Abstract
Background
The best data on prognosis comes from population-based incident cohorts but few such cohorts exist for Parkinson's disease and atypical parkinsonism.
Methods
The PINE study is a prospective follow-up study of an incident cohort of people with degenerative or vascular parkinsonism and age-sex matched controls. Participants have annual follow-up from diagnosis until death with review of primary/secondary care records and linkage to the UK death register. Data are collected on survival, disability (dependency on others for activities of daily living) and institutionalization. Research criteria are used to guide the clinical diagnosis, which is updated annually. We compared all-cause mortality, disability and institutionalization in patients (subdivided by diagnosis) and controls, adjusted for important confounders.
Results
323 incident parkinsonian patients (199 Parkinson's disease, 124 atypical parkinsonism, mean age at diagnosis 75yrs) and 262 controls (mean age 75yrs) had 1349 and 1334 person-years follow-up respectively (maximum follow-up 10 years). All outcomes were worse in parkinsonian patients than controls, especially in atypical parkinsonism (adjusted mortality hazards ratios Parkinson's disease 2.49, 95%CI 1.72–3.58, atypical parkinsonism, 6.85, 95%CI 4.78–9.81). Median survival times for Parkinson's disease and atypical parkinsonism were 7.8 and 2.7 years respectively but were very age-dependent. At three years the rates of death or dependency were controls 21%, Parkinson's disease 46%, atypical parkinsonism 96% whilst overall institutionalization rates were 5%, 15% and 55% respectively.
Conclusion
The prognosis of Parkinson's disease and atypical parkinsonism in this unselected incident cohort was significantly worse than previously reported. This has important implications for patient management.
Keywords: Parkinson's disease, Parkinsonian disorders, Prognosis, Survival, Cohort study
Highlights
-
•
323 incident parkinsonian patients were followed for up to 10 years.
-
•
Mortality was 2.5–6.8 times higher in patients compared to controls.
-
•
After 3 years 46% (PD) and 96% (atypical parkinsonism) were dead or dependent.
-
•
14% (PD) and 55% (atypical parkinsonism) were institutionalized during follow-up.
-
•
Prognosis was significantly worse than in previous non-incident younger cohorts.
1. Introduction
Prognosis is a fundamental aspect in understanding any disease. Only with a clear knowledge of prognosis, and factors that influence it, can clinicians give patients appropriate information and plan management, whilst healthcare providers and researchers need this information to develop appropriate services and plan new trials [1]. For neurodegenerative diseases like Parkinson's disease (PD), prognostic studies should assess all important aspects of prognosis including survival, disease progression in terms of impairment, disability and quality of life, the development of motor and non-motor complications and the risk of long-term care in a nursing/residential home, a major driver of overall costs of care [2].
The optimal design for a prognostic study is prospective follow-up of a representative group of patients from diagnosis to death, ideally an incident cohort of patients [3]. Unfortunately, to our knowledge, only one true incidence study of PD or any other parkinsonian disorder has provided long-term prognostic data [4], although a few population-based studies using inception cohorts from the time of diagnosis have published survival data [5]. Hence, it is not surprising that there is still substantial uncertainty about important aspects of PD prognosis, including the degree to which mortality is increased: studies have found the relative risk of mortality in PD varies between 0.95 and 3.79 and median survival ranges from six to 22 years [5].
The Parkinsonism Incidence in North-East Scotland (PINE) study prospectively identified and followed up a population-based incident cohort of PD and other degenerative or presumed vascular parkinsonian conditions along with an age-sex matched community-based control group. The incidence results have been reported previously [6]. This paper describes the medium-term prognosis of the patients (subdivided by diagnosis) versus controls with respect to survival, disability (dependency on others for activities of daily living), and institutionalization.
2. Methods
The PINE study recruited all patients with a newly diagnosed presumed degenerative or vascular parkinsonian syndrome over 4.5 years from a baseline population of about 315,000 registered with 37 primary care practices in and around Aberdeen, Scotland (pilot study 2002-04, main study 2006-09) [6]. Multiple overlapping searches were used to minimize the risk of missing patients, including direct referral from all primary and secondary care physicians serving this population who were sent regular reminders, hand-searching of secondary care referrals, regular electronic searches of primary and secondary care databases and limited screening of the population over 65 years old. Parkinsonism was defined as two or more cardinal motor signs (bradykinesia, rigidity, rest tremor, otherwise unexplained postural instability). Patients with drug-induced parkinsonism (resolved within six to 12 months of stopping the responsible drug or, if the drug could not be stopped, when 123I ioflupane (FP-CIT) single photon emission computed tomography was normal) were excluded. Eligible patients and their carers were offered ongoing life-long yearly follow-up with linkage to the national death register. Clinical care was not altered by participation in the study.
At each annual review the parkinsonian syndrome was classified by a single consultant neurologist with movement disorders expertise (CEC) using all available information (clinical syndrome, atypical features, response to dopamine replacement therapy, development of motor complications, results of structural (CT or MRI) or FP-CIT brain scans where undertaken on the basis of clinical need), by applying the appropriate research criteria available at the time for PD [7], dementia with Lewy bodies (DLB) [8], multiple system atrophy (MSA) [9], progressive supranuclear palsy (PSP) [10], corticobasal degeneration (CBD) [11] and vascular parkinsonism [12]. If patients fulfilled criteria for more than one condition, the diagnosis that fitted best was assigned. In those who died the final diagnosis was made after reviewing all the clinical and imaging information held in their research files and the annual videotaped examinations or from pathology in those who had given consent for post-mortems.
For each eligible patient who consented to follow-up we tried to identify an age-sex matched control from the same primary care practice or a register of elderly people who had taken part in a previous community-based screening project [13]. We have previously shown that the controls had similar health indices to the general population and those who consented were not significantly healthier than those who did not [13]. For some patients we failed to recruit a control.
