Superior inhibition of viral replication by CD8+ T cells is associated with better retention of functional qualities ex-vivo, and is enhanced by IL-15.
Keywords: HIV control, polyfunctionality, apoptosis, IL-15
Abstract
Mechanisms modulating HIV-specific CD8+ T cell-mediated viral inhibition are not well defined. To delineate features of effective control, we compared the ability of CD8+ T cells from HIV ECs and CPs to inhibit HIV ex vivo. ECs showed superior inhibition compared to HAART-treated or untreated CPs in a typical VIA in which CD8+ T cells are rested 3 d before use (P = 0.025). In contrast, comparable antiviral activity was observed in freshly thawed cells. Rested CD8+ T cells underwent apoptosis with preferential loss of HIV-specific cells. EC CD8+ T cells showed greater capacity to sustain polyfunctionality ex vivo compared with those of CPs, and incubation of CD8+ T cells with IL-15 augmented inhibition. These results indicate that superior ex vivo inhibition of viral replication by CD8+ T cells from ECs is associated with enhanced retention of functional qualities and that in vitro antiviral function is enhanced by IL-15.
Introduction
The central role of CD8+ T cells in containment of HIV infection has been repeatedly demonstrated. Numerous studies have shown a strong genetic association of specific class I HLA alleles with spontaneous control of HIV [1–3]. Depletion of CD8+ T cells blunts viral control in acute and chronic SIV infection [4, 5], and the appearance of these responses (measured by IFNγ ELISPOT) coincides with the decline of postpeak viremia [6, 7]. A handful of studies examining ECs have demonstrated a central role for CD8+ T cells in natural control of HIV, including superior cytotoxic abilities [8, 9], proliferation capacity [10–12], preferential TCR usage [13, 14], and polyfunctionality [12, 15–17].
An additional quality thought to be associated with HIV control is the ability of CD8+ T cells to inhibit HIV replication ex vivo in infected target cells [reviewed in (18)]. A VIA in which infected CD4+ T cells are cocultured with autologous CD8+ T cells has been in use for nearly 3 decades [19] and has been shown to be an important correlate of HIV control [20, 21]. Superior CD8 inhibition has been found in EC individuals, as compared with persons with uncontrolled progressive infection [22–25], and the degree of inhibition correlates inversely with the slope of CD4 loss (both in acute and chronic HIV) and with the viral set point achieved at the resolution of acute HIV [26]. Both cytolytic (infected cell elimination) [22] and noncytolytic [27, 28] mechanisms have been attributed to this inhibition.
Nevertheless, close examination of the studies comparing inhibition capacity in different cohorts reveals several caveats. First, the differences between ECs and those with progressive infection (CPs) are relatively small [23, 26, 28]. For example, one study revealed inhibitory activities of 90% in ECs vs. 74% in CPs (P = 0.04), respectively [26], and the observed differences were even smaller in another study [28]. Second, in a fraction of EC (up to 50% in some studies), CD8+ T cells lack the ability to inhibit HIV replication ex vivo [23]. Third, the standard assay examining CD8-mediated inhibition capacity uses CD8+ T cells that are rested in vitro, unstimulated and without the addition of exogenous cytokines for a few days before their inhibition is tested, during which time infected CD4+ cells are prepared for the assay [20]. Another approach has been to stimulate CD8+ T cells with mitogens or mAbs [28, 29], which may alter the function of these cells and may skew results if there are underlying differences in proliferative potential. These approaches have consistently shown decreased viral inhibitory capacity using cells derived from CPs compared with ECs, but have not determined the mechanism that accounts for this difference.
To define mechanisms that may be involved in differential CD8 viral inhibition, we examined the quality of the CD8+ T cells and to what extent their function is affected by the in vitro “rest” period. To minimize induction of ex vivo alterations in function, we avoided in vitro stimulation with viral antigen or mitogens. Our results demonstrate that freshly isolated CD8+ T cells from ECs and CPs have comparable intrinsic inhibition capacity, but marked differences in sustainability of their functional properties ex vivo. We also show that in vitro addition of γ-chain cytokines, especially IL-15, restored the inhibitory capacity of rested CD8+ T cells, suggesting that therapeutic intervention targeting sustainability of T cell homeostasis may have a beneficial effect on CD8 immunity [30]. These new insights strengthen the notion that the ability of CD8+ T cells to successfully inhibit HIV replication is a complex and multifactorial state that is heavily influenced by the ability of the cells to survive and retain functional properties.
MATERIALS AND METHODS
Study subjects
Samples from a total of 21 HIV ECs, 16 antiretroviral naïve CPs, 10 HAART-treated patients, and 5 HIV-uninfected individuals were analyzed. ECs had had an HIV VL of <50 copies/ml for an average of 8 yr (3.4–12.8), and a median CD4 count of 1035 (704–1,163, IQR) cells/mm3. CPs had a median VL of 17,893 (8,087–81,585, IQR) copies/ml and a median CD4 count of 549 (471–713, IQR) cells/mm3. HAART-treated individuals had had an undetectable VL for a minimum of 12 mo and a median CD4 count of 846 (623–1023, IQR) cells/mm3. All subjects gave written informed consent per the Declaration of Helsinki under Massachusetts General Hospital Institutional Review Board–approved protocols. PBMCs were isolated from whole blood by Ficoll-Hypaque density gradient centrifugation, frozen (90% FBS-10% DMSO), and stored at −180°C until analyzed.
VIA
The ability of CD8+ T cells to inhibit HIV replication was assessed after a published protocol, with minor modifications [20]. In brief, preparation of CD4+ target cells was begun 3 d before infection. PBMCs were depleted of CD8+ T cells using positive-selection magnetic beads (Miltenyi Biotech, San Diego, CA, USA), then stimulated in T cell medium containing IL-2 (50 IU/ml) and a bispecific anti-CD3, anti-CD8 mAb [31]. The positively selected CD8+ T cells were maintained in RPMI medium supplemented with 10% FBS (R10) for 3 d (“rested” CD8+ T cells) until target cells were ready for infection [20]. To prevent autologous virus production, all cultures were maintained in medium containing 1 μM of the nonnucleoside reverse transcriptase inhibitor nevirapine (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA). Patients harboring virus resistant to nevirapine were excluded. In a second protocol, CD8+ T cells were separated on d 0 (the day of infection and coculture establishment) by using positive-selection magnetic beads (freshly thawed CD8+ T cells). Cell purity of >98% was confirmed by flow cytometry. On d 0, CD4+ targets cells were incubated with a ×4-tropic nevirapine-resistant HIV-1 strain (National Institute of Allergy and Infectious Diseases Reference Reagent Program, N119, Dr. Douglas Richman), at an MOI of 0.001 or as otherwise specified. After 4 h of incubation with the virus, infected cells were washed twice and resuspended at 1 × 106 cells/ml in R10 with IL-2 (50 IU/ml) and were cultured in triplicate in flat bottom 96-well plates at 1 × 105 cells/well, alone (positive control) or together with CD8+ effector cells at an effector-to-target ratio of 1:1 or as otherwise specified. Culture medium was changed at d 3 and 5. The level of p24 antigen in the supernatant at d 3, 5, and 7 was determined by ELISA (PerkinElmer, Waltham, MA). Log inhibition units were calculated as −log10 (p24 with CD8/p24 without CD8) at d 7. To examine the effect of γ-chain cytokines on viral inhibition capacity of rested CD8+ T cells, the rested cells were incubated with IL-2 (50 IU/ml), IL-7 (10 ng/ml), or IL-15 (10 ng/ml).
