Dex suppresses IL-33-stimulated mast cell functions in vitro and in vivo, where the predominant mechanism appears to be a blockade of transcriptional activity.
Keywords: glucocorticoid, inflammation, NFκB, AP-1, ST2, neutrophil recruitment
Abstract
Mast cells are critical effectors of allergic disease and can be activated by IL-33, a proinflammatory member of the IL-1 cytokine family. IL-33 worsens the pathology of mast cell–mediated diseases, but therapies to antagonize IL-33 are still forthcoming. Because steroids are the mainstay of allergic disease treatment and are well known to suppress mast cell activation by other stimuli, we examined the effects of the steroid dexamethasone on IL-33-mediated mast cell function. We found that dexamethasone potently and rapidly suppressed cytokine production elicited by IL-33 from murine bone marrow–derived and peritoneal mast cells. IL-33 enhances IgE-mediated mast cell cytokine production, an activity that was also antagonized by dexamethasone. These effects were consistent in human mast cells. We additionally observed that IL-33 augmented migration of IgE-sensitized mast cells toward antigen. This enhancing effect was similarly reversed by dexamethasone. Simultaneous addition of dexamethasone with IL-33 had no effect on the phosphorylation of MAP kinases or NFκB p65 subunit; however, dexamethasone antagonized AP-1- and NFκB-mediated transcriptional activity. Intraperitoneal administration of dexamethasone completely abrogated IL-33-mediated peritoneal neutrophil recruitment and prevented plasma IL-6 elevation. These data demonstrate that steroid therapy may be an effective means of antagonizing the effects of IL-33 on mast cells in vitro and in vivo, acting partly by suppressing IL-33-induced NFκB and AP-1 activity.
Introduction
IL-33 is now appreciated as an important early inducer of both protective and pathologic inflammation. A nuclear cytokine belonging to the IL-1 family, IL-33 is unusual, in that it functions as both a proinflammatory cytokine and a regulator of transcription, because it can bind to chromatin [1]. IL-33 is constitutively expressed in epithelial barrier tissues, keratinocytes, endothelial cells, fibroblastic reticular cells, and lymphoid organs [2]. Although expression can be induced in macrophages and dendritic cells, tissue-derived IL-33 rather than immune cell-derived IL-33 has proved to be crucial for inflammation [3]. Biologically active full-length IL-33 is released as an “alarmin” after cell stress or injury. Secreted IL-33 binds a complex of ST2 (IL-1RL1) and IL-1 receptor accessory protein (IL-1RAcP), activating group 2 innate lymphoid cells, basophils, eosinophils, Th2 cells, NK cells, and MCs [4, 5]. IL-33 is important for protective type 2 responses to pathogens, such as parasites [2, 6], and it is also elevated in lung inflammation, atopic dermatitis, psoriasis, and many rheumatological diseases. In these inflammatory disorders, antagonizing IL-33 may be clinically beneficial.
MCs are present in mucosal and connective tissues, where they serve as master regulators of allergic responses [7]. IL-33, a survival factor for MCs [8], stimulates MCs to produce proinflammatory cytokines and chemokines, independent of IgE-mediated stimulation. IL-33 also amplifies IgE-induced MC function, exacerbating allergic disease [9–11]. Collectively, these effects have been shown to be functionally important. For example, recent studies have indicated that IL-33-induced MC activation worsens airway hyperreactivity during asthma [12, 13]. The IL-33-MC axis also exacerbates collagen-induced arthritis and inflammatory arthritis in murine models [10, 14]. Neutralizing IL-33 has been shown to reduce late-phase responses during antigen-induced passive cutaneous anaphylaxis [15], supporting the rationale for targeting IL-33 in MC mediated diseases.
Asano et al. [16] recently showed that dexamethasone suppresses IL-33-mediated acute lung inflammation. First made in 1957, Dex is a synthetic glucocorticoid. An analog of cortisone, Dex is the most potent of the corticosteroids. It has been widely and effectively used for the treatment of rheumatoid arthritis, rheumatic fever, bronchial asthma, allergy, lupus, and ulcerative colitis [17–19]. Dexamethasone suppresses IgE-mediated MC functions including degranulation, lipid mediator release, and cytokine production in vitro and passive cutaneous anaphylaxis and wheal-and-flare reactions in vivo [20–24]. These studies prompted us to investigate the effects of Dex on IL-33-induced MC function. Dex was a potent suppressor of IL-33 effects in vitro and in vivo in mouse and human MCs. These effects were rapid and selective, acting to inhibit the proinflammatory transcription factors AP-1 and NFκB. Our data suggest that this and other corticosteroids would be a reliable means of antagonizing pathologic inflammatory responses involving the IL-33-MC axis.
MATERIALS AND METHODS
Reagents
Recombinant mouse IL-3, stem cell factor, mature, cleaved human and mouse IL-33, and FITC-conjugated anti-mouse CD11b [(cat. no.) 101206], PE-conjugated anti-mouse Gr-1 (108408), APC-conjugated anti-mouse TNF (506308), APC-conjugated anti-mouse IL-6 (504508) were purchased from BioLegend (San Diego, CA, USA). FITC-conjugated anti-mouse T1/ST2 (101001F) was purchased from MD Biosciences (St. Paul, MN, USA). Anti-mouse CD16/32 Fc block (clone 2.4G2), and purified mouse IgE (clone C38-2, k isotype) were purchased from BD Biosciences (San Diego, CA, USA). DNP-HSA was purchased from Sigma-Aldrich (St. Louis, MO, USA). Dexamethasone and RU-486 were purchased from Tocris Bioscience (Bristol, UK). All Western blot antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Phospho-p38 MAPK (T180/Y182) (D3F9) rabbit 4511, p38 MAPK rabbit (9212), phospho-p44/42 MAPK (T202/Y204) rabbit (9101), p-44/42 MAPK (Erk1/2) (L34F12) mouse (4696), phospho-NFκB p65 (Ser536) (93H1) rabbit (3033), NFκB p65 (L8F6) mouse (6956), phospho-SAPK/JNK (T183/Y185) rabbit (9251), and SAPK/JNK rabbit (9252) were used as primary antibodies. Anti-rabbit IgG (H+L) (DyLight 800 4X PEG Conjugate) (5151) and anti-mouse IgG (H+L) (DyLight 680 4X PEG conjugate) (5470) were used as secondary antibodies. Luciferase reporter assay reagents were purchased from Promega (Madison, WI, USA).
Animals
C57BL/6J and 129/SvJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and used at a minimum of 10 wk old, with approval from the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Mouse MC cultures
Mouse bone marrow-derived MCs (BMMCs) were cultured as published [25]. Peritoneal lavage cells were cultured in complete RPMI containing 10% FBS, IL-3 (10 ng/ml), and stem cell factor (10 ng/ml) for 10 d before use.