2.1. Assessments/outcome measures
Patients and controls who gave consent had a standardized baseline visit at diagnosis and annually thereafter including clinical examination looking for features of an atypical parkinsonian syndrome and assessment of: (i) parkinsonian impairment (UPDRS part III motor score, hand tapping test); (ii) mobility (timed 6 m get-up-and-go walk); (iii) disease stage (Hoehn-Yahr), (iv) disability (Schwab & England [S&E], Barthel index); (v) quality of life (Parkinson's Disease Questionnaire −39 item [PDQ-39], Euroquol-5D [EQ-5D]); (vi) motor complications (UPDRS part IV); (vii) cognitive function (mini-mental state examination (MMSE), mini-mental Parkinson's [MMP]); (viii) mood (Geriatric Depression Scale 15 item version [GDS-15]); (ix) other non-motor complications including falls and fractures, pain, autonomic and sleep problems using a symptom checklist. The measurement scales were selected on the basis of clinical relevance, validity and reliability. Some patients only consented to limited assessment including UPDRS motor score, S&E score, MMSE and the checklist of motor and non-motor complications. Those who were unable to come to clinic were visited in the community in their home/institution.
Each year we also updated information about other medical conditions and their medication by reviewing each participant's hospital and primary care record. We also collected information about place of residence for data on institutionalization (admission to a nursing or residential care home) and for those who died we collected details about the date, place and cause of death from death certificates and primary and secondary care records. Parkinsonism-related deaths were defined as those due to end-stage parkinsonism or due to complications of parkinsonism such as immobility, aspiration pneumonia, or falls.
2.2. Analysis
Outcome data were extracted on 31st March 2013 when all participants had at least three years follow-up. Baseline characteristics were described using frequency/percentage for categorical variables, mean/standard deviation for continuous variables with a normal distribution and median/interquartile range if skewed. Time-to-death from date of diagnosis censored at last known follow-up date was plotted with a Kaplan-Meier curve and compared between three diagnostic groups (control, PD, atypical parkinsonism which combined the diagnoses other than PD) using Cox regression. Adjusted hazard ratios (HRs) and 95% CIs were calculated with fixed entry of a pre-defined set of potential confounders measured at the baseline/diagnostic assessment, which were selected on the basis of clinical plausibility and previous literature reviews. These were age, sex, living alone [yes/no], socioeconomic deprivation category based on the Carstairs index from the 2001 Scottish census (1 = most affluent, 6 = least affluent) [14], vascular co-morbidity [any previous symptomatic stroke/transient ischaemic attack/ischaemic heart disease/peripheral vascular disease or diabetes], and smoking status [ever versus never]). Time-to-institutionalization for those not institutionalized at baseline was assessed between diagnostic groups using a competing risk model to account for the competing risk of death prior to institutionalization with adjustment for same confounder variables as for death. The Fine-Gray approach was used to model the cumulative incidence function, which was plotted rather than a standard Kaplan-Meier plot because of the competing risk for death.
Significant disability was defined as S&E score <80, which was defined in PINE as being dependent on others for basic activities of daily living (washing, dressing, toileting, feeding, walking). Dead or dependent (defined as dead or S&E < 80) at three years follow-up was analysed using logistic regression with adjustment as per the time-to-event models.
The overall sample size was defined by the cohort sizes. The survival model was fitted on those with complete confounder information (n = 573); the time-to-institutionalization model was fitted on those with complete confounder information who were not institutionalized at baseline (n = 545) and the logistic model for death or dependency at three years was fitted on all those with Schwab and England scores at three years who were independent at baseline (n = 393). No imputation of missing data was performed. Analysis was carried out using SAS v9.3 with the competing risk analysis undertaken in STATA 13.
The study was approved by the NHS Grampian Research Ethics Committee and the Multicentre Research Ethics Committee A for Scotland, which gave agreement to include patients with dementia who lacked capacity to consent with a guardian's assent.
3. Results
3.1. Patient characteristics
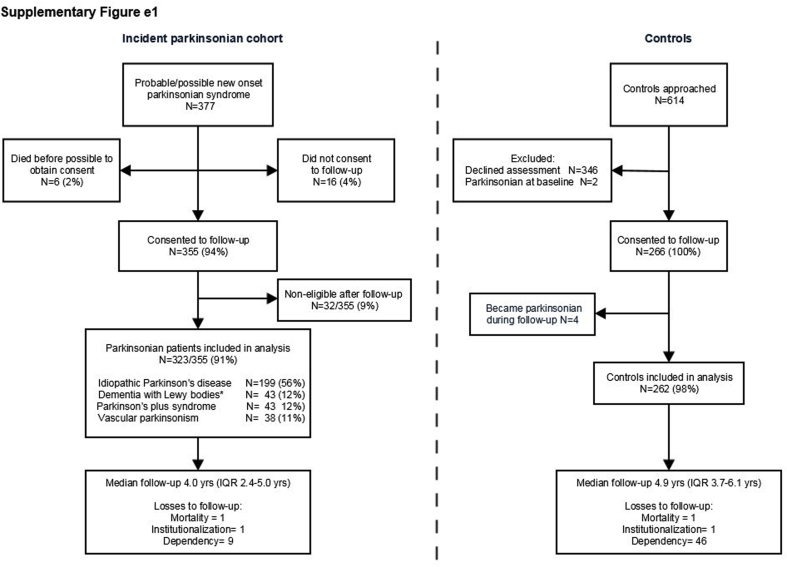
Of 377 patients with suspected incident parkinsonism, 355 patients (94%) gave consent for follow-up who were subdivided into six diagnostic groups: PD (n = 199), DLB (n = 43) [one person with parkinsonism associated with Alzheimer's was included in this group rather than excluded], MSA (n = 16), PSP (n = 24) combined with CBD (n = 3), vascular parkinsonism (n = 38) and non-eligible (n = 32), where it became clear with follow-up that either they were not parkinsonian (such as those with essential or dystonic tremors) or probably had drug-induced parkinsonism. The latter were excluded, leaving 323 parkinsonian patients for analysis. Of 614 controls approached, 266 (43%) were recruited, of whom 262 were included in analysis as four became parkinsonian during follow-up. There were very few losses after 1349 and 1334 person-years of follow-up in patients and controls respectively (follow-up range 0.5–10.1 years) [Table 1, supplementary Fig. e1].