Tetramer and annexin V staining
CD8+ T cells were washed and stained for 20 min at room temperature with APC- or PE-conjugated MHC class I tetramer folded with the corresponding peptide (A2-SL9, A2-IV9, B27-KK10, B57-IW9, or B57-TW10; Beckman Coulter, Brea CA, USA). Cells were then washed and stained for 20 min at 4°C with the dead cell dye 7-AAD (BD Pharmingen, San Diego, CA, USA) and the following Abs against surface markers: CD3-Alexa Fluor 700, CD8-APC-Cy7, and exclusion channel that included CD4, CD14, CD19, and CD56-Pacific Blue (all Abs were from BD Biosciences, San Diego, CA, USA). After a wash, the cells were resuspended in 100 μl 1× annexin V binding buffer (BD Biosciences), and stained with annexin V conjugated with FITC or APC for 15 min, washed with 1× annexin V binding buffer and acquired unfixed on an FACSAria (BD Biosciences).
Surface and intracellular cytokine staining
CD4+ T cells were separated from the negative fraction after the freshly thawed CD8+ T cell separation, using CD4+ selection magnetic beads (Stemcell Technologies, Vancouver, BC, Canada). CD4+ T cells were either left unstimulated or were incubated with either an HIV-1 Gag peptide pool or SEB as a control. After 15 min of incubation, the cells were combined with the CD8+ T cells and incubated for 30 min. Brefeldin A 5 μg/ml (Sigma-Aldrich, St. MO, USA) and 1.5 μl/106 cells of GolgiStop (BD Biosciences) were added, and the cells were incubated overnight in the presence of CD107a-PE-Cy5 Ab. On the following day, cells were stained with blue viability dye (Thermo Fisher Scientific, Waltham, MA, USA), then with the following Abs against surface markers: CD3-APC-Cy 7, CD8-Qdot 605, and CD4, CD14, CD19-V500 (as an exclusion channel). Cells were then fixed and permeabilized using a Fix&Perm kit (Thermo Fisher Scientific), then stained for intracellular cytokines with the following Abs: IL-2-FITC, perforin-PE, IFNγ-PeCy7, MIP-1β-APC, TNFα-Alexa Fluor 700, and granzyme B-V450. Surface staining for memory subset phenotyping was performed on unstimulated CD8+ T cells with the following Abs: anti-CD3-Alexa Fluor 700, anti-CD8-Pacific Blue, anti-CD4-APC, Anti-CD197-PeCy7, anti-CD45RA-APC-H7, anti-CD27-Qdot 605 and anti-CD14, CD19-V500. Cells were acquired on an LSRFortessa flow cytometer (BD Biosciences).
Statistical analysis
Flow cytometry data were analyzed with FlowJo version 9.6 (TreeStar; Ashland, OR, USA). Statistical analyses were performed using Prism 6.0 (GraphPad, San Diego, CA, USA). Pairwise comparisons of freshly thawed vs. rested CD8+ T cells were verified with the Wilcoxon signed rank test (inhibition capacity, effector functions). Comparisons between independent cohorts were made with the Mann-Whitney test (inhibition capacity and fold change in effector functions and memory subsets). Longitudinal data analysis with mixed models was used to examine the kinetics of tetramer-positive cells and annexin V-binding cells. All tests were 2-tailed, and P < 0.05 indicated significance.
RESULTS
Viral inhibition capacity of CD8+ T cells in controlled and progressive HIV infection
HIV antiviral capacity of CD8+ T cells was examined using 2 different preparations of CD8+ T cells. One was a standard protocol in which CD8+ T cells are rested for 3 d before being added back to the infected CD4+ T cells (a period during which the CD4+ T cells are stimulated to make them infectible) [20]; the other involved adding freshly isolated CD8+ T cells on the day of coculture formation with infected CD4+ T cells.
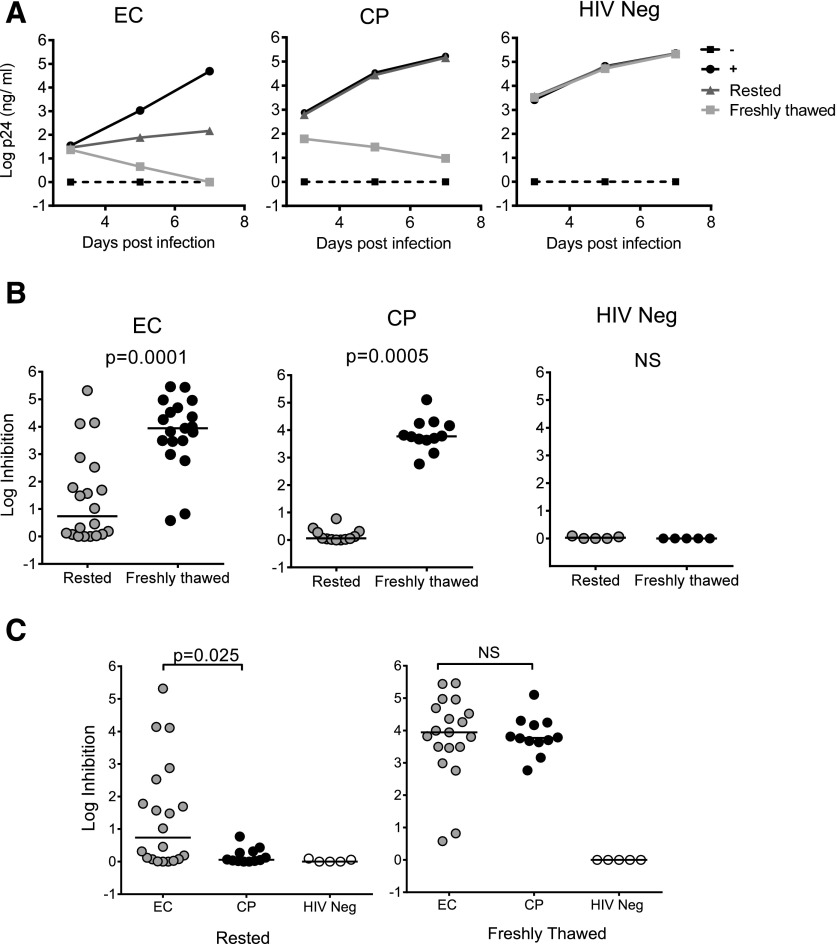
In agreement with previous studies using rested CD8+ T cells [21–23], ECs exhibited superior inhibition compared to CPs (Fig. 1; P = 0.025). As has been reported [23, 32], considerable variability in the degree of EC inhibition was observed, with some lacking this activity. There was no evidence of viral inhibition using CD8+ T cells from uninfected persons. However, when CD8+ T cells were used without a rest period, significant inhibition was detectable in most ECs and CPs (Supplemental Fig. S1), as well as in HAART-treated subjects (Supplemental Fig. S2). Comparable results were obtained when CD8+ T cells were separated by either positive or negative selection (Supplemental Fig. S3), when using freshly thawed CD8+ T cells at an MOI 10-fold higher (Supplemental Fig. S4A), and when the effector-to-target ratio was reduced as low as 1:20 (Supplemental Fig. S4B). These results indicate that the observed differences in viral inhibition capacity between ECs and CPs are related to the differential impact of a rest period in the absence of exogenous IL-2 on cells from these 2 groups.
