FcγR signaling activates the NLRP3 inflammasome via Syk to facilitate development of protective immunity to Ft LVS.
Keywords: macrophage, IL-1β, vaccine
Abstract
IgG (mAb)-opsonized, inactivated Francisella tularensis LVS (iFt-mAb) enhances TLR2-dependent IL-6 production by macrophages via Fcγ receptors (FcγR). In mice, vaccination with iFt-mAb provides IgA-dependent protection against lethal challenge with Ft LVS. Because inflammasome maturation of IL-1β is thought important for antibody-mediated immunity, we considered the possibility that iFt-mAb elicits an FcγR-dependent myeloid cell inflammasome response. Herein, we find that iFt-mAb enhances macrophage and dendritic cell IL-1β responses in a TLR2- and FcγR-dependent fashion. Although iFt-mAb complexes bind FcγR and are internalized, sensing of cytosolic DNA by absent in melanoma 2 (AIM2) is not required for the IL-1β response. In contrast, ASC, caspase-1, and NLR family pyrin domain-containing 3 (NLRP3) are indispensable. Further, FcγR-mediated spleen tyrosine kinase (Syk) signaling is required for this NLRP3-dependent IL-1β response, but the alternative IL-1β convertase caspase-8 is insufficient. Finally, iFt-mAb-vaccinated wild-type mice exhibit a significant delay in time to death, but IL-1R1– or Nlrp3-deficient mice vaccinated in this way are not protected and lack appreciable Francisella-specific antibodies. This study demonstrates that FcγR-mediated Syk activation leads to NLRP3 inflammasome-dependent IL-1β production in macrophages and suggests that an Nlrp3- and IL-1R–dependent process contributes to the IgA response important for protection against Ft LVS. These findings extend our understanding of cellular responses to inactivated pathogen-opsonized vaccine, establish FcγR-elicited Syk kinase-mediated NLRP3 inflammasome activation, and provide additional insight toward understanding crosstalk between TLR and FcγR signals.
Introduction
In response to pathogens, myeloid cells initiate innate inflammatory processes mediated in part by the production and secretion of cytokines. Cytokines, such as TNF-α, IL-6, and IL-1β, are important for neutrophil and macrophage recruitment, for activation of these phagocytes to clear invading organisms, and for facilitating development of adaptive immunity. Specifically, whereas IL-1β promotes innate defense against various pathogens [1–3], it also enhances pathogen-specific, protective Ab responses [4] and is required for adjuvants, such as alum [5], to marshal vaccine-elicited, Ag-specific Abs [6].
Elaboration of IL-1β requires two essential events: expression of pro-IL-1β and its subsequent enzymatic cleavage by caspase-1 to generate active IL-1β. Abundant expression of pro-IL1β, mediated via the NF-κB pathway, is initiated by various surface receptors, most commonly TLRs [7]. Caspase-1 maturation of IL-1β requires assembly of a multiprotein inflammasome complex, which promotes autocatalytic cleavage of procaspase-1 to active caspase-1 [8]. Assembly of the widely studied NLRP3 inflammasome is triggered by varied agonists ranging from pathogen-associated molecules (toxins) to environmental (asbestos) or cell damage–associated agonists (cholesterol, uric acid) [9]. The mechanisms leading to NLRP3 inflammasome activation are poorly understood. Other caspase-1–activating inflammasomes include those containing AIM2, activated upon binding cytosolic dsDNA; NLRP1, which responds to anthrax lethal toxin; and NLRC4, a sensor for flagellin [10]. Caspase-1–independent IL-1β processing has also been observed. For example, caspase-8–mediated IL-1β maturation follows dectin-1 recognition of Candida and subsequent Syk kinase signaling, initiating assembly of a caspase-8–activating complex containing MALT1, Bcl-10, and CARD9 [11]. Caspase-8 may also be involved in mechanisms contributing to NLRP3 inflammasome activity, including processing of procaspase-1 [12].
Francisella tularensis subsp. tularensis is the causative agent of lethal human tularemia in North America. The current live-vaccine strain (Ft LVS) is an attenuated strain derived from Francisella tularensis subsp. holarctica, the more common disease-causing subspecies in Europe and Asia. The inflammatory cytokine responses of in vitro macrophages to both live and killed Ft are weak, and even after i.n. challenge, proinflammatory responses in the lung are markedly delayed [13, 14]. TLR2 [14–16] and AIM2 [17–19], sensing bacterial lipoproteins and dsDNA, respectively, are involved in recognizing live Ft and appear important for Ft-elicited inflammatory cytokine production and IL-1β processing. Accordingly, despite seemingly modest inflammatory cytokine responses, TLR2- and Aim2-deficient mice are more susceptible to Ft LVS and Fn U112 infection, respectively, than WT mice [14, 17]. Antioxidant enzymes present in live Ft are thought to limit necessary activation of the TLR2 signaling cascade, leading to blunted TNF-α and IL-6 production [20], whereas reduced host-mediated bacterial lysis appears to limit the inflammasome response [21]. Although capable of engaging TLR2, killed Ft and phagolysome-escape mutants fail to access cytosolic pattern recognition receptors, such as AIM2 and NLRP3, leading to diminished inflammasome activation [22, 23].
Protection against Ft LVS requires both humoral and cellular immune responses [24]. Vaccination with live Ft LVS affords incomplete protection in humans [25], which may be explained by limited proinflammatory responses insufficient to support necessary Th cell responses [26]. However, in mice, killed Ft LVS opsonized with an anti–iFt-mAb resulted in an IgA-dependent protection against respiratory tularemia that required the common γ chain of FcγR [27]. The mechanism by which this iFt-mAb strategy confers protection is unknown but is likely important for developing FcγR-targeted vaccine strategies.
FcγRs are expressed by hematopoietic cells and bind the constant portion of IgG Abs, mediating either activating or inhibitory signals important for phagocytosis, Ag presentation, and promoting or restricting lymphocyte functions (for a review, see [28]). Human and mouse activating FcγRs include FcγRI (CD64) and FcγRIII (CD16). Humans also have activating FcγRIIa/c (CD32), whereas FcγRIV is exclusive to mice. All these contain either an ITAM motif (FcγRIIa/c) or associate with the ITAM-containing common γ chain (FcγRI, FcγRIII, and FcγRIV) essential for FcγR activation of the Src/Syk kinase pathway. Upon ligation of FcγR, the ITAM motifs are phosphorylated by members of the Src family of tyrosine kinases, leading to the recruitment of Syk, which is then activated by phosphorylation [29]. In contrast, human or mouse FcγRIIb (CD32) inhibits the Src/Syk pathway through an integral, phosphatase-activating ITIM motif.
Targeting Ag to FcγR improves humoral and cellular immune responses in the absence of adjuvant and, as such, is a valuable vaccine strategy, especially for mucosal immunity [30–32]. In the case of iFt-mAb vaccination, IgA-mediated protection against Ft LVS requires the FcR common γ chain, suggesting involvement of FcγRI, FcγRIII, and/or FcγRIV [27]. However, how FcγR mediates the enhanced vaccine response of iFt-mAb is not fully understood. At the cellular level, iFt-mAb enhances IL-6 secretion and the Ag-presentation capacity of macrophages and DCs in an FcγR-dependent fashion [33, 34]. In addition, immunization with iFt-mAb–pulsed DC was also protective against lethal Ft LVS challenge and enhanced Ab production [35]. iFt-mAb appears to influence other parameters important for protection, including activation of lung DCs, increased DC production of IL-12, and expansion of IFN-γ-producing effector memory CD4+ T cells [36], which appears to require inflammatory macrophages and a greater proinflammatory cytokine response [37]. How FcγR signaling promotes these cellular effects is unclear.
Engagement of FcγR by immune complexes has different outcomes depending on the signal context. Immune complex costimulation of TLR and FcγR can activate or inhibit signaling pathways leading to cytokine responses, depending on the tonicity of the signals [38]. For example, immune complexes elicit a synergistic TLR- and FcγR-mediated DC activation promoting a proinflammatory Th17 response [39]. Similar activating crosstalk has been demonstrated using other human myeloid cells [40, 41]. In contrast, the proinflammatory cytokine response of mouse bone-marrow macrophages to various inflammasome agonists was inhibited by the presence of immune complexes [42].
Given that inflammasome-dependent IL-1β appears necessary for successful vaccination [43, 44] and that FcγR engagement enhances macrophage production of other proinflammatory cytokines [33, 37], we examined the capacity of the iFt-mAb vaccine platform to elicit an IL-1β response. We find that, in cooperation with TLR2, FcγR engagement directs the activation of an unanticipated, kinase signal-dependent, NLRP3 inflammasome response mechanism. We also observe that the NLRP3/IL-1 axis is important for protective Ab responses following iFt-mAb vaccination, implicating FcγR-mediated activation of the NLRP3 inflammasome as both a relevant adjuvant pathway and a potential target for treating immune-complex disease.
MATERIALS AND METHODS
Cell culture
The human monocytic cell lines THP-1 (ATCC, Manassas, VA, USA) and THP-1 deficient in NLRP3 (THP-1defNLRP3; InvivoGen, San Diego, CA, USA) were cultured, as recommended by the suppliers, in RPMI-1640 (HyClone; GE Healthcare Bio-Sciences, Piscataway, NJ, USA) with 10% FBS (Atlanta Biologicals, Flowery Branch, GA, USA) and 0.1% Pen/Strep (CellGro; Corning Life Sciences, Tewksbury, MA, USA). Hygromycin (200 µg/ml; Thermo Fisher Scientific) was added to THP-1defNLRP3 culture every other passage to maintain selective pressure. THP-1defNLRP3 cells were passaged once without selective antibiotic before seeding. Unless otherwise indicated, THP-1 cells were seeded at 2.5 × 105 cells/ml in 24-well tissue culture dishes and stimulated with 100 nM PMA (Sigma-Aldrich, St. Louis, MO, USA) overnight (16–18 h) to elicit adherence and a more macrophage-like phenotype. BMDM were isolated from femurs of 8-wk-old mice as described previously [45]. Briefly, femurs were flushed with DMEM and red blood cells lysed with ACK buffer (Lonza Inc., Allendale, NJ, USA). Cells were cultured in DMEM containing 10% L929-cell supernatants for 1 wk before use. For all experiments, BMDM cells were seeded and allowed to settle and attach overnight (about 18 h) before stimulation and were stimulated without LPS. BMDCs from femurs processed as for BMDMs were differentiated with mouse recombinant Flt3 ligand (50 ng/ml; R&D Systems, Minneapolis, MN, USA) for 9 d.