Table 1.
Baseline characteristics of the participants with outcomes in each diagnostic group.
| Control (n = 262) | PD (n = 199) | Vascular (n = 38) | DLB (n = 43) | Parkinson plus syndromes |
Atypical Parkinsonism combined (n = 124) | ||
|---|---|---|---|---|---|---|---|
| PSP/CBD (n = 27) | MSA (n = 16) | ||||||
|
Baseline characteristics | |||||||
| Male | 162 (62%) | 115 (58%) | 27 (71%) | 29 (67%) | 13 (67%) | 12 (75%) | 81 (65%) |
| Mean age yrs (SD) | 74.9 (9.3) | 71.9 (10.4) | 78.5 (7.4) | 79.0 (6.2) | 78.5 (8.5) | 78.3 (9.3) | 78.6 (7.4) |
| Caucasian | 251 (96%) | 198 (99%) | 38 (100%) | 43 (100%) | 27 (100%) | 16 (100%) | 124 (100%) |
| Living Alone a | 86 (33%) | 53 (27%) | 15 (39%) | 13 (30%) | 6 (22%) | 5 (31%) | 39 (31%) |
| Carstairs deprivation category‡ | |||||||
| 1–2 (most affluent) | 120 (46%) | 104 (52%) | 15 (37%) | 29 (67%) | 13 (48%) | 10 (63%) | 66 (53%) |
| 3-4 | 84 (32%) | 56 (28%) | 20 (53%) | 10 (23%) | 8 (30%) | 3 (19%) | 41 (33%) |
| 5–6 (least affluent) | 58 (22%) | 39 (20%) | 4 (11%) | 4 (9%) | 6 (22%) | 3 (19%) | 17 (14%) |
| Family history of PDb | 12 (5%) | 31 (16%) | 2 (5%) | 4 (9%) | 1 (4%) | 3 (19%) | 10 (8%) |
| Current/ex-smokerc | 159 (61%) | 85 (43%) | 23 (61%) | 26 (63%) | 17 (63%) | 8 (50%) | 74 (60%) |
| Vascular co-morbidity† | 83 (32%) | 62 (31%) | 21 (55%) | 16 (37%) | 13 (48%) | 9 (56%) | 59 (47%) |
| Median duration symptoms at diagnosis (months, IQR)d | – | 14 (9, 24) | 20 (6, 26.5) | 12 (6, 24) | 24 (8, 37) | 13 (12, 24) | 15 (7, 26) |
| Symptoms at diagnosis | |||||||
| Tremore | 35 (14%) | 171 (86%) | 18 (47%) | 30 (70%) | 13 (48%) | 6 (38%) | 67 (54%) |
| Postural Instabilitye | 62 (25%) | 60 (30%) | 27 (71%) | 29 (67%) | 25 (93%) | 10 (63%) | 91 (74%) |
| Prior fallsf | 50 (20%) | 76 (38%) | 25 (66%) | 32 (75%) | 23 (85%) | 9 (56%) | 89 (72%) |
| Mean motor UPDRS (SD)g | 3.5 (3.8) | 24.9 (11.9) | 31.4 (14.6) | 32.8 (11.5) | 35.6 (17.7) | 30.9 (12.2) | 32.8 (14.0) |
| Hoehn–Yahr ≥3h | n/a | 51 (26%) | 19 (50%) | 25 (58%) | 17 (65%) | 10 (63%) | 71 (57%) |
| GDS-15 ≥ 5 (depressed)f | 26 (11%) | 61 (31%) | 21 (55%) | 16 (37%) | 16 (59%) | 8 (50%) | 61 (49%) |
| MMSE<24i | 2 (0.8%) | 13 (7%) | 11 (29%) | 18 (62%) | 5 (26%) | 3 (23%) | 37 (30%) |
| Schwab & England <80j |
16 (6%) |
51 (26%) |
23 (60%) |
28 (65%) |
18 (67%) |
7 (44%) |
76 (61%) |
|
Outcomes | |||||||
| Person-years of follow-up | 1334 | 972 | 113 | 136 | 74 | 54 | 377 |
| Total deaths | 57 (22%) | 73 (37%) | 28 (74%) | 35 (81%) | 23 (85%) | 10 (63%) | 96 (77%) |
| Parkinsonian-related deaths∗ | – | 29 (40%) | 13 (46%) | 29 (83%) | 19 (83%) | 7 (70%) | 68 (71%) |
| Median survival yrs (95% CI) # | NE | 7.8 (6.5, 9.4) | 2.7 (1.7, 4.2) | 3.3 (2.4, 4.1) | 2.7 (1.1, 3.8) | 3.3 (1.7, 6.9) | 2.7 (2.3, 3.6) |
| Institutionalized at baseline | 2 (0.8%) | 3 (1.5%) | 5 (13%) | 8 (19%) | 9 (33%) | 2 (13%) | 24 (19%) |
| Institutionalized during follow-up | 11 (4%) | 26 (13%) | 8 (21%) | 24 (56%) | 9 (33%) | 3 (19%) | 44 (35%) |
| Median time to institution yrs (95% CI) # | NE | NE | NE | 1.8 (0.6, 2.6) | 3.3 (1.0, 6.2) | NE | 3.1 (2.3, 4.3) |
| Dead at 3 yrs | 28 (11%) | 32 (16%) | 21 (55%) | 22 (51%) | 16 (59%) | 8 (50%) | 67 (54%) |
| Dead or dependent at 3 yrs | 46/216 (21%) | 90/197 (46%) | 32/34 (94%) | 39/40 (98%) | 26/27 (96%) | 15/16 (94%) | 112/117 (96%) |
Figures given are numbers of patients and percentages unless otherwise stated.
