In this review paper, Rodero and Crow outline the current understanding of the type I interferonopathies.
Abstract
Type I interferon is a potent substance. As such, the induction, transmission, and resolution of the type I interferon–mediated immune response are tightly regulated. As defined, the type I interferonopathies represent discrete examples of a disturbance of the homeostatic control of this system caused by Mendelian mutations. Considering the complexity of the interferon response, the identification of further monogenic diseases belonging to this disease grouping seems likely, with the recognition of type I interferonopathies becoming of increasing clinical importance as treatment options are developed based on an understanding of disease pathology and innate immune signaling. Definition of the type I interferonopathies indicates that autoinflammation can be both interferon and noninterferon related, and that a primary disturbance of the innate immune system can “spill over” into autoimmunity in some cases. Indeed, that several non-Mendelian disorders, most particularly systemic lupus erythematosus and dermatomyositis, are also characterized by an up-regulation of type I interferon signaling suggests the possibility that insights derived from this work will have relevance to a broader field of clinical medicine.
Introduction
In 2003 we highlighted aspects of phenotypic overlap between the rare Mendelian encephalopathy Aicardi-Goutières syndrome (AGS), the complex autoimmune disease systemic lupus erythematosus (SLE), and certain congenital viral infections, including transplacentally acquired human immunodeficiency virus (HIV-1), and postulated that this overlap might result from the common pathological feature of an up-regulation of interferon α activity (Crow et al., 2003). The subsequent partial dissection of the genetic basis of AGS (Crow et al., 2006a,b; Rice et al., 2009), the molecular definition of a monogenic form of SLE associated with up-regulated type I interferon (Briggs et al., 2011; Lausch et al., 2011), and the developing understanding of a primary link between nucleic acid metabolism and interferon induction led to the proposition, in 2011, of the grouping of Mendelian disorders associated with an up-regulation of type I interferon signaling as a novel set of human inborn errors of immunity, in which such constitutive up-regulation is central to pathogenesis (Crow, 2011). In 2015, a framework was proposed for the consideration of the pathogenesis of this group of diseases (Crow, 2015; Crow and Manel, 2015), which can be viewed as analogous to previously described single-gene defects in immune signaling pathways leading to primary immunodeficiency (Casanova et al., 2005) and monogenic autoinflammation (Kastner et al., 2010).
At the outset, it is important to state that, as strictly defined, the central tenet of the type I interferonopathy concept remains unproven; i.e., definitive evidence that pathology is determined by an up-regulation of type I interferon signaling is lacking. Indeed, it will not be until we have therapeutic agents that specifically target type I interferon signaling, and use them in putative type I interferonopathy patients, that the contribution of type I interferon to clinical phenotype will become clear. Simply put, at this time, it is still possible that the finding of up-regulated type I interferon signaling in certain phenotypes represents an association rather than a pathologically causal relationship. That being said, as we argue below, the observation of phenotypic overlap, the elucidation of shared pathomechanisms through human genetics, in vitro and in vivo experimentation, and the first results of early treatment trials all give support to the scientific validity of the type I interferonopathy grouping.
Because this is a field in its infancy, it is necessary to avoid being overly didactic. Thus, except in a few cases, most notably perhaps disease related to mutations in TREX1 and RNASEH2B, it is important to acknowledge that the true clinical spectrum and frequency of features associated with particular genotypes is likely not known. This point is well illustrated by the expansion of the phenotype associated with mutations in TMEM173 in a period of a little over two years, now spanning early-onset systemic inflammation with mutilating skin lesions and lethal pulmonary inflammation (Jeremiah et al., 2014; Liu et al., 2014), through to “idiopathic” lung fibrosis (Clarke et al., 2016; Picard et al., 2016) and isolated chilblain lupus inherited stably across several generations (König et al., 2016). Similar uncertainty exists in regard to questions central to disease pathogenesis, e.g., the exact source and nature of the endogenous ligands considered to induce a type I interferon response in certain of the type I interferonopathies. As such, the aim here is to draw out general themes relating to phenotype and pathology, fully expecting that our understanding of detail will change over the short to medium term.
Which diseases should be considered as type I interferonopathies?
Given that the acid test of response to anti-interferon therapy is not yet available, here we base our inclusion of distinct monogenic disorders as type I interferonopathies on evidence indicating a persistent up-regulation of type I interferon signaling, assessed by measuring the expression of interferon-stimulated genes (ISGs) and/or in vivo (animal)/ex vivo/in vitro experimental evidence. This group thus comprises 18 genotypes in which we consider that the link to enhanced interferon signaling is established (Table 1 and Fig. 1). The somewhat imprecise nature of these criteria means that we do not include, for example, gain-of-function mutations in STAT1, although it may be that future studies will demonstrate a functional relationship of ISG production to phenotype. For similar reasons, we exclude discussion of DNASE1L3, chronic granulomatous disease, prolidase deficiency, and some early components of the complement cascade, all of which are associated with an increased risk of SLE, so that one might predict an up-regulation of type I interferon signaling but where the point rests unproven. Other diseases that may also come to be considered in this grouping, but where we feel that the evidence currently remains uncertain, are those caused by mutations in CECR1 (Belot et al., 2014; Uettwiller et al., 2016), TRNT1 (Frans et al., 2016), and RNASET2 (Tonduti et al., 2016).
Table 1. Genotypes considered as type I interferonopathies in this manuscript, with protein function, link to interferon signaling, proposed molecular mechanism, and currently recognized associated clinical phenotypes.
| Gene | Protein function | Sensing/activation pathway related to type I interferon signaling | Mutation effect | Major patient phenotypes |
|---|---|---|---|---|
| TREX1 | Deoxyribonuclease | Cytosolic DNA | LOF (recessive or dominant-negative) | AGS, FCL, SLE |
| SAMHD1 | Control of dNTP pool (±nuclease) | Cytosolic DNA (±cytosolic RNA) | LOF (recessive) | AGS, FCL, CVD |
| TMEM173 | Transduction of cytosolic type I interferon signal | Cytosolic DNA (±cytosolic RNA) | GOF (dominant) | SAVI, FCL |
| RNASEH2A | Ribonuclease | Cytosolic RNA:DNA hybrids | LOF (recessive) | AGS |
| RNASEH2B | Ribonuclease | Cytosolic RNA:DNA hybrids | LOF (recessive) | AGS, SP |
| RNASEH2C | Ribonuclease | Cytosolic RNA:DNA hybrids | LOF (recessive) | AGS |
| POLA1 | Polymerase | Cytosolic RNA:DNA hybrids | X-linked recessive | XLPDR |
| ADAR1 | RNA editing | Cytosolic RNA | LOF (recessive or dominant-negative) | AGS, DSH, BSN, SP |
| IFIH1 | dsRNA sensor | Cytosolic RNA | GOF (dominant) | AGS, SP, SMS |
| RIG-I | dsRNA sensor | Cytosolic RNA | GOF (dominant) | Atypical SMS |
| SKIV2L | RNA helicase | Cytosolic RNA | LOF (recessive) | THES |
| UPS18 | Inhibition of ISG transcription | IFNAR1 signaling | LOF (recessive) | pseudo-TORCH |
| ISG15 | Inhibition of ISG transcription | IFNAR1 signaling | LOF (recessive) | MSMD, ICC |
| PSMB8 | Proteasome | Unknown | LOF (recessive) | PRAAS |
| PSMB4 | Proteasome | Unknown | LOF (recessive) | PRAAS |
| PSMA3 | Proteasome | Unknown | LOF (recessive) | PRAAS |
| ACP5 | Phosphatase activity related to osteopontin | Unknown | LOF (recessive) | SPENCD, SLE, cytopenias |
| C1q | Alternative complement pathway activity | Unknown | LOF (recessive) | SLE |
BSN, bilateral striatal necrosis; CVD, cerebrovascular disease; DSH, dyschromatosis symmetrica hereditaria; FCL, familial chilblain lupus; GOF, gain-of-function; ICC, intracranial calcification; LOF, loss-of-function; MSMD, Mendelian susceptibility to mycobacterial disease; PRAAS, proteasome-associated autoinflammatory syndrome; SAVI, STING-associated vasculopathy with onset in infancy; SMS, Singleton-Merten syndrome; SP, spastic paraparesis; SPENCD, spondyloenchondrodysplasia; XLPDR, X-linked reticulate pigmentary disorder.
