Abstract
Adolescent depression is common and has become a major public health concern in China, yet little research has examined the etiology of depression in Chinese adolescents. In the present study, genetic and environmental influences on Chinese adolescent depressive symptoms were investigated in 1181 twin pairs residing in Beijing, China (ages 11 to 19 years). Child- and parent-versions of the Children’s Depression Inventory (CDI) were used to measure adolescents’ depressive symptoms. For self-reports, genetic factors, shared environmental factors, and non-shared environmental factors accounted for 50%, 5%, and 45% of the variation in depressive symptoms, respectively; for parent-reports, genetic factors, shared environmental factors, and non-shared environmental factors accounted for 51%, 18%, and 31% of the variation, respectively. These estimates are generally consistent with previous findings in Western adolescents, supporting the cross-cultural generalizability of etiological model of adolescent depression. Neither qualitative nor quantitative sex differences were found in the etiological model. Future studies are needed to investigate how genes and environments work together (gene-environment interaction, gene-environment correlation) to influence depression in Chinese adolescents.
Keywords: Adolescent, Depressive symptoms, Genetic and environmental influences, Chinese twins
Epidemiological studies suggest that the prevalence of child and adolescent depression in China is rising in recent years (Dennis 2004; Lee et al. 2009; Lu et al. 2008; Ma et al. 2009; Phillips et al. 2009). For example, in a community-based sample of 6–14 year-old children in Wuhan, a large metropolitan city in south-central China, the prevalence of current clinically-diagnosed depressive disorder was found to be 2.8% (Zhong et al. 2013), which is comparable with the results of surveys in Western countries (Costello et al. 2003; Demir et al. 2011; Ford et al. 2003). Several questionnaire-based studies reported similar or even higher prevalence rates of depression in Chinese adolescents (Abela et al. 2011; Greenberger et al. 2000; Hesketh et al. 2002; Liu et al. 1999; Sun et al. 2010) than in Western adolescents (Merikangas et al. 2011; Rushton et al. 2002; Thapar et al. 2012). Emerging evidence therefore suggests that adolescent depression has become a major public health concern in China, yet little research has examined the etiology of depression in Chinese adolescents, and in particular, how genetic and environmental factors influence adolescent depression.
Etiological research in Western populations suggests that both genetic and social factors contribute to adolescent depression (Rice et al. 2002; Rice and Thapar 2009). One meta-analysis of twin and adoption studies (including 17 samples with 21,027 sibling pairs) found that genetic factors accounted for 44% of the variance, while shared and non-shared environments accounted for 14% and 42% of the total variation in child and adolescent depression, respectively (Burt 2009). Albeit extensive, previous studies have almost entirely employed Western samples, thus, it is still unknown whether prior findings can be generalized to other ethnic groups, and specifically to a population with genetic and cultural differences compared to their Western counterparts, such as residents of China, as detailed below.
The first aim of the present study was to examine the genetic and environmental contributions to adolescent depressive symptoms in a large Chinese adolescent twin sample. There are good reasons to expect somewhat different genetic and environmental influences on depression in Chinese adolescents relative to Western populations. Genetically, evidence has shown that the frequencies of some depression-related genetic variants (e.g. BDNF val66met polymorphism and 5-HTTLPR) are different between Eastern and Western populations (Chiao and Blizinsky 2010; Verhagen et al. 2008). In addition, the same allele may serve as a risk allele in one population (Western or Eastern), but as a protective allele in the other (Eastern or Western) (Chen et al. 2012; Long et al. 2013; Zhang et al. 2009). The environmental factors associated with depression may also differ for youth living in different cultures (Western vs. Eastern). Chinese adolescents live in a very different social context than Western adolescents. For example, students in China bear heavier academic stress and higher levels of parental expectations, which may render them more depressed than their Western counterparts (Hou et al. 2012). The mental health service utilization rates, however, are lower in Chinese children and adolescents compared with those in Western countries (Ryder et al. 2012). According to the bioecological model (Bronfenbrenner and Ceci 1994) and some empirical studies (Hicks et al. 2009; Lau and Eley 2008; Rice et al. 2006), social factors, such as family, school, and the peer group, can influence the expression of genetic predispositions to depression. Because there are differences in proximal social contexts surrounding adolescents between Eastern and Western cultures (Greenberger et al. 2000; Zhou et al. 2008), the extent to which genetic and environmental factors contribute to Chinese adolescents’ depression may differ from Western populations.
The second aim of the present study was to examine sex differences with regard to genetic and environmental influences on adolescent depression. Studies in Western samples have shown that girls start to exhibit more depressive symptoms than boys at approximately age 13, and that this sex difference continues into adulthood (Ge et al. 1994; Hankin et al. 1998; Nolen-Hoeksema and Girgus 1994). This robust finding, however, has not been well replicated in samples of Chinese adolescents. Some studies have find more depressive symptoms in girls than in boys (Abela et al. 2011; Greenberger et al. 2000), but some studies have found no significant sex differences in mean levels of depressive symptoms (Liu et al. 1999; Tepper et al. 2008) and other studies have found greater rates of depressive symptoms in boys than in girls (Hong et al. 2009; Sun et al. 2010). These inconsistent findings prompted our interest exploring whether the genetic and environmental etiology of depression in Chinese adolescents differs by sex.
Behavioral genetic research on sex differences in the etiology of depression has considered both qualitative (i.e., there are some sex-specific genetic and/or environmental contributors to adolescent depression) and quantitative (i.e., the same genetic and environmental factors contribute to boys’ and girls’ depression, but these contributors influence the two sexes unequally) sex differences. After reviewing 34 twin studies, Franic et al. (2010) concluded that there were no qualitative sex differences in genetic and environmental etiologies of childhood and adolescent depression. However, the results of quantitative sex differences varied across studies. For example, Rice et al. (2002) and Eley and Stevenson (1999) found stronger genetic effects on adolescent boys’ than on adolescent girls’ self-reported depressive symptoms. Conversely, Scourfield et al. (2003) found a greater genetic influence for girls than for boys using parent-report data. Studies based on data from the National Longitudinal Study of Adolescent Health Add Health also reported larger genetic influences on self-reported depressive symptoms for female than for male adolescents (Cho et al. 2006; Jacobson and Rowe 1999; McCaffery et al. 2008). In addition, three studies reported no sex differences in the magnitude of genetic and environmental influences on adolescent depressive symptoms (Eaves et al. 1997; Happonen et al. 2002; Lau and Eley 2006).