Mouse strains
WT C57Bl/6 mice were obtained from Taconic Biosciences (Hudson, NY, USA). IL1R1 KO (B6.129S7-Il1r1tm1Imx/J) and Aim2 KO (B6.129P2-Aim2Gt(CSG445)Byg/J) mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Caspase-1/11 KO [46], Asc KO [47], and Nlrp3 KO mice on the C57Bl/6 background [48] were kindly provided by Dr. Timothy Sellati (Caspase-1/11 and ASC KO) and Dr. Jenny Ting (Nlrp3 KO). For some experiments, WT mice were directly supplied from Taconic Biosciences, otherwise all mice were bred in house. All mice were housed in the Animal Resources Facility at Albany Medical College under specific-pathogen–free conditions for at least 2 wk to facilitate equilibrium of intestinal microbiota. All experimental procedures were approved by the Albany Medical College Institutional Animal Care and Use Committee and the Institutional Biosafety Committee.
Reagents and Abs
Mouse IgG2a monoclonal anti–F. tularensis LPS (mAb) was purchased from Fitzgerald Industries International (Acton, MA, USA). Mouse anti-human CD32 (clone AT10) and anti-human CD64 (clone 10.1) Abs were from Abcam (Cambridge, MA, USA) and were used at 10 and 5 µg/ml, respectively. Rat anti-human TLR2 polyclonal Ab (Thermo Fisher Scientific) was used at 100 ng/ml. PP1 (10 µM), PP2 (10 µM), and piceatannol (5 µM) (all from EMD Millipore, Billerica, MA, USA) were dissolved in DMSO. Caspase-8 inhibitor (z-IETD-fmk, 50 µM in DMSO) was obtained from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). Polybead carboxylate microspheres (1 µm, PolySciences, Inc., Warrington, PA, USA) were coated with 200-µg mAb using the PolyLink Protein Coupling kit (PolySciences, Inc.), according to the included protocol. Uncoated beads were washed twice in 0.4 ml PolyLink Wash/Storage buffer (from the kit) and resuspended in 0.4 ml PolyLink Wash/Storage buffer. Coated or uncoated beads were diluted in media before cell stimulation.
Growth of Ft LVS, inactivation of Ft, and immune complex formation
Live Ft LVS was obtained from the Albany Medical College Microbiology Core Facility. Bacteria were grown in modified Mueller-Hinton broth (Difco MH broth; BD Biosciences, East Rutherford, NJ, USA) with ferric pyrophosphate and IsoVitalex (BD Biosciences), and aliquots of midlog-phase growth cultures were stored in liquid nitrogen [49]. The viability of frozen aliquots of bacteria and the inocula dosage after serial dilution in PBS were confirmed by colony counting. Formalin-inactivated Ft LVS (iFt) and immune complexes were prepared as described in Rawool et al. [27]. In brief, 1 × 1010 CFU/ml live bacteria were fixed for 2 h with 2% paraformaldehyde in sterile PBS, followed by 3 washes with PBS, and were resuspended in a final volume of 1 ml, generating a final approximate iFt stock concentration of 1 × 107 iFt/µl. For vaccination with 2 × 107 iFt, 20 µl of a 1:10 dilution of the stock was used. Anti-Ft mAb (5 µg/ml except as indicated) was added to iFt in 100 µl sterile PBS and incubated overnight at 4°C with rocking. Cell cultures were stimulated with iFt immune complexes diluted with RPMI 1640.
Stimulation of cell cultures
BMDM-, BMDC-, or PMA-treated THP-1 were stimulated for 24 h with iFt or iFt-mAb in RPMI 1640 media at 100 MOI (based on the stock concentration of 1 × 107 iFt/μL). Cells were incubated with blocking Abs (30 min at 4°C) or chemical inhibitors (30 min at 37°C) before stimulation. ATP (5 mM, Sigma-Aldrich) was used as an NLRP3 inflammasome activator and added to cells for the final 30 min of stimulation. Cell supernatants were harvested 24 h poststimulation and clarified by centrifugation. Human IL-1β or TNF-α (Thermo Fisher Scientific) or mouse IL-1β or TNF-α (eBioscience, San Diego, CA, USA) in cell-free supernatants was assayed by ELISA. Human IL-18 was assayed using ProcartaPlex Simplex bead sets (eBioscience) and a Bio-Plex instrument (Bio-Rad, Hercules, CA, USA).
Immunofluorescence staining
PMA-treated THP-1 was cultured on sterile glass coverslips overnight in RPMI media. The following day, cells were stimulated at 37°C with iFt-mAb for 0, 15, and 60 min. At each time point, cover slips were removed from medium and fixed using 4% paraformaldehyde in PBS. Fixed coverslips were stained with anti-IgG2a–Alexa Fluor 488 (1:250 dilution) for 1 h, washed using fixative containing media, permeabilized with 0.02% saponin, and subsequently stained with anti-IgG2a–Alexa Fluor 594 (1:250 dilution). Because the mAb used in the iFt-mAb complex is an IgG2a isotype, this immunofluorescence staining procedure labels extracellular iFt-mAb complexes green and the intracellular complexes red. Coverslips were mounted onto slides using FluoroGel II containing DAPI (Electron Microscopy Sciences, Hatfield, PA, USA). Images were taken using an Olympus 1X81 microscope (Olympus, Tokyo, Japan) and Fluoview FV1000 laser (Olympus). Images were analyzed using Olympus Fluoview FV10-ASW software.
Caspase-8 activity assay
BMDM were seeded at 5 × 104/ml in a 96-well plate and either treated with vehicle or caspase-8 inhibitor Z-IETD-FMK in DMSO for 30 min before stimulation with iFt or iFt-mAb for 24 h. All treatments were performed in triplicate, and assays were conducted on 3 separate occasions. Caspase-8 activity was determined using a luminescence detection kit from Promega (Madison, WI, USA) per the manufacturer’s supplied protocol. Background luminescence was subtracted from sample values. Average activity was normalized to WT BMDM stimulated with iFt.
Immunization and challenge studies
WT B6 mice, Nlrp3 KO mice, and IL1R1 KO mice (2 sets of mice, 10 each genotype per set, age 6–8 wk with equal numbers of males and females) were subjected to the immunization and challenge strategy, as described previously [27]. In brief, iFt-mAb (equivalent to 2 × 107 bacteria with 1 μg of anti-LVS mAb) was delivered by drop-wise i.n. administration in a total volume of 20 µl, 10 µl per nare on day 0 and again 21 d later. At 35 d (14 d postboost), mice were challenged i.n. with 20,000 CFU of Ft LVS in a 40-µl bolus to a single nare. Following challenge, mice were observed for survival twice daily. For challenge studies, 2 sets of WT or IL1R1 KO mice (6 of each genotype per set, age 7 wk) were challenged i.n. with an LD50 of Ft LVS (500 CFU) in a single 40-µl bolus and monitored for survival twice daily.
Serum anti-Ft LVS Ab determination
Serum from the mice prechallenge was analyzed for Ft LVS-specific IgA or IgG. A 96-well plate was coated with Ft LVS (1 × 107 CFU/ml) in 100 μl carbonate buffer [4.3 g/L sodium bicarbonate (Sigma-Aldrich) and 5.3 g/L sodium carbonate (Sigma-Aldrich), at pH 9.4]. Following overnight incubation at 4°C, plates were washed (PBS/0.05% Tween 20) and blocked with PBS/10% BSA. Two-fold dilutions of serum, starting with 1:25, were applied in duplicate to the ELISA plate and incubated at 22°C for 2 h. Captured serum Abs were detected with an anti-IgA or anti-IgG Ab conjugated to HRP and incubated 1 h at 22°C. Following washes, TMB substrate (Sigma-Aldrich) was applied, incubated for 20 min at 22°C, and stopped by the addition of 25 μl of HCl; absorbance at 450 nm was read on a Epoch plate reader (Biotek, Winooski, VT, USA). Absorbance at 450 nm was used as a relative measure of Ab levels. IgA and IgG A450 values were normalized to unvaccinated serum Ab values.
Statistical analysis
All experiments represent at least 3 independent experiments, unless otherwise mentioned. A P value of ≤0.05, calculated using a 2-tailed, unpaired Student’s t test, was considered statistically significant. Survival curves were compared using the log-rank test (Mantel-Cox).
Supplemental Fig. 1 demonstrates that FcγR crosslinking by mAb-coated beads is insufficient to elicit IL-1β production in the absence of iFt.
Supplemental Fig. 2 shows that Syk can activate the NLRP3 inflammasome in a reconstituted system in the absence of an activator.