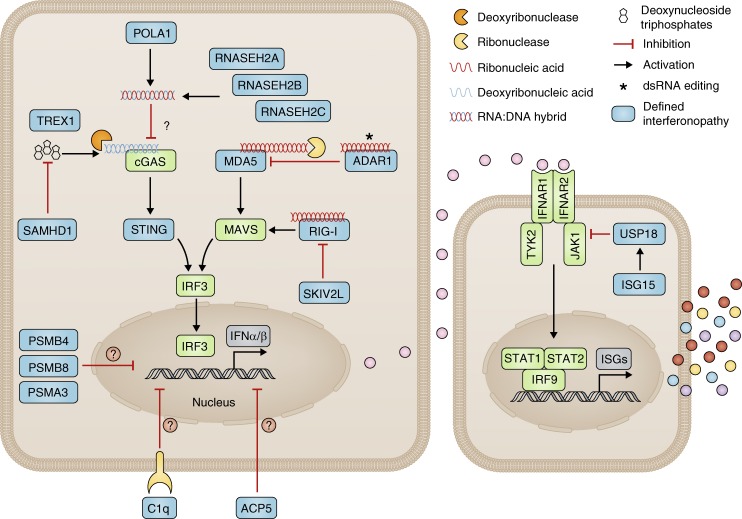
Figure 1.
Type I interferon signaling and type I interferonopathies as currently assigned. Diseases considered as monogenic interferonopathies are represented by blue boxes. This schema alludes to at least seven possible cellular mechanisms resulting in sustained activation of interferon signaling caused by the following: (1) loss-of-function mutations leading to increased cytosolic DNA (TREX1 [Stetson et al., 2008] and SAMHD1 [Behrendt et al., 2013; Rehwinkel et al., 2013]) or RNA/DNA hybrid (RNASEH2A, RNASEH2B and RNASEH2C, POLA1) sensing (Hiller et al., 2012; Mackenzie et al., 2016; Starokadomskyy et al., 2016); (2) loss-of-function mutations leading to a defect in RNA editing and abnormal sensing of self–nucleic acid RNA species in the cytosol (ADAR1 [Liddicoat et al., 2015; Pestal et al., 2015]); (3) gain-of-function mutations leading to constitutive activation of cytosolic interferon signaling pathways/increased sensitivity to cytosolic nucleic acid ligands (MDA5 [Rice et al., 2014], RIG-I [Jang et al., 2015], and STING [Liu et al., 2014]); (4) loss-of-function mutations leading to aberrant RNA signaling via MAVS caused by a disturbance of the unfolded protein response (SKIV2L [Eckard et al., 2014]); (5) loss-of-function mutations in molecules responsible for limiting interferon receptor (IFNAR1/2) signaling leading to uncontrolled ISG production (USP18 [Meuwissen et al., 2016] and ISG15 [Zhang et al., 2015]); (6) proteasomal dysfunction leading to increased interferon signaling through an unknown mechanism (PSMA3, PSMB4, and PSMB8 [Brehm et al., 2015]; we do not include the so-far single-published mutations in PSMB9 and POMP); and (7) loss-of-function mutations in TRAP/ACP5 (Briggs et al., 2011; Lausch et al., 2011) and C1q (Lood et al., 2009; Santer et al., 2010) where we consider the mechanisms leading to type I interferon signaling are yet to be fully clarified (we do not include mutations in other molecules of the complement pathway as a clear demonstration of enhanced interferon signaling has not been established).
It is of course the case that dysfunction of certain proteins can have more than one biological consequence. For example, in the context of the putative type I interferonopathies, ADAR1 has both an interferon-related and a distinct developmental (MDA5/MAVS independent) function (Pestal et al., 2015). Of possible note also, Rnaseh2b knockout confers embryonic lethality in the mouse (Hiller et al., 2012; Reijns et al., 2012), in the absence of the induction of an interferon response, which is seen in the corresponding hypomorphic model (Mackenzie et al., 2016; Pokatayev et al., 2016). Whether or not this difference reflects distinct biological roles of the RNase H2 complex or the timing/degree of a shared disturbance of ribonucleotide excision repair remains unknown. The situation appears clearer with respect to mutations in SKIV2L causing tricho-hepato-enteric syndrome (THES). Here, there is a markedly elevated ISG expression and a proven link to interferon signaling via a disturbance of the unfolded protein response (Eckard et al., 2014). However, the same disease phenotype can be caused by mutations in TTC37, where no such interferon signature is present, and where, in contrast to SKIV2L, in vitro assays do not suggest a role in interferon signaling. These data lead to the conclusion that most of the features of THES are the consequence of a loss of cytosolic RNA exosome function in RNA turnover, rather than an aberrant interferon response that is apparently specific to SKIV2L deficiency.
It is interesting to view the aforementioned observations in regards to the potential efficacy of future anti-interferon therapy. Thus, the involvement/dysfunction of noninterferon-related disease pathways in a phenotype would variably limit the efficacy of such treatment. On this basis then, one can conceptualize the existence of more “pure” interferonopathies, where anti-interferon therapy would be expected to have the greatest benefit, and “mixed” phenotypes, where such therapies would be of more limited or, indeed, no utility.
Is it appropriate to use the term type I interferonopathies (I)?
In 1957 Isaacs and Lindenmann described a soluble factor that protects cells from viral infection, which agent, in consideration of its antiviral interfering properties, they termed interferon (Isaacs and Lindenmann, 1957; Isaacs et al., 1957). We now know that multiple species of type I interferon exist, with this heterogeneity arising from the presence of 13 functional α genes and single genes for interferon β, ε, κ, and ω. Despite almost 60 years of active research, an understanding of the role of these different interferon species has been hampered by the inability to directly measure type I interferon protein in biological samples using available ELISAs. Correspondingly, type I interferon mRNA is usually unrecordable in peripheral blood from healthy individuals, even after vaccination (Sobolev et al., 2016), or in patients with putative type I interferonopathies. Such low levels of circulating interferons likely reflect the very high biological potency of these cytokines, with most cell types expressing a type I interferon receptor. As a practical work-around of this problem, researchers have made extensive use of the measurement of the mRNA of genes that are induced by interferon (ISGs), thus effectively capturing an amplified signal consequent upon the interferon stimulus (Baechler et al., 2003; Bennett et al., 2003). Indeed, we have shown that the measurement of six ISGs represents a powerful screening tool for the identification of several of the putative type I interferonopathies (Rice et al., 2013).
The repertoire of ISGs produced in response to type I, II, or III interferons show considerable overlap, begging the legitimate question as to whether or not it is type I interferons that are relevant—solely, partially, or not at all—to the up-regulation of ISGs seen in the diseases discussed here. At least in the context of AGS, data have been published to indicate that interferon activity in patient material, as measured using an antiviral cytopathic protection assay, can be neutralized by antiserum against interferon α but not β (Lebon et al., 2002; Rice et al., 2013). Furthermore, levels of interferon γ were below the detectable range of a sensitive ELISA, and thus possibly inconsistent with the degree of interferon activity recorded in certain of these samples. Although these data are important, the field awaits the availability of high-sensitivity protein assays allowing the quantification of discrete interferons, at least in circulation. Such a tool could be usefully combined with measures of ISG production and/or interferon activity, thus capturing the relationship between the inducing (protein) signal and the response (ISGs/antiviral activity) to that signal and thereby enabling an exploration of interferon signaling dynamics. As we have previously noted, the absence of a reliable, high-throughput measure of type I interferon in routine medical practice goes some way to explaining why the concept of the type I interferonopathies as a discrete pathological grouping has only recently been mooted.
Is it appropriate to use the term type I interferonopathies (II)?
Returning to an earlier point, the question arises as to whether the enhanced interferon signaling identified in the proposed type I interferonopathies represents a true pathological factor or simply a disease biomarker. The answer to this question is of fundamental importance because the former possibility implies the potential utility of therapeutic approaches targeting interferon up- and downstream signaling.
Considering clinically derived observations, the fact that, in its classical form (Aicardi and Goutières, 1984), AGS is such a remarkable Mendelian mimic of certain congenital infections provides circumstantial support to the up-regulation of interferon signaling, recorded in both situations, representing a common pathogenic link. Second, numerous reports describe the occurrence, after treatment with interferon, of features such as digital vasculitis (Al-Zahrani et al., 2003), SLE (Rönnblom et al., 1990), and glaucoma (Kwon et al., 2001), which are also seen in the putative type I interferonopathies. As a final point, and as discussed in more detail below, the recognition of a shared set of clinical signs, most particularly intracranial calcification and skin inflammation, across several of these genotypes is further evidence in favor of pathogenic overlap.