To our knowledge, only two twin studies in Eastern cultures have examined genetic and environmental influences on depression and sex differences associated with these influences. Using 490 pairs of adolescent and young adult twins (aged 13–23 years) from the South Korean Twin Registry (SKTR), Hur (2008) reported significant genetic and non-shared environmental effects on depressive symptoms in females (explained 41% and 59% of total variance, respectively), but no significant genetic influences in males (shared and non-shared environmental effects explained 34% and 66% of total variance, respectively). In another study with using 602 pairs of adolescent twins (ages: 11–19 years) from the Qingdao Twin Registry (QTR) in China, Unger et al. (2011) found no significant genetic influences on depressive symptoms in either girls or boys, while shared and non-shared environmental factors significantly explained 38% and 62% of total variance.
These findings seemed to suggest higher shared environmental effects and lower genetic effects (both males and females in QTR, males in SKTR) on depression in Eastern cultures, compared with the findings in Western populations. However, several limitations should be kept in mind when interpreting the results of the two previous studies. First, both studies were based on relatively small samples, especially when considering the number of dizygotic (DZ) twin pairs (QTR: 81 pairs of female DZ twins, 81 pairs of male DZ twins, 119 pairs of opposite-sex DZ twins; SKTR: 56 pairs of female DZ twins, 41 pairs of male DZ twins, 51 pairs of opposite-sex DZ twins). The small sample size limited the statistical power to draw reliable conclusions. Second, both studies only used self-report method to assess adolescents’ depressive symptoms. Previous research has suggested that, twin studies based on parent-report data can provide additional information regarding the etiology of adolescent depression (Happonen et al., 2002).
In order to get a more accurate estimation of genetic and environmental effects in the two sexes, the present study used the data from the Beijing Twin Study (BeTwiSt) (Chen et al. 2013). The BeTwiSt is a longitudinal twin study based on a large and representative twin sample in Beijing, China. Both self-reported and parent-reported adolescent depressive symptoms were collected to provide perspectives from multiple informants. Both qualitative and quantitative sex differences in the etiological models were examined by employing both same-sex and opposite-sex twins.
Method
Sample
This study was based on data from the Beijing Twin Study (BeTwiSt), a longitudinal twin study of Chinese adolescents. The primary aim of the BeTwiSt was to examine how genes, environment, and their interplay influence psychological development and mental health problems among Chinese adolescents. Approximately 1400 pairs of twins aged 10 to 19 years were recruited from elementary and secondary schools randomly selected from all 18 countries or districts in the Beijing municipality. Detailed information about recruitment and assessment procedures are described elsewhere (Chen et al. 2013).
The sample for the present study included 1181 pairs of twins. The breakdown by zygosity is as follows: 338 pairs of monozygotic male (MZM) twins, 122 pairs of dizygotic male (DZM) twins, 359 pairs of monozygotic female MZF twins, 148 pairs of dizygotic female (DZF) twins, and 214 pairs of opposite-sex dizygotic (DZOS) twins. The age of the study sample ranged from 11 to 19 years, with a mean age of 14.17 years (SD = 2.28 years). Ninety-two percent of participants were of Han ethnicity. Regarding fathers’ highest educational attainment, 6.8% had a primary school degree, 32.8% had a junior high school degree, 31.8% had a senior high school degree, 26.1% had a college degree, and 2.5% had a graduate degree. For the mothers’ highest educational attainment, 5.4% had a primary school degree, 35.3% had a junior high school degree, 29.5% had a senior high school degree, 25.2% had a college degree, and 4.6% had a graduate degree. Self- and parent-reported adolescent depressive symptoms during Wave 1 were used in the present study. We asked one parent from each family, the one who knew their twin children better, to complete the parent-report questionnaire. Sixty-five percent of parent respondents were mothers, 34% were fathers, and 1% were other caregivers. The representativeness of the twin sample is described in a previous study (Chen et al. 2013). Specifically, we compared the basic demographic characteristics of the BeTwiSt sample to a population-based representative youth population in Beijing (Chen et al. 2009). No significant differences were detected in terms of perceived family social economic status, fathers’ educational attainment, or parents’ marital status or marital quality. The mothers’ educational levels were significantly higher in the twin sample than in the general youth sample. Overall, these comparisons suggest that the representativeness of the twin sample was acceptable.
Measures
Depressive symptoms
Self-reported depressive symptoms were assessed with the Children’s Depression Inventory (CDI; Kovacs & Staff 2003), one of the most widely used measures of depression in children and adolescents (Mash & Hunsley 2005). The youth were asked to assess their behaviors over the previous two weeks. Each of the CDI items consists of three choices, with higher scores indicating higher severity: “1” = the absence of symptoms; “2” = mild symptoms; “3” = definite symptoms. The total scores ranged from 27 to 81. Psychometric studies of the CDI have shown a high degree of internal consistency, test-retest reliability, and construct validity, especially in nonclinical populations (Cole & Martin 2005). The Chinese version of the CDI (Chen et al. 2000) was used in this study. The internal consistency of the CDI in our sample was acceptable, with Cronbach’s alpha of .86.
Parents reported on children’s depressive symptoms via the CDI Parent Form (CDI-PF) (Kovacs & Staff 2003). The CDI-PF retains the original content of the self-report CDI items, but is reworded for parental use. For example, the item “I feel sad all the time” is rephrased into “My child feels sad all the time.” Previous studies show satisfactory psychometric characteristics of the CDI-PF (Cole et al. 2002; Tram & Cole 2006). In the current study, Cronbach’s alpha was .87.
