Abstract
Type 2 immunity is characterized by expression of the cytokines interleukin (IL)-4, IL-5, IL-9 and IL-13, which can function in mediating protective immunity in the host or possess a pathogenic role. T helper (Th) 2 cells have emerged to play a beneficial role in mediating anti-parasitic immunity and are also known to be key players in mediating allergic diseases. In addition to the Th2 cells, recent studies have identified T follicular helper (Tfh) cells as an alternative source of IL-4 to regulate type 2 humoral immune responses, indicating that Th2 and Tfh cells exhibit overlapping phenotypical and functional characteristics. Th2 and Tfh cells appear to utilize unique mechanisms for regulation of IL-4 expression; however unlike Th2 cells, the regulation and function of Tfh-derived IL-4 is not yet fully understood. Understanding of the molecular mechanisms for IL-4 expression and function in both cell subsets will lead to development of pharmacological approaches to regulate the function of IL-4 in different disease settings.
Keywords: Th2, Tfh, Interleukin-4, transcription, type-2 immunity
1. Introduction
Type 2 immune responses are typically characterized by the differentiation of CD4+ T helper (Th)2 cells and the production of classical type 2 signature cytokines including IL-4, IL-5, IL-9 and IL-13 [1]. The major effector responses in type 2 immunity include B cell mediated humoral responses such as IgG1 and IgE antibody class switching and recruitment of inflammatory effector cells such as eosinophils, basophils and mast cells [2, 3]. Type 2 immunity is beneficial in mediating anti-parasitic immunity, wound-repair, suppressing autoimmune-type disease and maintaining tissue homeostasis [2, 4]. However on the other hand, type 2 immune responses are also known to be responsible for the development of asthma and other allergic inflammatory diseases [5].
In human and mice, Th2 cells have critical functions in immune responses against extracellular parasites and are involved in asthma and other allergic diseases [2, 6, 7]. Together with T cell receptor (TCR) ligation, IL-4-mediated signaling promotes Th2 differentiation. IL-4 activates the transcription factor STAT6 (signal transduction and activator of transcription 6) leading to expression and activation of GATA3, the Th2 master regulator and consequently to production of signature Th2 cytokines including IL-4, IL-5, and IL-13. In addition, Nuclear factor of activated T cells (NFAT), AP-1, Jun B, c-Maf and Interferon regulatory factor 4 (IRF4) are important for IL-4 production [8, 9]. Th2-derived IL-4 acts in an autocrine feedback loop to promote further Th2 differentiation.
Recent findings have led to identification of a new Th subset called T follicular helper (Tfh) cells because of their location in B cell follicles [10, 11]. Tfh cells are known to provide help for formation and maintenance of germinal center B cell reactions. Some of the distinct features of Tfh cells are expression of CXCR5, PD-1, ICOS, IL-21 and the requirement of the transcription factor Bcl6 [11, 12]. Of importance is the recent data using mouse models of helminth parasite infection as well as asthma model demonstrating that Tfh cells produce the Th2 signature cytokine IL-4 that regulates isotype class switching of B cells toward IgG1 and IgE [13–16], indicating that Th2 and Tfh cells, although distinct subset, yet display similar phenotypical and functional properties; however, the regulation and function of Tfh-derived IL-4 is not fully understood. In contrast to Th2 cells, regulation of IL-4 expression in Tfh cells exclusively depends on the 3′ conserved non coding sequence (CNS)2 region in the IL-4locus [17, 18]. SLAM-SAP pathway and Notch and RBP-J signaling are potential candidates for regulation of IL-4 expression in Tfh cells through the CNS2 enhancer [19]. Furthermore, our recent study identifies a crucial role of the transcription factor basic leucine zipper transcription factor ATF-like (Batf) in controlling IL-4 expression in Tfh cells through direct binding and controlling access of the chromatin machinery in the CNS2 region [16]. Further understanding of the molecular details of IL-4 gene regulation in Tfh cells may facilitate the development of novel approaches to control IL-4 production in different disease settings.
In this review, we discuss the molecular mechanisms driving IL-4 production in Th2 and Tfh cells. Further, we elaborate on the current understanding of the physiological role of IL-4 derived from both subsets in shaping the type 2 immune responses and the current implications of IL-4 in a disease setting.