Experimental evidence also supports a primary role for interferon in the diseases discussed here. Likely indicative of intrathecal synthesis, levels of interferon activity in the cerebrospinal fluid of AGS patients are consistently higher than in matched serum samples (Crow et al., 2015). Undoubtedly, interferon is a neurotoxin, and experiments undertaken in mice demonstrate that overexpression of interferon in the central nervous system (CNS) results in neuropathology reminiscent of that seen in certain type I interferonopathies (Akwa et al., 1998; Campbell et al., 1999; Kavanagh et al., 2016). Relevant crosses in other mouse models, in particular double knockouts involving the type I interferon receptor, provide unequivocal evidence of the importance of type I interferon signaling in these settings (Stetson et al., 2008; Goldmann et al., 2015). Furthermore, given that cytosolic nucleic acid recognition represents a principal trigger for the induction of type I interferon, it is of note that 11 of the 18 diseases discussed here involve mutations in genes known to play a role in nucleic acid metabolism/signaling. We highlight particularly that gain-of-function mutations in MDA5 (IFIH1), RIG-I (DDX58), and STING (TMEM173), essential components of cytosolic nucleic acid signaling to a type I interferon response, and biallelic loss-of-function mutations in USP18 or ISG15, both involved in the negative regulation of ISG expression, are all associated with currently recognized type I interferonopathy phenotypes.
Phenotypic overlap and differences, variable expression, and nonpenetrance
As touched on above, there is a striking overlap of clinical features, particularly the involvement of the CNS and the skin, across several of the disorders classified here as type interferonopathies (Fig. 2). Indeed, we would highlight the value of searching for the presence of intracranial calcification, most easily appreciated on computed tomography, even in the absence of overt neurological signs, and the presence of vasculitic/chilblain-like skin lesions as highly useful clinical markers of this disease grouping. At the same time, major phenotypic differences exist between certain of these genotypes. Thus, the severe lung disease that frequently accompanies mutations in TMEM173 has not been reported in the context of other putative type I interferonopathies, whereas the essentially distinct complex diseases SLE, systemic sclerosis, and dermatomyositis all correlate with the presence of a type I interferon signature. Such observations apparently challenge the suggestion of the primacy of interferon as a shared pathogenic molecule. While acknowledging this point, we have already highlighted that dysfunction of certain proteins can have more than one biological consequence, which might explain different phenotypic characteristics across genotypes. Furthermore, distinct expression patterns of relevant disease-associated proteins, their protein partners, interferon-inducing signaling components, and proteins involved in alternative (“redundant”) signaling pathways according to the gene/protein mutated might also be relevant. Finally, we draw attention to the possible importance of the timing of a putative interferon-related insult. Perhaps instructive here, similar to AGS, congenital HIV-1 infection is characterized by intracranial calcification, white matter abnormalities, cerebral atrophy, and high levels of interferon α (Kauffman et al., 1992; Krivine et al., 1992; DeCarli et al., 1993), whereas these radiological signs are not seen with postnatally acquired HIV-1 infections, suggesting that the developing brain is specifically susceptible to intrauterine viral exposure/the host interferon response (Tardieu et al., 2000).
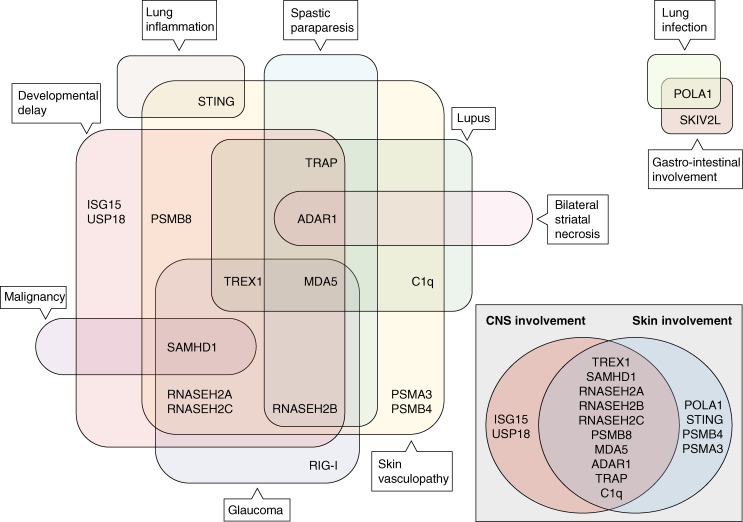
Figure 2.
Specific and overlapping features of monogenic type I interferonopathies. In the broadest sense, CNS and skin disease are the most common features of the type I interferonopathies. Discrete neurological phenotypes associated with mutations in AGS-associated genes include “nonsyndromic” spastic paraparesis (RNASEH2B, ADAR1, and IFIH1 [Crow et al., 2014]) and bilateral striatal necrosis (ADAR1 [Livingston et al., 2014]). Glaucoma is a common feature of AGS (Crow et al., 2015) and is also seen in the Singleton-Merton syndrome phenotype associated with gain-of-function mutations in IFIH1 (Bursztejn et al., 2015; Rutsch et al., 2015) and DDX58 (RIG-I [Jang et al., 2015]). SLE (lupus) is most frequently associated with mutations in ACP5 (An et al., 2016; Briggs et al., 2016) and C1q (Lood et al., 2009; Santer et al., 2010). Malignancy has only been reported in the context of SAMHD1 (Clifford et al., 2014; Merati et al., 2015). Lung inflammation is so far restricted to patients with mutations in TMEM173 (STING; Liu et al., 2014; Clarke et al., 2016; Picard et al., 2016). The phenotypes associated with mutations in POLA1 and SKIV2L appear distinct.
It is also pertinent to acknowledge the variable expression, and even nonpenetrance, seen in certain type I interferonopathies, particularly relating to the recurrent dominant-negative mutations in ADAR1 (Gly1007Arg; Rice et al., 2012; Livingston et al., 2014) and TREX1 (p.Asp18Asn; Abe et al., 2013) and dominant gain-of-function mutations in IFIH1 (Rice et al., 2014) and TMEM173 (Jeremiah et al., 2014). To explain these observations, we need to invoke differential exposure to environmental triggers such as infection or the effect of genetic modifiers. Indeed, considering a putative role of physical stressors, note should be made of the cold dependency of the skin lesions seen in the type I interferonopathies and of a striking temporal relationship between the onset of ADAR1-related bilateral striatal necrosis and preceding infection (Livingston et al., 2014). Whether vaccination represents a disease trigger is an important, and currently unanswered, question. Meanwhile, the possibility of a “cumulative” genetic burden contributing to cellular pathology is notable in light of recently published data on the group of type I interferonopathies caused by loss-of-function mutations in proteasome subunits (Brehm et al., 2015).
As an allied but distinct point, the identification of dominant gain-of-function mutations in IFIH1 has highlighted the possibility of clinical nonpenetrance into old age in the presence of life-long, marked overexpression of ISGs (Rice et al., 2014). This situation is reminiscent of a mouse model where transgenic expression of a picornavirus RNA-dependent RNA polymerase (RdRP) leads to a dramatic up-regulation of ISG stimulation and profound viral resistance via an MDA5/MAVS-dependent pathway, but where the mice are entirely healthy (Painter et al., 2015). The contrast with mouse models also showing type I interferon up-regulation, but demonstrating a clear link between interferon expression and phenotype, might relate to the involvement of other inflammatory cytokines in the latter cases. A further possibility is that the constitutively augmented RdRP-induced antiviral network is balanced by up-regulation of type I interferon negative regulators such as Usp18, and which effect might be tissue specific. Interestingly, polymorphisms across TMEM173 (Yi et al., 2013) and IFIH1 (Shigemoto et al., 2009), some of which occur at relatively high population frequencies, can be associated with marked differential interferon induction in vitro. It may be that such genetic variation reflects an evolutionary balance between the response to infection and the risk of inflammatory disease (Sharma et al., 2015). The observation of heritable high interferon production in certain families demonstrating an increased risk of SLE possibly represents a further piece of evidence in favor of this hypothesis (Niewold et al., 2007).
Autoinflammation or autoimmunity?
At least in regard to AGS, different authors variably refer to the associated pathology as either autoimmune or autoinflammatory in basis, with the use of the latter term being particularly favored where the emphasis is being placed on the link to SLE. A suggested definition of autoinflammation relates to disorders characterized by abnormally increased inflammation, mediated predominantly by cells and molecules of the innate immune system, with a significant host predisposition (Kastner et al., 2010). Thus, according to the schema outlined above, we propose that, as a group, the type I interferonopathies can reasonably be considered as autoinflammatory in origin, with “spill-over” into autoimmunity in some cases. Having made this point, we would add that rather than being overly concerned by questions of classification, the cornerstone of the type I interferonopathy concept, as envisaged here, relates to a primary role of type I interferon in disease pathogenesis, irrespective of the relative involvement of innate/adaptive immune components.