Zygosity determination
The twins’ zygosity was determined by a validated method combining DNA analysis (89.5% of twins) and questionnaire data (10.5% of twins) (Chen et al. 2010). For the DNA analyses, nine short tandem repeat loci, which are highly heterogeneous in the Chinese population, were used. Same-sex twins with at least one different genetic marker were classified as dizygotic twins; otherwise, the twins were classified as monozygotic (MZ) twins. The posterior probability of being MZ for same-sex twins with the same genotype in all nine loci was estimated to be 99.99%. The validity of the zygosity determination questionnaire was examined by comparing it with the results of the DNA analyses. The predictive accuracy of the questionnaire method used in this study reached 91% (Chen et al. 2010).
Assessment Procedure
All twins and their parents signed informed consents before participation. Arrangements were made for the twins to stay in their classrooms after school. After describing the purpose and procedures of the study, trained research staff distributed the questionnaires to the twin participants and instructed them to complete the questionnaires independently. Research staff members were present to answer any questions that the students might have about the questionnaires. Participants were assured of the confidentiality of their responses and the voluntary nature of their participation. All procedures had been approved by the Institutional Review Board.
Data Analysis
The twin design relies on different levels of genetic relatedness between MZ twin pairs who are genetically identical, and DZ twin pairs who share one-half of the additive genetic effects. This difference is used to estimate the contribution of genetic and environmental factors to the individual differences in the phenotype of interest. As the current study aimed to clarify both qualitative and quantitative sex differences in genetic and environmental influences on adolescent depressive symptoms, the Pearson correlations of five twin groups (i.e., MZM, MZF, DZM, DZF, and DZOS) were computed first. To obtain parameter estimates of genetic, shared, and non-shared environmental effects, and to test for sex difference in etiological model, maximum-likelihood model fitting to the variance-covariance matrices using the structural equation modeling package Mx (Neal et al. 2006) was undertaken.
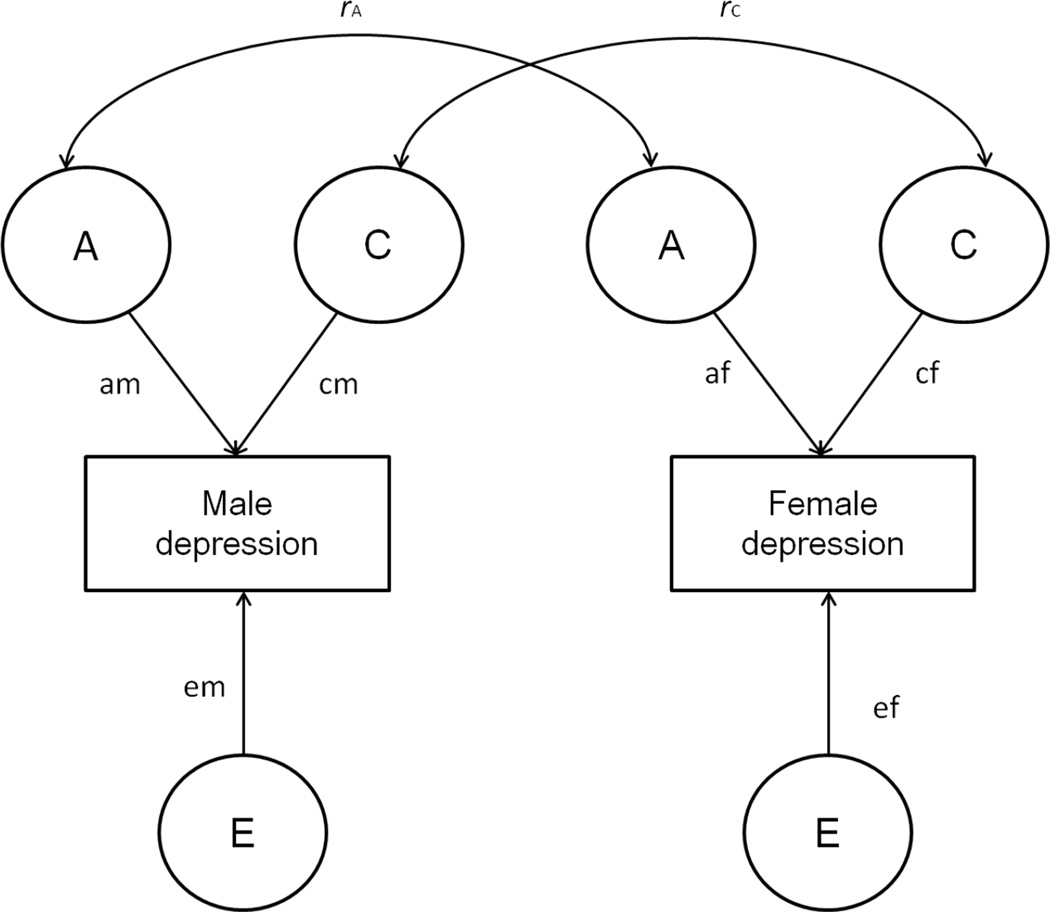
To examine both qualitative and quantitative sex differences, it is necessary to first fit a full sex-limitation model and then two nested sub-models, which progressively model fewer parameters (Figure 1). In the full sex-limitation model, additive genetic (A), shared (C) and nonshared environmental (E) parameters were allowed to differ between males and females, assuming that the magnitudes of influence of A, C, and E on depressive symptoms may vary in males and females. For DZOS twins, the correlations for the A factors (rA) and C factors (rC) were estimated freely. Because a model estimating both rA and rC simultaneously is not identifiable, the two correlations were estimated separately in two non-nested models. The fits of these two non-nested models were compared with the Akaike information criterion (AIC) and the model with the smaller AIC was selected as the best fitting model. Then, data were fit into the first nested sub-model, the common effect model, which constrained the DZOS twins’ rA to 0.5 or rC to 1.0 but allowed the A, C, E parameters for males and females to differ. The significance of difference in fits between the common effect model and the full sex-limitation model was tested to examine qualitative sex differences. The second nested sub-model is the scalar model, which constrains the DZOS twins’ rA to 0.5 or rC to 1.0 and constraint the A, C, E parameters for males and females to be equal. The scalar model also allows sex differences in the variance of the phenotype. The significance of difference in fits between the scalar model and the common effect model tested quantitative sex differences. The self- and parent-reported adolescent depressive symptoms were examined separately.