Figure 1. Comparable inhibition of HIV replication between ECs and CPs in freshly isolated CD8+ T cells.
(A) VIA of 3 representative subjects: an EC (left), a CP (center), and an HIV-uninfected person (HIV neg, right). CD8+ T cells examined in this assay were incubated for 3 d without stimulation (rested) or were isolated on the day of coculture establishment (freshly thawed). (B, C) viral inhibition capacity at d 7 comparing rested with freshly thawed CD8+ T cells for 20 ECs, 12 CPs, and 5 HIV-negative subjects. Log inhibition was calculated as −log10 (p24 in coculture/p24 infected CD4 only).
Preferential loss of HIV-specific CD8+ T cells and increased levels of apoptosis in rested CD8+ T cells
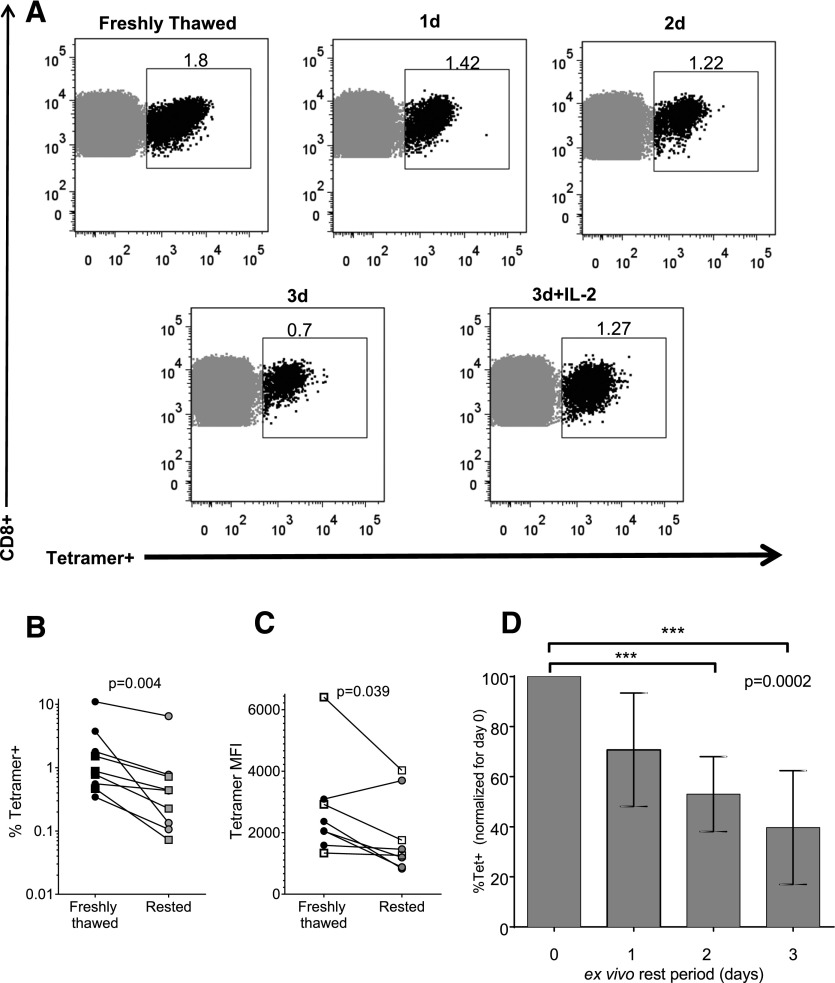
We next quantified HIV-specific CD8+ T cells during the 3-d rest period. Epitope-specific CD8+ T cells from ECs and CPs were stained with an HLA class I tetramer specific for an immunodominant response defined either by ELISPOT or tetramer staining of PBMCs. A total of 5 ECs and 4 CPs were examined. A gradual loss of tetramer-positive cells was observed during the rest period, with partial rescue by incubation with IL-2 (Fig. 2A). Freshly thawed CD8+ T cells were significantly more enriched with HIV-specific cells than were rested CD8+ T cells (5.6-fold more on average; P = 0.004, Supplemental Fig. S1; Fig. 2B). We also observed a significant decrease in tetramer staining per cell (reflected by the tetramer MFI) during the unstimulated incubation (Fig 2C), suggesting a decrease in TCR expression on the cells. We were not able to demonstrate significant differences in tetramer-positive cell loss between ECs and CPs, but our sample size, together with the small number of antigen-specific cells detectable with these methods, left us underpowered to address this question.
Figure 2. Preferential loss of rested HIV-specific CD8+ T cells.
CD8+ T cells were isolated and rested in vitro for 0–3 d, then stained with a class I HIV-specific tetramer to quantify the tetramer-positive population. (A) Flow plots for a representative subject: tetramer staining (B27-KK10) was performed at d 0 (freshly thawed) and daily until d 3 in culture, or incubated with IL-2 for 3 d. (B, C) Cumulative data for 9 subjects, both ECs (circles) and CPs (squares) at d 0 (freshly thawed) and after 3 d in culture (rested) using 1 tetramer per subject (A2-SL9, A2-IV9, B27-KK10, B57-IW9, or B57-TW10) according to each person’s most immunodominant response. (B) percentage of tetramer-positive CD8+ T cells. (C) Tetramer MFI gated on tetramer-positive cells. (D) Change in percentage tetramer-positive CD8+ T cells over time. The bars represent mean and sd. Values are normalized for d 0 (freshly thawed) as the maximum (100%).