There is a clear association of certain type I interferonopathies with autoimmunity, and SLE in particular, as indicated by the co-occurrence of such rare phenotypes (e.g., Dale et al., 2000; De Laet et al., 2005; Hacohen et al., 2015; Van Eyck et al., 2015). Furthermore, a broad spectrum of autoantibodies has been observed in patients with AGS (Cuadrado et al., 2015a; Zhang et al., 2015; Cattalini et al., 2016), albeit distinct from other autoimmune diseases. At the same time, it is striking that monogenic interferon-related inflammation, as so-far defined, is most frequently unaccompanied by frank autoimmunity (Crow et al., 2015), so that it is difficult to know if these autoantibodies are pathologically relevant or represent an epiphenomenon consequent upon a more general immune dysregulation. The (inconsistent) link to overt autoimmunity in certain type I interferonopathies might reflect variable engagement of the adaptive immune system secondary to an initial, interferon-associated, innate immune disturbance. Having said this, in contrast to AGS and STING-related disease for example, there is a remarkably high risk of autoimmune cytopenias and early-onset SLE in the context of mutations in ACP5 (Briggs et al., 2011, 2016; Lausch et al., 2011) and complement components (Troedson et al., 2013), suggesting that these encoded proteins have particular roles more closely aligned to the maintenance of self-tolerance.
The acceptance of the type I interferonopathies as autoinflammatory disorders implies that autoinflammation can be considered as both interferon and noninterferon related. From a clinical perspective, it is clear that certain type I interferonopathies can present as “classical” autoinflammatory phenotypes, demonstrating recurrent fevers and/or organ-specific involvement with elevated markers of systemic inflammation in the absence of autoimmunity and underlying infection. At the same time, most patients conforming to this clinical description that we have tested show no evidence of enhanced type I interferon signaling (unpublished data), lending support to the specificity of type I interferon–induced gene transcript measurement as a screening tool. This distinction is likely also reflected in the observation that noninterferon-related autoinflammation is not normally associated with a risk of lupus.
Cellular pathology and therapeutic approaches
The cellular pathology of the type I interferonopathies is associated with a diversity of mechanisms currently encompassing nucleic acid signaling in the broadest sense, proteasomal dysfunction and the unfolded protein response. Mouse work has been particularly instructive in defining the signaling pathways involved in several putative type I interferonopathies, so that we can now think of 11 of these genotypes as being transduced via cytosolic DNA (3), RNA (4) or RNA:DNA (4) hybrid sensing. Most type I interferonopathy genotypes relate to loss of protein function, whereas mutations in MDA5, RIG-I, and STING are associated with a gain-of-function resulting in either constitutive activation of the relevant molecule and/or enhanced sensitivity for endogenous ligands.
We make the point here that different treatment strategies are implied according to pathological mechanism relevant to the type I interferonopathy being considered (Fig. 3). Thus, based on the hypothesis that type I interferon might, in certain AGS-related genotypes, be induced by cytosolic recognition of DNA derived from endogenous retroelements (Stetson et al., 2008; Beck-Engeser et al., 2011), we are currently running the first ever clinical trial in AGS, using reverse transcription inhibitors (https://clinicaltrials.gov/ct2/show/NCT02363452). From a practical perspective, even if the precise nature of the interferon-inducing signal remains unclear in all cases, success in defining the signaling pathways involved in certain genotypes is already informing potential therapeutic strategies. Thus, as indicated in mice, inhibition of cGAS or STING would be predicted as relevant for TREX1- and RNASEH2-related disease (Gall et al., 2012; Gao et al., 2015; Gray et al., 2015; Mackenzie et al., 2016; Pokatayev et al., 2016), but not for disease consequent upon mutations in ADAR1 (Liddicoat et al., 2015; Pestal et al., 2015) or IFIH1 (Funabiki et al., 2014). We note recent work suggesting that antimalarial drugs such as hydroxychloroquine could be beneficial in this context, by antagonizing dsDNA stimulation of cGAS (An et al., 2015). In contrast, TBK1 inhibition might be relevant to mutant genotypes induced by either DNA or RNA (Hasan et al., 2015). Notably, crossing of the Trex1-null mouse with mice heterozygous for any of cGAS, Tmem173, or Irf3 significantly ameliorates the otherwise lethal phenotype. Similarly, crossing the ENU gain-of-function Mda5 mutant with a Mavs heterozygote was associated with a marked reduction in the severity of the associated nephritis. These data are important in suggesting a degree of “suppleness” in the response to pathological signaling. That is, they indicate that future therapies blocking these molecules might demonstrate clinical efficacy at doses that may not entail iatrogenic immunodeficiency consequent upon loss of signaling to viral nucleic acids. Perhaps relevant to this point also, our experience with JAK1/2 inhibition, see below, has so far been notable by the absence of an increased risk of infection.
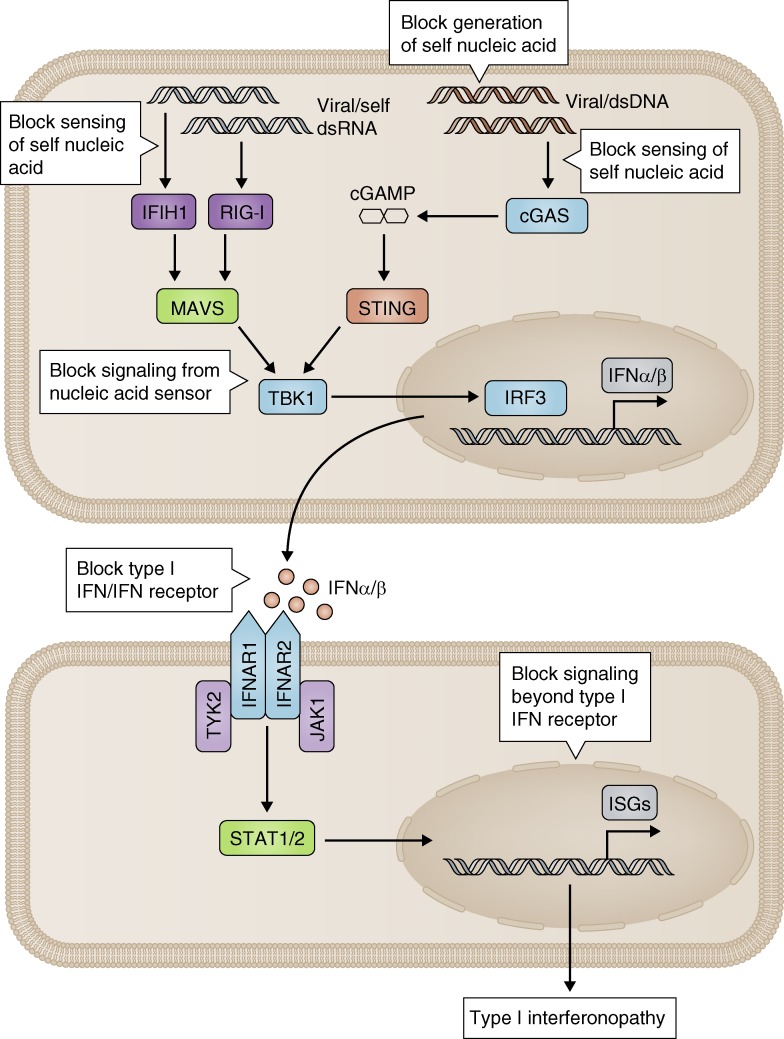
Figure 3.
Outline of treatment strategies in the type I interferonopathies. Dependent on the underlying pathological mechanism, therapeutic approaches in the type I interferonopathies might include blocking the generation (e.g., using reverse transcription inhibitors: https://clinicaltrials.gov/ct2/show/NCT02363452), sensing (e.g., cGAS inhibition by hydroxychloroquine [An et al., 2015]), or signaling (e.g., TBK1 inhibition [Hasan et al., 2015]) of putative self–nucleic acids engaging the type I interferon innate immune machinery and blocking of interferon itself (e.g., with anti–type I interferon antibodies), the IFNAR receptor, or the signaling cascades distal to interferon ligand binding (e.g., by JAK1 inhibition [Frémond et al., 2016]).