Human MC culture
Protocols involving human tissues were approved by the human studies Institutional Review Board at the University of South Carolina. Surgical skin samples were obtained from the Cooperative Human Tissue Network of the National Cancer Institute. Skin MCs were cultured as described elsewhere [26] and were used after 8–16 wk. MC purity was determined to be 100% pure by toluidine blue staining.
IgE-mediated activation
Human MCs and mouse BMMCs were sensitized overnight with DNP-specific mouse IgE (1.0 μg/ml for human MC; 0.5 μg/ml for BMMCs). Cells were washed, resuspended 1 × 106 cells/ml in complete medium with cytokines, and stimulated for 6 (mouse cells) or 16 (human cells) hours with DNP-HSA (50ng/ml) for assessment of cytokines in supernatant.
Cytokine measurement by ELISA
Murine IL-13 ELISA kit was purchased from Peprotech (Rocky Hill, NJ, USA). IL-6, MCP-1, and TNF ELISA kits were purchased from BioLegend. ELISAs were performed with culture supernatants according to the manufacturer’s protocols. ELISAs for human cytokines were developed using BD OptEIA reagents from BD Biosciences (Franklin Lakes, NJ,).
Cytokine mRNA analysis
BMMCs were activated with IL-33 for 2 h. Cells were harvested, and total RNA was extracted with TRIzol reagent (Thermo Fisher Scientific, Grand Island, NY, USA). RNA was quantified with the NanoDrop 1000 UV–vis spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s recommended protocol. For cytokine mRNA detection, cDNA was synthesized by using qScript cDNA Synthesis from Quanta Biosciences (Gaithersburg, MD, USA). The CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) was used to amplify message with PerfeCTa SYBR Green SuperMix (Quanta Biosciences). Primers for IL-6 (forward: 5′-TCCAGTTGCCTTCTTGGGAC-3′, reverse: 5′-TCCAGTTGCCTTCTTGGGAC-3′), TNF (forward: 5′-AGCACAGAAAGCATCATCCGC-3′, reverse: 5′-TGCCACAAGCAGGAATGAGAAG-3′), β-actin (forward: 5′-GATGACGATATCGCTGCGC-3′, reverse: 5′-CTCGTCACCCACATAGGAGTC-3′), were purchased from Eurofins MWG Operon (Huntsville, AL, USA). Amplification conditions for qPCR consisted of a heat-activation step at 95°C for 2 min followed by 40 cycles of 95°C for 15 s, 55°C for 30 s, and 60°C for 1 min. All melting curve analyses were performed between 50°C and 95°C. Results were normalized to housekeeping genes by using the relative Livak Method.
Western blot analysis
Western blot analysis was performed with 25 μg total cellular protein, as described previously [25]. Blots were visualized and quantified with an Odyssey CLx infrared imaging system (LiCor, Lincoln, NE, USA).
Luciferase assay
BMMCs (3 × 106/condition) were transfected with 1.2 μg of pGL4.74[hRluc/TK] vector encoding luciferase gene from Renilla reniformis under the HSV-TK promoter and 6 μg of either pGL4.44[luc2p/AP1 RE/Hygro] or pGL4.32[luc2p/NFkB RE/Hygro] vector encoding the luciferase gene from Photinus pyralis (firefly) under the AP-1 and NFκB response elements, respectively. All transfection experiments were performed with Amaxa Nucleofector (Lonza; Allendale, NJ, USA) with program T-5 in 20% FBS and 50 mM HEPES (pH 7.5) [27]. Cells were used 48 h after transfection. Luciferase activity among the lysates was measured with a Dual-Luciferase Reporter Assay System, by the GloMax 20/20 luminometer, program DLR-2-INJ (Promega).
Flow cytometry.
For surface staining, cells were washed in PBS after the appropriate treatment. For directly labeled antibody staining, cell pellets were incubated in Fc block+staining or isotype control antibodies for 30 min at 4°C, washed with PBS and resuspended in FACS buffer (PBS, 3% FBS, and 0.1% sodium azide), and analyzed by flow cytometry on a FACSCalibur (BD Biosciences) after propidium iodide exclusion staining.
In-cell staining for cytokines.
BMMCs were activated with IL-33 (50 ng/ml) ± Dex (1 μM) for 90 min, then treated with 5 µM monensin for 5 h, fixed 20 min in 4% paraformaldehyde, washed twice in PBS and stored overnight at 4°C. Cells were then pelleted and resuspended in saponin buffer (PBS, 0.1% BSA, 0.01M HEPES, and 0.5% saponin) for 20 min at room temperature. Washed cell pellets were incubated in Fc block+staining or isotype control antibodies for 30 min at 4°C.
Migration assay.
IgE-sensitized BMMCs were washed and resuspended at 2 × 106 cells/ml in migration medium [cRPMI in which FBS is replaced by 10 mg/ml BSA)+IL-3 (1 ng/ml)]±Dex (1 μM) or vehicle, 1-h before use. Polycarbonate (8 μm) 24-well Transwell inserts (Corning, Corning NY, USA) were coated in migration medium for 1 h at 37°C before use. Migration wells contained 900 μl migration medium±antigen (50 ng/ml)±IL-33 (50 ng/ml)±Dex (1 μM) or vehicle. Coated inserts were placed in the migration wells, and 200 µl of the resuspended cells was placed in the top chamber. Cells were incubated for 16 h at 37°C, after which cells from quadruplicate aliquots from the migration well were counted via flow cytometry with propidium-iodide exclusion staining.
Neutrophil recruitment assay
Age- and gender-matched groups of C57BL/6 mice (10–16 wk old) received intraperitoneal injections of Dex (2 mg/kg) or vehicle and 1 μg recombinant IL-33 in 200 μl of sterile PBS. Four h later, mice were euthanized by CO2 asphyxiation, and peritoneal lavage was performed. Cells in the peritoneal lavage were analyzed for surface expression of Gr-1 and CD11b by flow cytometry. Blood was collected via cardiac puncture. Plasma isolated from blood samples was analyzed for cytokines by ELISA.
Statistical analysis
Data shown in each figure are the sem of the indicated number of samples, unless specified otherwise. P-values were calculated by paired or unpaired Student’s t test unless mentioned otherwise. P < 0.05 indicated statistical significance (Prism software; GraphPad, San Diego, CA, USA).