RESULTS
Immune complexes trigger enhanced IL-1β production in macrophages and DCs
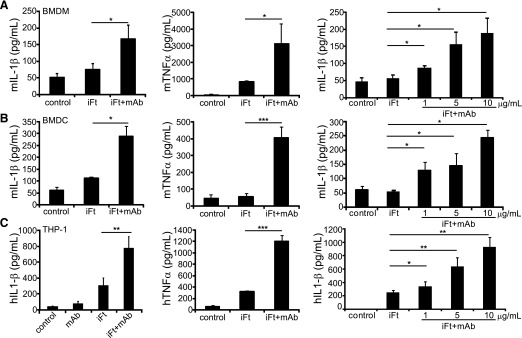
A novel Ab complex vaccine approach, using inactivated Ft LVS and anti-Ft LPS Ab (iFt-mAb), results in full protection against lethal respiratory challenge with Ft LVS and partial protection against the highly virulent Ft SchuS4 in mice [27]. The importance of the inflammasome and the IL-1 response for promoting adaptive immunity and subsequent Ab production [5, 43, 44, 50, 51] led us to consider whether iFt-mAb complexes elicit an inflammasome-dependent IL-1β response. Killed Ft does not escape the phagosome and fails to elicit much IL-1β from macrophages, suggesting limited induction of inflammasome activation/IL-1β processing activity by iFt alone [13, 23, 52]. Because engaging FcγR through addition of an opsonizing Ab to iFt stimulates protective immunity and greater TNF-α and IL-6 production, we reasoned that iFt-mAb might also stimulate a more-robust IL-1β response. Therefore, we examined the ability of iFt and iFt-mAb to elicit production of IL-1β from macrophages and DCs. We measured TNF-α production as an indicator of NF-κB activity and the subsequent induction of pro-IL-1β message [53]. Although IL-1β secretion by BMDM stimulated with iFt alone was not significantly greater than that of untreated cells, stimulation with iFt-mAb induced a significant, 3-fold increase in IL-1β secretion over media alone (Fig. 1A). Stimulation with the iFt-mAb complex similarly enhanced TNF-α production at 24 h (Fig. 1A). IL-1β and TNF-α production by BMDC was also enhanced by iFt-mAb, suggesting an analogous response to iFt-mAb complexes among myeloid cells (Fig. 1B). Inflammasome usage and the magnitude of the IL-1β response differed between mouse and human macrophages [49, 54]. Therefore, we further examined the IL-1β response to iFt-mAb using the human THP-1 monocytic/macrophage cell line. THP-1 not pretreated with PMA produced little IL-1β with iFt, iFt-mAb, or even live Ft LVS (data not shown and [49]); therefore, subsequent experiments used PMA-treated THP-1. Although PMA-treated THP-1 cells infected with live Ft LVS produced abundant IL-1β [49], quantities from iFt-stimulated THP-1 cells were limited (Fig. 1C). However, iFt-mAb complexes significantly enhanced IL-1β elaboration 2–3-fold, with a similar impact on TNF-α production. IL-1β production following stimulation with mAb alone or with mAb-coated beads was similar to untreated controls (Fig. 1C and Supplemental Fig. 1); thus, both iFt and mAb are required for the enhanced response. For all cells tested, the Ab-enhanced IL-1β response to iFt was dose dependent (Fig. 1A-C), and at 10 μg/ml Abs, the IL-1β response approached that previously reported for cells infected with live Ft LVS [49, 55]. Treatment with iFt-mAb for 24 h did not significantly diminish cell viability as measured by lactate dehydrogenase release (data not shown), indicating that the IL-1β detected in culture supernatants does not reflect pro-IL-1β released from dead cells. Thus, opsonization of iFt with mAb enhances IL-1β elaboration by both mouse and human myeloid cells
Figure 1. The iFt-mAb complex activates cytokine production in macrophages and BMDCs.
BMDM (A) and BMDC (B) from WT mice or PMA-treated THP-1 (C) were left untreated (media control) or stimulated for 24 h with iFt, mAb, or iFt-mAb at 5 µg/ml or at increasing concentrations. IL-1β and TNF-α were analyzed by ELISA using cell-free supernatants in duplicate. BMDM and THP-1 stimulations are means and sem of 3 independent experiments. BMDC results are means and sd of 2 independent experiments. Student’s t test was used to compare iFt to iFt-mAb. *P < 0.05; **P < 0.01; ***P < 0.001.
iFt-mAb–induced IL-1β production is dependent on NLRP3-inflammasome components
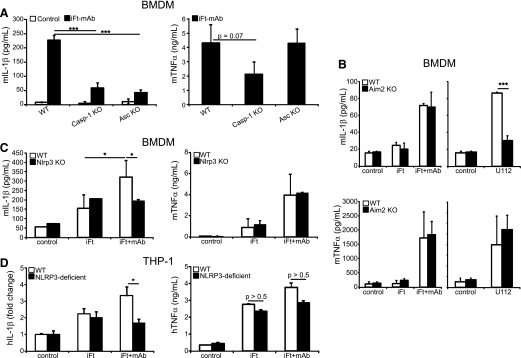
IL-1β maturation typically results from processing by activated caspase-1 [8], although some other proteases also enzymatically process IL-1β [56]. IL-1β production following iFt-mAb stimulation was dramatically diminished in caspase-1/11–deficient BMDM (Fig. 2A, left panel). Curiously, caspase-1–deficient BMDMs also produced less TNF-α than WT cells did after iFt-mAb stimulation (Fig. 2A, right panel), perhaps because of the NF-κB–activating properties of caspase-1 [57]. Nevertheless, caspase-1 or caspase-11 or both are important for the IL-1β response to iFt-mAb. As expected, the IL-1β response to iFt-mAb was diminished in ASC-deficient BMDMs, and TNF-α production was maintained (Fig. 2A). As expected, in iFt-mAb–stimulated, PMA-treated THP-1, caspase-1 activity was increased over iFt alone, as measured by p10 release, whereas mAbs alone did not appreciably activate caspase-1 (data not shown). Together, these results suggest involvement of an ASC-dependent inflammasome (e.g., NLRPs and/or AIM2) and demonstrate that diminished TNF-α production by iFt-mAb–stimulated Casp1/11 KO cells is not due to the lack of an ASC-dependent inflammasome.
Figure 2. The NLRP3 inflammasome is required for IL-1β production by iFt-mAb.
BMDM from WT, Casp-1 KO, or Asc KO (A), Aim2 KO (B), and Nlrp3 KO (C) mice were left untreated (media control) or stimulated with iFt or iFt-mAb or infected with Fn U112 (100 MOI) for 24 h before collection of cell-free culture supernatants for IL-1β and TNF-α ELISA. IL-1β and TNF-α analyzed by ELISA of THP-1– and NLRP3-deficient THP-1 24 h poststimulation with iFt or iFt-mAb. Control concentrations of IL-1β (pg/ml) for THP-1– and NLRP3-deficient THP-1 were 275.2 (sd 120.7) and 21.2 (sd 3.1), respectively (D). Results are means of 3 independent experiments with error bars indicating sem. *P < 0.05; ***P < 0.001.
In mice, recognition of cytoplasmic dsDNA by Aim2 leads to inflammasome-generated IL-1β and is essential for mouse survival following infection with Fn U112 [17, 18]. In contrast, Fn U112 and Ft LVS elicit both NLRP3- and AIM2-dependent inflammasome responses in human-derived macrophages [49]. However, all of these studies rely upon live bacteria, which must escape the phagosome to elicit IL-1β [23]. Greater than 80% of formalin-fixed Ft colocalize with CD63 (LAMP-1) in the late endosome and persist in this compartment for at least 16 h following removal of extracellular Ft [22]. Thus, it is unlikely that nonviable iFt or its products, such as the bacterial DNA, have significant access to the cytosol or Aim2. Indeed, IL-1β elaboration by Aim2-deficient (Aim2 KO mice) BMDM after iFt-mAb stimulation was comparable to that of WT BMDM (Fig. 2B, left panel). However, Aim2 is required for IL-1β production following Fn U112 infection (Fig. 2B, left panel), but Aim2 deficiency does not generally impair the inflammatory response of these cells because TNF-α was not reduced (Fig. 2B, right panel). Thus, in mouse cells, iFt-mAb likely triggers an IL-1β processing pathway distinct from the Aim2 inflammasome. In contrast to the dsDNA-restricted response of AIM2, the NLRP3 inflammasome is responsive to multiple microbial and environmental stimuli, including various endogenous agonists [9]. Nlrp3-deficient (Nlrp3 KO) BMDM treated with iFt-mAb failed to produce additional IL-1β above that seen with iFt alone (Fig. 2C, left panel) but remained responsive to live Fn U112 (data not shown and [49]), demonstrating that NLRP3 is required for iFt-mAb–mediated, enhanced IL-1β response. TNF-α elaboration was again unaffected (Fig. 2C, right panel), demonstrating an otherwise intact innate response. Consistently, NLRP3-deficient THP-1 responded with reduced IL-1β, but equivalent TNF-α (Fig. 2D), suggesting that mouse and human macrophage IL-1β responses to iFt-mAb are similarly dependent on NLRP3. Collectively, these data indicate a requirement for an Nlrp3, but not Aim2, inflammasome for iFt-mAb–elicited IL-1β production by macrophages.