PD- Parkinson's disease; DLB-dementia with Lewy bodies; MSA-multiple system atrophy; PSP-progressive supranuclear palsy; CBD-corticobasal degeneration; vascular-vascular parkinsonism.
UPDRS-Unified Parkinson's Disease Rating Scale; GDS-15-Geriatric Depression Scale; MMSE-mini-mental state examination; IQR–interquartile range; CI – confidence interval; NE – not estimable.
‡ Small postcode measure of socioeconomic status based on proportions of overcrowding, male unemployment, low occupational social class and car ownership.
† Previous history stroke/transient ischemic attack or ischemic heart disease or peripheral vascular disease or diabetes at baseline.
* Parkinsonian-related death defined as being due to end-stage disease or a complication of parkinsonism (e.g. a fall/fracture, immobility leading to pneumonia/venous thromboembolism, aspiration pneumonia etc); percentage refers to % of deaths that were parkinsonism related.
# Median time calculated from Kaplan Meier estimates.
Missing n = 5 controls.
Missing n = 16 controls, n = 2 vascular, n = 1 PSP/CBD.
Missing n = 3 controls, n = 1 PD, n = 1 vascular, n = 2 DLB, n = 1 PSP/CBD.
Missing n = 2 vascular, n = 1 MSA.
Missing n = 12 controls.
Missing n = 15 controls, n = 1 vascular.
Missing n = 16 controls, n = 1 PD.
Missing n = 1 PD, n = 1 PSP/CBD.
Missing n = 16 controls, n = 29 PD, n = 12 vascular, n = 14 DLB, n = 8 PSP/CBD, n = 3 MSA.
Missing n = 16 control, n = 1 PD.
Table 1 shows the baseline characteristics of the participants. The cohort was overwhelmingly Caucasian, reflecting the demography of the study area, and elderly. Patients were seen and diagnosed relatively soon after the onset of their symptoms (median delay 15 months) but despite this many were dependent at baseline (for example, 26% of PD at baseline). As expected, atypical parkinsonian disorders had more severe physical and cognitive impairments at baseline than PD with less tremor and more postural instability/falls. Thirty of the 169 (18%) patients who died had brain autopsies to confirm the diagnosis.
Ninety-two percent of PD patients (184/199) were treated with dopamine replacement therapy during follow-up (11% dopamine agonist monotherapy, 89% levodopa monotherapy/combination therapy). The remaining 15 patients either died before treatment was indicated or decided to remain off treatment up to the latest follow-up. Mean levodopa equivalent daily doses (LEDD) at one, three and five years of follow-up were 353 mg, 429 mg and 502 mg respectively. Sixty-one percent of the atypical parkinsonian patients (76/124) had a trial of treatment (levodopa in all except one) and 34 were still on treatment at three years (mean LEDD 428 mg).
3.2. All-cause mortality
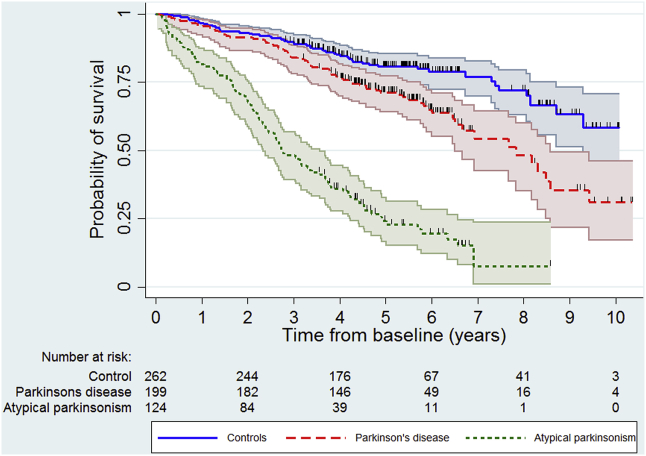
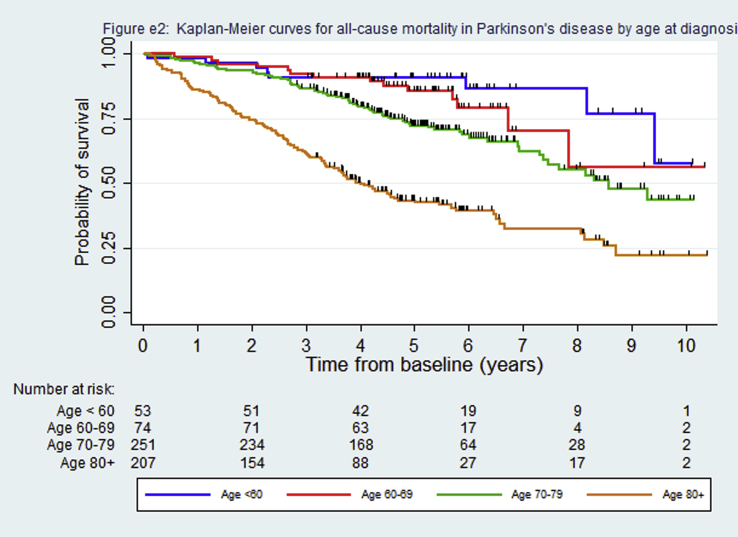
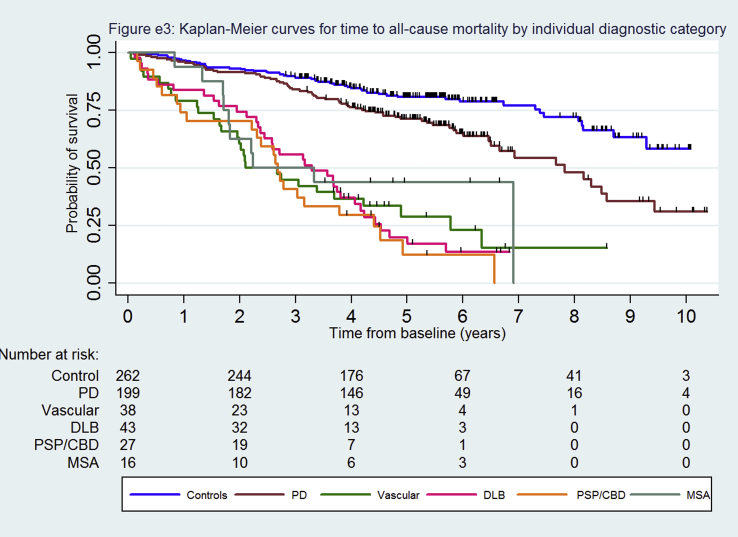