As postulated here, all type I interferonopathy pathology converges on up-regulated type I interferon signaling. Thus, any compounds that neutralize type I interferons, block the type I interferon receptor, or inhibit signaling downstream of the receptor might be of utility. Currently, no drugs are specifically licensed for any member of the type I interferonopathy grouping. We have recently described the effect of JAK1/2 inhibition using ruxolitinib in the context of mutations in TMEM173, where we observed highly promising efficacy in all aspects of the clinical phenotype (systemic inflammation, destructive skin lesions, and pulmonary disease; Frémond et al., 2016). The same may also be true of proteasome-associated autoinflammatory syndromes (Jabbari et al., 2015). Anti-interferon therapy is being actively pursued in the treatment of SLE (Wang et al., 2013; Oon et al., 2016), with antibodies against the type I interferon receptor showing particular promise. We have not been able to test these molecules in any monogenic interferonopathy. Interestingly, inactivated interferon α 2b coupled to a carrier protein can induce the production of a polyclonal antibody response against all 13 subtypes of interferon α, and a reduction of the associated interferon signature in high responders to vaccination (Ducreux et al., 2016). These data link nicely with those showing that patients with mutations in AIRE produce endogenous antibodies against all interferon α subtypes, but not interferon β, γ, or λ (Meyer et al., 2016). Despite the remarkably high affinity of these antibodies, considerably greater than those used in commercial trials, these patients do not suffer an increased burden of viral infection, perhaps because they maintain antiviral protection through interferon β. If it can be shown that any type I interferonopathy relates predominately/exclusively to interferon α, the therapeutic use of such antibodies might prove highly effective.
Finally, we highlight uncertainty regarding the cellular source of type I interferon production in distinct type I interferonopathies. Early data from the Trex1-null mouse indicated the importance of tissue-resident cells in disease pathology (Stetson et al., 2008), whereas more recent papers have emphasized a role for hematopoietic cells in driving disease (Ahn et al., 2014; Peschke et al., 2016). The latter results are important in pointing to a lack of current knowledge relating to the efficacy of bone marrow transplant in any of the type I interferonopathies. Allied to this issue is the question of which cell types drive the brain involvement characteristic of the majority of type I interferonopathies so far identified (Cuadrado et al., 2015b; Goldmann et al., 2015) and of blood–brain barrier penetration in regards to drug therapy.
Conclusion
There has been a rapid adoption of the type I interferonopathy paradigm, with the definition of 10 associated genotypes since the introduction of the term into the medical lexicon in 2011. The study of the monogenic type I interferonopathies provides an unprecedented opportunity to define the role of type I interferons in human health and disease—through the identification of patients showing discrete molecular perturbation of proteins essential to interferon homeostasis. The model outlined here predicts that such studies will be of real clinical value as therapies to reduce type I interferon levels and/or block interferon signaling become available.
Acknowledgments
We thank Gillian Rice, Isabelle Melki, and Marie-Louise Frémond for their critical reading of the manuscript.
Y.J. Crow acknowledges funding from the European Research Council (GA 309449: Fellowship to Y.J. Crow), ERA-NET Neuron (MR/M501803/1), and a state subsidy managed by the National Research Agency (France) under the “Investments for the Future” (ANR-10-IAHU-01).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- AGS
- Aicardi-Goutières syndrome
- CNS
- central nervous system
- ISG
- interferon-stimulated gene
- RdRP
- RNA-dependent RNA polymerase
- SLE
- systemic lupus erythematosus
- THES
- tricho-hepato-enteric syndrome
References
- Abe J., Izawa K., Nishikomori R., Awaya T., Kawai T., Yasumi T., Hiragi N., Hiragi T., Ohshima Y., and Heike T.. 2013. Heterozygous TREX1 p.Asp18Asn mutation can cause variable neurological symptoms in a family with Aicardi-Goutières syndrome/familial chilblain lupus. Rheumatology (Oxford). 52:406–408. 10.1093/rheumatology/kes181 [DOI] [PubMed] [Google Scholar]
- Ahn J., Ruiz P., and Barber G.N.. 2014. Intrinsic self-DNA triggers inflammatory disease dependent on STING. J. Immunol. 193:4634–4642. 10.4049/jimmunol.1401337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicardi J., and Goutières F.. 1984. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann. Neurol. 15:49–54. 10.1002/ana.410150109 [DOI] [PubMed] [Google Scholar]
- Akwa Y., Hassett D.E., Eloranta M.L., Sandberg K., Masliah E., Powell H., Whitton J.L., Bloom F.E., and Campbell I.L.. 1998. Transgenic expression of IFN-α in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J. Immunol. 161:5016–5026. [PubMed] [Google Scholar]
- Al-Zahrani H., Gupta V., Minden M.D., Messner H.A., and Lipton J.H.. 2003. Vascular events associated with alpha interferon therapy. Leuk. Lymphoma. 44:471–475. 10.1080/1042819021000055066 [DOI] [PubMed] [Google Scholar]
- An J., Woodward J.J., Sasaki T., Minie M., and Elkon K.B.. 2015. Cutting edge: Antimalarial drugs inhibit IFN-β production through blockade of cyclic GMP-AMP synthase-DNA interaction. J. Immunol. 194:4089–4093. 10.4049/jimmunol.1402793 [DOI] [PubMed] [Google Scholar]
- An J., Briggs T.A., Dumax-Vorzet A., Alarcón-Riquelme M.E., Belot A., Beresford M., Bruce I., Carvalho C., Chaperot L., Frostegård J., et al. 2016. Tartrate-resistant acid phosphatase deficiency in the predisposition to systemic lupus erythematosus. Arthritis Rheumatol. 10.1002/art.39810 [DOI] [PubMed] [Google Scholar]
- Baechler E.C., Batliwalla F.M., Karypis G., Gaffney P.M., Ortmann W.A., Espe K.J., Shark K.B., Grande W.J., Hughes K.M., Kapur V., et al. 2003. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA. 100:2610–2615. 10.1073/pnas.0337679100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck-Engeser G.B., Eilat D., and Wabl M.. 2011. An autoimmune disease prevented by anti-retroviral drugs. Retrovirology. 8:91 10.1186/1742-4690-8-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt R., Schumann T., Gerbaulet A., Nguyen L.A., Schubert N., Alexopoulou D., Berka U., Lienenklaus S., Peschke K., Gibbert K., et al. 2013. Mouse SAMHD1 has antiretroviral activity and suppresses a spontaneous cell-intrinsic antiviral response. Cell Reports. 4:689–696. 10.1016/j.celrep.2013.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belot A., Wassmer E., Twilt M., Lega J.C., Zeef L.A., Oojageer A., Kasher P.R., Mathieu A.L., Malcus C., Demaret J., et al. 2014. Mutations in CECR1 associated with a neutrophil signature in peripheral blood. Pediatr. Rheumatol. Online J. 12:44 10.1186/1546-0096-12-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett L., Palucka A.K., Arce E., Cantrell V., Borvak J., Banchereau J., and Pascual V.. 2003. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197:711–723. 10.1084/jem.20021553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm A., Liu Y., Sheikh A., Marrero B., Omoyinmi E., Zhou Q., Montealegre G., Biancotto A., Reinhardt A., Almeida de Jesus A., et al. 2015. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J. Clin. Invest. 125:4196–4211. (published erratum appears in J. Clin. Invest. 2016. 126:795) 10.1172/JCI81260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs T.A., Rice G.I., Daly S., Urquhart J., Gornall H., Bader-Meunier B., Baskar K., Baskar S., Baudouin V., Beresford M.W., et al. 2011. Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat. Genet. 43:127–131. 10.1038/ng.748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs T.A., Rice G.I., Adib N., Ades L., Barete S., Baskar K., Baudouin V., Cebeci A.N., Clapuyt P., Coman D., et al. 2016. Spondyloenchondrodysplasia due to mutations in ACP5: A comprehensive survey. J. Clin. Immunol. 36:220–234. (published erratum appears in J. Clin. Immunol. 2016. 36:529) 10.1007/s10875-016-0252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursztejn A.C., Briggs T.A., del Toro Duany Y., Anderson B.H., O’Sullivan J., Williams S.G., Bodemer C., Fraitag S., Gebhard F., Leheup B., et al. 2015. Unusual cutaneous features associated with a heterozygous gain-of-function mutation in IFIH1: overlap between Aicardi-Goutières and Singleton-Merten syndromes. Br. J. Dermatol. 173:1505–1513. 10.1111/bjd.14073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I.L., Krucker T., Steffensen S., Akwa Y., Powell H.C., Lane T., Carr D.J., Gold L.H., Henriksen S.J., and Siggins G.R.. 1999. Structural and functional neuropathology in transgenic mice with CNS expression of IFN-α. Brain Res. 835:46–61. 10.1016/S0006-8993(99)01328-1 [DOI] [PubMed] [Google Scholar]