IL-1β production by iFt-mAb is FcγR dependent
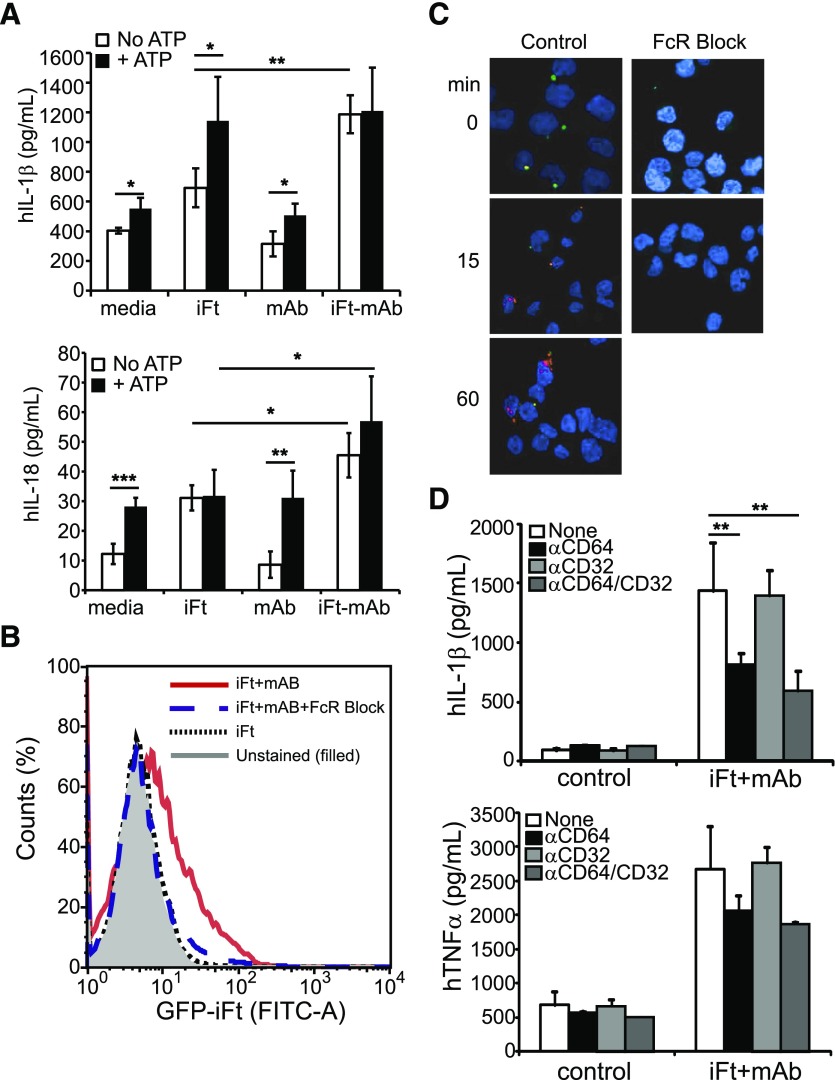
Maturation of IL-1β by activated macrophages requires proIL1B transcription to generate an available pool of pro-IL-1β for proteolytic processing. PMA treatment of THP-1 modestly increases pro-IL1B expression over non-PMA–treated control cells, but further stimulation is needed for robust expression of pro-IL-1β and subsequent maturation [49]. Stimulation of PMA-treated THP-1 cells with mAbs alone had no impact on pro-IL-1β mRNA levels; however, iFt elicited an approximately 8-fold increase (similar to that observed with live Ft LVS infection [49]), which is not enhanced further by iFt-mAbs (data not shown). Thus, the increased IL-1β protein secretion observed with iFt-mAb is most likely a consequence of proteolytic processing of the available pro-IL-1β and not a simple increase in available pro-IL-1β. Further, the NLRP3 inflammasome pathways appear fully activated by iFt-mAbs because the addition of the NLRP3 agonist ATP after stimulation of PMA–THP-1 for 24 h with iFt or iFt-mAbs elicited equivalent IL-1β (Fig. 3A, top panel). However, when cells were stimulated with mAbs followed by ATP, no significant increase over ATP alone was observed, indicating that mAb does not substitute for TLR-dependent signaling. Active caspase-1 also cleaves pro-IL-18, which, unlike IL-1β, is constitutively expressed; thus, IL-18 processing more directly indicates inflammasome activation. Macrophage IL-18 increases following iFt-mAb stimulation relative to iFt alone, confirming inflammasome activation by the immune complex (Fig. 3A, bottom panel). Addition of ATP following iFt stimulation did not increase IL-18 production over that of iFt alone, ATP alone, or mAb + ATP, suggesting that the full response requires both iFt and mAb. Addition of ATP did not significantly increase IL-18 after iFt-mAb stimulation, consistent with prior activation of the NLRP3 inflammasome. These results suggest that mAb acts at the level of inflammasome activation and is unlikely to drive the initial signals leading to pro-IL-1β expression.
Figure 3. FcγR mediates inflammasome activation to enhance IL-1β.
(A) THP-1 were stimulated with iFt, mAb, or iFt-mAb for 23.5 h followed by treatment for 30 min with ATP (5 mM) before collection of cell-free culture supernatants for IL-1β or IL-18 by multiplex immunoassay. (B) Binding of GFP-iFt-mAb to THP-1 with or without FcγR blocking Abs as indicated by flow cytometry counting of cells labeled with GFP-iFt-mAb. The 1-dimensional histogram is 1 of 3 independent experiments with similar results. The filled curve is unstained cells, the solid curve is GFP-iFt-mAb without blocking Abs, the dashed curved is GFP-iFt-mAb with blocking Abs, and the dotted curve is GFP-iFt without mAb and without blocking Abs. THP-1 stimulated with iFt-mAb with and without FcγR blocking Abs were stained with Abs against mAbs (C). Green fluorescence indicates extracellular iFt-mAb; red fluorescence indicates intracellular iFt-mAb. Images were obtained by confocal microscopy. (D) Images are representative of 3 independent experiments with similar results. IL-1β and TNF-α from cell-free supernatants of THP-1 in the presence or absence of anti-FcγR blocking Abs. Shown are means of 3 independent experiments with error bars indicating sem. *P < 0.05; **P < 0.01; ***P < 0.001.
It is noteworthy that iFt-mAb enhances in vitro maturation of murine BMDC by facilitating increased binding and uptake of iFt via the FcγR [34]. Given that the NLRP3 inflammasome is required (Fig. 2C and D), we considered whether binding and internalization of the iFt-mAb complex by FcγR was necessary to elicit the inflammasome response. Prior addition of a commercially available mixture of human serum–isolated FcγR-blocking Abs to THP-1 cells decreased binding resulting in a lack of internalization of iFt-mAb (Fig. 3B and C), confirming that iFt-mAb binds via an FcγR that facilitates phagocytic uptake in these cells. However, phagocytosed Ab complexes are degraded in the lysosome, and, beyond providing MHC II presented peptides to the endosomal compartment [34], are unlikely to facilitate iFt access to the cytosol, suggesting that FcγR-mediated signals might be involved. PMA-treated THP-1 cells express the kinase-activating receptors FcγRI (CD64) and FcγRII (CD32) but lack FcγRIII (CD16) [58]. To further confirm that FcγR contributes to inflammasome activation and to establish the involvement of CD64 and/or CD32 in iFt-mAb–elicited IL-1β production, specific blocking Abs [59, 60] were used. IL-1β production by iFt-mAb–stimulated THP-1 cells was significantly reduced in the presence of anti-CD64 alone and in combination with anti-CD32 (Fig. 3D, top panel), indicating involvement of FcγRI. In agreement with reports that FcγR signals enhance TNF-α and IL-6 secretion [33, 39], anti-CD64 treatment also diminished TNF-α production (Fig. 3D, bottom panel). Anti-CD32 (FcγRII) alone failed to reduce iFt-mAb–elicited IL-1β or TNF-α production, suggesting that FcγRII engagement is not required. However, anti-CD32 cannot distinguish between activating FcγRIIa/FcγRIIc and inhibitory FcγRIIb; thus, we cannot exclude the involvement of negative signals. Blockade of FcγRI/CD64, although not completely ablating production of IL-1β, did reduce IL-1β production to the approximate level seen with iFt alone (data not shown), consistent with an FcγRI-mediated signal promoting inflammasome activation.
TLR- and FcγR-dependent kinase signals are also required for iFt-mAb–mediated IL-1β production
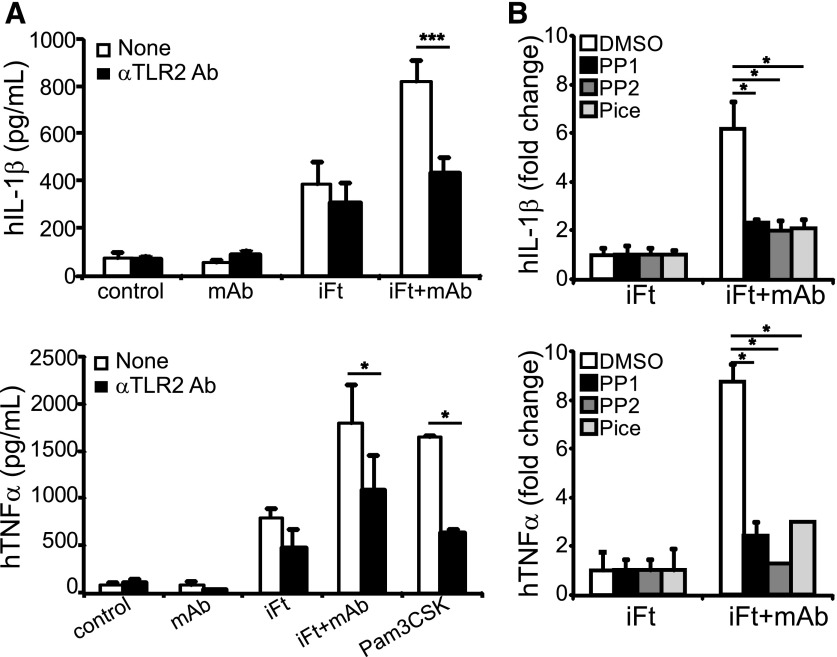
Production of active IL-1β requires expression of pro-IL-1β and inflammasome component proteins in an NF-κB–dependent step, typically provided by TLR signaling [7]. Consistent with recognition of live Ft by TLR2 [14–16], pretreatment of THP-1 cells with TLR2 blocking Abs significantly reduced iFt-mAb–stimulated IL-1β production to levels approximating those seen with iFt alone (Fig. 4A, top panel). TLR2 blockade also reduced TNF-α production elicited by iFt, iFt-mAb, or the TLR2 agonist PAM3CSK (Fig. 4A, bottom panel). Antibody blockade had a minimal impact on iFt-elicited IL-1β production; however, the anti-TLR2 Ab also failed to completely block activation with PAM3CSK, suggesting that the incomplete blockade of TLR2 by the Ab is more likely than activation of other receptors by iFt. Stimulation with the mAb alone resulted in no increase in either IL-1β or TNF-α secretion (Fig. 4A), consistent with the TLR2 dependence of these responses. Thus, TLR2 signaling is necessary for iFt-mAb–enhanced IL-1β production.
Figure 4. TLR2 and Src/Syk signaling are important for cytokine production with iFt-mAb.
IL-1β and TNF-α from cell-free supernatants of THP-1 stimulated for 24 h in the presence or absence of anti-TLR2 blocking Ab (A), with or without 30 min pretreatment with Syk (Pice, piceattenol, 5 µM) and Src inhibitors (PP1 and PP2, 10 μM) (B). Shown are means of 3 independent experiments with error bars indicating sem, except TNF-α in (B), which is the means of 2 independent experiments with triplicate samples and error bars indicating sd. *P < 0.05.