RESULTS
Dex rapidly suppresses IL-33-induced cytokine production
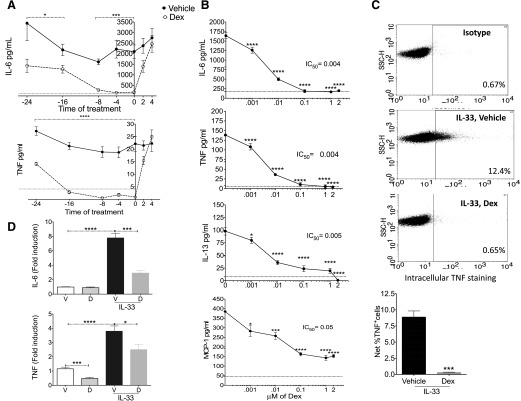
Previous studies indicated that the length of pretreatment is critical for Dex to maximally suppress IgE-induced mediator release in MCs [20, 21]. Hence, as a first step toward characterizing the effects of Dex on IL-33-mediated MC activation, we investigated the effects of pretreatment timing before IL-33 stimulation. BMMCs isolated from C57BL/6J mice were given Dex for up to 24 h before IL-33. Although all pretreatment times reduced IL-33-mediated TNF and IL-6 secretion, maximum suppression was noted, with 0–8 h pretreatment (Fig. 1A). To determine whether Dex exhibits its suppressive effects after activation begins, we treated MCs 2 or 4 h after IL-33 addition. Although drug-treated cells tended to produce less IL-6 and TNF when Dex was added 2 h after IL-33, suppression did not reach significance.
Figure 1. Dex suppresses IL-33-induced cytokine production in mouse BMMCs.
BMMCs were treated with 2 µM (A) or the indicated concentrations (B) of Dex at the indicated times in (A) or simultaneously with IL-33 (50 ng/ml) in (B). Cells were stimulated with IL-33 for 6 h, and supernatants were analyzed by ELISA. Dotted line: background cytokine production without activation. (C) BMMCs were treated with 1 µM Dex as in (B) and assessed for TNF production by intracellular staining and flow cytometry. Dot plots are representative of 3 BMMC populations. Numbers indicate percentage of cells positive for TNF. Quantification of vehicle- or Dex-treated cells (bottom). (D) RT-qPCR of cytokine mRNAs isolated from BMMCs 2 h after treatment with 1 µM Dex as in (B). Fold induction was calculated by normalizing treatment groups to the vehicle-treated, unactivated group. Data shown are representative of 3 (A–C) or an average of 2 (D) independent experiments performed in triplicate. Unpaired Student t tests were performed to compare the vehicle-treated cells to the drug-treated cells at each time point in (A). Dunnett’s multiple-comparison test was performed to compare each group treated with a particular dose of the drug with the control group that was treated only with the vehicle in (B). Tukey’s multiple-comparison test was used to calculate the P-values in (D) . *P < 0.05; ***P < 0.001; ****P < 0.0001.
These data indicated rapid and relatively short-acting effects of Dex, prompting our use of simultaneous addition in further experiments. We first calculated the IC50 for Dex-mediated effects. Using a broad range of the drug, we found that IC50 for suppression of IL-6, TNF, and IL-13 was ≤5 nM, whereas for MCP-1, it was ∼50 nM (Fig. 1B). Previous studies in our lab indicated that TGFβ1 or fluvastatin suppress MC-mediator release on C57BL/6 background but not on the 129/SvJ background [25, 28] indicating differential sensitivity among different strains. However, 129/SvJ BMMCs showed no significant difference in IC50 for Dex responsiveness when compared to C57BL/6 BMMCs (data not shown).
Reduced cytokine release can be related to a decrease in production or secretion. To differentiate this, we performed in-cell staining and flow cytometry for TNF and IL-6 on IL-33-activated BMMCs treated with vehicle or Dex (Fig. 1C; data not shown). We observed that the fraction of TNF-producing cells became negligible in the presence of Dex. These data suggest that Dex acts at the level of cytokine induction rather than its secretion. To further investigate this, we measured cytokine mRNA synthesis by RT-qPCR. IL-33 induced IL-6 and TNF mRNA 4–8-fold vs. control cells, an effect that was significantly reduced by Dex. We also noted reduced basal TNF mRNA levels among Dex-treated BMMCs (Fig. 1D).
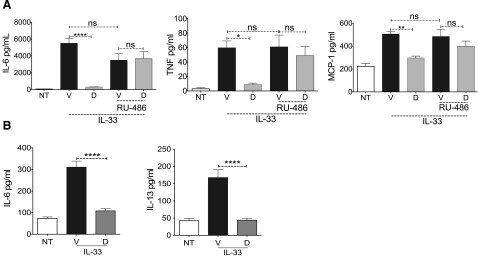
To confirm that Dex acts via the GR, BMMCs were treated with the GR antagonist RU-486 before adding Dex. RU-486 completely reversed Dex-mediated suppression of cytokines (Fig. 2A), indicating that the suppressive effects required GR function. Although BMMCs are primary cells, their in vitro differentiation may alter responsiveness to Dex. Therefore, we employed murine peritoneal MCs expanded ex vivo. As with BMMCs, IL-33-mediated IL-13 and -6 production was significantly reduced by Dex (Fig. 2B). These collective data show that Dex is a potent and rapid inhibitor of IL-33-mediated MC cytokine production, acting at least partly to suppress IL-33-mediated cytokine transcription.
Figure 2. Effects of Dex are specific and are consistent with peritoneal MCs.
(A) BMMCs were treated with RU-486 (0.5 µM) for 1 h before IL-33 (50 ng/ml) and Dex (0.1 µM) were added. Supernatants collected 6 h after activation were analyzed by ELISA. (B) Supernatants obtained from murine peritoneal MCs activated with IL-33 (50 ng/ml) and simultaneously treated with vehicle or Dex (1 µM) for 6 h were analyzed by ELISA. Data shown are representative of 3 independent experiments performed in triplicate. Tukey’s multiple-comparison test was used to calculate the P-values. ****P < 0.0001.
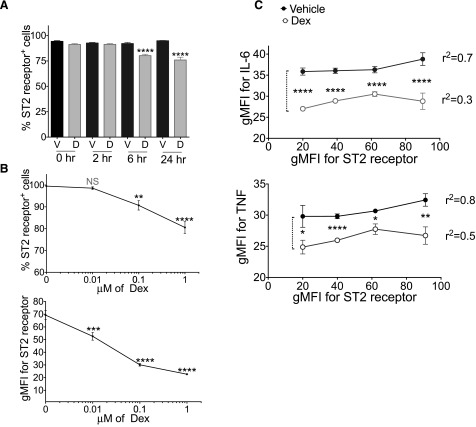
Dex-mediated suppression of cytokine production is independent of changes in ST2 receptor surface expression
A possible explanation for the effects of Dex is that it suppresses expression of the receptor ST2, which is required for MC responses to IL-33 [11]. To assess this possibility, BMMCs were treated with Dex for up to 24 h before flow cytometric analysis. The results revealed modest surface ST2 receptor down-regulation starting 6 h after Dex treatment (Fig. 3A). Because dexamethasone effects on ST2 receptor expression occurred more slowly than its effects on cytokine production, ST2 suppression cannot be the main explanation for inhibition caused by simultaneous addition of the drug with IL-33. However, regulation of ST2 receptor is poorly understood, and its down-regulation may be partly responsible for the effects of Dex that occur at later time points. Using the 24 h time point, we first found that the effects of Dex were dose dependent, with ST2 receptor suppression more apparent at concentrations greater than 10 nM (Fig. 3B). Dex had little impact on the percentage of cells expressing ST2 receptor, which stayed at or above 80%. Rather, drug-treated BMMCs showed a nearly 60% reduction in ST2 receptor staining intensity, indicating fewer surface receptors/cell were available after Dex exposure.