Figure 1. Sex-limitation model for adolescent depressive symptoms.
The magnitude of additive genetic (A), shared environmental (C), nonshared environmental (E) influences may differ for males and females (am ≠ af, cm ≠ cf, em ≠ ef), and/or the genetic (rA) or shared environmental (rC) correlation among opposite-sex twins may fall below the expected genetic (.50) and shared environmental (1.00) correlations for same sex dizygotic twins.
Results
The descriptive statistics for self- and parent-reported adolescent depressive symptoms in groups by zygosity and sex are shown in Table 1. Sex and age differences in the mean level of depressive symptoms were first examined. We found no significant sex differences in the means of either self- (t = −1.26, p = .21) or parent-reported adolescent depressive symptoms (t = −1.24, p = .18). Adolescent depressive symptoms increased significantly with age (self-report: r = .12, p < .001; parent-report: r = .11, p < .01). The correlation between self- and parent-report data was moderate and significant (r = .50, p < .001). The residuals after regressing out the effects of sex and age were used in following analyses (McGue & Bouchard 1984).
Table 1.
Means and standard deviations of depressive symptoms grouped by zygosity and sex
| Self-reports | Parent-reports | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| MZT Female | 36.82 | 6.40 | 33.04 | 4.69 |
| DZT Female | 37.55 | 6.62 | 33.62 | 5.18 |
| MZT Male | 37.70 | 6.77 | 33.72 | 5.75 |
| DZT Male | 37.73 | 6.87 | 34.65 | 5.37 |
| OST Female | 37.52 | 6.38 | 33.85 | 5.90 |
| OST Male | 37.23 | 6.69 | 33.54 | 4.97 |
| Total | 37.35 | 6.60 | 33.58 | 5.26 |
Notes: MZT: Monozygotic Twins, DZT: Dizygotic Twins, OST: Opposite-sex Twins, SD: Standard deviation
The Pearson correlations for the five groups are shown in Table 2. For both self- and parent-report data, the twin correlations of MZ twins were larger than those of the same-sex and opposite-sex DZ twins, suggesting substantial genetic influences. Furthermore, the twin correlations of DZOS twins were similar to those of same-sex DZ twins, suggesting that there may be trivial qualitative sex differences.
Table 2.
Twin correlations for age-regressed Chinese adolescents’ depressive symptoms
| Twin Correlations | |||||
|---|---|---|---|---|---|
| Monozygotic Twins | Dizygotic Twins | ||||
| Depressive symptoms | Male | Female | Male | Female | Opposite-sex |
| Child-report | .52 (285) | .56 (342) | .27 (106) | .38 (142) | .27 (187) |
| Parent-report | .74 (225) | .60 (278) | .41 (87) | .47 (117) | .48 (150) |
Note. All correlation coefficients were significant at p < 0.01 level. The number of twin pairs is shown in brackets.
Table 3 provides the model-fitting results and parameter estimates for self- and parent-reported adolescent depressive symptoms. For the self-report data, the fits of the full sex-limitation models (full rA and rC) were very similar; we selected the full rA model as the baseline model. When the freely estimated rA was set to 0.5 (common effect model), there was no significant change in chi-square (Δχ2 (1) = 0, p > .5), suggesting no qualitative sex differences. Allowing the genetic and environmental estimates to be equal between the sexes (scalar model) resulted in a non-significant change in chi-square (Δχ2 (3) = 3.10, p > .2), indicating no quantitative sex differences. Thus, the best-fitting model for self-reported depressive symptoms was the scalar model with no qualitative and quantitative sex differences, in which genetic factors accounted for 50%, while non-shared environmental factors accounted for 45% of individual differences in adolescent depression. The shared environmental effect was negligible and not significant statistically.
Table 3.
Parameter estimates and fit indices for Chinese adolescents’ twin data
| Male | Female | Opposite-sex twins | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | df | χ2 | AIC | A | C | E | A | C | E | rA | rC |
| Self-report | |||||||||||
| Full rA | 8 | 22.18 | 6.18 | .49(.22,.59) | .03(.00,.27) | .48(.40,.56) | .50(.25,.64) | .07(.00,.30) | .42(.36,.50) | .50(.12,.50) | 1.0 |
| Full rC | 8 | 22.18 | 6.18 | .49(.22,.59) | .03(.00,.27) | .48(.40,.56) | .50(.25,.64) | .07(.00,.30) | .42(.36,.50) | .50 | 1.0(.00,1.0) |
| Common | 9 | 22.18 | 4.18 | .49(.22,.59) | .03(.00,.27) | .48(.40,.56) | .50(.25,.64) | .07(.00,.30) | .42(.36,.50) | .50 | 1.0 |
| Scalar | 12 | 25.28 | 1.28 | .50(.32,.60) | .05(.00,.21) | .45(.40,.51) | .50(.32,.60) | .05(.00,.21) | .45(.40,.51) | .50 | 1.0 |
| Parent-report | |||||||||||
| Full rA | 8 | 25.62 | 9.62 | .54(.29,.74) | .19(.06,.43) | .27(.23,.34) | .48(.24,.67) | .17(.01,.38) | .35(.29,.42) | .50(.25,.50) | 1.0 |
| Full rC | 8 | 25.62 | 9.62 | .54(.29,.74) | .19(.06,.43) | .27(.23,.34) | .48(.24,.67) | .17(.01,.38) | .35(.29,.42) | .50 | 1.0(.40,1.0) |
| Common | 9 | 25.62 | 7.62 | .54(.29,.74) | .19(.06,.43) | .27(.23,.34) | .48(.24,.67) | .17(.01,.38) | .35(.29,.42) | .50 | 1.0 |
| Scalar | 12 | 28.72 | 4.72 | .51(.35,.69) | .18(.01,.32) | .31(.27,.36) | .51(.35,.69) | .18(.01,.32) | .31(.27,.36) | .50 | 1.0 |
Note. Full rA = full sex-limitation model allowing for quantitative sex differences, qualitative genetic sex differences; full rC = full sex-limitation model allowing for quantitative sex differences, qualitative shared environmental sex differences; Common = common effects model allowing for quantitative sex differences; Scalar=scalar model. AIC=Akaike’s information criterion; A, C, and E = the proportion of variance in depressive symptoms explained by additive genetic, shared environmental, and nonshared environmental influences, respectively; rA= the genetic correlation among opposite-sex twin pairs; rC= the shared environmental correlation among opposite-sex twin pairs; 95% confidence intervals are shown in brackets.