FcγR engagement also elicits a tyrosine kinase-dependent signal cascade [28, 61], which might drive or enhance inflammasome activation. Indeed, Syk, a kinase activated by FcγR engagement, has been implicated in the activation of Nlrp3-dependent IL-1β maturation, although this role is controversial [2, 62–65]. However, even exposure to mAb-coated beads expected to cross-link FcγR and activate Syk was insufficient to elicit an IL-1β response without the addition of iFt (Supplemental Fig. 1). Thus, to examine whether signals downstream of FcγR elicit IL-1β maturation in conjunction with TLR2, THP-1 cells were incubated with kinase inhibitors targeting Syk (piceatannol) and Src (PP1 and PP2) before iFt-mAb stimulation. All the inhibitors significantly decreased mAb-enhanced IL-1β and TNF-α, returning them to iFt-alone stimulated levels (Fig. 4B). Thus, the increase in IL-1β and TNF-α resulting from immune complex stimulation requires both Syk and Src kinase activity. Collectively, the production of IL-1β, subsequent to iFt-mAb stimulation requires TLR2 signaling, is FcγR dependent and requires activation of the FcγR-associated Src and Syk kinases.
Caspase-8 activity is necessary but insufficient for FcγR-mediated IL-1β
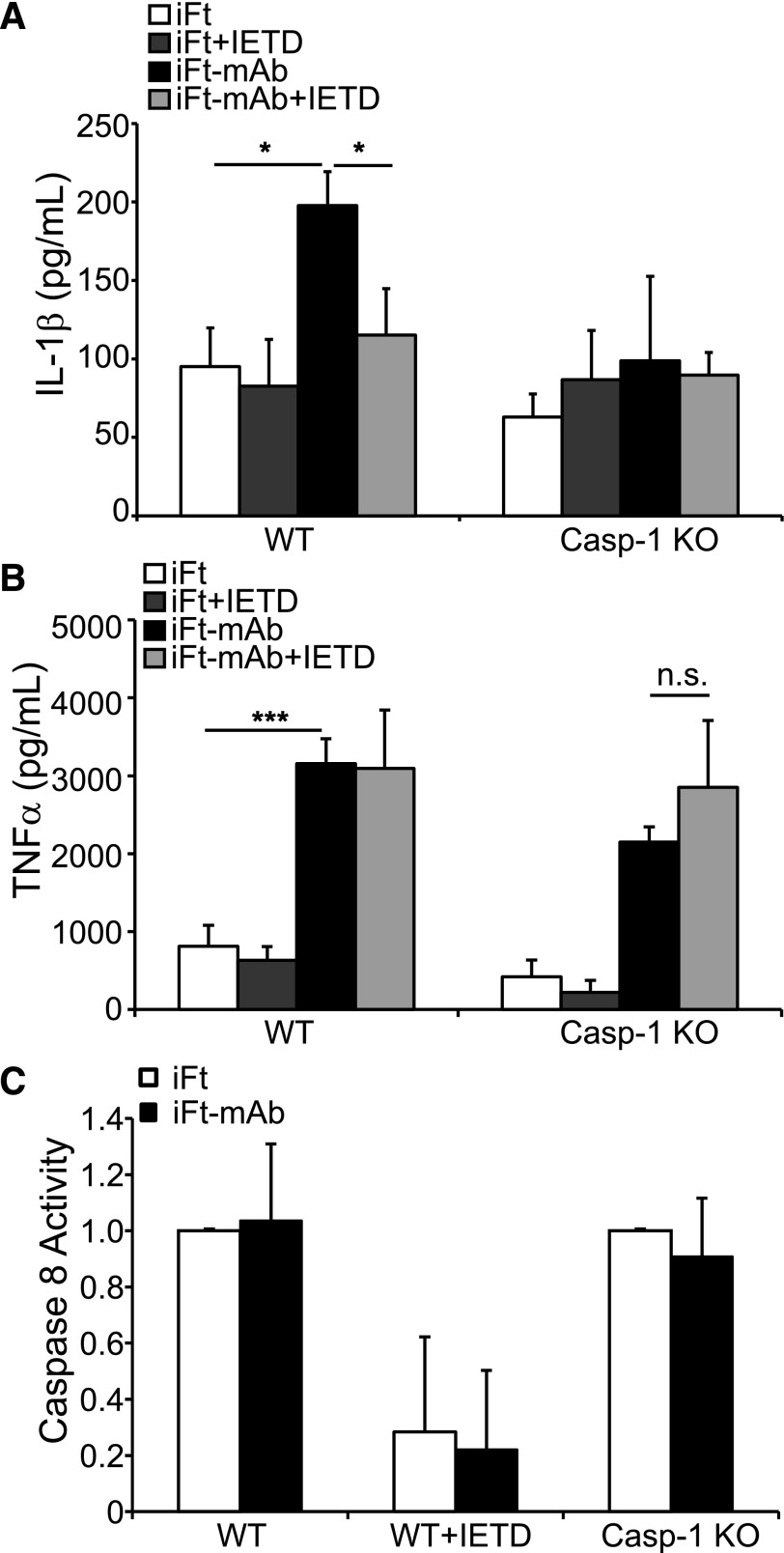
Our data strongly support a role for Syk in iFt-mAb–elicited IL-1β production. Of note, internalization-independent fungal recognition by dectin-1 activates Syk leading to a caspase-8–dependent IL-1β-processing complex comprising Malt1, Bcl10, Card9, and caspase-8 [11]. This Syk-dependent fungal response is independent of caspase-1. We, therefore, considered the intriguing possibility that iFt-mAb–elicited IL-1β might also result from Syk-dependent caspase-8 activation. As caspase-8 deficiency results in embryonic lethality, we used the caspase-8 inhibitor Z-IETD-FMK to address this question. Treatment of WT BMDM with Z-IETD-FMK markedly reduced IL-1β after 24 h of iFt-mAb stimulation (Fig. 5A), suggesting the involvement of caspase-8. Because caspase-8 can influence caspase-1 activity [12] and possibly affect IL-1β production, we examined the effect of caspase-8 inhibition using BMDM deficient in caspase-1. IL-1β production was not increased in Casp-1 KO BMDMs following iFt-mAb stimulation (Fig. 5A), and caspase-8 inhibitor had no further effect on IL-1β production by Casp-1 KO cells. TNF-α production was not significantly impaired by the caspase-8 inhibitor, indicating that deficient IL-1β production is unlikely to result from nonspecific effects of the inhibitor (Fig. 5B). These results suggested that caspase-8 might be responsible for IL-1β processing in response to iFt-mAb, but it is dependent on caspase-1. To establish whether caspase-8 activity is dependent on caspase-1, we measured caspase-8 activity after iFt or iFt-mAb stimulation. It is interesting that caspase-8 was active in both iFt- and iFt-mAb–stimulated WT BMDM, and addition of caspase-8 inhibitor nearly abrogated this activity (Fig. 5C). Because caspase-8 is active in the absence of FcγR engagement, this suggests that Syk activation is not required for caspase-8 activity in iFt-stimulated BMDMs and, thus, that caspase-8 does not correlate with the iFt-mAb enhancement of IL-1β production. Further, in Casp-1 KO cells, caspase-8 activity was comparable to that of WT BMDM (Fig. 5C). Therefore, because caspase-8 activity is maintained under conditions in which iFt-mAb–elicited IL-1β maturation is lost, caspase-8 activation is not sufficient for IL-1β processing. Instead, IL-1β processing appears to be dependent on both caspase-1 and caspase-8, whereas caspase-8 activation is independent of caspase-1. This suggests a more-complex relationship between these caspases than described previously. However, if caspase-8 cleavage of pro-IL-1β was the principal mechanism, as in the dectin-1 system, sustained caspase-8 activity in iFt-mAb–stimulated Casp-1 KO BMDM should have resulted in full production of IL-1β. The insufficiency of caspase-8 and the dependence on caspase-1, together with the need for NLRP3, supports the conclusion that IL-1β cleavage in this system relies on the canonical NLPR3 inflammasome.
Figure 5. Caspase-8 activity is necessary but insufficient for FcγR-mediated IL-1β.
BMDM from WT or Casp-1 KO mice were stimulated for 24 h with iFt or iFt-mAb, with or without pretreatment with a caspase-8 inhibitor (IETD), and IL-1β (A) and TNF-α (B) were analyzed by ELISA in cell-free supernatants. Caspase-8 activity in BMDM from WT or Casp-1 KO mice assayed using a luminescence detection system (C). Data shown reflect the means and sd of triplicate samples and are representative of 3 independent experiments with similar results. *P < 0.05; n.s., not significant.
IL-1 and NLRP3 have a role in iFt-mAb vaccination response
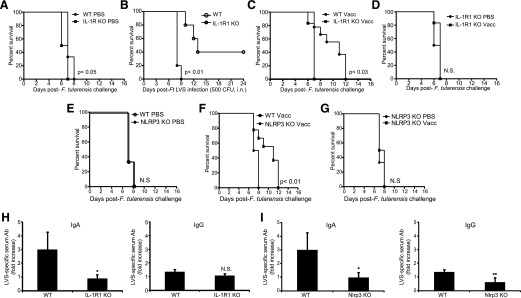
Inflammasome and IL-1 responses are thought important for adaptive Ab responses [26]. Further, mice vaccinated with iFt-mAbs exhibit resistance to Ft LVS challenge, a protection that requires FcRγ and the generation of protective IgA Abs [27, 66]. Because we observed, an FcγR signal-driven, NLRP3 inflammasome-dependent macrophage IL-1β response to iFt-mAb, we considered that IL-1β and NLRP3 might be important for the protective Ab response elicited by the iFt-mAb vaccine. Following the published iFt-mAb vaccine strategy [27], we challenged either unvaccinated or iFt-mAb vaccinated WT, IL-1R1–, or Nlrp3-deficient mice i.n. with 20,000 CFU of Ft LVS. All mice exhibited similar weight loss throughout the experiments, indicating successful infection (data not shown). Unvaccinated IL-1R1 KO mice died approximately 1 d earlier than unvaccinated WT mice (Fig. 6A), suggesting that mice lacking IL-1R1 may be more susceptible to Ft LVS infection. Indeed, without vaccination, no IL-1R1 KO mice survived past d 9 after sublethal challenge with Ft LVS (Fig. 6B), demonstrating that IL-1R1, and by extension IL-1, contributes to resistance in naïve mice. Moreover, vaccinated IL-1R1 KO mice exhibited 100% mortality by d 7, whereas vaccinated WT mice showed a significant increase in mean time to death upon challenge (Fig. 6C). Unvaccinated and vaccinated IL-1R KO mice were equally susceptible to Ft LVS (Fig. 6D). Unvaccinated Nlrp3 KO and WT mice all died by 8 d postchallenge, suggesting similar susceptibility to the challenge dose (Fig. 6E). However, as with IL-1R1 KO mice, the mean time to death of vaccinated Nlrp3 KO mice was significantly shorter than it was for vaccinated WT mice (Fig. 6F) and was comparable to unvaccinated Nlrp3 KO mice (Fig. 6G). These results suggest that both IL-1R and Nlrp3 are necessary for responsiveness to the iFt-mAb vaccine.