Figure 3. Dex decreases ST2 receptor expression.
(A, B) Flow cytometry analysis of surface expression of ST2 receptor on BMMCs treated with 1 µM (A) or indicated concentrations of Dex (B) for the indicated times (A) or for 24 h (B). (C) Staining intensities for cytokines vs. ST2 receptor expression on BMMCs pretreated with Dex (1 µM) for 24 h and activated with IL-33 (50 ng/ml). Data are representative of 3 independent experiments performed in triplicates. Dunnett’s multiple-comparison test was performed to compare drug and vehicle-treated groups in (B). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
We next determined the relationship between ST2 receptor and cytokine protein expression. The staining intensity of IL-6 or TNF was plotted as a function of ST2 receptor expression among IL-33-activated cells treated with vehicle or Dex (Fig. 3C). Cells were gated by groups based on ST2 receptor expression, generating a plot of average ST2 vs. average cytokine staining intensity. These plots showed that the change in cytokine staining was very gradual as ST2 receptor expression increased. This result indicates little relationship between ST2 receptor expression and cytokine production, suggesting a low threshold for ST2-induced cytokine production that changes little if ST2 receptor expression is altered. If ST2 inhibition was critical for Dex-induced effects, cells that maintained high ST2 receptor expression would be more resistant to the drug. However, Dex-treated cells had lower cytokine staining vs. vehicle control cells, even among groups that maintained high ST2 receptor levels. These data suggest that Dex-mediated antagonism of cytokine production is independent of its effects on ST2 receptor expression.
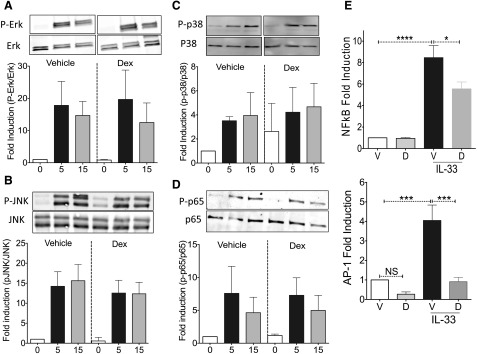
Rapid Dex effects are largely at the level of transcription factor activity
If Dex does not act by inhibiting receptor expression, it is likely that it antagonizes IL-33 signaling. The hallmark of IL-1 family receptor signaling, including ST2, is recruitment of the adaptor protein MyD88 followed by IRAK and TRAF6 activation. These events lead to MAPK phosphorylation and activation of the transcription factors AP-1 and NFκB [4, 5, 9]. We measured Dex effects on ERK, JNK, p38, and NFκB subunit-p65 activation by IL-33. Phosphorylation of these signal transducers peaked 5 min after IL-33 addition (Fig. 4A–D). However, simultaneous addition of Dex had no effect on phosphorylation. To determine whether Dex suppresses downstream transcription factor activity during IL-33 stimulation, we used luciferase reporter vectors. These assays showed that Dex reduced IL-33-elicited NFκB-mediated transcription and completely abrogated AP-1-mediated transcription (Fig. 4E). These results indicate that Dex largely acts at late stages in IL-33 signaling, antagonizing transcription factor activity without affecting upstream phosphorylation events.
Figure 4. Dex suppresses NFκB and AP-1 transcriptional activity.
(A–D) BMMCs were left unactivated (0 min) or activated with IL-33 (100 ng/ml) plus simultaneous addition of vehicle or Dex (2 µM). Lysates collected at 5 and 15 min after activation were used for Western blot analysis. Normalized signals for individual time points were plotted vs. untreated cells. Data are representative of 2 independent experiments performed in triplicate. (E) Transfected BMMCs were activated with IL-33 (50 ng/ml) and simultaneously treated with vehicle or Dex (2 μM) for 2 h. Ratios of signal for firefly luciferase to that of Renilla luciferase for individual samples were normalized to that of vehicle-treated cells. Data are the average of 2 independent experiments performed in triplicate. Tukey’s multiple-comparison test was used to determine the P values. *P < 0.05; ***P < 0.001; ****P < 0.0001.
Dex suppressed IL-33-induced enhancement of IgE-mediated responses
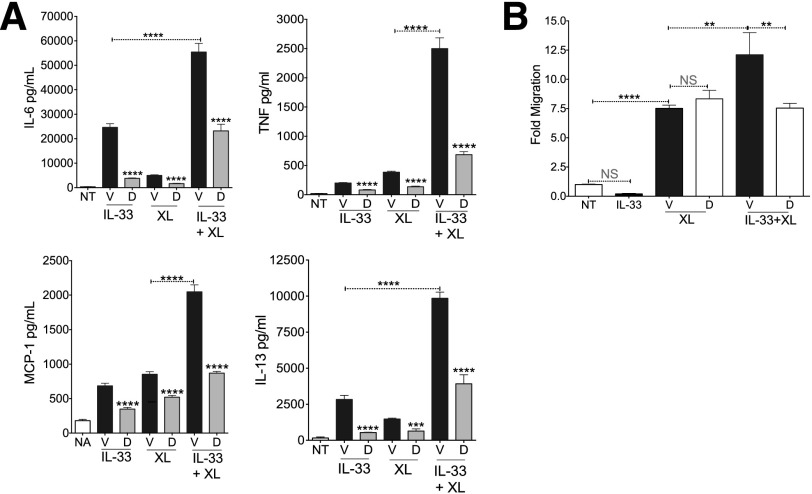
Andrade et al. [29] showed that IL-33 enhances FcεRI-mediated cytokine and chemokine production, an effect that may be critical to allergic inflammation. Therefore, we assessed the ability of Dex to suppress this synergy. Using relatively high antigen and IL-33 concentrations (50 ng/ml each), we too observed strong additive effects (Fig. 5A). Dex inhibited cytokine and chemokine secretion by the same magnitude under all conditions, reducing FcεRI+IL-33-mediated cytokine production nearly to levels of IL-33 alone.
Figure 5. Dexamethasone suppresses IL-33-induced enhancement of IgE-mediated responses.
