compromised size selectivity in the glomerular filtration barrier that was similar to, but milder than the phenotype seen with loss of nephrin [Ebarasi et al., 2015].
Mechanism
It is unclear if the same mechanism underlying the renal defects associated with CRB2 loss of function is also responsible for the cerebral malformations. The extracellular domain of Crb is important for stabilizing the protein at the plasma membrane and in the zebrafish retinae, the extracellular domains of the 2 paralogous genes, crb2a and crb2b, form hetero- and homodimers [Thompson et al., 2013]. The Crb proteins directly mediate cell-cell adhesion through these extracellular domains [Thompson et al., 2013]. The extracellular domain is also important for the regulation of Notch signaling, as Crb can mask Notch from interactions with activating ligands [Thompson et al., 2013]. In zebrafish, simultaneous knockdown of crb1, crb2a, and crb2b results in severe polarization defects of the apicobasal neuroepithelium [Zou et al., 2013]. Conditional ablation of Crb2 in dorsal telencephalon of the mouse also disrupts the Crb and partioning-defective apical polarity protein complexes [Dudok et al., 2016].
The above functions suggest several possible mechanisms for the hydrocephalus and retinal defects in CRB2-related syndrome. As the variants causing CRB2-related syndrome affect the extracellular domain, it is possible that the clinical findings are related to disrupted cell-cell adhesion because of altered dimerization of the extracellular domains. Disruption to planar polarity and compromised ciliogenesis and ciliary function can also cause severe hydrocephalus [Al-Dosari et al., 2013; Narita and Takeda, 2015], and thus altered cell polarity and perturbation of ciliary function may also be relevant. However, in D. rerio, antisense morpholinos targeting crb2b did not disturb the overall polarity of podocytes [Ebarasi et al., 2009]. Finally, the hydrocephalus could result from aberrant signaling through the Notch or Hippo pathways. The similarities between the renal pathophysiology in Finnish nephrosis and CRB2-related syndrome suggest that a similar mechanism might be possible and, as nephrin is primarily an extracellular protein, it is plausible that both proteins are required for cell-cell interactions or cell-matrix interactions that ensure tight cellular contact.
Conclusion
Recently, mutations in CRB2 have been detected in patients with CRB2-related syndrome and steroid resistant nephrotic syndrome, adding to the phenotypic manifestations associated with deleterious variants in genes of the Crb complex. Although the visual phenotype associated with CRB1 loss of function has been well described, CRB2-related syndrome is still evolving, and further information regarding the ocular manifestations, systemic findings, prognosis, and the underlying mechanisms is still being determined. CRB2-related syndrome has clinical overlap with the ciliopathies and the function of CRB2 is likely to be modified by pathogenic variants in ciliopathy genes, meaning that a broader testing strategy that takes into account the possibility of modifying genes may be needed for accurate clinical information and prognosis.
Disclosure Statement
The author has no conflicts of interest to declare.
References
- 1.Al-Dosari MS, Al-Owain M, Tulbah M, Kurdi W, Adly N, et al. Mutation in MPDZ causes severe congenital hydrocephalus. J Med Genet. 2013;50:54–58. doi: 10.1136/jmedgenet-2012-101294. [DOI] [PubMed] [Google Scholar]