Median survival was 7.8 years for the PD cohort and 2.7–3.3 years for the other parkinsonian syndromes (Table 1, Fig. 1). However, the median survival in PD was heavily influenced by age, being inestimable in those aged under 70 at diagnosis because 50% had not died during follow-up and about 4 years in those aged 80 or more (supplementary Fig. e2). Fifty-seven percent (97/169) of deaths were directly or indirectly related to parkinsonism, significantly higher (p < 0.0001, Chi-squared test) in the atypical syndromes (71%) than PD (40%). Kaplan-Meier survival curves by specific diagnostic group showed no difference in mortality (log rank test p = 0.998) between the DLB, Parkinson's plus (PSP/CBD or MSA) and vascular groups (supplementary Fig. e3) and so these were combined into one atypical parkinsonism group for subsequent analyses to improve power because of the small number of people in these diagnostic groups (Fig. 1, supplementary Table e1). In the Cox regression models, people with PD (HR 2.49, 95% CI 1.72–3.58) and atypical parkinsonism (HR 6.85, 95% CI 4.78–9.81) had significantly worse survival than controls (Table 2) and those with atypical parkinsonism had worse survival compared to PD (HR 3.02, 95% CI 2.15–4.24). Age, vascular co-morbidity and socioeconomic category were independently associated with mortality (supplementary Table e2).
Fig. 1.
Kaplan-Meier curves for all-cause mortality by grouped diagnostic category.
Table 2.
Adjusted risk ratios for different outcomes by diagnostic group.
| Diagnostic groupd | Na (ne) | All-cause mortalitya (N = 573) |
Na (ne) | Institutionalizationa,b (N = 545) |
Na (ne) | Dead or dependent at 3 years and independent at baselinea,c (N = 393) |
|||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | OR | 95% CI | ||||
| Control | 255 (54) | 1.00 | – | 253 (11) | 1.00 | – | 202 (35) | 1.00 | – |
| Parkinson's disease | 198 (72) | 2.49 | (1.72, 3.58) ∗ | 195 (26) | 3.98 | (1.98, 8.00) ∗ | 147 (50) | 3.87 | (2.18, 6.86) ∗ |
| Atypical parkinsonismd |
120 (92) |
6.85 |
(4.78, 9.81) ∗ |
97 (42) |
14.2 |
(7.17, 28.2) ∗ |
44 (39) |
45.3 |
(15.3, 134.5)∗ |
| Vascular Parkinsonism | 37 (27) | 6.32 | (3.91, 10.2)∗ | 32 (8) | 6.72 | (2.47, 18.2)∗ | 13 (11) | 24.6 | (4.90, 123.4)∗ |
| Dementia with Lewy bodies | 41 (33) | 7.75 | (4.88, 12.3)∗ | 33 (22) | 27.7 | (13.3, 57.6)∗ | 13 (12) | 73.1 | (8.80, 606.7)∗ |
| Parkinson Plus | 42 (32) | 6.58 | (4.13.10.5) ∗ | 32 (12) | 11.4 | (4.70, 27.7) ∗ | 18 (16) | 55.2 | (9.80, 311.5) ∗ |
N = total number of patients and controls included in analysis. Na = number of people included in analysis; Ne = number of events; HR hazards ratio; OR odds ratio.
* p-value <0.001.
Analysis adjusted for: age, sex, living alone, vascular co-morbidity, smoking, deprivation category.
Analysis with competing risk for death on those not institutionalized at baseline.
Analysis only includes those independent at baseline, N = 393 due to missing baseline Schwab & England scores.
Atypical parkinsonian patients analysed all together and repeated below subdivided into separate diagnostic groups.
4. Institutionalization
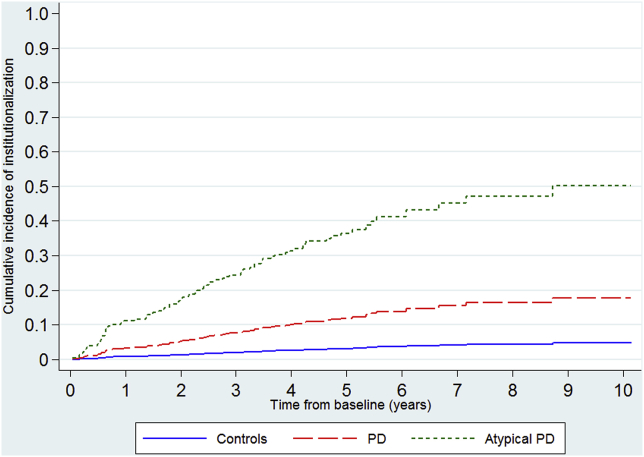
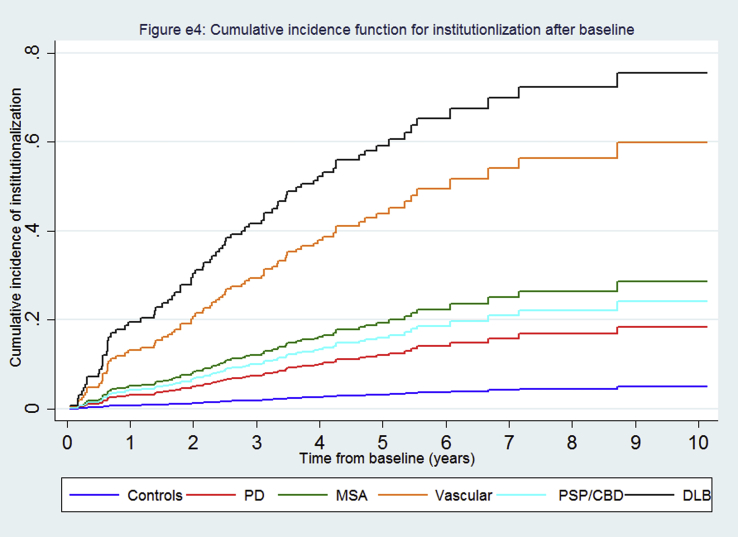
There were high rates of institutionalization (Fig. 2, supplementary Table e1), especially in the DLB group where the median time-to-institutionalization was 1.8 years (Table 1, supplementary Fig. e4). The rates for PD varied by age (supplementary Table e3). People with atypical parkinsonism and PD were respectively nearly 14 and four fold more likely to be institutionalized than controls in the competing risk analyses (Table 2), whilst those with atypical parkinsonism were about four times more likely to be institutionalized than PD (HR 3.78, 95% CI 2.25–6.34).