(A) After IgE sensitization, BMMCs were activated with IL-33 (50 ng/ml) alone, 50 ng/ml of antigen alone (XL), or both together, and simultaneously treated with vehicle or Dex (1 µM). Supernatants collected 6 h after activation were analyzed by ELISA. Data are representative of 3 independent experiments performed in triplicate. (B) BMMCs treated as in (A) were assessed for migration. Fold migration was normalized to that of medium alone. Data are representative of 3 independent experiments performed in triplicate. Tukey’s multiple-comparisons test was performed to calculate the P-values. **P < 0.01; ***P < 0.001; ****P < 0.0001.
MCs migrate toward many stimuli, including antigen. However, it is not known whether IL-33 also enhances FcεRI-mediated migration or if Dex can alter these effects. Using an in vitro Transwell migration assay, we found no evidence that MCs migrate toward IL-33 alone. IgE-loaded BMMCs migrated toward antigen as anticipated, an effect that was significantly augmented by the presence of IL-33 in the bottom chamber. Dex did not suppress migration toward antigen, but did suppress IL-33-mediated enhancement, reducing migration to the level of antigen alone (Fig. 5B). These data indicate that Dex suppresses IL-33 effects, including its ability to enhance FcεRI function.
Dex blocks IL-33-induced inflammation in vivo
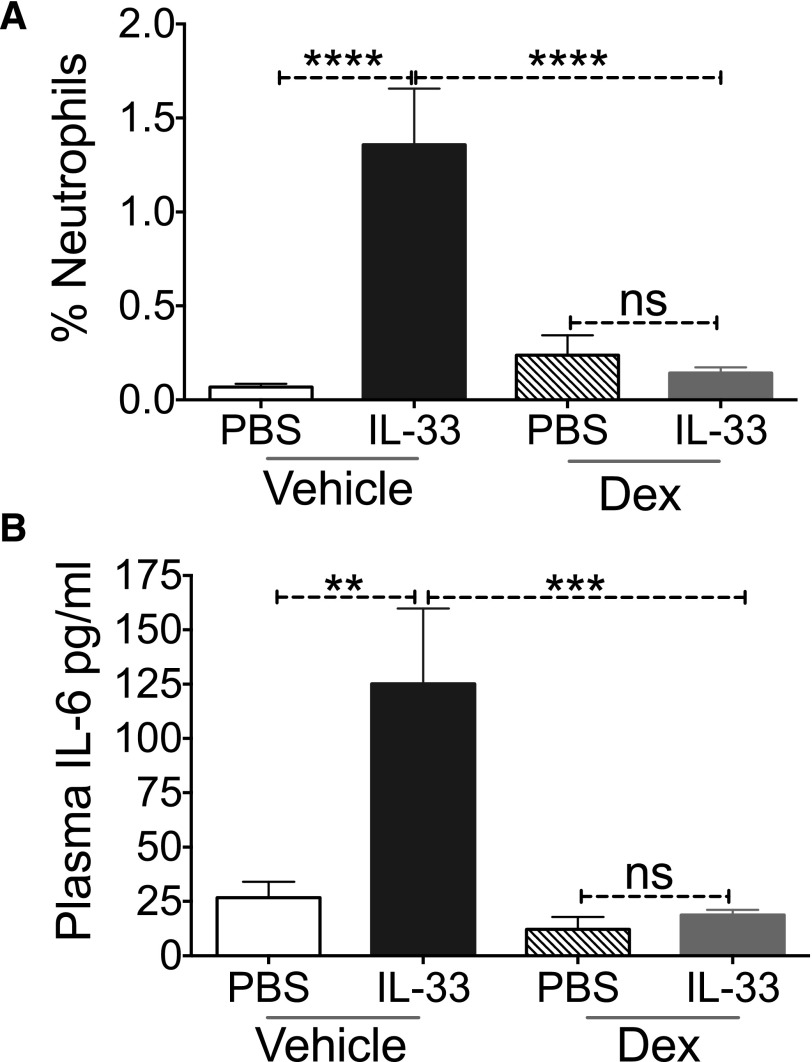
A recent study observed that intraperitoneal IL-33 injection led to neutrophil recruitment, an effect that was dependent on IL-33-induced-MC activation [30]. To determine whether Dex alters this in vivo response, mice were injected intraperitoneally with vehicle or Dex, followed by PBS or IL-33. As expected, PBS-injected mice had negligible levels of neutrophils in the peritoneal cavity. This percentage increased significantly in vehicle/IL-33-treated mice. In contrast, Dex/IL-33 treatment completely prevented neutrophil infiltration (Fig. 6A). We also noted that IL-33 injection increased plasma IL-6 concentration, an effect that was ablated by Dex (Fig. 6B). These data are consistent with our in vitro findings and demonstrate that Dex effectively antagonizes IL-33 functions in vivo.
Figure 6. Dex blocks IL-33-induced inflammation in vivo.
C57BL/6 mice were injected with vehicle or Dex followed by PBS or IL-33. Four hours later, neutrophil recruitment was determined by flow cytometry (A) and plasma IL-6 levels were measured by ELISA (B). Data are representative of 2 independent experiments performed with 5 mice/treatment group. Tukey’s multiple-comparisons test was performed to calculate P-values. **P < 0.01; ***P < 0.001; ****P < 0.0001.
Dex suppresses IL-33-induced cytokine production from human skin MCs
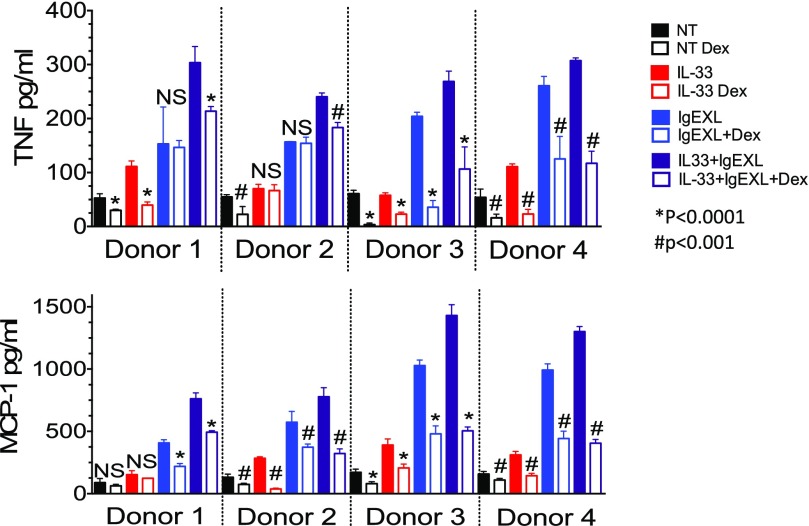
Previous studies have noted that in contrast to murine MCs, Dex is unable to suppress the release of early mediators from human MCs after IgE cross-linking, indicating that human MCs may be less sensitive to Dex [31]. Hence, it was important to determine whether IL-33 suppression similarly varies between species. Human skin MCs derived from 4 healthy donors were activated with IL-33 and/or IgE+antigen (IgE XL). IL-33 alone only weakly induced TNF or MCP-1 from human MCs, effects that were significantly suppressed by Dex in 3 of 4 donors (Fig. 7). Dex generally suppressed IgE XL-induced cytokine production as expected, although we noted donor-to-donor variability. Costimulation with IL-33+IgE XL elicited the greatest cytokine secretion, an effect that was consistently suppressed by Dex. These data suggest that IL-33 signaling in human or mouse MCs is similarly sensitive to the effects of Dex.