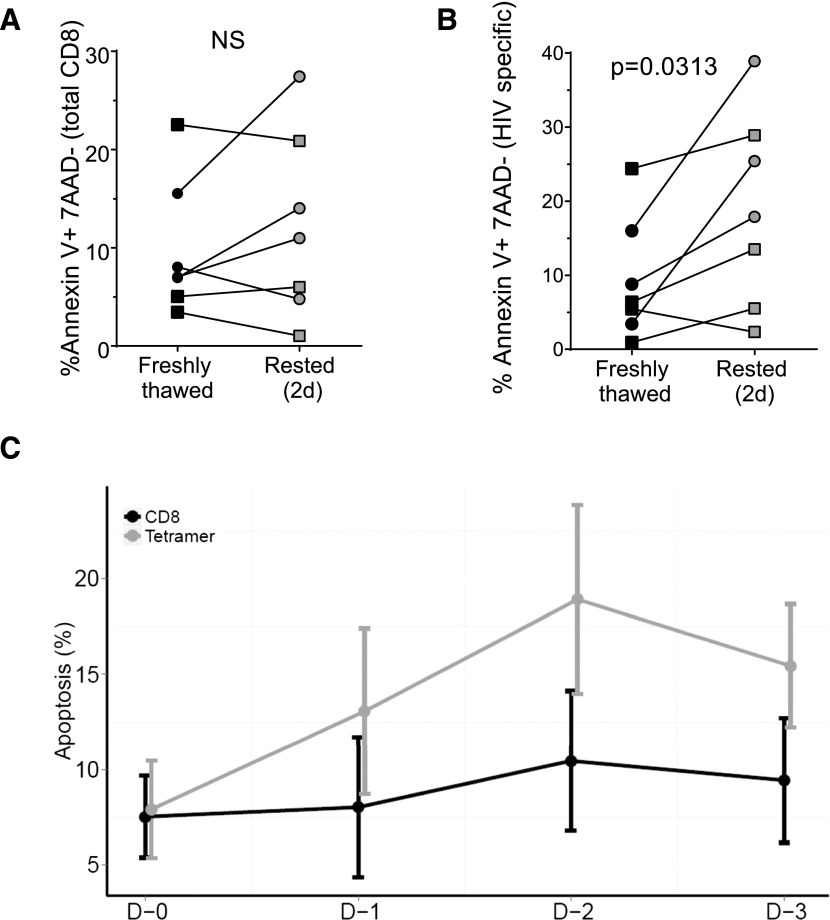
We also examined levels of apoptosis of these cells during in vitro incubation in annexin V-binding assays. We focused on the apoptotic cells that were still viable (excluding late apoptotic and necrotic cells by gating out annexin V-positive cells that also stained with the dead cell dye 7AAD). Whereas no significant changes in the level of annexin V binding in the total CD8+ T cell population were observed (Fig. 3A), the tetramer-positive cells showed a significant increase in annexin V binding (Fig. 3B; P = 0.0313). Maximum apoptosis was observed after 2 d of incubation without stimulation (Fig. 3C), after which the percentage of apoptotic cells seemed to decrease, probably as the result of deletion of that cell population.
Figure 3. Increasing levels of apoptosis (annexin V binding) in rested HIV-specific CD8+ T cells.
CD8+ T cells were isolated and rested in vitro for 0–3 d, stained with 1 class I HIV-specific tetramer and with annexin V, and measured by polychromatic flow cytometry. Late apoptotic/necrotic cells were excluded by gating out cells that were positive for the dead cell dye 7AAD. (A) Annexin V binding by the total CD8 population. (B) Annexin V binding by the HIV-specific CD8 population (tetramer-positive cells). ECs (circles), CPs (squares). (C) Kinetics of Annexin V binding over 3 d of in vitro incubation in the HIV-specific and total CD8 population.
Loss of effector functions and reduction of the TEM subset in unstimulated CD8+ T cells from CPs but not ECs
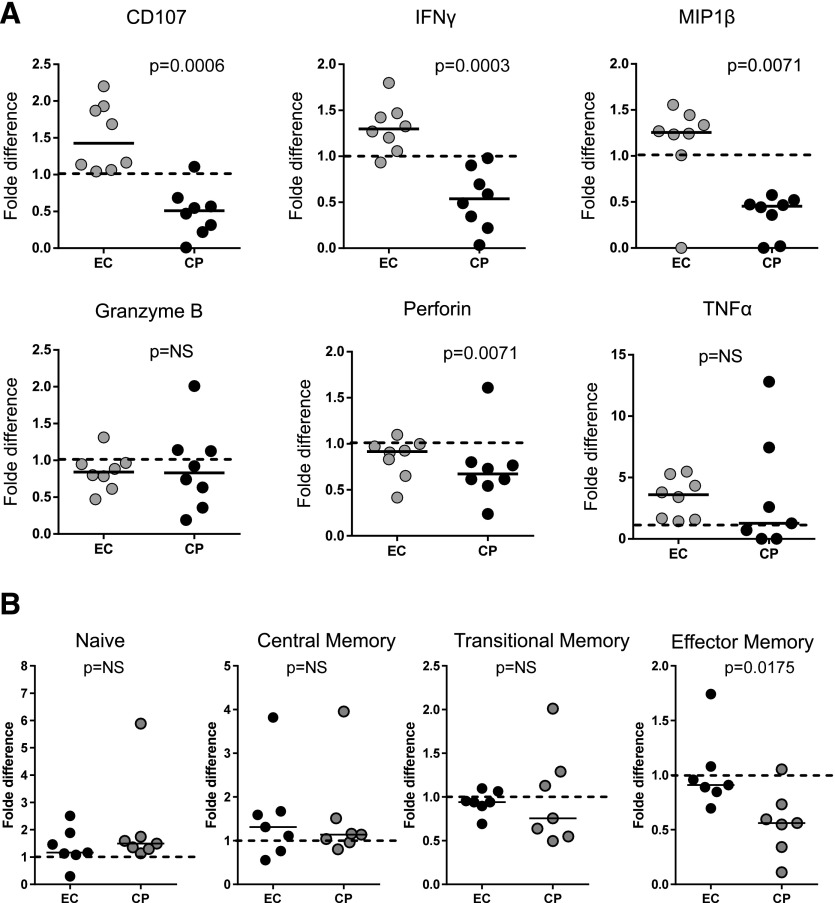
The above data indicate quantitative differences in CD8+ T cells that occurred in both EC and CP CD8+ T cells when cultured in vitro, but do not explain the observed differential antiviral function. We therefore next evaluated the impact of incubation on effector functions of HIV-specific CD8+ T cells. CD8+ T cells were stained for IFNγ, CD107a, MIP-1β, TNFα, perforin, and granzyme B, after stimulation with a pool of HIV Gag overlapping peptides. The percentage of cells expressing these effector functions was retained or even increased in ECs over the incubation period, whereas a significant decrease in the levels of CD107a, IFNγ, MIP-1β, and perforin-expressing cells was observed in CPs (Fig 4; Supplemental Fig. S5). We also stained unstimulated CD8+ T cells for CD45RA, CD27, and CCR7 to define memory subsets, using a gating strategy as described before: naive (CD45RA+, CD27+, CCR7+), central memory (CD45RA−, CD27+, CCR7+), transitional memory (CD45RA−, CD27+, CCR7-), effector memory (CD45RA−, CD27-, CCR7-), and terminally differentiated effector (CD45RA+, CD27−, CCR7−) cells [33]. We observed a trend toward enrichment in more differentiated memory subsets in CPs (mainly terminally differentiated effector cells) than in ECs and a significant shrinkage of the TEM subset in CPs after 3 d of incubation without stimulation (Fig. 4B). The ex vivo incubation did not lead to any significant change in the composition of the different memory subsets in ECs.
Figure 4. Preferential loss of effector functions and effector memory cells in rested CD8+ T cells from CPs compared to ECs.
(A) CD8+ T cells from EC and CP (freshly thawed or rested) were cocultured with Gag-pulsed autologous CD4s. Degranulation (CD107a) and 5 effector functions (production of IFN-γ, MIP-1β, TNFα, perforin, and granzyme B) were measured by polychromatic flow cytometry. Background levels (measured under no antigen stimulation) were subtracted. Granzyme B and perforin were quantified gating on HIV-specific CD8+ T cells (IFN-γ+). Fold difference: freshly thawed vs. rested. (B) Unstimulated CD8+ T cells from 7 ECs and 7 CPs were stained for CD45RA, CD27, and CCR7 for memory subset profiling. Fold difference: freshly thawed vs. rested CD8+ T cells.