- Casanova J.L., Fieschi C., Bustamante J., Reichenbach J., Remus N., von Bernuth H., and Picard C.. 2005. From idiopathic infectious diseases to novel primary immunodeficiencies. J. Allergy Clin. Immunol. 116:426–430. 10.1016/j.jaci.2005.03.053 [DOI] [PubMed] [Google Scholar]
- Cattalini M., Galli J., Andreoli L., Olivieri I., Ariaudo G., Fredi M., Orcesi S., Tincani A., and Fazzi E.. IAGSA study group . 2016. Exploring autoimmunity in a cohort of children with genetically confirmed Aicardi-Goutières syndrome. J. Clin. Immunol. 36:693–699. 10.1007/s10875-016-0325-y [DOI] [PubMed] [Google Scholar]
- Clarke S.L., Pellowe E.J., de Jesus A.A., Goldbach-Mansky R., Hilliard T.N., and Ramanan A.V.. 2016. Interstitial lung disease caused by STING-associated vasculopathy with onset in infancy. Am. J. Respir. Crit. Care Med. 194:639–642. 10.1164/rccm.201510-2102LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford R., Louis T., Robbe P., Ackroyd S., Burns A., Timbs A.T., Wright Colopy G., Dreau H., Sigaux F., Judde J.G., et al. 2014. SAMHD1 is mutated recurrently in chronic lymphocytic leukemia and is involved in response to DNA damage. Blood. 123:1021–1031. 10.1182/blood-2013-04-490847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow Y.J. 2011. Type I interferonopathies: a novel set of inborn errors of immunity. Ann. N. Y. Acad. Sci. 1238:91–98. 10.1111/j.1749-6632.2011.06220.x [DOI] [PubMed] [Google Scholar]
- Crow Y.J. 2015. Type I interferonopathies: Mendelian type I interferon up-regulation. Curr. Opin. Immunol. 32:7–12. 10.1016/j.coi.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Crow Y.J., and Manel N.. 2015. Aicardi-Goutières syndrome and the type I interferonopathies. Nat. Rev. Immunol. 15:429–440. 10.1038/nri3850 [DOI] [PubMed] [Google Scholar]
- Crow Y.J., Black D.N., Ali M., Bond J., Jackson A.P., Lefson M., Michaud J., Roberts E., Stephenson J.B., Woods C.G., and Lebon P.. 2003. Cree encephalitis is allelic with Aicardi-Goutiéres syndrome: implications for the pathogenesis of disorders of interferon alpha metabolism. J. Med. Genet. 40:183–187. 10.1136/jmg.40.3.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow Y.J., Hayward B.E., Parmar R., Robins P., Leitch A., Ali M., Black D.N., van Bokhoven H., Brunner H.G., Hamel B.C., et al. 2006a Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutières syndrome at the AGS1 locus. Nat. Genet. 38:917–920. 10.1038/ng1845 [DOI] [PubMed] [Google Scholar]
- Crow Y.J., Leitch A., Hayward B.E., Garner A., Parmar R., Griffith E., Ali M., Semple C., Aicardi J., Babul-Hirji R., et al. 2006b Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutières syndrome and mimic congenital viral brain infection. Nat. Genet. 38:910–916. 10.1038/ng1842 [DOI] [PubMed] [Google Scholar]
- Crow Y.J., Zaki M.S., Abdel-Hamid M.S., Abdel-Salam G., Boespflug-Tanguy O., Cordeiro N.J.V., Gleeson J.G., Gowrinathan N.R., Laugel V., Renaldo F., et al. 2014. Mutations in ADAR1, IFIH1, and RNASEH2B presenting as spastic paraplegia. Neuropediatrics. 45:386–391. 10.1055/s-0034-1389161 [DOI] [PubMed] [Google Scholar]
- Crow Y.J., Chase D.S., Lowenstein Schmidt J., Szynkiewicz M., Forte G.M.A., Gornall H.L., Oojageer A., Anderson B., Pizzino A., Helman G., et al. 2015. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am. J. Med. Genet. A. 167:296–312. 10.1002/ajmg.a.36887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado E., Michailidou I., van Bodegraven E.J., Jansen M.H., Sluijs J.A., Geerts D., Couraud P.O., De Filippis L., Vescovi A.L., Kuijpers T.W., and Hol E.M.. 2015a Phenotypic variation in Aicardi-Goutières syndrome explained by cell-specific IFN-stimulated gene response and cytokine release. J. Immunol. 194:3623–3633. 10.4049/jimmunol.1401334 [DOI] [PubMed] [Google Scholar]
- Cuadrado E., Vanderver A., Brown K.J., Sandza A., Takanohashi A., Jansen M.H., Anink J., Herron B., Orcesi S., Olivieri I., et al. 2015b Aicardi-Goutières syndrome harbours abundant systemic and brain-reactive autoantibodies. Ann. Rheum. Dis. 74:1931–1939. 10.1136/annrheumdis-2014-205396 [DOI] [PubMed] [Google Scholar]
- Dale R.C., Tang S.P., Heckmatt J.Z., and Tatnall F.M.. 2000. Familial systemic lupus erythematosus and congenital infection-like syndrome. Neuropediatrics. 31:155–158. 10.1055/s-2000-7492 [DOI] [PubMed] [Google Scholar]
- DeCarli C., Civitello L.A., Brouwers P., and Pizzo P.A.. 1993. The prevalence of computed tomographic abnormalities of the cerebrum in 100 consecutive children symptomatic with the human immune deficiency virus. Ann. Neurol. 34:198–205. 10.1002/ana.410340216 [DOI] [PubMed] [Google Scholar]
- De Laet C., Goyens P., Christophe C., Ferster A., Mascart F., and Dan B.. 2005. Phenotypic overlap between infantile systemic lupus erythematosus and Aicardi-Goutières syndrome. Neuropediatrics. 36:399–402. 10.1055/s-2005-873058 [DOI] [PubMed] [Google Scholar]
- Ducreux J., Houssiau F.A., Vandepapelière P., Jorgensen C., Lazaro E., Spertini F., Colaone F., Roucairol C., Laborie M., Croughs T., et al. 2016. Interferon α kinoid induces neutralizing anti-interferon α antibodies that decrease the expression of interferon-induced and B cell activation associated transcripts: analysis of extended follow-up data from the interferon α kinoid phase I/II study. Rheumatology (Oxford). 55:1901–1905. 10.1093/rheumatology/kew262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckard S.C., Rice G.I., Fabre A., Badens C., Gray E.E., Hartley J.L., Crow Y.J., and Stetson D.B.. 2014. The SKIV2L RNA exosome limits activation of the RIG-I-like receptors. Nat. Immunol. 15:839–845. 10.1038/ni.2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frans G., Moens L., Schaballie H., Wuyts G., Liston A., Poesen K., Janssens A., Rice G.I., Crow Y.J., Meyts I., and Bossuyt X.. 2016. Homozygous N-terminal missense mutation in TRNT1 leads to progressive B-cell immunodeficiency in adulthood. J. Allergy Clin. Immunol. 10.1016/j.jaci.2016.06.050 [DOI] [PubMed] [Google Scholar]
- Frémond M.L., Rodero M.P., Jeremiah N., Belot A., Jeziorski E., Duffy D., Bessis D., Cros G., Rice G.I., Charbit B., et al. 2016. Efficacy of the Janus kinase 1/2 inhibitor ruxolitinib in the treatment of vasculopathy associated with TMEM173-activating mutations in 3 children. J. Allergy Clin. Immunol. 10.1016/j.jaci.2016.07.015 [DOI] [PubMed] [Google Scholar]
- Funabiki M., Kato H., Miyachi Y., Toki H., Motegi H., Inoue M., Minowa O., Yoshida A., Deguchi K., Sato H., et al. 2014. Autoimmune disorders associated with gain of function of the intracellular sensor MDA5. Immunity. 40:199–212. 10.1016/j.immuni.2013.12.014 [DOI] [PubMed] [Google Scholar]