Fig. 2.
Cumulative incidence curves for institutionalization by grouped diagnostic category.
4.1. Dead or dependent at three years
Nearly all patients with atypical parkinsonian syndromes and 50% of those with PD were dead or required help from others in basic activities of daily living by three years compared to about 20% of controls. The rates for controls and PD were age-dependent (supplementary Table e3). In those independent at baseline (n = 393) there was an increased odds of death or dependency of about four-fold for PD (odds ratio [OR] 3.87 95%CI 2.18–6.86) and 45-fold (OR 45.3 95%CI 15.3–134.5) for atypical parkinsonism (Table 2, supplementary Table e2) compared to controls, whilst the OR was 3.02 (95% CI 2.15–4.24) for atypical parkinsonism relative to PD.
5. Discussion
This prospective incident cohort study found that mortality, institutionalization and dependency rates were significantly higher in PD and especially in atypical parkinsonian syndromes compared to controls. The median survival times of the atypical syndromes were comparable to motor neuron disease [15]. Institutionalization was especially common for those with DLB and nearly all of those with atypical syndromes and 50% of those with PD were dead or dependent three years from diagnosis.
The HR for mortality in PD in this study (2.49) was higher than the standardized mortality ratios (range 1.1–2.9) and HRs (1.76–2.32) found in most previous cohorts studied from diagnosis [5] and our median survival of 7.8 years was shorter than previous studies [5]. However, most of these studies were not cohorts based on all incident patients identified from a population over a given time-period. Therefore, they may have suffered from selection bias such that those with poorer outcomes (e.g. the elderly) were under-represented. It is also possible that our control group was healthier than the general population, which would have inflated the HR although a previous analysis had not supported this [13]. The only previous data from an incident PD cohort [4] also found a lower mortality ratio than our study (1.29) and longer median survival (10.3 years), which may be partly explained by a lower mean age at diagnosis (70 years) and longer follow-up (mean 7.2 years).
Our data on the rate for death and dependency in PD are novel since there are no data from incident cohorts. The 3-year rate was much higher than expected: a previous hospital-based inception cohort found only 16% of patients had disability/dependency at three years [16], but this study included younger patients (mean age at diagnosis 65 years) and had a lower S&E cut-off for dependency (≤70) than we used. Only one small (n = 89) incident PD cohort has reported data on institutionalization and found a higher relative risk (6.7) than we did but the 95% confidence interval was wide (3.7–12.1) and overlapped with ours [17].
There are very few data on the prognosis of atypical degenerative and vascular parkinsonian disorders from incident cohorts and, therefore, our data are important. These disorders were associated with a much worse prognosis than controls and a poorer prognosis than has generally been reported in the literature. Previous non-incident cohorts have shown mean/median survival times from diagnosis ranging from 1.8 to 9.7 years for PSP [18], [19], 5.7–9 years for MSA [20], [21], [22] and 4.4–7.3 years for DLB [23], [24] with a HR for survival in DLB versus a control population of 1.64 (95% CI 1.39–1.95) [25]. However, these cohorts were often relatively young at diagnosis. The single incident cohort of PSP (n = 16) and MSA (n = 9) showed median survival times of 5.3 and 8.5 years respectively [26], longer than we found. Our median time to institutionalization in DLB (1.8 years) was the same as one previous study [27] but much shorter than another (6.1 years) [24].
The main strength of this study is its design, which follows best practice for studying prognosis [3]: namely a population-based incident cohort gathered using multiple methods of case-ascertainment to maximize recruitment, which was then followed up forwards in time to collect pre-specified information on a number of different aspects of prognosis. There were few exclusions due to lack of consent and few losses to follow-up, partly because patients were seen at home when they were unable to come to the clinic. There was also consistent application of diagnostic criteria, reviewed by a single principal investigator and confirmed, where possible, by pathology at death. Therefore, our data are likely to be less biased and more representative than much of the previous published prognostic information on these conditions.
There are also several limitations of our study. Although one of the largest incidence studies of parkinsonism, it remains relatively small and would benefit from being combined with other similar incidence studies with follow-up. Median follow-up is currently only about five years but is continuing and so there will be further data on longer term outcomes, especially for PD, in the future. As an incidence study we had few patients with young-onset Parkinson's disease (only nine patients were under 50 at diagnosis) and so the findings cannot be generalized to young patients or predominantly non-Caucasian populations. As with any clinical study of parkinsonism without 100% pathological confirmation, there may be some diagnostic inaccuracy but we applied robust diagnostic criteria with yearly review of all available clinical data to minimize errors.
Despite the fact that this was an incident cohort, the parkinsonian cohort appeared to have quite advanced disease at baseline. We do not believe this reflects late diagnosis because median time from symptom onset to diagnosis, as determined by patient recall at their baseline interview, was only 15 months. Instead this may reflect the older age of our cohort compared to other studies, which in turn may reflect improved case-ascertainment in the elderly. This highlights the importance of studying prognosis in representative rather than highly selected patient cohorts such as those recruited from neurology clinics or trials [28]. Our controls were not a random sample of the general population and had to have capacity to provide informed consent and so may have been less prone to dementia despite evidence showing that they were not overly healthy [13].