For the parent-report data, the full rA model was also selected as the baseline model, as the fits of the two full sex-limitation models (full rA and rC) were very similar. When the freely estimated rA was set to 0.5 (common effect model), there was no significant change in chi-square (Δχ2 (1) = 0, p > .5). Additionally, allowing the genetic and environmental estimates to be equal across sexes (scalar model) resulted in a nonsignificant change in chi-square (Δχ2 (3) = 3.10, p > .2). The best-fitting model for parent-reported depressive symptoms was also the scalar model, in which genetic factors accounted for 51%, while non-shared environmental factors accounted for 31% of individual differences in adolescent depression. Furthermore, unlike the self-reports, the shared environmental effect for parent-reported adolescent depression was modest and significant, explaining 18% of variation.
Discussion
Given the differences in genetic makeup and social contexts between Asian and Western countries, it is necessary to re-examine the genetic and environmental influences on adolescent depression and sex differences in Asian samples. Adolescent depression is common and has become a major public health concern in China, the country with the largest youth population. However, little research has examined the etiology of depression in Chinese adolescents. Thus, investigating the genetic and environmental etiology of depression has potential implications, especially for prevention and intervention of Chinese adolescent depression.
Behavioral genetic research in Western populations has demonstrated that adolescent depression was moderately heritable and non-shared environmental factors were an important contributor. We obtained similar findings in the present study. Our results showed that the heritability of depressive symptoms in Chinese adolescents was moderate (genetic factors explained about 50% of variation); non-shared environmental factors accounted for a large amount of individual differences (45% for self-reports, and 31% for parent-reports). The consistency between findings of this study and the previous studies that had used Western population samples supports a cross-cultural generalizability of etiological models of adolescent depression.
Shared environmental effects were found to be modest in parent-reports, but negligible in self-reports. The higher estimates of shared environmental effects in parent-reported data might be due to shared rater effects (Bartels et al. 2004). We found similar moderate estimate of genetic influences on parent-report compared to self-report, which was consistent with previous studies with multiple informants (Happonen et al. 2002). As a whole, the genetic and environmental estimates in the current study were similar to findings in samples of Western adolescents (Burt 2009; Rice 2009; Rice 2010).
However, our results were inconsistent with the previous two twin studies utilizing Asian samples. The study of the QTR in China suggested no genetic influences on depressive symptoms, but significant shared and non-shared environmental influences (Unger et al. 2011). Hur (2008) did find significant genetic influences on depression among South Korean females, but no genetic influences on depression for South Korean males. Different measurements might account for the discrepancy between the current study and two previous Asian studies (CDI vs. CES-D). Studies including multi-wave surveys showed that CDI scores are more stable and represent more stable trait component, compared with CES-D scores (Cole & Martin 2005; Kenny & Zautra 2001; Tram & Cole 2006; Windle & Dumenci 1998). The differential stabilities may owe to the different extents of transient characteristic of two measures, i.e., the CES-D assesses individual’s feelings or behaviors during past one week, while the CDI measures individual’s mood or behaviors during past two weeks. Thus, it is possible that the depressive symptoms measured by the CDI have more genetic origins than symptoms assessed by the CES-D because they reflect a more stable and persistent set of symptoms. Similarly, previous empirical twin studies using Western populations have also found higher heritability of CDI scores (Eley 1997; Eley & Stevenson 1999; Happonen et al. 2002; Lau & Eley 2008; O'Connor et al. 1998) than CES-D scores (Byers et al. 2009; Jacobson & Rowe 1999; Jansson et al. 2004; McCaffery et al. 2003; McCaffery et al. 2008). This finding reminds us that the specific measurements selected might affect heritability estimates of adolescent depression.
Consistent with findings in Western adolescents (Rice & Thapar 2009), we found no evidence of qualitative sex differences in the genetic and environmental influences on depressive symptoms in Chinese adolescents. We also found no sex differences in the quantity of genetic and environmental effects on Chinese adolescents’ depression, which is consistent with several studies of Western adolescents (Eaves et al. 1997; Happonen et al. 2002; Lau & Eley 2006; Burt 2009). This finding, however, is inconsistent with the findings of Hur (2008). She reported a significant sex difference, with moderate genetic effects (41% of variation) in females, no genetic effects but modest shared environmental effects (34% of variation) in males. The discrepancy of evidence regarding sex differences in magnitude of genetic and environmental influences on adolescent depression has also been demonstrated in Western twin studies. Some studies have found greater genetic influences on depression in girls than in boys (Cho et al. 2006; Jacobson & Rowe 1999; McCaffery et al. 2008; Scourfield et al. 2003), whereas other studies have demonstrated opposite findings (Eley & Stevenson 1999; Rice et al. 2002). Additional studies are needed to clarify the sex difference in genetic and environmental etiologies of adolescent depression, especially for Asians.