These results imply that the difference between the 2 patient populations in VIAs may be explained, at least partially, by the greater loss of effector functions in CP CD8+ T cells, as well as the change in the composition of the different memory subsets when the cells are cultured ex vivo in the absence of mitogen or antigen stimulation.
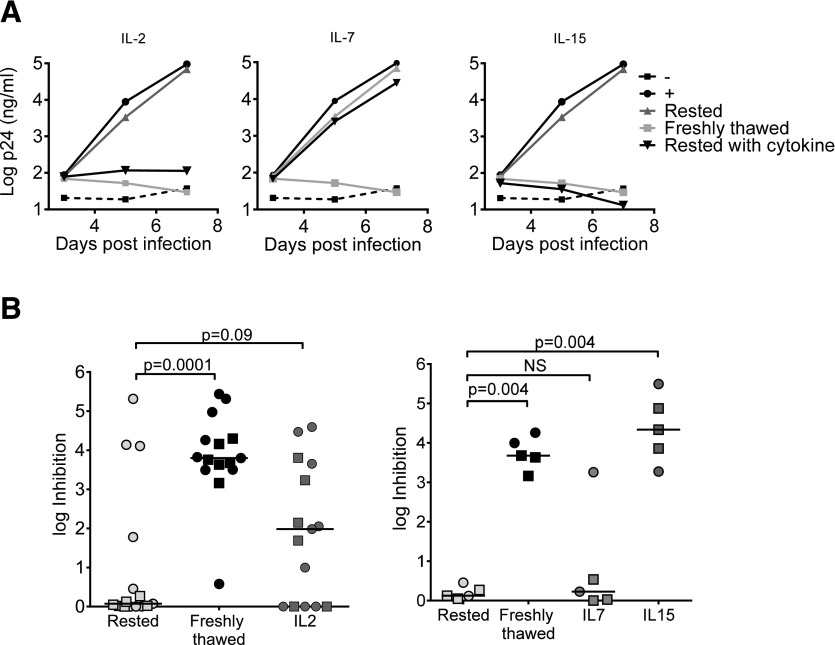
Restoration of viral inhibitory capacity of unstimulated CD8+ T cells by IL-2, -7, and -15
Because several studies have highlighted the importance of γ-chain cytokines in sustaining function and survival of CD8+ T cells [34–40], we evaluated the ability of IL-2, -7, and -15 to restore viral inhibitory capacity. CD8+ T cells from 15 patients (9 ECs and 6 CPs) were supplemented with IL-2 (50 IU/ml) immediately after their separation, or 1 or 2 d after separation (d 0, 1, or 2, respectively, referring to the day IL-2 was added to the resting cells). In 53% (8/15) of subjects, inhibition capacity increased by 0.3–3.8 logs, if IL-2 was added at d 0 (compared to rested CD8+ T cells), and reached 50–100% of the maximum inhibition observed with freshly thawed CD8+ T cells (Fig. 5). In 47% (7/15), stimulation of rested CD8+ T cells with IL-2 did not increase their inhibition capacity at all (Supplemental Fig. S6A; 1 representative subject, and Fig. 5B). We did not observe a difference in effects of IL-2 on CD8+ T cells from ECs and CPs. The effect of IL-2 was time sensitive: it was maximum if added at d 0, and negligible if added at d 2 (Supplemental Fig. S6B). Performing the entire assay in the absence of IL-2 led to significantly lower levels of infection [as previously reported (22)], but similar trends of inhibition were observed with significantly higher inhibition capacity of freshly thawed vs. rested CD8+ T cells (data not shown). Incubation of CD8+ T cells from 5 patients with IL-7 (10 ng/ml) for 3 d led to rescue of inhibition in only 1 patient (an EC), whereas incubation of CD8+ T cells with IL-15 (10 ng/ml) augmented the inhibitory capacity in all subjects tested, both ECs and CPs (Fig. 5). These data suggest that addition of the γ-chain cytokine IL-15 can restore the inhibitory capacity of rested CD8+ T cells, even from CP subjects.
Figure 5. Influence of IL-2, -7, and -15 on CD8+ T cell inhibitory capacity.
(A) VIA for a representative subject. CD8+ T cells were incubated with IL-2, -7, or -15 for 3 d before inhibition was examined. (B) Left: The effect of IL-2; cumulative data for 15 subjects (9 ECs and 6 CPs). Right: effect of IL-7 and -15; cumulative data for 5 subjects. ECs (circles), CPs (squares).
DISCUSSION
We examined the inhibition capacity of CD8+ T cells from HIV-infected persons. When examined directly ex vivo, CD8+ T cells from ECs and CPs show impressive and comparable ability to inhibit HIV replication. The superiority of CD8+ T cells from ECs to inhibit HIV is revealed only when examined after in vitro incubation of unstimulated CD8+ T cells and is associated with retained polyfunctionality. Culture of CD8+ T cells in the presence of IL-15 during this rest period enhanced viral inhibition capacity. Together, these data indicate that the previously observed lack of viral inhibition by CD8+ T cells from CPs lies is their inability to sustain effector function in vitro.
Although substantial heterogeneity in methodologies exist among different studies that examined the viral inhibition capacity of CD8+ T cells, most used variations on a protocol [20] in which CD8+ T cells were rested without stimulation for a few days before their viral inhibition capacity was tested [21–23, 32]. Earlier reports using this approach demonstrated significantly superior inhibition capacity when examining CD8+ T cells from ECs as compared to patients with progressive infection. In some early studies in small numbers of subjects, 90% of ECs exhibited near-complete inhibition of HIV-1 replication by autologous unstimulated CD8+ T cells [22], but larger cohorts revealed 25% of ECs to show either <2 log inhibition or no inhibition [23], and the proportion of noninhibiting ECs reached 50% in a report published more recently [32]. This proportion is similar to our findings when using the same protocol. On the other hand, Freel et al. [28] demonstrated impressive CD8 inhibition capacity, both in HIV controllers (mostly viremic controllers) and CPs (mostly HAART treated), but in their assay, effector cells were stimulated with OKT3 and anti-CD28 in the presence of IL-2 before inhibition was examined. In one of the only studies using unstimulated freshly thawed CD8+ T cells as effectors [25], superior inhibition was found in ECs as compared to CPs, but in that study, macrophages were used as target cells (as opposed to CD4+ T cells in most other studies) and percentage change in p24 levels was calculated (as opposed to the log changes presented in our assay) which may accentuate subtle differences in inhibition levels.