This study fulfils criteria for a combined level 1 and 2 prognostic study and has several implications for clinical practice, healthcare planning and future research [1]. Firstly, atypical parkinsonism syndromes are aggressive diseases with high levels of mortality, dependency and institutionalization and require appropriate care planning from an early stage. Secondly, despite available medical treatments, PD also carries a high risk of death or dependency at three years, especially in the elderly. Optimising therapy early with levodopa may be the most appropriate strategy for many elderly patients who may not survive long enough to develop motor complications. Further research should concentrate on prognostic modelling to try to predict outcomes or treatment responses in individual patients, which may allow personalized approaches to management [29]. Finally, it is important to include elderly parkinsonian patients in future research to ensure that results can be generalized to them and, given their worse prognoses, it may also be more efficient to study potentially disease-modifying drugs in them.
Acknowledgements
We thank all the participants who took part, the research fellows (Kate Taylor, Robert Caslake, David McGhee), the study nurses (Clare Harris, Joanna Gordon, Anne Hayman, Hazel Forbes), the secretaries (Susan Kilpatrick, Pam Rebecca), the data management team (Katie Wilde, David Ritchie) and the clinicians who referred patients to the PINE study.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.parkreldis.2016.08.010.
Funding
This work was supported by Parkinson's UK (grant numbers G0502, G0914), BMA Doris Hillier Award, the BUPA Foundation, NHS Grampian Endowments, RS MacDonald Trust.
Authors' contributions
Shona Fielding checked the data, performed the statistical analysis and drafted the first version of the manuscript and contributed to subsequent revisions.
Angus Macleod helped review the diagnoses, contributed to the design of the statistical analysis and helped draft and revise the manuscript.
Carl Counsell conceived and designed the study, obtained funding, oversaw the conduct of the study, collected some data, reviewed the clinical diagnoses, checked the data prior to analysis and helped draft and revise the manuscript. All authors approved the final version before submission.
Competing interests
None.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Fig. e1: Study flow diagram.
Fig. e2: Kaplan-Meier curves for all-cause mortality in Parkinson's disease by age at diagnosis.
Fig. e3: Kaplan-Meier curves for time to all-cause mortality by individual diagnostic category.
Fig. e4: Cumulative incidence curves for institutionalization by individual diagnostic category.
References
- 1.Hemingway H., Croft H.P., Perel P., Hayden J.A., Abrams K., Timmis A., Briggs A., Udumyan R., Moons K.G., Steyerberg E.W., Roberts I., Schroter S., Altman D.G., Riley R.D. PROGESS group, Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. Br. Med. J. 2013;346:e5595. doi: 10.1136/bmj.e5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindgren P., von Campenhausen S., Spottke E., Siebert U., Dodel R. Cost of Parkinson's disease in europe. Eur. J. Neurol. 2005;12(Suppl 1):68–73. doi: 10.1111/j.1468-1331.2005.01197.x. [DOI] [PubMed] [Google Scholar]
- 3.Laupacis A., Wells G., Richardson W.S., Tugwell P. Users' guides to the medical literature. V. How to use an article about prognosis. JAMA. 1994;272:234–237. doi: 10.1001/jama.272.3.234. [DOI] [PubMed] [Google Scholar]
- 4.Williams-Gray C.H., Mason S.L., Evans J.R., Foltynie T., Brayne C., Robbins T.W., Barker R.A. The CamPaIGN study of Parkinson's disease: 10 year outlook in an incident population-based cohort. J. Neurol. Neurosurg. Psychiatry. 2013;84:1258–1264. doi: 10.1136/jnnp-2013-305277. [DOI] [PubMed] [Google Scholar]
- 5.Macleod A.D., Taylor K.S.M., Counsell C.E. Mortality in Parkinson's disease: a systematic review and meta-analysis. Mov. Disord. 2014;29:1615–1622. doi: 10.1002/mds.25898. [DOI] [PubMed] [Google Scholar]
- 6.Caslake R., Taylor K., Scott N., Gordon J., Harris C., Wilde K., Murray A., Counsell C. Age-, gender-, and socioeconomic status-specific incidence of Parkinson's disease and parkinsonism in North East Scotland: the PINE study. Park. Relat. Disord. 2013;19:515–521. doi: 10.1016/j.parkreldis.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease. A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKeith I.G., Dickson D.W., Lowe J., Emre M., O'Brien J.T., Feldman H., Cummings J., Duda J.E., Lippa C., Perry E.K., Aarsland D., Arai H., Ballard C.G., Boeve B., Burn D.J., Costa D., Del Ser T., Dubois B., Galasko D., Gauthier S., Goetz C.G., Gomez-Tortosa E., Halliday G., Hansen L.A., Hardy J., Iwatsubo T., Kalaria R.N., Kaufer D., Kenny R.A., Korczyn A., Kosaka K., Lee V.M., Lees A., Litvan I., Londos E., Lopez O.L., Minoshima S., Mizuno Y., Molina J.A., Mukaetova-Ladinska E.B., Pasquier F., Perry R.H., Schulz J.B., Trojanowski J.Q., Yamada M. Consortium on DLB, Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 9.Gilman S., Wenning G.K., Low P.A., Brooks D.J., Mathias C.J., Trojanowski J.Q., Wood N.W., Colosimo C., Dürr A., Fowler C.J., Kaufmann H., Klockgether T., Lees A., Poewe W., Quinn N., Revesz T., Robertson D., Sandroni P., Seppi K., Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litvan I., Agid Y., Calne D., Campbell G., Dubois B., Duvoisin R.C., Goetz C.G., Golbe L.I., Grafman J., Growdon J.H., Hallett M., Jankovic J., Quinn N.P., Tolosa E., Zee D.S. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP International Workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Bak T.H., Hodges J.R. Corticobasal degeneration: clinical aspects. Handb. Clin. Neurol. 2008;89:509–521. doi: 10.1016/S0072-9752(07)01247-X. [DOI] [PubMed] [Google Scholar]