Conclusion
The heritability of Chinese adolescents’ depressive symptoms was moderate, and the remainder of variance was mainly explained by nonshared environmental factors. Modest shared environmental influences were found in parent-reported adolescent depressive symptoms. The same genetic and environmental factors influence depressive symptoms in Chinese adolescent males and females, with similar quantity. These findings generally support the cross-cultural generalizability of the etiological model of adolescent depression. Future studies can be conducted to investigate the underlying mechanisms of how genes and environments act together to influence depression in Chinese adolescents.
Acknowledgments
This study was supported by the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-EW-J-8), and the National Natural Science Foundation of China (31170993, 91132728 and 31300841). This research was also supported by Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences and the BeTwiSt of Institute of Psychology, Chinese Academy of Sciences. The original principal investigator, Dr. Xiaojia Ge, passed away during the course of this study and the authors dedicate this paper to his memory and for his great contribution in founding the BeTwiSt. We are also grateful to participating twin families and schools.
References
- Abela JRZ, Stolow D, Mineka S, Yao S, Zhu XZ, Hankin BL. Cognitive vulnerability to depressive symptoms in adolescents in urban and rural Hunan, China: A multiwave longitudinal study. J Abnorm Psychol. 2011;120(4):765–778. doi: 10.1037/a0025295. [DOI] [PubMed] [Google Scholar]
- Bartels M, Boomsma DI, Hudziak JJ, Rietveld MJ, van Beijsterveldt TC, van den Oord EJ. Disentangling genetic, environmental, and rater effects on internalizing and externalizing problem behavior in 10-year-old twins. Twin Res Hum Genet. 2004;7(2):162–175. doi: 10.1375/136905204323016140. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U, Ceci SJ. Nature-nurture reconceptualized in developmental perspective: A bioecological model. Psychol Rev. 1994;101(4):568–586. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: A meta-analysis of shared environmental influences. Psychol Bull. 2009;135(4):608–637. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- Byers AL, Levy BR, Kasl SV, Bruce ML, Allore HG. Heritability of depressive symptoms: a case study using a multilevel approach. Int J Methods Psychiatr Res. 2009;18(4):287–296. doi: 10.1002/mpr.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah C, Rubin K. European American and Mainland Chinese mothers’ responses to aggression and social withdrawal in preschoolers. Int J Behav Dev. 2004;28(1):83–94. [Google Scholar]
- Chen J, Li X, Chen Z, Yang X, Zhang J, Ge X, et al. Optimization of zygosity determination by questionnaire and DNA genotyping in Chinese adolescent twins. Twin Res Hum Genet. 2010;13(2):194–200. doi: 10.1375/twin.13.2.194. [DOI] [PubMed] [Google Scholar]
- Chen J, Li X, McGue M. Interacting effect of BDNF Val66Met polymorphism and stressful life events on adolescent depression. Genes Brain Behav. 2012;11(8):958–965. doi: 10.1111/j.1601-183X.2012.00843.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Li X, Zhang J, Natsuaki MN, Leve LD, Harold GT, et al. The Beijing Twin Study (BeTwist): A longitudinal study of child and adolescent development. Twin Res Hum Genet. 2013;16(1):91–97. doi: 10.1017/thg.2012.115. [DOI] [PubMed] [Google Scholar]
- Chen X, Liu M, Li D. Parental warmth, control, and indulgence and their relations to adjustment in Chinese children: A longitudinal study. J Fam Psychol. 2000;14(3):401–419. doi: 10.1037//0893-3200.14.3.401. [DOI] [PubMed] [Google Scholar]
- Chen Z, Guo F, Yang X, Li X, Duan Q, Zhang J, Ge X. Emotional and behavioral effects of romantic relationships in Chinese adolescents. J Youth Adolesc. 2009;38(10):1282–1293. doi: 10.1007/s10964-009-9405-0. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Blizinsky KD. Culture–gene coevolution of individualism–collectivism and the serotonin transporter gene. Proc Biol Sci. 2010;277(1681):529–537. doi: 10.1098/rspb.2009.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Guo G, Iritani BJ, Hallfors DD. Genetic contribution to suicidal behaviors and associated risk factors among adolescents in the US. Prev Sci. 2006;7(3):303–311. doi: 10.1007/s11121-006-0042-5. [DOI] [PubMed] [Google Scholar]
- Cole DA, Martin NC. The longitudinal structure of the Children's Depression Inventory: testing a latent trait-state model. Psychol Assess. 2005;17(2):144–155. doi: 10.1037/1040-3590.17.2.144. [DOI] [PubMed] [Google Scholar]
- Cole DA, Tram JM, Martin JM, Hoffman KB, Ruiz MD, Jacquez FM, et al. Individual differences in the emergence of depressive symptoms in children and adolescents: A longitudinal investigation of parent and child reports. J Abnorm Psychol. 2002;111(1):156–165. [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Demir T, Karacetin G, Demir DE, Uysal O. Epidemiology of depression in an urban population of Turkish children and adolescents. J Affect Disord. 2011;134(1):168–176. doi: 10.1016/j.jad.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Dennis C. Mental health: Asia's tigers get the blues. Nature. 2004;429(6993):696–698. doi: 10.1038/429696a. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Silberg JL, Meyer JM, Maes HH, Simonoff E, Pickles A, et al. Genetics and developmental psychopathology 2: The main effects of genes and environment on behavioral problems in the Virginia Twin Study of Adolescent Behavioral Development. J Child Psychol Psychiatry. 1997;38(8):965–980. doi: 10.1111/j.1469-7610.1997.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Eley TC. Depressive symptoms in children and adolescents: Etiological links between normality and abnormality: A research note. J Child Psychol Psychiatry. 1997;38(7):861–865. doi: 10.1111/j.1469-7610.1997.tb01604.x. [DOI] [PubMed] [Google Scholar]