Figure 6. IL-1 and NLRP3 are important for survival against respiratory Ft-LVS in mice.
Groups of 10 WT, IL-1r1 KO, or Nlrp3 KO mice were immunized i.n. with either 20 μl of vehicle (PBS) or 20 μl of 2 × 107 inactivated organisms complexed with 1 μg/ml mAb (iFt-mAb) in PBS (Vacc). Mice were immunized on d 0 and d 21 and then challenged i.n. on d 35 with 20,000 CFU live Ft-LVS and monitored for survival (A, C, D, E, F, and G). Survival curves in all 6 of these panels are from the same groups of mice and are separated for ease of comparison. Results from 1 of 2 vaccination studies with similar results are shown. Groups of 6 WT or IL-1R1 KO mice were infected i.n. with 500 CFU Ft LVS and observed for survival (B). Serum from WT and IL-1r1 mice was collected before challenge, and relative Ft LVS-specific Ab levels for IgA and IgG were measured by ELISA (H). Serum from WT and Nlrp3 mice was collected before challenge, and relative Ft LVS-specific Ab levels for IgA and IgG were measured by ELISA (I). The bars represent the average of Ft LVS-specific Ab detected in serum of iFt-mAb treated mice normalized to the corresponding average unvaccinated serum Ab level. Survival curves were compared using the log-rank test (Mantel-Cox). *P < 0.05; N.S., not significant.
It has been shown previously that iFt-mAb vaccination results in FcγR-dependent serum Ft LVS-specific IgA and IgG responses and that the IgA response is protective [27]. Mice lacking IL-1R1 mount a comparable Ft LVS-specific IgG response but fail to mount the important IgA response (Fig. 6H). However, similar to mice lacking FcγR [27], iFt-mAb–vaccinated Nlrp3 KO mice fail to mount Ft LVS-specific IgA and IgG Ab responses (Fig. 6I). Thus, both IL-1R1 and Nlrp3 are required for the IgA response known to be required for protection against Ft LVS after vaccination with iFt-mAb, suggesting that the FcγR signal-dependent activation of the Nlrp3 inflammasome observed in macrophages may reflect a similar in vivo mechanism important for FcγR-targeted vaccination.
DISCUSSION
Inflammasome-dependent elaboration of IL-1β during infection or vaccination is thought to be important, in part, for the development of protective Ab responses. Protection against pneumonic tularemia afforded by an opsonized-iFt vaccine in mice requires FcγR and concomitant production of pathogen-specific IgA [27], suggesting that engagement of FcγR might activate a necessary IL-1β response. Indeed, iFt-mAb–elicited IL-1β from macrophages and DCs was substantially increased over iFt alone implicating a role for FcγR, leading to subsequent enzymatic processing of IL-1β. In macrophages, iFt-mAb–elicited IL-1β relies on TLR2 and FcγR, and Src and Syk kinase activities downstream of FcγR engagement. Intriguingly, the alternative caspase-8 pathway of IL-1β cleavage, previously linked to Syk activity, appears to be necessary but insufficient, whereas caspase-1/11 and ASC are required. Surprisingly, the IL-1β response to iFt-mAb appears independent of the well-established Aim2 axis that is important for the response to live Ft LVS and Fn U112, requiring NLRP3 instead. Together, our data support the conclusion that FcγR kinase signals represent a pathway to NLRP3 inflammasome activation. Finally, this FcγR–Nlrp3–inflammasome–IL-1 axis appears critical for the enhanced survival response afforded by iFt-mAb vaccination.
The enhanced IL-1β, TNF-α, and IL-6 cytokine response of macrophages and DCs to iFt-mAb requires signals derived from engagement of both TLR2 and activating FcγR ([27, 33] and this report). Murine IgG2a binds strongly to the activating human FcγRI receptor (CD64) [67], consistent with the ability of anti-CD64 to inhibit enhanced IL-1β production. Other IgG subclasses have different affinities for the various FcγRs and, thus, may be less effective in activation of the requisite kinases [68]. Both cooperative and inhibitory crosstalk between TLR and FcγR signals has been proposed [33, 38, 39]. Here, TLR2 stimulation with iFt or FcγR engagement by the mAb alone was not sufficient to provoke the full IL-1β response, the latter likely because of limited pro-IL-1β expression. FcγRI and TLR2 cooperation via NF-κB and MAPK signaling likely account for increased TNF-α production by iFt-mAb–stimulated macrophages, DCs (Fig. 1), and IL-6 [33]. However, whether these pathways contribute to inflammasome activation in conjunction with Syk is unclear.
For some proinflammatory cytokines, such as IL-6 and TNF-α, no posttranslational processing is required for activity, but for IL-1β, activity is dependent on enzymatic cleavage of its proform by caspase-1. FcγR and TLR2 stimulation by iFt-mAb activates caspase-1 via the NLRP3 inflammasome. Although precisely how convergent FcγR/TLR2 signals drive NLRP3 inflammasome activation remains unknown, Syk activity is clearly involved. Interestingly, although in our study iFt-mAb activated the NLRP3 inflammasome, Janczy et al. [42] reported that IgG-complexed erythrocytes inhibited NLRP3, AIM2, and NLRC4 inflammasomes. In their study, instead of stimulating with an opsonized Ag, BMDMs were treated with LPS to induce pro-IL-1β expression, immune complexes were added, and were then exposed to inflammasome agonists. Although it remains unclear how signal order might influence inflammasome activation, activation or inhibition of Syk kinase, depending on whether activating or inhibitory FcγRs are engaged, might account for this difference.
Although the role of TLR signals in driving pro-IL-1β expression is well appreciated [69], how FcγR signals and Syk contribute to NLRP3 inflammasome activation is less clear. NLRP3 inflammasome activation in response to select pathogens, including mycobacteria, fungi, and protozoa, has been reported to involve Syk [2, 62, 64, 65]. However, whether Syk is absolutely required to activate NLRP3 and under which circumstances is still unresolved. For instance, in 1 study, nigericin-induced caspase-1 activation and IL-1β production were reduced in the absence of Syk [70]. However, in 2 other reports, Syk was not required for nigericin to act as an NLRP3-activating signal [2, 71] but was suggested, instead, to be necessary for up-regulation of the pro-IL-1β message [71]. Moreover, although Syk binds to NLRP3 and ASC in THP-1 cells stimulated with hemozoin (another NLRP3 agonist) [65], consistent with a potential direct activation of this inflammasome, Syk can also indirectly lead to IL-1β cleavage through dectin-1 activation of caspase-8, an NLRP3- and caspase-1–independent process [11]. In our system, it is unclear whether NLRP3 inflammasome activation via Syk is direct or indirect. Syk may directly activate the inflammasome by phosphorylating 1 or more of the components. Notably, Syk phosphorylation of ASC is required for subsequent caspase-1 activation; however, because ASC associates with NLRP3 in the absence of ASC phosphorylation, phosphorylation of ASC does not appear to be a critical inflammasome-activating signal [70]. Indeed, an initial experiment to examine the impact of Syk activity using NLRP3 inflammasome reconstitution showed constitutively active, Syk-enhanced IL-1β production (Supplemental Fig. 2). Although the precise mechanism remains unclear, Syk can lead to NLRP3 inflammasome activation. NLRP3 could also be a direct target of Syk phosphorylation. NLRC4 requires phosphorylation for activation, however, not by Syk [72]. Alternatively, Syk may provide an indirect signal through induction of reactive oxygen species or calcium flux (both of which have been implicated in activation of the NLRP3 inflammasome) [73, 74]. The Syk–NLRP3 connection appears quite complex and needs more in-depth studies designed to better parse the targets and requirement for Syk.
Syk may also lead to caspase-1/inflammasome-independent IL-1β processing through dectin-1–mediated activation of caspase-8 [11]. In this study, however, although caspase-8 appears necessary, its activity was not sufficient for IL-1β processing after iFt-mAb stimulation. One explanation is that caspase-8 is essential for capsase-1 activation, but not involved directly with IL-1β processing [12]. This seeming caspase-8/caspase-1 codependent elaboration of IL-1β deserves further consideration as a potentially distinct type of NLRP3 inflammasome response. We also did not observe caspase-8–mediated inhibition of the NLRP3 inflammasome as reported by Kang et al. [75] because IL-1β was not increased after caspase-8 inhibition.
Although IL-1R and Nlrp3 are clearly important for the Ab response to iFt-mAb vaccination and the delayed time to death observed with WT mice, we did not observe the previously demonstrated complete protection against lethal challenge with Ft LVS. The initial iFt-mAb study [27] used a challenge dose of 2 × 104 CFU Ft LVS, and this dose served as the basis for our study. However, our challenge inoculation methods were based on the work of Miller et al. [76], and we have found that the LD100 of the Ft LVS challenge inoculum used may be closer to 1000 CFU, rather than 1 × 104 CFU and, thus, represents a challenge dose of 20 LD100. Even at this dose, iFt-mAb–vaccinated WT mice display a prolonged survival time that correlates with the production of Ft LVS-specific Abs. Vaccinated mice challenged with a lower dose (4 × 103 CFU; 4 LD100) yielded comparable results (data not shown). In addition, Miller et al. [76] demonstrated that greater inoculum volumes are needed to ensure Ft infection of the lung. Thus, our inoculum volumes are greater than those typically used before the Miller et al. [76] study. This dissimilarity with the earlier study may mean that this modification to the inoculation method or greater infectious dose affects the ability to survive challenge postvaccination, despite similar Ab response. Nevertheless, in contrast to WT mice, neither IL-1R1 KO mice nor Nlrp3 KO mice displayed any degree of prolonged survival at either challenge dose following vaccination.