Figure 7. Dex suppresses IL-33 induced cytokine production from human skin MCs.
After IgE sensitization, human skin MCs from 4 healthy donors were activated with IL-33 (100 ng/ml), antigen (50 ng/ml), or both together and simultaneously treated with vehicle or Dex (1 µM). (A) Supernatants collected 16 h after activation were analyzed by ELISA. Data are ± sd of 6 replicates per sample. P-values indicate differences between relevant pairs of samples ± dexamethasone. *P < 0.0001, #P < 0.001.
DISCUSSION
Research in the past decade has cast light on the proinflammatory effects of IL-33. MC activation by IL-33 exacerbates the pathology of MC-mediated diseases. However, the effects of steroids such as Dex on IL-33-induced MC function have not been examined. Because steroids are a mainstay of allergic disease treatment, we examined the effects of Dex in vitro and in vivo.
According to time course experiments, Dex significantly suppressed IL-33-mediated cytokine production when added up to 8 h before or simultaneously with IL-33 stimulation. In fact, suppression obtained with simultaneous addition of the drug and IL-33 was greater than that obtained with 24-h pretreatment (Fig. 1A), indicating that Dex acts rapidly but has transient effects. Because a longer pretreatment includes the variables associated with autocrine/paracrine receptor signaling, we focused our study on simultaneous addition of IL-33 and Dex.
GR is almost ubiquitously expressed and is present in the cytoplasm of cells under basal conditions. Passive diffusion of Dex inside the cell is followed by translocation of the ligand-bound GR into the nucleus. Inside the nucleus, the receptor can directly bind to glucocorticoid response elements to activate transcription of target genes (trans-activation) or can interact with other transcription factors to block transcription (trans-repression) [32]. It is also possible that Dex can alter receptor-proximal phosphorylation events.
Studies have shown that Dex-inactivated MAPKs via de novo MAPK phosphatase-1 transcription, requiring several hours of Dex treatment [33–37]. When added simultaneously to MCs, we noted that Dex reduced AP-1 and NFκB-mediated transcriptional activation (Fig. 4E), without altering MAPK or p65 phosphorylation. Reduced transcriptional activity could be the result of direct physical interaction between ligand-bound GR and the AP-1 and NFκB complexes, as has been observed [32, 38–40]. Our luciferase reporter assays were reinforced by the decline in TNF and IL-6 mRNA expression during Dex treatment (Fig. 1D). These data suggest that simultaneous addition of Dex with IL-33 rapidly suppresses proinflammatory transcription factors.
IL-33 enhances IgE-mediated MC cytokine production from human and murine MCs [9, 29], an effect we noted in our study. Dex suppressed this synergy, reducing the amount of cytokines produced to levels comparable to individual stimuli. Dex exposure for 6–48 h has been reported to suppress FcεRI expression ∼50% [41]. Under the conditions used in our study, we noted <10% decrease in surface FcεRI when measured up to 6 h after Dex addition (not shown). Because Dex was added simultaneously with antigen and IL-33, FcεRI suppression is an unlikely explanation for reduced cytokine production. These data are important, because they demonstrate that steroid therapy can repress the enhancing effects of IL-33, but they also show the limitations of Dex, which did not reduce cytokine production to background levels.
Antigen and some chemokines act as chemotactic factors for MCs, leading to their accumulation in asthma, atopic dermatitis, psoriasis, and rheumatoid arthritis [7, 42]. Increased expression of IL-33 in these inflammatory foci [6] creates a niche rich in both antigen and IL-33, but how IL-33 affects MC migration is unknown. We observed for the first time that IL-33 enhances MC migration to antigen, similar to its effects on cytokine/chemokine secretion. In our experimental setting, Dex did not suppress migration toward antigen, but did suppress the IL-33-mediated enhancement. MC migration toward antigen involves distinct signaling proteins including p38 MAPK-, ERK-, Syk-, Rho GTpase-, S1P-, and Orail 1-mediated calcium influx [7, 42–44]. The effects of Dex on these pathways needs separate attention to explain the inability of Dex to block migration toward the antigen, while inhibiting the effects of IL-33. The current data suggest that Dex reduces MC infiltration in pathology associated with IL-33 function.
Autoimmune and allergic diseases, including bullous pemphigoid, experimental autoimmune encephalomyelitis, and urticaria, involve MC-mediated neutrophil recruitment [45–47]. Recent studies have implicated IL-33 in this infiltration. For example, local MC activation and neutrophil recruitment both contribute to the development of psoriatic skin lesions after IL-33 injections in mice [48]. Similarly, a previous study showed that MCs are critical for IL-33-induced neutrophil recruitment in a peritoneal inflammation model [30]. Using this model, we found that Dex blocks IL-33-mediated neutrophil recruitment to the peritoneum, concomitant with reduced systemic IL-6. Because IL-33 has been shown to induce airway eosinophilia, we anticipated peritoneal eosinophil recruitment, but that effect did not occur. The likely explanation is that, in the airways, IL-33 activates innate lymphoid cells (ILC2) to recruit eosinophils [2] and that ILC2 cells are less abundant in the peritoneum. Although previous data support the importance of MCs in IL-33-mediated neutrophil recruitment, it should be stressed that Dex affects many cell types in our assay. Our data do show, however, that Dex is an effective IL-33 antagonist in vivo, completely repressing both neutrophil migration and plasma IL-6.
Because MC phenotype varies with anatomic location and microenvironment [7], it was important to assess the effects of Dex beyond BMMCs. Peritoneal MCs, which differentiate in vivo before being expanded in vitro, responded quite similarly to BMMCs (Fig. 2B). We also noted consistent responses among BMMCs isolated from C57BL/6 and 129/SvJ mice (data not shown), which have many polymorphisms. Human skin MCs, however, are poorly activated by IL-33 alone, forcing us to examine IL-33 enhancement of FcεRI-mediated responses. Furthermore, human donors have far more genetic diversity than inbred mice, making it more likely that Dex and/or IL-33 responses may vary from donor to donor. Although we did note these variations, overall, Dex suppressed IL-33 effects among MCs from 4 donors.