- Eley TC, Stevenson J. Exploring the covariation between anxiety and depression symptoms: a genetic analysis of the effects of age and sex. J Child Psychol Psychiatry. 1999;40(8):1273–1282. [PubMed] [Google Scholar]
- Ford T, Goodman R, Meltzer H. The British Child and Adolescent Mental Health Survey 1999: The Prevalence of DSM-IV Disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(10):1203–1211. doi: 10.1097/00004583-200310000-00011. [DOI] [PubMed] [Google Scholar]
- Franic S, Middeldorp CM, Dolan CV, Ligthart L, Boomsma DI. Childhood and adolescent anxiety and depression: Beyond heritability. J Am Acad Child Adolesc Psychiatry. 2010;49(8):820–829. doi: 10.1016/j.jaac.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, Simons RL. Trajectories of stressful life events and depressive symptoms during adolescence. Dev Psychol. 1994;30(4):467–483. [Google Scholar]
- Greenberger E, Chen C, Tally SR, Dong Q. Family, peer, and individual correlates of depressive symptomatology among US and Chinese adolescents. J Consult Clin Psychol. 2000;68(2):209–219. doi: 10.1037//0022-006x.68.2.209. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107(1):128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, Roesch L. Sex differences in adolescent depression: Stress exposure and reactivity models. Child Dev. 2007;78(1):279–295. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- Happonen M, Pulkkinen L, Kaprio J, Van der Meere J, Viken RJ, Rose RJ. The heritability of depressive symptoms: multiple informants and multiple measures. J Child Psychol Psychiatry. 2002;43(4):471–479. doi: 10.1111/1469-7610.00038. [DOI] [PubMed] [Google Scholar]
- Hicks BM, DiRago AC, Iacono WG, McGue M. Gene–environment interplay in internalizing disorders: consistent findings across six environmental risk factors. J Child Psychol Psychiatry. 2009;50(10):1309–1317. doi: 10.1111/j.1469-7610.2009.02100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X, Li JQ, Xu F, Tse L, Liang YQ, Wang ZY, et al. Physical activity inversely associated with the presence of depression among urban adolescents in regional China. BMC public health. 2009;9(1):148–157. doi: 10.1186/1471-2458-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou XY, Sun J, Dunne MP. Academic stress among adolescents in China. Australasian Epidemiologist. 2012;19(1):9–12. [Google Scholar]
- Hudziak JJ. Chapter 13: Importance of Phenotype Definition in Genetic Studies of Child Psychopathology. Defining psychopathology in the 21st century: DSM-V and beyond, American Psychiatric Publishing, Inc.; 1400K Street N.W. Washington, DC 20005. 2002. pp. 211–230. [Google Scholar]
- Hur YM. Sex differences in genetic and environmental contributions to depression symptoms in South Korean adolescent and young adult twins. Twin Res Hum Genet. 2008;11(3):306–313. doi: 10.1375/twin.11.3.306. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: Integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychol Rev. 2008;115(2):291–313. doi: 10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- Jacobson KC, Rowe DC. Genetic and environmental influences on the relationships between family connectedness, school connectedness, and adolescent depressed mood: sex differences. Dev Psychol. 1999;35(4):926–939. doi: 10.1037//0012-1649.35.4.926. [DOI] [PubMed] [Google Scholar]
- Jansson M, Gatz M, Berg S, Johansson B, Malmberg B, McClearn GE, et al. Gender differences in heritability of depressive symptoms in the elderly. Psychol Med. 2004;34(3):471–479. doi: 10.1017/s0033291703001375. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Zautra A. Trait–state models for longitudinal data. In: Collins LM, Sayer AG, editors. New methods for the analysis of change: Decade of behavior. Washington, DC: American Psychological Association; 2001. pp. 243–263. [Google Scholar]
- Kovacs M, Staff MHS. Children's Depression Inventory (CDI): Technical Manual Update. North Tonawanda, NY, USA: Multi-Health Systems. Inc.; 2003. [Google Scholar]
- Lau JYF, Eley TC. Disentangling gene-environment correlations and interactions on adolescent depressive symptoms. J Child Psychol Psychiatry. 2008;49(2):142–150. doi: 10.1111/j.1469-7610.2007.01803.x. [DOI] [PubMed] [Google Scholar]
- Lau JYF, Eley TC. Changes in genetic and environmental influences on depressive symptoms across adolescence and young adulthood. Br J Psychiatry. 2006;189(5):422–427. doi: 10.1192/bjp.bp.105.018721. [DOI] [PubMed] [Google Scholar]
- Lee S, Tsang A, Huang Y, He Y, Liu Z, Kessler R, et al. The epidemiology of depression in metropolitan China. Psychol Med. 2009;39(5):735–747. doi: 10.1017/S0033291708004091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XC, Ma DD, Kurita H, Tang MQ. Self-reported depressive symptoms among Chinese adolescents. Soc Psychiatry Psychiatr Epidemiol. 1999;34(1):44–47. doi: 10.1007/s001270050110. [DOI] [PubMed] [Google Scholar]
- Long H, Liu B, Hou B, Wang C, Li J, Yu C, et al. The long rather than the short allele of 5-HTTLPR predisposes Han Chinese to anxiety and reduced connectivity between prefrontal cortex and amygdala. Neurosci Bull. 2013;29(1):4–15. doi: 10.1007/s12264-013-1299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Ruan Y, Huang Y, Yao J, Dang W, Gao C. Major depression in Kunming: prevalence, correlates and co-morbidity in a south-western city of China. J Affect Disord. 2008;111(2):221–226. doi: 10.1016/j.jad.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Ma X, Xiang YT, Cai ZJ, Li SR, Xiang YQ, Guo HL, et al. Prevalence and socio-demographic correlates of major depressive episode in rural and urban areas of Beijing, China. J Affect Disord. 2009;115(3):323–330. doi: 10.1016/j.jad.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Mash EJ, Hunsley J. Evidence-based assessment of child and adolescent disorders: Issues and challenges. J Clin Child Adolesc Psychol. 2005;34(3):362–379. doi: 10.1207/s15374424jccp3403_1. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Niaura R, Swan GE, Carmelli D. A study of depressive symptoms and smoking behavior in adult male twins from the NHLBI twin study. Nicotine Tob Res. 2003;5(1):77–83. doi: 10.1080/14622200307259. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Papandonatos GD, Stanton C, Lloyd-Richardson EE, Niaura R. Depressive symptoms and cigarette smoking in twins from the National Longitudinal Study of Adolescent Health. Health Psychol. 2008;27(3S):S207–S215. doi: 10.1037/0278-6133.27.3(suppl.).s207. [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ., Jr Adjustment of twin data for the effects of age and sex. Behav Genet. 1984;14(4):325–343. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- Merikangas K, Nakamura E, Tsuang M, Tohen M, Jones P. The epidemiology of depression and anxiety in children and adolescents. Textbook in Psychiatric Epidemiology. (3) 2011:435–448. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. Richmond, VA: Department of Psychiatry. Virginia Institute for Psychiatric and Behavior Genetics, Virginia Commonwealth University; 2006. [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115(3):424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, McGuire S, Reiss D, Hetherington EM, Plomin R. Co-occurrence of depressive symptoms and antisocial behavior in adolescence: a common genetic liability. J Abnorm Psychol. 1998;107(1):27–37. doi: 10.1037//0021-843x.107.1.27. [DOI] [PubMed] [Google Scholar]
- Phillips MR, Zhang J, Shi Q, Song Z, Ding Z, Wang Z, et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001–05: an epidemiological survey. The Lancet. 2009;373(9680):2041–2053. doi: 10.1016/S0140-6736(09)60660-7. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Thapar A. Assessing the effects of age, sex and shared environment on the genetic aetiology of depression in childhood and adolescence. J Child Psychol Psychiatry. 2002;43(8):1039–1051. doi: 10.1111/1469-7610.00231. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Thapar A. The genetic aetiology of childhood depression: a review. J Child Psychol Psychiatry. 2002;43(1):65–79. doi: 10.1111/1469-7610.00004. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Shelton KH, Thapar A. Family conflict interacts with genetic liability in predicting childhood and adolescent depression. J Am Acad Child Adolesc Psychiatry. 2006;45(7):841–848. doi: 10.1097/01.chi.0000219834.08602.44. [DOI] [PubMed] [Google Scholar]
- Rice F, Thapar A. Depression and anxiety in childhood and adolescence: Developmental pathways, genes and environment. Handbook of behavior genetics, Spring Science. 2009;Chapter 26:379–396. [Google Scholar]
- Rushton JL, Forcier M, Schectman RM. Epidemiology of depressive symptoms in the National Longitudinal Study of Adolescent Health. J Am Acad Child Adolesc Psychiatry. 2002;41(2):199–205. doi: 10.1097/00004583-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Ryder AG, Sun J, Zhu X, Yao S, Chentsova-Dutton YE. Depression in China: Integrating developmental psychopathology and cultural-clinical psychology. J Clin Child Adolesc Psychol. 2012;41(5):682–694. doi: 10.1080/15374416.2012.710163. [DOI] [PubMed] [Google Scholar]
- Scourfield J, Rice F, Thapar A, Harold GT, Martin N, McGuffin P. Depressive symptoms in children and adolescents: changing aetiological influences with development. J Child Psychol Psychiatry. 2003;44(7):968–976. doi: 10.1111/1469-7610.00181. [DOI] [PubMed] [Google Scholar]
- Sun Y, Tao F, Hao J, Wan Y. The mediating effects of stress and coping on depression among adolescents in China. J Child Adolesc Psychiatr Nurs. 2010;23(3):173–180. doi: 10.1111/j.1744-6171.2010.00238.x. [DOI] [PubMed] [Google Scholar]
- Tepper P, Liu X, Guo C, Zhai J, Liu T, Li C. Depressive symptoms in Chinese children and adolescents: Parent, teacher, and self reports. J Affect Disord. 2008;111(2):291–298. doi: 10.1016/j.jad.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. The Lancet. 2012;379(11):60871–60874. doi: 10.1016/S0140-6736(11)60871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tram JM, Cole DA. A multimethod examination of the stability of depressive symptoms in childhood and adolescence. J Abnorm Psychol. 2006;115(4):674–686. doi: 10.1037/0021-843X.115.4.674. [DOI] [PubMed] [Google Scholar]
- Unger JB, Lessov-Schlaggar CN, Pang Z, Guo Q, Ning F, Gallaher P, Lee L, Cao W, Conti D, Johnson CA. Heritability of Smoking, Alcohol Use, and Psychological Characteristics Among Adolescent Twins in Qingdao, China. Asia Pac J Public Health. 2011;23(4):568–565. doi: 10.1177/1010539509351052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen M, Van Der Meij A, van Deurzen P, Janzing J, Arias-Vasquez A, Buitelaar J, et al. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol Psychiatry. 2008;15(3):260–271. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- Windle M, Dumenci L. An investigation of maternal and adolescent depressed mood using a latent trait-state model. J Res Adolesc. 1998;8(4):461–484. [Google Scholar]
- Zhang K, Xu Q, Xu Y, Yang H, Luo J, Sun Y, et al. The combined effects of the 5-HTTLPR and 5-HTR1A genes modulates the relationship between negative life events and major depressive disorder in a Chinese population. J Affect Disord. 2009;114(1–3):224–231. doi: 10.1016/j.jad.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Zhong BL, Ding J, Chen HH, Li Y, Xu HM, Tong J, et al. Depressive disorders among children in the transforming China: An epidemiological survey of prevalence, correlates, and service use. Depress Anxiety. 2013 doi: 10.1002/da.22109. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang Y, Deng XL, Eisenberg N, Wolchik SA, Tein JY. Relations of parenting and temperament to Chinese children’s experience of negative life events, coping efficacy, and externalizing problems. Child Dev. 2008;79(3):493–513. doi: 10.1111/j.1467-8624.2008.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]