Although FcγR-, TLR-, and NLRP3-mediated cytokine production can be beneficial in combating infections or stimulating protective immunity after vaccination, these same signaling pathways may also lead to undesirable consequences in autoinflammatory diseases, such as RA or SLE, in which immune complexes are known to be present. Indeed, FcγR crosstalk with TLRs may be a central mechanism for driving proinflammatory cytokine production [77]. Further, Syk inhibition has been suggested as a therapeutic for RA and SLE, but this approach appears problematic [78]. A better understanding of how Syk mediates NLRP3 inflammasome activation would provide additional insight that may prove useful in developing therapies targeting inflammation associated with immune complex–related autoimmune diseases.
AUTHORSHIP
E.B.D. designed and performed experiments, analyzed data, generated figures, and wrote the manuscript. S.P. and D.H designed and performed experiments and analyzed data. J.R.D. helped with data interpretation, discussed the hypotheses, and participated in manuscript preparation. J.A.H. supervised the project, assisted with experimental design, helped with data interpretation, participated in data analysis, and wrote the manuscript.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health National Institute of Allergy and Infectious Diseases Grant P01AI056320. The authors thank Dr. William O’Connor, Jr., for critical review of this manuscript, Dr. Timothy Sellati for generously providing caspase-1/11- and ASC-deficient mice, and Dr. Jenny Ting for generously providing Nlrp3-deficient mice.
Glossary
- AIM2
absent in melanoma 2
- ASC
apoptosis-associated speck-forming complex containing a CARD
- BMDC
bone marrow-derived dendritic cell
- BMDM
bone marrow-derived macrophage
- DC
dendritic cell
- FcγR
Fcγ receptor
- Fn U112
Francisella novicida U112
- Ft LVS
Francisella tularensis LVS
- iFt
inactivated Francisella tularensis LVS
- iFt-mAb
inactivated Francisella tularensis monoclonal antibody complex
- i.n.
intranasal
- KO
knockout
- MOI
multiplicity of infection
- NLRP3
NACHT, LRR, and PYD domains-containing protein 3
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- Syk
spleen tyrosine kinase
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Allen I. C., Scull M. A., Moore C. B., Holl E. K., McElvania-TeKippe E., Taxman D. J., Guthrie E. H., Pickles R. J., Ting J. P. (2009) The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30, 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross O., Poeck H., Bscheider M., Dostert C., Hannesschläger N., Endres S., Hartmann G., Tardivel A., Schweighoffer E., Tybulewicz V., Mocsai A., Tschopp J., Ruland J. (2009) Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459, 433–436. [DOI] [PubMed] [Google Scholar]
- 3.He X., Mekasha S., Mavrogiorgos N., Fitzgerald K. A., Lien E., Ingalls R. R. (2010) Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J. Immunol. 184, 5743–5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitz N., Kurrer M., Bachmann M. F., Kopf M. (2005) Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 79, 6441–6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenbarth S. C., Colegio O. R., O’Connor W., Sutterwala F. S., Flavell R. A. (2008) Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wüthrich M., LeBert V., Galles K., Hu-Li J., Ben-Sasson S. Z., Paul W. E., Klein B. S. (2013) Interleukin 1 enhances vaccine-induced antifungal T-helper 17 cells and resistance against Blastomyces dermatitidis infection. J. Infect. Dis. 208, 1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B. G., Fitzgerald K. A., Hornung V., Latz E. (2009) Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinon F., Burns K., Tschopp J. (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426. [DOI] [PubMed] [Google Scholar]
- 9.Lamkanfi M., Kanneganti T. D. (2010) Nlrp3: an immune sensor of cellular stress and infection. Int. J. Biochem. Cell Biol. 42, 792–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Zoete M. R., Palm N. W., Zhu S., Flavell R. A. (2014) Inflammasomes. Cold Spring Harb. Perspect. Biol. 6, a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gringhuis S. I., Kaptein T. M., Wevers B. A., Theelen B., van der Vlist M., Boekhout T., Geijtenbeek T. B. (2012) Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat. Immunol. 13, 246–254. [DOI] [PubMed] [Google Scholar]
- 12.Gurung P., Anand P. K., Malireddi R. K., Vande Walle L., Van Opdenbosch N., Dillon C. P., Weinlich R., Green D. R., Lamkanfi M., Kanneganti T. D. (2014) FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol. 192, 1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanneganti T. D., Lamkanfi M., Kim Y. G., Chen G., Park J. H., Franchi L., Vandenabeele P., Núñez G. (2007) Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26, 433–443. [DOI] [PubMed] [Google Scholar]
- 14.Malik M., Bakshi C. S., Sahay B., Shah A., Lotz S. A., Sellati T. J. (2006) Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect. Immun. 74, 3657–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz J., Zhang P., Martin M., Vogel S. N., Michalek S. M. (2006) Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect. Immun. 74, 2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H., Nookala S., Bina X. R., Bina J. E., Re F. (2006) Innate immune response to Francisella tularensis is mediated by TLR2 and caspase-1 activation. J. Leukoc. Biol. 80, 766–773. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes-Alnemri T., Yu J. W., Juliana C., Solorzano L., Kang S., Wu J., Datta P., McCormick M., Huang L., McDermott E., Eisenlohr L., Landel C. P., Alnemri E. S. (2010) The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones J. W., Kayagaki N., Broz P., Henry T., Newton K., O’Rourke K., Chan S., Dong J., Qu Y., Roose-Girma M., Dixit V. M., Monack D. M. (2010) Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. U. S. A. 107, 9771–9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rathinam V. A., Jiang Z., Waggoner S. N., Sharma S., Cole L. E., Waggoner L., Vanaja S. K., Monks B. G., Ganesan S., Latz E., Hornung V., Vogel S. N., Szomolanyi-Tsuda E., Fitzgerald K. A. (2010) The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melillo A. A., Bakshi C. S., Melendez J. A. (2010) Francisella tularensis antioxidants harness reactive oxygen species to restrict macrophage signaling and cytokine production. J. Biol. Chem. 285, 27553–27560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng K., Broz P., Jones J., Joubert L. M., Monack D. (2011) Elevated AIM2-mediated pyroptosis triggered by hypercytotoxic Francisella mutant strains is attributed to increased intracellular bacteriolysis. Cell. Microbiol. 13, 1586–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemens D. L., Lee B. Y., Horwitz M. A. (2004) Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect. Immun. 72, 3204–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole L. E., Santiago A., Barry E., Kang T. J., Shirey K. A., Roberts Z. J., Elkins K. L., Cross A. S., Vogel S. N. (2008) Macrophage proinflammatory response to Francisella tularensis live vaccine strain requires coordination of multiple signaling pathways. J. Immunol. 180, 6885–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirimanjeswara G. S., Olmos S., Bakshi C. S., Metzger D. W. (2008) Humoral and cell-mediated immunity to the intracellular pathogen Francisella tularensis. Immunol. Rev. 225, 244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conlan J. W. (2011) Tularemia vaccines: recent developments and remaining hurdles. Future Microbiol. 6, 391–405. [DOI] [PubMed] [Google Scholar]
- 26.Mills K. H., Dungan L. S., Jones S. A., Harris J. (2013) The role of inflammasome-derived IL-1 in driving IL-17 responses. J. Leukoc. Biol. 93, 489–497. [DOI] [PubMed] [Google Scholar]
- 27.Rawool D. B., Bitsaktsis C., Li Y., Gosselin D. R., Lin Y., Kurkure N. V., Metzger D. W., Gosselin E. J. (2008) Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J. Immunol. 180, 5548–5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nimmerjahn F., Ravetch J. V. (2008) Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8, 34–47. [DOI] [PubMed] [Google Scholar]
- 29.Majeed M., Caveggion E., Lowell C. A., Berton G. (2001) Role of Src kinases and Syk in Fcγ receptor-mediated phagocytosis and phagosome-lysosome fusion. J. Leukoc. Biol. 70, 801–811. [PubMed] [Google Scholar]
- 30.Bitsaktsis C., Iglesias B. V., Li Y., Colino J., Snapper C. M., Hollingshead S. K., Pham G., Gosselin D. R., Gosselin E. J. (2012) Mucosal immunization with an unadjuvanted vaccine that targets Streptococcus pneumoniae PspA to human Fcγ receptor type I protects against pneumococcal infection through complement- and lactoferrin-mediated bactericidal activity. Infect. Immun. 80, 1166–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gosselin E. J., Bitsaktsis C., Li Y., Iglesias B. V. (2009) Fc receptor-targeted mucosal vaccination as a novel strategy for the generation of enhanced immunity against mucosal and non-mucosal pathogens. Arch. Immunol. Ther. Exp. (Warsz.) 57, 311–323. [DOI] [PubMed] [Google Scholar]