In summary, our data show that Dex, a member of the clinically important steroid family, is a potent inhibitor of IL-33-mediated MC function, including cytokine production, migration, and neutrophil recruitment. Our understanding of how IL-33 contributes to allergic and inflammatory disease is still unfolding. Deciphering which interventions disrupt this pathway will be relevant for clinical care and may offer new molecular targets for treatment.
AUTHORSHIP
All authors contributed to generating the data shown in this document. A.P., O.C., and J.R. wrote the manuscript, which was reviewed by all authors.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health, National Institute of Allergy and Infectious Diseases Grants 1R01AI101153 and 2R01AI059638 (to J.J.R.) and Grant 1R01 AI095494 (to C.A.O.)
Glossary
- AP-1
activator protein-1
- BMMC
bone marrow-derived mast cell
- Dex
dexamethasone
- DNP-HAS
dinitrophenyl-coupled human serum albumin
- gMFI
geometric mean fluorescence intensity
- GR
glucocorticoid receptor
- MC
mast cell
DISCLOSURE
The authors report no conflicts of interest.
REFERENCES
- 1.Carriere V., Roussel L., Ortega N., Lacorre D.-A., Americh L., Aguilar L., Bouche G., Girard J.-P. (2007) IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. USA 104, 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cayrol C., Girard J.-P. (2014) IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 31, 31–37. [DOI] [PubMed] [Google Scholar]
- 3.Nakanishi W., Yamaguchi S., Matsuda A., Suzukawa M., Shibui A., Nambu A., Kondo K., Suto H., Saito H., Matsumoto K., Yamasoba T., Nakae S. (2013) IL-33, but not IL-25, is crucial for the development of house dust mite antigen-induced allergic rhinitis. PLoS One 8, e78099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T. K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D. M., Bazan J. F., Kastelein R. A. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490. [DOI] [PubMed] [Google Scholar]
- 5.Kakkar R., Lee R. T. (2008) The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat. Rev. Drug Discov. 7, 827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller A. M. (2011) Role of IL-33 in inflammation and disease. J. Inflamm. (Lond.) 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okayama Y., Kawakami T. (2006) Development, migration, and survival of mast cells. Immunol. Res. 34, 97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J. X., Kaieda S., Ameri S., Fishgal N., Dwyer D., Dellinger A., Kepley C. L., Gurish M. F., Nigrovic P. A. (2014) IL-33/ST2 axis promotes mast cell survival via BCLXL. Proc. Natl. Acad. Sci. USA 111, 10281–10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iikura M., Suto H., Kajiwara N., Oboki K., Ohno T., Okayama Y., Saito H., Galli S. J., Nakae S. (2007) IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab. Invest. 87, 971–978. [DOI] [PubMed] [Google Scholar]
- 10.Xu D., Jiang H.-R., Kewin P., Li Y., Mu R., Fraser A. R., Pitman N., Kurowska-Stolarska M., McKenzie A. N. J., McInnes I. B., Liew F. Y. (2008) IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc. Natl. Acad. Sci. USA 105, 10913–10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saluja R., Khan M., Church M. K., Maurer M. (2015) The role of IL-33 and mast cells in allergy and inflammation. Clin. Transl. Allergy 5, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur D., Gomez E., Doe C., Berair R., Woodman L., Saunders R., Hollins F., Rose F. R., Amrani Y., May R., Kearley J., Humbles A., Cohen E. S., Brightling C. E. (2015) IL-33 drives airway hyper-responsiveness through IL-13-mediated mast cell: airway smooth muscle crosstalk. Allergy 70, 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjöberg L. C., Gregory J. A., Dahlén S. E., Nilsson G. P., Adner M. (2015) Interleukin-33 exacerbates allergic bronchoconstriction in the mice via activation of mast cells. Allergy 70, 514–521. [DOI] [PubMed] [Google Scholar]
- 14.Xu D., Jiang H. R., Li Y., Pushparaj P. N., Kurowska-Stolarska M., Leung B. P., Mu R., Tay H. K., McKenzie A. N. J., McInnes I. B., Melendez A. J., Liew F. Y. (2010) IL-33 exacerbates autoantibody-induced arthritis. J. Immunol. 184, 2620–2626. [DOI] [PubMed] [Google Scholar]
- 15.Hsu C.-L., Neilsen C. V., Bryce P. J. (2010) IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS One 5, e11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabata H., Moro K., Fukunaga K., Suzuki Y., Miyata J., Masaki K., Betsuyaku T., Koyasu S., Asano K. (2013) Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat. Commun. 4, 2675. [DOI] [PubMed] [Google Scholar]
- 17.Bunim J. J. (1959) A decade of anti-inflammatory steroids, from cortisone to dexamethasone: summary. Ann. N. Y. Acad. Sci. 82, 1012–1013. [DOI] [PubMed] [Google Scholar]
- 18.Bunim J. J., Black R. L., Lutwak L., Peterson R. E., Whedon G. D. (1958) Studies on dexamethasone, a new synthetic steroid, in rheurheumatoid arthritis: a preliminary report—adrenal cortical, metabolic and early clinical effects. Arthritis Rheum. 1, 313–331. [DOI] [PubMed] [Google Scholar]
- 19.Hart F. D. 1960. Dexamethasone. Postgrad. Med. J. 36, 226–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robin J. L., Seldin D. C., Austen K. F., Lewis R. A. (1985) Regulation of mediator release from mouse bone marrow-derived mast cells by glucocorticoids. J. Immunol. 135, 2719–2726. [PubMed] [Google Scholar]
- 21.Smith S. J., Piliponsky A. M., Rosenhead F.. 2002. Dexamethasone inhibits maturation, cytokine production and FcyRI expression of human cord blood‐derived mast cells. Clin. Exp. Immunol. 32, 906–913. [DOI] [PubMed] [Google Scholar]
- 22.Andrade M., Hiragun T.. 2004. Dexamethasone suppresses antigen-induced activation of phosphatidylinositol 3-kinase and downstream responses in mast cells. J. Immunol. 172, 7254–7262. [DOI] [PubMed] [Google Scholar]
- 23.Cole Z. A., Clough G. F., Church M. K. (2001) Inhibition by glucocorticoids of the mast cell-dependent weal and flare response in human skin in vivo. Br. J. Pharmacol. 132, 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wershil B. K., Furuta G. T., Lavigne J. A., Choudhury A. R., Wang Z. S., Galli S. J. (1995) Dexamethasone or cyclosporin A suppress mast cell-leukocyte cytokine cascades: multiple mechanisms of inhibition of IgE- and mast cell-dependent cutaneous inflammation in the mouse. J. Immunol. 154, 1391–1398. [PubMed] [Google Scholar]