- 12.Zijlmans J.C.M., Daniel S.E., Hughes A.J., Revesz T., Lees A.J. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov. Disord. 2004;19:630–640. doi: 10.1002/mds.20083. [DOI] [PubMed] [Google Scholar]
- 13.Taylor K.S.M., Gordon J.C., Harris C.E., Counsell C.E. Recruitment bias resulted in poorer overall health status in a community-based control group. J. Clin. Epidemiol. 2008;61:890–895. doi: 10.1016/j.jclinepi.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 14.McLoone P. MRC Social and Public Health Sciences Unit; Glasgow: 2004. Carstairs Scores for Scottish Postcode Sectors from the 2001 Census.www.sphsu.mrc.ac.uk/library/other%20reports/Carstairs_report.pdf Available at: (accessed 18.05.15) [Google Scholar]
- 15.Chio A., Logroscino G., Hardiman O., Swingler R., Mitchell D., Beghi E., Traynor B.G. Eurals consortium, prognostic factors in ALS: a critical review. Amyotroph. Lateral Scler. 2009;10:310–323. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Post B., Muslimovic D., van Geloven N., Speelman J.D., Schmand B., de Haan R.J. CARPA-study group, Progression and prognostic factors of motor impairment, disability and quality of life in newly diagnosed Parkinson's disease. Mov. Disord. 2011;26:449–456. doi: 10.1002/mds.23467. [DOI] [PubMed] [Google Scholar]
- 17.Parashos S.A., Maraganore D.M., O'Brien P.C., Rocca W.A. Medical services utilization and prognosis in Parkinson disease: a population-based study. Mayo Clin. Proc. 2002;77:918–925. doi: 10.4065/77.9.918. [DOI] [PubMed] [Google Scholar]
- 18.Maher E.R., Lees A.J. The clinical features and natural history of the Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1986;36:1005–1008. doi: 10.1212/wnl.36.7.1005. [DOI] [PubMed] [Google Scholar]
- 19.Golbe L.I., Davis P.H., Schoenberg B.S., Duvoisin R.C. Prevalence and natural history of progressive supranuclear palsy. Neurology. 1988;38:1031–1034. doi: 10.1212/wnl.38.7.1031. [DOI] [PubMed] [Google Scholar]
- 20.Bjornsdottir A., Gudmundsson G., Blondal H., Olafsson E. Incidence and prevalence of multiple system atrophy: a nationwide study in Iceland. J. Neurol. Neurosurg. Psychiatry. 2013;84:136–140. doi: 10.1136/jnnp-2012-302500. [DOI] [PubMed] [Google Scholar]
- 21.Vanacore N., Bonifati V., Fabbrini G., Colosimo C., De Michele G., Marconi R., Nicholl D., Locuratolo N., Talarico G., Romano S., Stocchi F., Bonuccelli U., De Mari M., Vieregge P., Meco G. European study group on atypical parkinsonism (ESGAP), epidemiology of multiple system atrophy. Neurol. Sci. 2001;22:97–99. doi: 10.1007/s100720170064. [DOI] [PubMed] [Google Scholar]
- 22.Figueroa J.J., Singer W., Parsaik A., Benarroch E.E., Ahlskog J.E., Fealey R.D., Parisi J.E., Sandroni P., Mandrekar J., Iodice V., Low P.A., Bower J.H. Multiple system atrophy: prognostic indicators of survival. Mov. Disord. 2014;29:1151–1157. doi: 10.1002/mds.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oesterhus R., Soennesyn H., Rongve A., Ballard C., Aarsland D., Vossius C. Long-term mortality in a cohort of home-dwelling elderly with mild Alzheimer's disease and Lewy body dementia. Dement. Geriatr. Cogn. Disord. 2014;38:161–169. doi: 10.1159/000358051. [DOI] [PubMed] [Google Scholar]
- 24.Williams M.M., Xiong C., Morris J.C., Galvin J.E. Survival and mortality differences between dementia with Lewy bodies vs Alzheimer disease. Neurology. 2006;67:1935–1941. doi: 10.1212/01.wnl.0000247041.63081.98. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Ptacek S., Farahmand B., Kåreholt I., Religa D., Cuadrado M.L., Eriksdotter M. Mortality risk after dementia diagnosis by dementia type and underlying factors: a cohort of 15,209 patients based on the Swedish Dementia Registry. J. Alzheimers Dis. 2014;41:467–477. doi: 10.3233/JAD-131856. [DOI] [PubMed] [Google Scholar]
- 26.Bower J.H., Maraganore D.M., McDonnell S.K., Rocca W.A. Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976 to 1990. Neurology. 1997;49:1284–1288. doi: 10.1212/wnl.49.5.1284. [DOI] [PubMed] [Google Scholar]
- 27.Rongve A., Vossius C., Nore S., Testad I., Aarsland D. Time until nursing home admission in people with mild dementia: comparison of dementia with Lewy bodies and Alzheimer's dementia. Int. J. Geriatr. Psychiatry. 2014;29:392–398. doi: 10.1002/gps.4015. [DOI] [PubMed] [Google Scholar]
- 28.Rybicki B.A., Johnson C.C., Gorell J.M. Demographic differences in referral rates to neurologists of patients with suspected Parkinson's disease: implications for case-control study design. Neuroepidemiology. 1995;14:72–81. doi: 10.1159/000109781. [DOI] [PubMed] [Google Scholar]
- 29.Hingorani A.D., Windt D.A., Riley R.D., Abrams K., Moons K.G., Steyerberg E.W., Schroter S., Sauerbrei W., Altman D.G., Hemingway H. PROGRESS Group, Prognosis research strategy (PROGRESS) 4: stratified medicine research. Br. Med. J. 2013;346:e5793. doi: 10.1136/bmj.e5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.