Our results show that prolonged incubation of CD8+ T cells led to gradual and selective loss of HIV-specific CD8+ T cells, as reflected by the progressive loss of tetramer-binding cells during the first 2 d in culture. In addition, HIV-specific CD8+ T cells showed a gradual increase in apoptosis, as measured by binding of annexin V. Taking into account the small sample size, the limited number of available class I tetramers, and the generally low percentage of tetramer binding, especially in CPs, our study was not powered to address potential differences in tetramer-positive cell loss and apoptosis levels between ECs and CPs. Nonetheless, we saw a significant difference in maintenance of polyfunctionality [a quality previously shown as one of the best correlates for HIV control (12, 15, 16, 41)], as measured by the ability of ECs to maintain expression of CD107a, IFN-γ, and MIP-1β during the 3 d rest. Preferential loss of HIV-specific CD8+ T cells has been demonstrated [42, 43], as well as excessive necroptosis and HIV-specific CD8+ T cell loss in CPs [44]. Studies have shown that HIV-specific CD8+ T cells are 3 times more prone to apoptosis, as compared to CMV-specific CD8+ T cells of the same individual [42], and that levels of apoptosis markers in HIV-specific CD8+ T cells from ECs are comparable to the levels found in HIV-negative individuals, whereas significantly higher levels are found in viremic patients [43]. These results support our hypothesis that superior performance of CD8+ T cells of ECs in VIAs reflects a survival advantage of the cells rather than enhanced intrinsic inhibition capacity. These differences may also be the result of the specific composition and qualities of the memory cell subpopulations in ECs and CPs, as reflected in our results showing superior preservation of the TEM subset in ECs as compared to CPs. Broader and better maintained central and effector memory responses in ECs was previously demonstrated by our group and others [24, 45] and a potent TEM response was associated with viral control in a nonhuman primate model involving the use of rhesus CMV-SIV recombinant vaccine [46]. The potential contribution of the different memory subsets to HIV control deserves further investigation.
Prolonged incubation also led to a decrease in TCR density, as reflected by the decrease in the tetramer staining MFI (Fig. 2C). Previous studies have shown that HIV-specific CD8+ T cells, as well as other virus-specific cells, downregulate the expression of the CD8 molecule, a process of T cell nonresponsiveness in an environment of high viremia [43, 47]. The reduced TCR density observed in our work may be part of the same process. The reduced tetramer staining and MFI may also be due to loss of high avidity TCR, a consequence of lack of antigenic stimulation. Previous works have demonstrated that dominant clonotypes with superior functional properties and TCR avidity fail to survive in culture in an environment of low antigenic stimulus [48, 49].
This study indicates that defects in viral inhibition capacity can be reversible: CD8+ T cells incubated with the γ-chain cytokine IL-15 retained function (Fig. 5). γ-Chain cytokines have been shown to have stimulatory, proliferative, and antiapoptotic properties, and to play an essential role in T-lymphocyte homeostasis [34, 35, 37]. Addition of IL-15 to long-term cultures of PBMCs of HIV-infected individuals leads to significant reduction of HIV-specific CD8+ T cell death and apoptosis and improvement in effector function [36, 40]. Taken together, these findings support the hypothesis that the better performance in VIAs reflects better survival and preservation of effector functions of the cells rather than a mere superior capacity to inhibit HIV replication ex vivo.
Our study has several limitations. First, in our experiments we used cryopreserved cells. Using fresh cells to examine viral inhibition according to the modified protocol would require drawing blood from patients twice in the same week, as target cells need several days of stimulation to become infectible. Furthermore, as CD8+ T cells exhibited near complete inhibition of HIV replication even after freeze-thawing, we do not expect greater inhibition using freshly isolated cells. Second, in the modified assay we examined inhibition in one strain of HIV. Ideal experimental design would be one using the patient’s autologous virus. Freel et al. [28] examined viral inhibition using 6 different viral strains, both lab strain and founder viruses, from patients. In their study, all viruses showed similar magnitudes of suppression, which led them to the conclusion that sequence variation alone did not account for differences in inhibition. Sequence variation may, however, compromise the effect of γ-chain cytokines in vivo, as dominant immune responses may be ineffective, even when augmented, if targeted against escaped epitopes.
In summary, in our study, CD8+ T cells from EC, CP, and HAART-treated patients had comparable capacity to inhibit HIV replication when examined immediately after isolation, avoiding in vitro incubation in the absence of stimulation, which led to gradual loss of HIV-specific CD8+ T cells and increased apoptosis. In CPs we also observed loss of several important effector functions. The higher tendency of cytotoxic cells of patients with uncontrolled HIV infection to undergo necroptosis and cell death has been described [44]. The superior performance of cells from ECs in ex vivo assays reflects their ability to better survive and retain their functional abilities. Such insights may be a step forward in the quest for an HIV vaccine and in the development of new treatment modalities for HIV, as interventions to block apoptosis and exhaustion or to support cell survival may augment efficient and desirable immune responses. In that regard, IL-15 may prove to be an important target of intervention.
AUTHORSHIP
D.S. contributed to the project design, performed the experiments, analyzed the data, and wrote the manuscript. D.K., O.A., and A.G. performed experiments. M.G. performed the statistical analysis. X.Y. contributed intellectually and to interpretation of the data. F.P. contributed to the project design, performed experiments, analyzed the data, and contributed to writing the manuscript. B.D.W. contributed to the conception and design of the overall project, analyzed the data, and contributed to writing the manuscript.
ACKNOWLEDGMENTS
This work was supported by U.S. NIH National Institute of Allergy and Infectious Diseases Grant AI30914. The authors thank laboratory manager Dr. Alicja Piechocka-Trocha for her thorough contribution to this project.