- 2.Aleman TS, Cideciyan AV, Aguirre GK, Huang WC, Mullins CL, et al. Human CRB1-associated retinal degeneration: comparison with the rd8 Crb1-mutant mouse model. Invest Ophthalmol Vis Sci. 2011;52:6898–6910. doi: 10.1167/iovs.11-7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves CH, Bossers K, Vos RM, Essing AH, Swagemakers S, et al. Microarray and morphological analysis of early postnatal CRB2 mutant retinas on a pure C57BL/6J genetic background. PLoS One. 2013;8:e82532. doi: 10.1371/journal.pone.0082532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alves CH, Pellissier LP, Wijnholds J. The CRB1 and adherens junction complex proteins in retinal development and maintenance. Prog Retin Eye Res. 2014;40:35–52. doi: 10.1016/j.preteyeres.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Bernal S, Calaf M, Garcia-Hoyos M, Garcia-Sandoval B, Rosell J, et al. Study of the involvement of the RGR CRPB1, and CRB1 genes in the pathogenesis of autosomal recessive retinitis pigmentosa. J Med Genet. 2003;40:e89. doi: 10.1136/jmg.40.7.e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bujakowska K, Audo I, Mohand-Saïd S, Lancelot ME, Antonio A, et al. CRB1 mutations in inherited retinal dystrophies. Hum Mutat. 2012;33:306–315. doi: 10.1002/humu.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulgakova NA, Knust E. The Crumbs complex: from epithelial-cell polarity to retinal degeneration. J Cell Sci. 2009;122:2587–2596. doi: 10.1242/jcs.023648. [DOI] [PubMed] [Google Scholar]
- 8.Carmi S, Hui KY, Kochav E, Lui X, Xue J, et al. Sequencing an Ashkenazi reference panel supports population-targeted personal genomics and illuminates Jewish and European origins. Nat Commun. 2014;5:4835. doi: 10.1038/ncomms5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.den Hollander AI, Ghiani M, De Kok YJ, Wijnholds J, Ballabio A, et al. Isolation of Crb1, a mouse homologue of Drosophila crumbs, and analysis of its expression pattern in eye and brain. Mech Dev. 2002;110:203–207. doi: 10.1016/s0925-4773(01)00568-8. [DOI] [PubMed] [Google Scholar]
- 10.den Hollander AI, Davis J, van der Velde-Visser SD, Zonneveld MN, Pierrottet CO, et al. CRB1 mutation spectrum in inherited retinal dystrophies. Hum Mutat. 2004;24:355–369. doi: 10.1002/humu.20093. [DOI] [PubMed] [Google Scholar]
- 11.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Dudok JJ, Murtaza M, Henrique Alves C, Rashbass P, Wijnholds J. Crumbs 2 prevents cortical abnormalities in mouse dorsal telencephalon. Neurosci Res. 2016;108:12–23. doi: 10.1016/j.neures.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Ebarasi L, He L, Hultenby K, Takemoto M, Betsholtz C, et al. A reverse genetic screen in the zebrafish identifies crb2b as a regulator of the glomerular filtration barrier. Dev Biol. 2009;334:1–9. doi: 10.1016/j.ydbio.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Ebarasi L, Ashraf S, Bierzynska A, Gee HY, McCarthy HJ, et al. Defects of CRB2 cause steroid-resistant nephrotic syndrome. Am J Hum Genet. 2015;96:153–161. doi: 10.1016/j.ajhg.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gosens I, den Hollander AI, Cremers FP, Roepman R. Composition and function of the Crumbs protein complex in the mammalian retina. Exp Eye Res. 2008;86:713–726. doi: 10.1016/j.exer.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Henderson RH, Mackay DS, Li Z, Moradi P, Sergouniotis P, et al. Phenotypic variability in patients with retinal dystrophies due to mutations in CRB1. Br J Ophthalmol. 2011;95:811–817. doi: 10.1136/bjo.2010.186882. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson SG, Cideciyan AV, Aleman TS, Pianta MJ, Sumaroka A, et al. Crumbs homolog 1 (CRB1) mutations result in a thick human retina with abnormal lamination. Hum Mol Genet. 2003;12:1073–1078. doi: 10.1093/hmg/ddg117. [DOI] [PubMed] [Google Scholar]
- 18.Jaron R, Rosenfeld N, Zahdeh F, Carmi S, Beni-Adani L, et al. Expanding the phenotype of CRB2 mutations - a new ciliopathy syndrome? Clin Genet. 2016 doi: 10.1111/cge.12764. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, Song JY, Karnam S, Park JY, Lee JJ, et al. Common and distinctive localization patterns of Crumbs polarity complex proteins in the mammalian eye. Gene Expr Patterns. 2015;17:31–37. doi: 10.1016/j.gep.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamont RE, Tan W-H, Innes AM, Parboosingh JS, Schneidman-Duhovny D, et al. Expansion of phenotype and genotypic data in CRB2-related syndrome. Eur J Hum Genet. 2016 doi: 10.1038/ejhg.2016.24. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon TT, Kim LS, Fishman GA, Stone EM, Zhao XC, et al. CRB1 gene mutations are associated with keratoconus in patients with Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2009;50:3185–3187. doi: 10.1167/iovs.08-2886. [DOI] [PubMed] [Google Scholar]