- Gall A., Treuting P., Elkon K.B., Loo Y.M., Gale M. Jr., Barber G.N., and Stetson D.B.. 2012. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 36:120–131. 10.1016/j.immuni.2011.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Li T., Li X.D., Chen X., Li Q.Z., Wight-Carter M., and Chen Z.J.. 2015. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl. Acad. Sci. USA. 112:E5699–E5705. 10.1073/pnas.1516465112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T., Zeller N., Raasch J., Kierdorf K., Frenzel K., Ketscher L., Basters A., Staszewski O., Brendecke S.M., Spiess A., et al. 2015. USP18 lack in microglia causes destructive interferonopathy of the mouse brain. EMBO J. 34:1612–1629. 10.15252/embj.201490791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E.E., Treuting P.M., Woodward J.J., and Stetson D.B.. 2015. Cutting Edge: cGAS is required for lethal autoimmune disease in the Trex1-deficient mouse model of Aicardi-Goutières syndrome. J. Immunol. 195:1939–1943. 10.4049/jimmunol.1500969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacohen Y., Zuberi S., Vincent A., Crow Y.J., and Cordeiro N.. 2015. Neuromyelitis optica in a child with Aicardi-Goutières syndrome. Neurology. 85:381–383. 10.1212/WNL.0000000000001792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M., Dobbs N., Khan S., White M.A., Wakeland E.K., Li Q.Z., and Yan N.. 2015. Cutting Edge: Inhibiting TBK1 by compound II ameliorates autoimmune disease in mice. J. Immunol. 195:4573–4577. 10.4049/jimmunol.1500162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller B., Achleitner M., Glage S., Naumann R., Behrendt R., and Roers A.. 2012. Mammalian RNase H2 removes ribonucleotides from DNA to maintain genome integrity. J. Exp. Med. 209:1419–1426. 10.1084/jem.20120876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs A., and Lindenmann J.. 1957. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 147:258–267. 10.1098/rspb.1957.0048 [DOI] [PubMed] [Google Scholar]
- Isaacs A., Lindenmann J., and Valentine R.C.. 1957. Virus interference. II. Some properties of interferon. Proc. R. Soc. Lond. B Biol. Sci. 147:268–273. 10.1098/rspb.1957.0049 [DOI] [PubMed] [Google Scholar]
- Jabbari A., Dai Z., Xing L., Cerise J.E., Ramot Y., Berkun Y., Sanchez G.A., Goldbach-Mansky R., Christiano A.M., Clynes R., and Zlotogorski A.. 2015. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EBioMedicine. 2:351–355. 10.1016/j.ebiom.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M.A., Kim E.K., Now H., Nguyen N.T., Kim W.J., Yoo J.Y., Lee J., Jeong Y.M., Kim C.H., Kim O.H., et al. 2015. Mutations in DDX58, which encodes RIG-I, cause atypical Singleton-Merten syndrome. Am. J. Hum. Genet. 96:266–274. 10.1016/j.ajhg.2014.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremiah N., Neven B., Gentili M., Callebaut I., Maschalidi S., Stolzenberg M.C., Goudin N., Frémond M.L., Nitschke P., Molina T.J., et al. 2014. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J. Clin. Invest. 124:5516–5520. 10.1172/JCI79100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner D.L., Aksentijevich I., and Goldbach-Mansky R.. 2010. Autoinflammatory disease reloaded: a clinical perspective. Cell. 140:784–790. 10.1016/j.cell.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman W.M., Sivit C.J., Fitz C.R., Rakusan T.A., Herzog K., and Chandra R.S.. 1992. CT and MR evaluation of intracranial involvement in pediatric HIV infection: a clinical-imaging correlation. AJNR Am. J. Neuroradiol. 13:949–957. [PMC free article] [PubMed] [Google Scholar]
- Kavanagh D., McGlasson S., Jury A., Williams J., Scolding N., Bellamy C., Gunther C., Ritchie D., Gale D.P., Kanwar Y.S., et al. 2016. Type I interferon causes thrombotic microangiopathy by a dose-dependent toxic effect on the microvasculature. Blood. 10.1182/blood-2016-05-715987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König N., Fiehn C., Wolf C., Schuster M., Cura Costa E., Tüngler V., Alvarez H.A., Chara O., Engel K., Goldbach-Mansky R., et al. 2016. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann. Rheum. Dis. 10.1136/annrheumdis-2016-209841 [DOI] [PubMed] [Google Scholar]
- Krivine A., Tovey M., Taty-Taty R., and Lebon P.. 1992. Endogenous interferon-alpha in newborns from HIV seropositive mothers. J. Interferon Res. 12:S151. [Google Scholar]
- Kwon Y.S., Choe Y.H., and Chin H.S.. 2001. Development of glaucoma in the course of interferon alpha therapy for chronic hepatitis B. Yonsei Med. J. 42:134–136. 10.3349/ymj.2001.42.1.134 [DOI] [PubMed] [Google Scholar]
- Lausch E., Janecke A., Bros M., Trojandt S., Alanay Y., De Laet C., Hübner C.A., Meinecke P., Nishimura G., Matsuo M., et al. 2011. Genetic deficiency of tartrate-resistant acid phosphatase associated with skeletal dysplasia, cerebral calcifications and autoimmunity. Nat. Genet. 43:132–137. 10.1038/ng.749 [DOI] [PubMed] [Google Scholar]
- Lebon P., Meritet J.F., Krivine A., and Rozenberg F.. 2002. Interferon and Aicardi-Goutières syndrome. Eur. J. Paediatr. Neurol. 6:A47–A53. 10.1053/ejpn.2002.0574 [DOI] [PubMed] [Google Scholar]
- Liddicoat B.J., Piskol R., Chalk A.M., Ramaswami G., Higuchi M., Hartner J.C., Li J.B., Seeburg P.H., and Walkley C.R.. 2015. RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself. Science. 349:1115–1120. 10.1126/science.aac7049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jesus A.A., Marrero B., Yang D., Ramsey S.E., Montealegre Sanchez G.A., Tenbrock K., Wittkowski H., Jones O.Y., Kuehn H.S., et al. 2014. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371:507–518. 10.1056/NEJMoa1312625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston J.H., Lin J.P., Dale R.C., Gill D., Brogan P., Munnich A., Kurian M.A., Gonzalez-Martinez V., De Goede C.G., Falconer A., et al. 2014. A type I interferon signature identifies bilateral striatal necrosis due to mutations in ADAR1. J. Med. Genet. 51:76–82. 10.1136/jmedgenet-2013-102038 [DOI] [PubMed] [Google Scholar]
- Lood C., Gullstrand B., Truedsson L., Olin A.I., Alm G.V., Rönnblom L., Sturfelt G., Eloranta M.L., and Bengtsson A.A.. 2009. C1q inhibits immune complex-induced interferon-α production in plasmacytoid dendritic cells: A novel link between C1q deficiency and systemic lupus erythematosus pathogenesis. Arthritis Rheum. 60:3081–3090. 10.1002/art.24852 [DOI] [PubMed] [Google Scholar]
- Mackenzie K.J., Carroll P., Lettice L., Tarnauskaitė Ž., Reddy K., Dix F., Revuelta A., Abbondati E., Rigby R.E., Rabe B., et al. 2016. Ribonuclease H2 mutations induce a cGAS/STING-dependent innate immune response. EMBO J. 35:831–844. 10.15252/embj.201593339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merati M., Buethe D.J., Cooper K.D., Honda K.S., Wang H., and Gerstenblith M.R.. 2015. Aggressive CD8+ epidermotropic cutaneous T-cell lymphoma associated with homozygous mutation in SAMHD1. JAAD Case Rep. 1:227–229. 10.1016/j.jdcr.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen M.E., Schot R., Buta S., Oudesluijs G., Tinschert S., Speer S.D., Li Z., van Unen L., Heijsman D., Goldmann T., et al. 2016. Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J. Exp. Med. 213:1163–1174. 10.1084/jem.20151529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S., Woodward M., Hertel C., Vlaicu P., Haque Y., Kärner J., Macagno A., Onuoha S.C., Fishman D., Peterson H., et al. APECED patient collaborative . 2016. AIRE-deficient patients harbor unique high-affinity disease-ameliorating autoantibodies. Cell. 166:582–595. 10.1016/j.cell.2016.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold T.B., Hua J., Lehman T.J., Harley J.B., and Crow M.K.. 2007. High serum IFN-α activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 8:492–502. 10.1038/sj.gene.6364408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oon S., Wilson N.J., and Wicks I.. 2016. Targeted therapeutics in SLE: emerging strategies to modulate the interferon pathway. Clin. Transl. Immunology. 5:e79 10.1038/cti.2016.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter M.M., Morrison J.H., Zoecklein L.J., Rinkoski T.A., Watzlawik J.O., Papke L.M., Warrington A.E., Bieber A.J., Matchett W.E., Turkowski K.L., et al. 2015. Antiviral protection via RdRP-mediated stable activation of innate immunity. PLoS Pathog. 11:e1005311 10.1371/journal.ppat.1005311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschke K., Achleitner M., Frenzel K., Gerbaulet A., Ada S.R., Zeller N., Lienenklaus S., Lesche M., Poulet C., Naumann R., et al. 2016. Loss of Trex1 in dendritic cells is sufficient to trigger systemic autoimmunity. J. Immunol. 197:2157–2166. 10.4049/jimmunol.1600722 [DOI] [PubMed] [Google Scholar]