Glossary
- APC
allophycocyanin
- CD
cluster of differentiation
- CP
chronic progressor
- EC
elite controller
- ELISPOT
enzyme-linked immunospot assay
- HAART
highly active antiretroviral therapy
- IQR
interquartile range
- MFI
mean fluorescence intensity
- PBMC
peripheral blood mononuclear cell
- SEB
staphylococcal enterotoxin B
- SIV
simian immunodeficiency virus
- TEM
effector memory T cells
- VIA
viral inhibition assay
- VL
viral load
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- 1.Kaslow R. A., Carrington M., Apple R., Park L., Muñoz A., Saah A. J., Goedert J. J., Winkler C., O’Brien S. J., Rinaldo C., Detels R., Blattner W., Phair J., Erlich H., Mann D. L. (1996) Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2, 405–411. [DOI] [PubMed] [Google Scholar]
- 2.Fellay J., Shianna K. V., Ge D., Colombo S., Ledergerber B., Weale M., Zhang K., Gumbs C., Castagna A., Cossarizza A., Cozzi-Lepri A., De Luca A., Easterbrook P., Francioli P., Mallal S., Martinez-Picado J., Miro J. M., Obel N., Smith J. P., Wyniger J., Descombes P., Antonarakis S. E., Letvin N. L., McMichael A. J., Haynes B. F., Telenti A., Goldstein D. B. (2007) A whole-genome association study of major determinants for host control of HIV-1. Science 317, 944-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The International HIV Controllers Study. (2010) The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330, 1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitz J. E., Kuroda M. J., Santra S., Sasseville V. G., Simon M. A., Lifton M. A., Racz P., Tenner-Racz K., Dalesandro M., Scallon B. J., Ghrayeb J., Forman M. A., Montefiori D. C., Rieber E. P., Letvin N. L., Reimann K. A. (1999) Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283, 857–860. [DOI] [PubMed] [Google Scholar]
- 5.Jin X., Bauer D. E., Tuttleton S. E., Lewin S., Gettie A., Blanchard J., Irwin C. E., Safrit J. T., Mittler J., Weinberger L., Kostrikis L. G., Zhang L., Perelson A. S., Ho D. D. (1999) Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques (published correction in J. Exp. Med. (1999) 189, 1999–2000). J. Exp. Med. 189, 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koup R. A., Safrit J. T., Cao Y., Andrews C. A., McLeod G., Borkowsky W., Farthing C., Ho D. D. (1994) Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68, 4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borrow P., Lewicki H., Hahn B. H., Shaw G. M., Oldstone M. B. (1994) Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68, 6103–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hersperger A. R., Martin J. N., Shin L. Y., Sheth P. M., Kovacs C. M., Cosma G. L., Makedonas G., Pereyra F., Walker B. D., Kaul R., Deeks S. G., Betts M. R. (2011) Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood 117, 3799–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Migueles S. A., Osborne C. M., Royce C., Compton A. A., Joshi R. P., Weeks K. A., Rood J. E., Berkley A. M., Sacha J. B., Cogliano-Shutta N. A., Lloyd M., Roby G., Kwan R., McLaughlin M., Stallings S., Rehm C., O’Shea M. A., Mican J., Packard B. Z., Komoriya A., Palmer S., Wiegand A. P., Maldarelli F., Coffin J. M., Mellors J. W., Hallahan C. W., Follman D. A., Connors M. (2008) Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29, 1009–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Migueles S. A., Laborico A. C., Shupert W. L., Sabbaghian M. S., Rabin R., Hallahan C. W., Van Baarle D., Kostense S., Miedema F., McLaughlin M., Ehler L., Metcalf J., Liu S., Connors M. (2002) HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3, 1061–1068. [DOI] [PubMed] [Google Scholar]
- 11.Day C. L., Kiepiela P., Leslie A. J., van der Stok M., Nair K., Ismail N., Honeyborne I., Crawford H., Coovadia H. M., Goulder P. J., Walker B. D., Klenerman P. (2007) Proliferative capacity of epitope-specific CD8 T-cell responses is inversely related to viral load in chronic human immunodeficiency virus type 1 infection. J. Virol. 81, 434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ndhlovu Z. M., Chibnik L. B., Proudfoot J., Vine S., McMullen A., Cesa K., Porichis F., Jones R. B., Alvino D. M., Hart M. G., Stampouloglou E., Piechocka-Trocha A., Kadie C., Pereyra F., Heckerman D., De Jager P. L., Walker B. D., Kaufmann D. E. (2013) High-dimensional immune monitoring models of HIV-1-specific CD8 T cell responses accurately identify subjects achieving spontaneous viral control. Blood 121, 801–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H., Ndhlovu Z. M., Liu D., Porter L. C., Fang J. W., Darko S., Brockman M. A., Miura T., Brumme Z. L., Schneidewind A., Piechocka-Trocha A., Cesa K. T., Sela J., Cung T. D., Toth I., Pereyra F., Yu X. G., Douek D. C., Kaufmann D. E., Allen T. M., Walker B. D. (2012) TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nat. Immunol. 13, 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladell K., Hashimoto M., Iglesias M. C., Wilmann P. G., McLaren J. E., Gras S., Chikata T., Kuse N., Fastenackels S., Gostick E., Bridgeman J. S., Venturi V., Arkoub Z. A., Agut H., van Bockel D. J., Almeida J. R., Douek D. C., Meyer L., Venet A., Takiguchi M., Rossjohn J., Price D. A., Appay V. (2013) A molecular basis for the control of preimmune escape variants by HIV-specific CD8+ T cells. Immunity 38, 425–436. [DOI] [PubMed] [Google Scholar]
- 15.Betts M. R., Nason M. C., West S. M., De Rosa S. C., Migueles S. A., Abraham J., Lederman M. M., Benito J. M., Goepfert P. A., Connors M., Roederer M., Koup R. A. (2006) HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107, 4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferre A. L., Hunt P. W., Critchfield J. W., Young D. H., Morris M. M., Garcia J. C., Pollard R. B., Yee H. F. Jr., Martin J. N., Deeks S. G., Shacklett B. L. (2009) Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood 113, 3978–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peris-Pertusa A., López M., Rallón N. I., Restrepo C., Soriano V., Benito J. M. (2010) Evolution of the functional profile of HIV-specific CD8+ T cells in patients with different progression of HIV infection over 4 years. J. Acquir. Immune Defic. Syndr. 55, 29–38. [DOI] [PubMed] [Google Scholar]
- 18.Walker B. D., Yu X. G. (2013) Unravelling the mechanisms of durable control of HIV-1. Nat. Rev Immunol. 13, 487–498. [DOI] [PubMed] [Google Scholar]
- 19.Walker C. M., Moody D. J., Stites D. P., Levy J. A. (1986) CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science 234, 1563–1566. [DOI] [PubMed] [Google Scholar]
- 20.Sáez-Cirión A., Shin S. Y., Versmisse P., Barré-Sinoussi F., Pancino G. (2010) Ex vivo T cell-based HIV suppression assay to evaluate HIV-specific CD8+ T-cell responses. Nat. Protoc. 5, 1033–1041. [DOI] [PubMed] [Google Scholar]
- 21.Julg B., Williams K. L., Reddy S., Bishop K., Qi Y., Carrington M., Goulder P. J., Ndung’u T., Walker B. D. (2010) Enhanced anti-HIV functional activity associated with Gag-specific CD8 T-cell responses. J. Virol. 84, 5540–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agence Nationale de Recherches sur le Sida EP36 HIV Controllers Study Group (2007) HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. USA 104, 6776–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ANRS EP36 HIV Controllers Study Group (2009) Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J. Immunol. 182, 7828–7837. [DOI] [PubMed] [Google Scholar]