- 25.Fernando J., Faber T. W., Pullen N. A., Falanga Y. T., Kolawole E. M., Oskeritzian C. A., Barnstein B. O., Bandara G., Li G., Schwartz L. B., Spiegel S., Straus D. B., Conrad D. H., Bunting K. D., Ryan J. J. (2013) Genotype-dependent effects of TGF-β1 on mast cell function: targeting the Stat5 pathway. J. Immunol. 191, 4505–4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kambe N., Kambe M., Kochan J. P., Schwartz L. B. (2001) Human skin-derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes. Blood 97, 2045–2052. [DOI] [PubMed] [Google Scholar]
- 27.Kim H. S., Kim A.-R., Kim D. K., Kim H. W., Park Y. H., Jang G. H., Kim B., Park Y. M., You J. S., Kim H. S., Beaven M. A., Kim Y. M., Choi W. S. (2015) Interleukin-10-producing CD5+ B cells inhibit mast cells during immunoglobulin E-mediated allergic responses. Sci. Signal. 8, ra28. [DOI] [PubMed] [Google Scholar]
- 28.Kolawole E. M., McLeod J. J. A., Ndaw V., Abebayehu D., Barnstein B. O., Faber T., Spence A. J., Taruselli M., Paranjape A., Haque T. T., Qayum A. A., Kazmi Q. A., Wijesinghe D. S., Sturgill J. L., Chalfant C. E., Straus D. B., Oskeritzian C. A., Ryan J. J. (2016) Fluvastatin suppresses mast cell and basophil IgE responses: genotype-dependent effects. J. Immunol. 196, 1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrade M. V., Iwaki S., Ropert C., Gazzinelli R. T., Cunha-Melo J. R., Beaven M. A. (2011) Amplification of cytokine production through synergistic activation of NFAT and AP-1 following stimulation of mast cells with antigen and IL-33. Eur. J. Immunol. 41, 760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enoksson M., Möller-Westerberg C., Wicher G., Fallon P. G., Forsberg-Nilsson K., Lunderius-Andersson C., Nilsson G. (2013) Intraperitoneal influx of neutrophils in response to IL-33 is mast cell-dependent. Blood 121, 530–536. [DOI] [PubMed] [Google Scholar]
- 31.Schleimer R. P., Schulman E. S., MacGlashan D. W. Jr., Peters S. P., Hayes E. C., Adams G. K. III, Lichtenstein L. M., Adkinson N. F. Jr (1983) Effects of dexamethasone on mediator release from human lung fragments and purified human lung mast cells. J. Clin. Invest. 71, 1830–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van der Velden V. H. (1998) Glucocorticoids: mechanisms of action and anti-inflammatory potential in asthma. Mediators Inflamm. 7, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rider L. G., Hirasawa N., Santini F., Beaven M. A. (1996) Activation of the mitogen-activated protein kinase cascade is suppressed by low concentrations of dexamethasone in mast cells. J. Immunol. 157, 2374–2380. [PubMed] [Google Scholar]
- 34.Hirasawa N., Sato Y., Fujita Y., Mue S., Ohuchi K. (1998) Inhibition by dexamethasone of antigen-induced c-Jun N-terminal kinase activation in rat basophilic leukemia cells. J. Immunol. 161, 4939–4943. [PubMed] [Google Scholar]
- 35.Lasa M., Brook M., Saklatvala J., Clark A. R. (2001) Dexamethasone destabilizes cyclooxygenase 2 mRNA by inhibiting mitogen-activated protein kinase p38. Mol. Cell. Biol. 21, 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lasa M., Abraham S. M., Boucheron C., Saklatvala J., Clark A. R. (2002) Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol. Cell. Biol. 22, 7802–7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kassel O., Sancono A., Krätzschmar J., Kreft B., Stassen M., Cato A. C. (2001) Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 20, 7108–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paliogianni F., Raptis A., Ahuja S. S., Najjar S. M., Boumpas D. T. (1993) Negative transcriptional regulation of human interleukin 2 (IL-2) gene by glucocorticoids through interference with nuclear transcription factors AP-1 and NF-AT. J. Clin. Invest. 91, 1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukaida N., Morita M., Ishikawa Y., Rice N., Okamoto S., Kasahara T., Matsushima K. (1994) Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-kappa B is target for glucocorticoid-mediated interleukin 8 gene repression. J. Biol. Chem. 269, 13289–13295. [PubMed] [Google Scholar]
- 40.Ray A., Prefontaine K. E. (1994) Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 91, 752–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaguchi M., Hirai K., Komiya A., Miyamasu M., Furumoto Y., Teshima R., Ohta K., Morita Y., Galli S. J., Ra C., Yamamoto K. (2001) Regulation of mouse mast cell surface Fc epsilon RI expression by dexamethasone. Int. Immunol. 13, 843–851. [DOI] [PubMed] [Google Scholar]
- 42.Ishizuka T., Okajima F., Ishiwara M., Iizuka K., Ichimonji I., Kawata T., Tsukagoshi H., Dobashi K., Nakazawa T., Mori M. (2001) Sensitized mast cells migrate toward the antigen: a response regulated by p38 mitogen-activated protein kinase and Rho-associated coiled-coil-forming protein kinase. J. Immunol. 167, 2298–2304. [DOI] [PubMed] [Google Scholar]
- 43.Lee J., Veatch S. L., Baird B., Holowka D. (2012) Molecular mechanisms of spontaneous and directed mast cell motility. J. Leukoc. Biol. 92, 1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung I. D., Lee H.-S., Lee H. Y., Choi O. H. (2009) FcepsilonRI-mediated mast cell migration: signaling pathways and dependence on cytosolic free Ca2+ concentration. Cell. Signal. 21, 1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen R., Ning G., Zhao M.-L., Fleming M. G., Diaz L. A., Werb Z., Liu Z. (2001) Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J. Clin. Invest. 108, 1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura Y., Kambe N., Saito M., Nishikomori R., Kim Y.-G., Murakami M., Núñez G., Matsue H. (2009) Mast cells mediate neutrophil recruitment and vascular leakage through the NLRP3 inflammasome in histamine-independent urticaria. J. Exp. Med. 206, 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christy A. L., Walker M. E., Hessner M. J., Brown M. A. (2013) Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE. J. Autoimmun. 42, 50–61. [DOI] [PubMed] [Google Scholar]
- 48.Hueber A. J., Alves-Filho J. C., Asquith D. L., Michels C., Millar N. L., Reilly J. H., Graham G. J., Liew F. Y., Miller A. M., McInnes I. B. (2011) IL-33 induces skin inflammation with mast cell and neutrophil activation. Eur. J. Immunol. 41, 2229–2237. [DOI] [PubMed] [Google Scholar]