- 32.Heijnen I. A., van Vugt M. J., Fanger N. A., Graziano R. F., de Wit T. P., Hofhuis F. M., Guyre P. M., Capel P. J., Verbeek J. S., van de Winkel J. G. (1996) Antigen targeting to myeloid-specific human Fc gamma RI/CD64 triggers enhanced antibody responses in transgenic mice. J. Clin. Invest. 97, 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franchini A. M., Hunt D., Melendez J. A., Drake J. R. (2013) FcγR-driven release of IL-6 by macrophages requires NOX2-dependent production of reactive oxygen species. J. Biol. Chem. 288, 25098–25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iglesias B. V., Bitsaktsis C., Pham G., Drake J. R., Hazlett K. R., Porter K., Gosselin E. J. (2013) Multiple mechanisms mediate enhanced immunity generated by mAb-inactivated F. tularensis immunogen. Immunol. Cell Biol. 91, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pham G. H., Iglesias B. V., Gosselin E. J. (2014) Fc receptor-targeting of immunogen as a strategy for enhanced antigen loading, vaccination, and protection using intranasally administered antigen-pulsed dendritic cells. Vaccine 32, 5212–5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bitsaktsis C., Babadjanova Z., Gosselin E. J. (2015) In vivo mechanisms involved in enhanced protection utilizing an Fc receptor-targeted mucosal vaccine platform in a bacterial vaccine and challenge model. Infect. Immun. 83, 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babadjanova Z., Wiedinger K., Gosselin E. J., Bitsaktsis C. (2015) Targeting of a fixed bacterial immunogen to Fc receptors reverses the anti-inflammatory properties of the Gram-negative bacterium, Francisella tularensis, during the early stages of infection. PLoS One 10, e0129981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivashkiv L. B. (2008) A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat. Rev. Immunol. 8, 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.den Dunnen J., Vogelpoel L. T., Wypych T., Muller F. J., de Boer L., Kuijpers T. W., Zaat S. A., Kapsenberg M. L., de Jong E. C. (2012) IgG opsonization of bacteria promotes Th17 responses via synergy between TLRs and FcγRIIa in human dendritic cells. Blood 120, 112–121. [DOI] [PubMed] [Google Scholar]
- 40.Bakema J. E., Tuk C. W., van Vliet S. J., Bruijns S. C., Vos J. B., Letsiou S., Dijkstra C. D., van Kooyk Y., Brenkman A. B., van Egmond M. (2015) Antibody-opsonized bacteria evoke an inflammatory dendritic cell phenotype and polyfunctional Th cells by cross-talk between TLRs and FcRs. J. Immunol. 194, 1856–1866. [DOI] [PubMed] [Google Scholar]
- 41.Vogelpoel L. T., Hansen I. S., Visser M. W., Nagelkerke S. Q., Kuijpers T. W., Kapsenberg M. L., de Jong E. C., den Dunnen J. (2015) FcγRIIa cross-talk with TLRs, IL-1R, and IFNγR selectively modulates cytokine production in human myeloid cells. Immunobiology 220, 193–199. [DOI] [PubMed] [Google Scholar]
- 42.Janczy J. R., Ciraci C., Haasken S., Iwakura Y., Olivier A. K., Cassel S. L., Sutterwala F. S. (2014) Immune complexes inhibit IL-1 secretion and inflammasome activation. J. Immunol. 193, 5190–5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen M., Wang H., Chen W., Meng G. (2011) Regulation of adaptive immunity by the NLRP3 inflammasome. Int. Immunopharmacol. 11, 549–554. [DOI] [PubMed] [Google Scholar]
- 44.Ichinohe T., Lee H. K., Ogura Y., Flavell R., Iwasaki A. (2009) Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X., Goncalves R., Mosser D. M. (2008) The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 83, 14.1.1–14.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuida K., Lippke J. A., Ku G., Harding M. W., Livingston D. J., Su M. S., Flavell R. A. (1995) Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267, 2000–2003. [DOI] [PubMed] [Google Scholar]
- 47.Ozören N., Masumoto J., Franchi L., Kanneganti T. D., Body-Malapel M., Ertürk I., Jagirdar R., Zhu L., Inohara N., Bertin J., Coyle A., Grant E. P., Núñez G. (2006) Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J. Immunol. 176, 4337–4342. [DOI] [PubMed] [Google Scholar]
- 48.Mariathasan S., Weiss D. S., Newton K., McBride J., O’Rourke K., Roose-Girma M., Lee W. P., Weinrauch Y., Monack D. M., Dixit V. M. (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232. [DOI] [PubMed] [Google Scholar]
- 49.Atianand M. K., Duffy E. B., Shah A., Kar S., Malik M., Harton J. A. (2011) Francisella tularensis reveals a disparity between human and mouse NLRP3 inflammasome activation. J. Biol. Chem. 286, 39033–39042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben-Sasson S. Z., Hogg A., Hu-Li J., Wingfield P., Chen X., Crank M., Caucheteux S., Ratner-Hurevich M., Berzofsky J. A., Nir-Paz R., Paul W. E. (2013) IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J. Exp. Med. 210, 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ben-Sasson S. Z., Hu-Li J., Quiel J., Cauchetaux S., Ratner M., Shapira I., Dinarello C. A., Paul W. E. (2009) IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc. Natl. Acad. Sci. U. S. A. 106, 7119–7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gavrilin M. A., Bouakl I. J., Knatz N. L., Duncan M. D., Hall M. W., Gunn J. S., Wewers M. D. (2006) Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc. Natl. Acad. Sci. U. S. A. 103, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cogswell J. P., Godlevski M. M., Wisely G. B., Clay W. C., Leesnitzer L. M., Ways J. P., Gray J. G. (1994) NF-kappa B regulates IL-1 beta transcription through a consensus NF-κB binding site and a nonconsensus CRE-like site. J. Immunol. 153, 712–723. [PubMed] [Google Scholar]
- 54.Gavrilin M. A., Wewers M. D. (2011) Francisella recognition by inflammasomes: differences between mice and men. Front. Microbiol. 2, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolger C. E., Forestal C. A., Italo J. K., Benach J. L., Furie M. B. (2005) The live vaccine strain of Francisella tularensis replicates in human and murine macrophages but induces only the human cells to secrete proinflammatory cytokines. J. Leukoc. Biol. 77, 893–897. [DOI] [PubMed] [Google Scholar]
- 56.Netea M. G., Simon A., van de Veerdonk F., Kullberg B. J., Van der Meer J. W., Joosten L. A. (2010) IL-1β processing in host defense: beyond the inflammasomes. PLoS Pathog. 6, e1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong G. S., Jung Y. K. (2002) Caspase recruitment domain (CARD) as a bi-functional switch of caspase regulation and NF-κB signals. J. Biochem. Mol. Biol. 35, 19–23. [DOI] [PubMed] [Google Scholar]
- 58.Fleit H. B., Kobasiuk C. D. (1991) The human monocyte-like cell line THP-1 expresses FcγRI and FcγRII. J. Leukoc. Biol. 49, 556–565. [DOI] [PubMed] [Google Scholar]
- 59.Dougherty G. J., Selvendran Y., Murdoch S., Palmer D. G., Hogg N. (1987) The human mononuclear phagocyte high-affinity Fc receptor, FcRI, defined by a monoclonal antibody, 10.1. Eur. J. Immunol. 17, 1453–1459. [DOI] [PubMed] [Google Scholar]
- 60.Greenman J., Tutt A. L., George A. J., Pulford K. A., Stevenson G. T., Glennie M. J. (1991) Characterization of a new monoclonal anti-Fcγ RII antibody, AT10, and its incorporation into a bispecific F(ab′)2 derivative for recruitment of cytotoxic effectors. Mol. Immunol. 28, 1243–1254. [DOI] [PubMed] [Google Scholar]
- 61.Swanson J. A., Hoppe A. D. (2004) The coordination of signaling during Fc receptor-mediated phagocytosis. J. Leukoc. Biol. 76, 1093–1103. [DOI] [PubMed] [Google Scholar]
- 62.Lee H. M., Yuk J. M., Kim K. H., Jang J., Kang G., Park J. B., Son J. W., Jo E. K. (2012) Mycobacterium abscessus activates the NLRP3 inflammasome via Dectin-1-Syk and p62/SQSTM1. Immunol. Cell Biol. 90, 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ritter M., Gross O., Kays S., Ruland J., Nimmerjahn F., Saijo S., Tschopp J., Layland L. E., Prazeres da Costa C. (2010) Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc. Natl. Acad. Sci. U. S. A. 107, 20459–20464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saïd-Sadier N., Padilla E., Langsley G., Ojcius D. M. (2010) Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One 5, e10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shio M. T., Eisenbarth S. C., Savaria M., Vinet A. F., Bellemare M. J., Harder K. W., Sutterwala F. S., Bohle D. S., Descoteaux A., Flavell R. A., Olivier M. (2009) Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases [Published correction in PLoS Pathog (2009) 5, ] PLoS Pathog. 5, e1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baron S. D., Singh R., Metzger D. W. (2007) Inactivated Francisella tularensis live vaccine strain protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion. Infect. Immun. 75, 2152–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lubeck M. D., Steplewski Z., Baglia F., Klein M. H., Dorrington K. J., Koprowski H. (1985) The interaction of murine IgG subclass proteins with human monocyte Fc receptors. J. Immunol. 135, 1299–1304. [PubMed] [Google Scholar]
- 68.Nimmerjahn F., Ravetch J. V. (2005) Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 310, 1510–1512. [DOI] [PubMed] [Google Scholar]
- 69.Latz E., Xiao T. S., Stutz A. (2013) Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hara H., Tsuchiya K., Kawamura I., Fang R., Hernandez-Cuellar E., Shen Y., Mizuguchi J., Schweighoffer E., Tybulewicz V., Mitsuyama M. (2013) Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat. Immunol. 14, 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He Y., Varadarajan S., Muñoz-Planillo R., Burberry A., Nakamura Y., Núñez G. (2014) 3,4-methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J. Biol. Chem. 289, 1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qu Y., Misaghi S., Izrael-Tomasevic A., Newton K., Gilmour L. L., Lamkanfi M., Louie S., Kayagaki N., Liu J., Kömüves L., Cupp J. E., Arnott D., Monack D., Dixit V. M. (2012) Phosphorylation of NLRC4 is critical for inflammasome activation. Nature 490, 539–542. [DOI] [PubMed] [Google Scholar]
- 73.Horng T. (2014) Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol. 35, 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. (2010) Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140. [DOI] [PubMed] [Google Scholar]
- 75.Kang T. B., Yang S. H., Toth B., Kovalenko A., Wallach D. (2013) Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 38, 27–40. [DOI] [PubMed] [Google Scholar]
- 76.Miller M. A., Stabenow J. M., Parvathareddy J., Wodowski A. J., Fabrizio T. P., Bina X. R., Zalduondo L., Bina J. E. (2012) Visualization of murine intranasal dosing efficiency using luminescent Francisella tularensis: effect of instillation volume and form of anesthesia. PLoS One 7, e31359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vogelpoel L. T., Baeten D. L., de Jong E. C., den Dunnen J. (2015) Control of cytokine production by human fc gamma receptors: implications for pathogen defense and autoimmunity. Front. Immunol. 6, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghosh D., Tsokos G. C. (2010) Spleen tyrosine kinase: an Src family of non-receptor kinase has multiple functions and represents a valuable therapeutic target in the treatment of autoimmune and inflammatory diseases. Autoimmunity 43, 48–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.