- 24.Buckheit R. W. III, Salgado M., Silciano R. F., Blankson J. N. (2012) Inhibitory potential of subpopulations of CD8+ T cells in HIV-1-infected elite suppressors. J. Virol. 86, 13679–13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker-Sperling V. E., Buckheit R. W. III, Blankson J. N. (2014) Comparative analysis of the capacity of elite suppressor CD4+ and CD8+ T cells to inhibit HIV-1 replication in monocyte-derived macrophages. J. Virol. 88, 9789–9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang H., Wu H., Hancock G., Clutton G., Sande N., Xu X., Yan H., Huang X., Angus B., Kuldanek K., Fidler S., Denny T. N., Birks J., McMichael A., Dorrell L. (2012) Antiviral inhibitory capacity of CD8+ T cells predicts the rate of CD4+ T-cell decline in HIV-1 infection. J. Infect. Dis. 206, 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackewicz C. E., Blackbourn D. J., Levy J. A. (1995) CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc. Natl. Acad. Sci. USA 92, 2308–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freel S. A., Lamoreaux L., Chattopadhyay P. K., Saunders K., Zarkowsky D., Overman R. G., Ochsenbauer C., Edmonds T. G., Kappes J. C., Cunningham C. K., Denny T. N., Weinhold K. J., Ferrari G., Haynes B. F., Koup R. A., Graham B. S., Roederer M., Tomaras G. D. (2010) Phenotypic and functional profile of HIV-inhibitory CD8 T cells elicited by natural infection and heterologous prime/boost vaccination. J. Virol. 84, 4998–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freel S. A., Picking R. A., Ferrari G., Ding H., Ochsenbauer C., Kappes J. C., Kirchherr J. L., Soderberg K. A., Weinhold K. J., Cunningham C. K., Denny T. N., Crump J. A., Cohen M. S., McMichael A. J., Haynes B. F., Tomaras G. D. (2012) Initial HIV-1 antigen-specific CD8+ T cells in acute HIV-1 infection inhibit transmitted/founder virus replication. J. Virol. 86, 6835–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leone A., Picker L. J., Sodora D. L. (2009) IL-2, IL-7 and IL-15 as immuno-modulators during SIV/HIV vaccination and treatment. Curr. HIV Res. 7, 83–90. [DOI] [PubMed] [Google Scholar]
- 31.Wilson C. C., Wong J. T., Girard D. D., Merrill D. P., Dynan M., An D. D., Kalams S. A., Johnson R. P., Hirsch M. S., D’Aquila R. T., et al. (1995) Ex vivo expansion of CD4 lymphocytes from human immunodeficiency virus type 1-infected persons in the presence of combination antiretroviral agents. J. Infect. Dis. 172, 88–96. [DOI] [PubMed] [Google Scholar]
- 32.ANRS 147 OPTIPRIM clinical trial (2013) CD8 T-cells from most HIV-infected patients lack ex vivo HIV-suppressive capacity during acute and early infection. PLoS One 8, e59767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breton G., Chomont N., Takata H., Fromentin R., Ahlers J., Filali-Mouhim A., Riou C., Boulassel M. R., Routy J. P., Yassine-Diab B., Sékaly R. P. (2013) Programmed death-1 is a marker for abnormal distribution of naive/memory T cell subsets in HIV-1 infection. J. Immunol. 191, 2194–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulfone-Paus S., Ungureanu D., Pohl T., Lindner G., Paus R., Rückert R., Krause H., Kunzendorf U. (1997) Interleukin-15 protects from lethal apoptosis in vivo. Nat. Med. 3, 1124–1128. [DOI] [PubMed] [Google Scholar]
- 35.Xiong Y., Luscher M. A., Altman J. D., Hulsey M., Robinson H. L., Ostrowski M., Barber B. H., MacDonald K. S. (2001) Simian immunodeficiency virus (SIV) infection of a rhesus macaque induces SIV-specific CD8(+) T cells with a defect in effector function that is reversible on extended interleukin-2 incubation. J. Virol. 75, 3028–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller Y. M., Bojczuk P. M., Halstead E. S., Kim A. H., Witek J., Altman J. D., Katsikis P. D. (2003) IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood 101, 1024–1029. [DOI] [PubMed] [Google Scholar]
- 37.Benczik M., Gaffen S. L. (2004) The interleukin (IL)-2 family cytokines: survival and proliferation signaling pathways in T lymphocytes. Immunol. Invest. 33, 109–142. [DOI] [PubMed] [Google Scholar]
- 38.Sato N., Patel H. J., Waldmann T. A., Tagaya Y. (2007) The IL-15/IL-15Ralpha on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc. Natl. Acad. Sci. USA 104, 588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vassena L., Proschan M., Fauci A. S., Lusso P. (2007) Interleukin 7 reduces the levels of spontaneous apoptosis in CD4+ and CD8+ T cells from HIV-1-infected individuals. Proc. Natl. Acad. Sci. USA 104, 2355–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White L., Krishnan S., Strbo N., Liu H., Kolber M. A., Lichtenheld M. G., Pahwa R. N., Pahwa S. (2007) Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T cells of patients with human immunodeficiency virus-1 (HIV). Blood 109, 3873–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ndhlovu Z. M., Proudfoot J., Cesa K., Alvino D. M., McMullen A., Vine S., Stampouloglou E., Piechocka-Trocha A., Walker B. D., Pereyra F. (2012) Elite controllers with low to absent effector CD8+ T cell responses maintain highly functional, broadly directed central memory responses. J. Virol. 86, 6959–6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller Y. M., De Rosa S. C., Hutton J. A., Witek J., Roederer M., Altman J. D., Katsikis P. D. (2001) Increased CD95/Fas-induced apoptosis of HIV-specific CD8(+) T cells. Immunity 15, 871–882. [DOI] [PubMed] [Google Scholar]
- 43.Yan J., Sabbaj S., Bansal A., Amatya N., Shacka J. J., Goepfert P. A., Heath S. L. (2013) HIV-specific CD8+ T cells from elite controllers are primed for survival. J. Virol. 87, 5170–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaiha G. D., McKim K. J., Woods M., Pertel T., Rohrbach J., Barteneva N., Chin C. R., Liu D., Soghoian D. Z., Cesa K., Wilton S., Waring M. T., Chicoine A., Doering T., Wherry E. J., Kaufmann D. E., Lichterfeld M., Brass A. L., Walker B. D. (2014) Dysfunctional HIV-specific CD8+ T cell proliferation is associated with increased caspase-8 activity and mediated by necroptosis. Immunity 41, 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ndhlovu Z. M., Stampouloglou E., Cesa K., Mavrothalassitis O., Alvino D. M., Li J. Z., Wilton S., Karel D., Piechocka-Trocha A., Chen H., Pereyra F., Walker B. D. (2015) The breadth of expandable memory CD8+ T cells inversely correlates with residual viral loads in HIV elite controllers. J. Virol. 89, 10735–10747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansen S. G., Ford J. C., Lewis M. S., Ventura A. B., Hughes C. M., Coyne-Johnson L., Whizin N., Oswald K., Shoemaker R., Swanson T., Legasse A. W., Chiuchiolo M. J., Parks C. L., Axthelm M. K., Nelson J. A., Jarvis M. A., Piatak M. Jr., Lifson J. D., Picker L. J. (2011) Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takada K., Jameson S. C. (2009) Self-class I MHC molecules support survival of naive CD8 T cells, but depress their functional sensitivity through regulation of CD8 expression levels. J. Exp. Med. 206, 2253–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conrad J. A., Ramalingam R. K., Smith R. M., Barnett L., Lorey S. L., Wei J., Simons B. C., Sadagopal S., Meyer-Olson D., Kalams S. A. (2011) Dominant clonotypes within HIV-specific T cell responses are programmed death-1high and CD127low and display reduced variant cross-reactivity. J. Immunol. 186, 6871–6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer-Olson D., Brady K. W., Bartman M. T., O’Sullivan K. M., Simons B. C., Conrad J. A., Duncan C. B., Lorey S., Siddique A., Draenert R., Addo M., Altfeld M., Rosenberg E., Allen T. M., Walker B. D., Kalams S. A. (2006) Fluctuations of functionally distinct CD8+ T-cell clonotypes demonstrate flexibility of the HIV-specific TCR repertoire. Blood 107, 2373–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]