- Pestal K., Funk C.C., Snyder J.M., Price N.D., Treuting P.M., and Stetson D.B.. 2015. Isoforms of RNA-editing enzyme ADAR1 Independently control nucleic acid sensor MDA5-driven autoimmunity and multi-organ development. Immunity. 43:933–944. 10.1016/j.immuni.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C., Thouvenin G., Kannengiesser C., Dubus J.C., Jeremiah N., Rieux-Laucat F., Crestani B., Belot A., Thivolet-Béjui F., Secq V., et al. 2016. Severe pulmonary fibrosis as the first manifestation of interferonopathy (TMEM173 mutation). Chest. 150:e65–e71. 10.1016/j.chest.2016.02.682 [DOI] [PubMed] [Google Scholar]
- Pokatayev V., Hasin N., Chon H., Cerritelli S.M., Sakhuja K., Ward J.M., Morris H.D., Yan N., and Crouch R.J.. 2016. RNase H2 catalytic core Aicardi-Goutières syndrome–related mutant invokes cGAS–STING innate immune-sensing pathway in mice. J. Exp. Med. 213:329–336. 10.1084/jem.20151464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehwinkel J., Maelfait J., Bridgeman A., Rigby R., Hayward B., Liberatore R.A., Bieniasz P.D., Towers G.J., Moita L.F., Crow Y.J., et al. 2013. SAMHD1-dependent retroviral control and escape in mice. EMBO J. 32:2454–2462. 10.1038/emboj.2013.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijns M.A., Rabe B., Rigby R.E., Mill P., Astell K.R., Lettice L.A., Boyle S., Leitch A., Keighren M., Kilanowski F., et al. 2012. Enzymatic removal of ribonucleotides from DNA is essential for mammalian genome integrity and development. Cell. 149:1008–1022. 10.1016/j.cell.2012.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G.I., Bond J., Asipu A., Brunette R.L., Manfield I.W., Carr I.M., Fuller J.C., Jackson R.M., Lamb T., Briggs T.A., et al. 2009. Mutations involved in Aicardi-Goutières syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 41:829–832. 10.1038/ng.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G.I., Kasher P.R., Forte G.M., Mannion N.M., Greenwood S.M., Szynkiewicz M., Dickerson J.E., Bhaskar S.S., Zampini M., Briggs T.A., et al. 2012. Mutations in ADAR1 cause Aicardi-Goutières syndrome associated with a type I interferon signature. Nat. Genet. 44:1243–1248. 10.1038/ng.2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G.I., Forte G.M., Szynkiewicz M., Chase D.S., Aeby A., Abdel-Hamid M.S., Ackroyd S., Allcock R., Bailey K.M., Balottin U., et al. 2013. Assessment of interferon-related biomarkers in Aicardi-Goutières syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case-control study. Lancet Neurol. 12:1159–1169. 10.1016/S1474-4422(13)70258-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G.I., del Toro Duany Y., Jenkinson E.M., Forte G.M., Anderson B.H., Ariaudo G., Bader-Meunier B., Baildam E.M., Battini R., Beresford M.W., et al. 2014. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat. Genet. 46:503–509. 10.1038/ng.2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnblom L.E., Alm G.V., and Oberg K.E.. 1990. Possible induction of systemic lupus erythematosus by interferon-α treatment in a patient with a malignant carcinoid tumour. J. Intern. Med. 227:207–210. 10.1111/j.1365-2796.1990.tb00144.x [DOI] [PubMed] [Google Scholar]
- Rutsch F., MacDougall M., Lu C., Buers I., Mamaeva O., Nitschke Y., Rice G.I., Erlandsen H., Kehl H.G., Thiele H., et al. 2015. A specific IFIH1 gain-of-function mutation causes Singleton-Merten syndrome. Am. J. Hum. Genet. 96:275–282. 10.1016/j.ajhg.2014.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer D.M., Hall B.E., George T.C., Tangsombatvisit S., Liu C.L., Arkwright P.D., and Elkon K.B.. 2010. C1q deficiency leads to the defective suppression of IFN-α in response to nucleoprotein containing immune complexes. J. Immunol. 185:4738–4749. 10.4049/jimmunol.1001731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Fitzgerald K.A., Cancro M.P., and Marshak-Rothstein A.. 2015. Nucleic acid-sensing receptors: Rheostats of autoimmunity and autoinflammation. J. Immunol. 195:3507–3512. 10.4049/jimmunol.1500964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto T., Kageyama M., Hirai R., Zheng J., Yoneyama M., and Fujita T.. 2009. Identification of loss of function mutations in human genes encoding RIG-I and MDA5: implications for resistance to type I diabetes. J. Biol. Chem. 284:13348–13354. 10.1074/jbc.M809449200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolev O., Binda E., O’Farrell S., Lorenc A., Pradines J., Huang Y., Duffner J., Schulz R., Cason J., Zambon M., et al. 2016. Adjuvanted influenza-H1N1 vaccination reveals lymphoid signatures of age-dependent early responses and of clinical adverse events. Nat. Immunol. 17:204–213. 10.1038/ni.3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starokadomskyy P., Gemelli T., Rios J.J., Xing C., Wang R.C., Li H., Pokatayev V., Dozmorov I., Khan S., Miyata N., et al. 2016. DNA polymerase-α regulates the activation of type I interferons through cytosolic RNA:DNA synthesis. Nat. Immunol. 17:495–504. 10.1038/ni.3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson D.B., Ko J.S., Heidmann T., and Medzhitov R.. 2008. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 134:587–598. 10.1016/j.cell.2008.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu M., Le Chenadec J., Persoz A., Meyer L., Blanche S., and Mayaux M.J.. French Pediatric HIV Infection Study and the SEROCO Group . 2000. HIV-1-related encephalopathy in infants compared with children and adults. Neurology. 54:1089–1095. 10.1212/WNL.54.5.1089 [DOI] [PubMed] [Google Scholar]
- Tonduti D., Orcesi S., Jenkinson E.M., Dorboz I., Renaldo F., Panteghini C., Rice G.I., Henneke M., Livingston J.H., Elmaleh M., et al. 2016. Clinical, radiological and possible pathological overlap of cystic leukoencephalopathy without megalencephaly and Aicardi-Goutières syndrome. Eur. J. Paediatr. Neurol. 20:604–610. 10.1016/j.ejpn.2016.03.009 [DOI] [PubMed] [Google Scholar]
- Troedson C., Wong M., Dalby-Payne J., Wilson M., Dexter M., Rice G.I., Crow Y.J., and Dale R.C.. 2013. Systemic lupus erythematosus due to C1q deficiency with progressive encephalopathy, intracranial calcification and acquired moyamoya cerebral vasculopathy. Lupus. 22:639–643. 10.1177/0961203313486950 [DOI] [PubMed] [Google Scholar]
- Uettwiller F., Sarrabay G., Rodero M.P., Rice G.I., Lagrue E., Marot Y., Deiva K., Touitou I., Crow Y.J., and Quartier P.. 2016. ADA2 deficiency: case report of a new phenotype and novel mutation in two sisters. RMD Open. 2:e000236 10.1136/rmdopen-2015-000236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eyck L., De Somer L., Pombal D., Bornschein S., Frans G., Humblet-Baron S., Moens L., de Zegher F., Bossuyt X., Wouters C., and Liston A.. 2015. IFIH1 mutation causes systemic lupus erythematosus with selective IgA deficiency. Arthritis Rheumatol. 67:1592–1597. 10.1002/art.39110 [DOI] [PubMed] [Google Scholar]
- Wang B., Higgs B.W., Chang L., Vainshtein I., Liu Z., Streicher K., Liang M., White W.I., Yoo S., Richman L., et al. 2013. Pharmacogenomics and translational simulations to bridge indications for an anti-interferon-α receptor antibody. Clin. Pharmacol. Ther. 93:483–492. 10.1038/clpt.2013.35 [DOI] [PubMed] [Google Scholar]
- Yi G., Brendel V.P., Shu C., Li P., Palanathan S., and Cheng Kao C.. 2013. Single nucleotide polymorphisms of human STING can affect innate immune response to cyclic dinucleotides. PLoS One. 8:e77846 10.1371/journal.pone.0077846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Bogunovic D., Payelle-Brogard B., Francois-Newton V., Speer S.D., Yuan C., Volpi S., Li Z., Sanal O., Mansouri D., et al. 2015. Human intracellular ISG15 prevents interferon-α/β over-amplification and auto-inflammation. Nature. 517:89–93. 10.1038/nature13801 [DOI] [PMC free article] [PubMed] [Google Scholar]