- 22.Narita K, Takeda S. Cilia in the choroid plexus: their roles in hydrocephalus and beyond. Front Cell Neurosci. 2015;9:39. doi: 10.3389/fncel.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omori Y, Malicki J. oko meduzy and related crumbs genes are determinants of apical cell features in the vertebrate embryo. Curr Biol. 2006;16:945–957. doi: 10.1016/j.cub.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 24.Pellissier LP, Lundvig DM, Tanimoto N, Klooster J, Vos RM, et al. CRB2 acts as a modifying factor of CRB1-related retinal dystrophies in mice. Hum Mol Genet. 2014;23:3759–3771. doi: 10.1093/hmg/ddu089. [DOI] [PubMed] [Google Scholar]
- 25.Pellissier LP, Quinn PM, Alves CH, Vos RM, Klooster J, et al. Gene therapy into photoreceptors and Müller glial cells restores retinal structure and function in CRB1 retinitis pigmentosa mouse models. Hum Mol Genet. 2015;24:3104–3118. doi: 10.1093/hmg/ddv062. [DOI] [PubMed] [Google Scholar]
- 26.Pocha SM, Knust E. Complexities of Crumbs function and regulation in tissue morphogenesis. Curr Biol. 2013;23:R289–293. doi: 10.1016/j.cub.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Slavotinek A, Kaylor J, Pierce H, Cahr M, DeWard SJ, et al. CRB2 mutations produce a phenotype resembling congenital nephrosis, Finnish type, with cerebral ventriculomegaly and raised alpha-fetoprotein. Am J Hum Genet. 2015;96:162–169. doi: 10.1016/j.ajhg.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson BJ, Pichaud F, Röper K. Sticking together the Crumbs - an unexpected function for an old friend. Nat Rev Mol Cell Biol. 2013;14:307–314. doi: 10.1038/nrm3568. [DOI] [PubMed] [Google Scholar]
- 29.Tsang SH, Burke T, Oll M, Yzer S, Lee W, et al. Whole exome sequencing identifies CRB1 defect in an unusual maculopathy phenotype. Ophthalmology. 2014;121:1773–1782. doi: 10.1016/j.ophtha.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallespin E, Cantalapiedra D, Riveiro-Alvarez R, Wilke R, Aguirre-Lamban J, et al. Mutation screening of 299 Spanish families with retinal dystrophies by Leber congenital amaurosis genotyping microarray. Invest Ophthalmol Vis Sci. 2007;48:5653–5661. doi: 10.1167/iovs.07-0007. [DOI] [PubMed] [Google Scholar]
- 31.van de Pavert SA, Sanz AS, Aartsen WM, Vos RM, Versteeg I, et al. Crb1 is a determinant of retinal apical Müller glia cell features. Glia. 2007;55:1486–1497. doi: 10.1002/glia.20561. [DOI] [PubMed] [Google Scholar]
- 32.van den Hurk JA, Rashbass P, Roepman R, Davis J, Voesenek KE, et al. Characterization of the Crumbs homolog 2 (CRB2) gene and analysis of its role in retinitis pigmentosa and Leber congenital amaurosis. Mol Vis. 2005;11:263–273. [PubMed] [Google Scholar]
- 33.Wolfson Y, Applegate CD, Strauss RW, Han IC, Scholl HP. CRB1-related maculopathy with cystoid macular edema. JAMA Ophthalmol. 2015;133:1357–1360. doi: 10.1001/jamaophthalmol.2015.2814. [DOI] [PubMed] [Google Scholar]
- 34.Xiao Z, Patrakka J, Nukui M, Chi L, Niu D, et al. Deficiency in Crumbs homolog 2 (Crb2) affects gastrulation and results in embryonic lethality in mice. Dev Dyn. 2011;240:2646–2656. doi: 10.1002/dvdy.22778. [DOI] [PubMed] [Google Scholar]
- 35.Zenteno JC, Buentello-Volante B, Ayala-Ramirez R, Villanueva-Mendoza C. Homozygosity mapping identifies the Crumbs homologue 1 (Crb1) gene as responsible for a recessive syndrome of retinitis pigmentosa and nanophthalmos. Am J Med Genet A. 2011;155A:1001–1006. doi: 10.1002/ajmg.a.33862. [DOI] [PubMed] [Google Scholar]
- 36.Zou J, Wen Y, Yang X, Wei X. Spatial-temporal expressions of Crumbs and Nagie oko and their interdependence in zebrafish central nervous system during early development. Int J Dev Neurosci. 2013;31:770–782. doi: 10.1016/j.ijdevneu.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]