2. Factors controlling Th2 differentiation and IL-4 expression
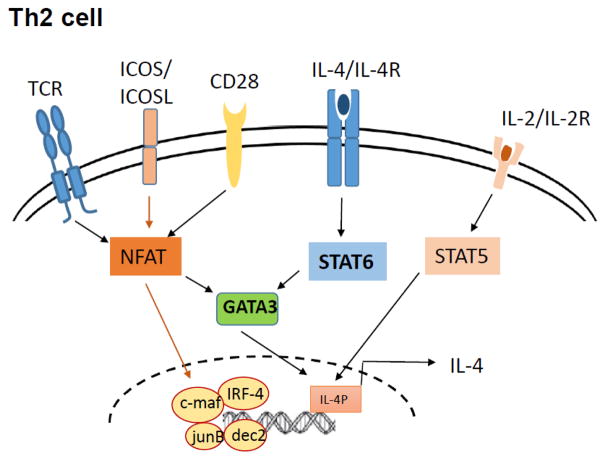
Activation and differentiation of CD4+ T cells into effector Th2 cells require the collaboration between multiple factors such as costimulatory molecules, cytokine signaling and transcription factors. TCR signaling and costimulation by CD28 and ICOS activate downstream transcription factors that regulate early IL-4 production which in an autocrine feedback loop promote further Th2 differentiation (Fig 1). In the section below, we discuss in detail the various signaling mechanisms and the cooperation between TCR, stimulatory molecules, cytokines signaling, transcription factors and epigenetic elements that are responsible for IL-4 production in Th2 cells.
Figure 1. Factors regulating IL-4 regulation in Th2 cells.
Weak TCR signalling induces expression of GATA3 in a IL-4/STAT6-independent manner and IL-2 production that in turn activates STAT5. GATA3 cooperates with STAT5 and regulates early TCR-dependent IL-4 production. Endogenously produced IL-4 activates GATA3 expression in a STAT6-dependent manner. In addition, NFAT, IRF-4, c-Maf, Jun-B and Dec-2 contribute to IL-4 production in the effector phase.
2.1 TCR and costimulation
Naïve CD4+ T cells are capable of producing IL-4 upon TCR activation and thereby endogenously produced IL-4 is sufficient for Th2 differentiation. Such early IL-4 production by naïve CD4+ T cells is caused by low-dose antigen stimulation leading to weak and transient extracellular signal-regulated kinase (ERK) activation, GATA3 induction and IL-2 production that in turn activates STAT5. GATA3 cooperates with STAT5 to induce TCR-dependent, IL-4/STAT6-independent early IL-4 transcription [20, 21]. The endogenously produced IL-4 in collaboration with continued STAT5 activation amplifies production of IL-4 and leads to completion of Th2 differentiation [21].
Triggering of Th2 differentiation via low strength TCR stimulation can bypass requirement for exogenous IL-4 but requires a second signal via costimulatory receptors [7, 22]. Of particular importance for Th2 development are CD28, inducible costimulator (ICOS) and the tumor necrosis factor receptor superfamily members (OX-40, GITR and CD30) [9]. CD28, the most important co-stimulatory receptor, binds to B7.1 (CD80) and B7.2 (CD86) expressed on activated APCs and plays a major role in early phase of T cell activation, including expression of IL-2 and bcl-xL upregulation [6]. CD28 ligation favors Th2 differentiation by enhancing NFATc1 nuclear localization and inducing GATA3 expression [6]; CD28 deficiency in T cells results in reduced proliferative responses and low production of IL-2 and IL-4. Another member of the CD28 superfamily, inducible costimulator (ICOS) is expressed in higher level on Th2 cells and its engagement is particularly important for IL-4 and IL-10 expression [23]. ICOS or ICOS ligand deficient mice possess deficiencies in T cell activation, Th2 cytokine production, immunoglobulin isotype switching (IgG1 and IgE), and germinal center formation [23, 24]. ICOS costimulation potentiates early IL-4 expression by enhancing NFATc1 expression and hence effector IL-4 production by regulating c-Maf expression in the effector phase [6, 23]. In addition to the CD28 superfamily, the tumor necrosis factor receptor family members, OX40, CD30 and glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR) play crucial roles in Th2 survival and expansion and thus for promoting Th2 mediated allergic responses [25, 26].
2.2 Cytokines
Although the TCR and costimulatory molecules dramatically influence the Th cell fate, cytokines are the major determinants of differentiation of CD4+ T cells. Many cytokines including IL-4, IL-2, IL-6, IL-25, IL-33 and thymic stromal lymphopoetin (TSLP) are involved in controlling Th2 differentiation [6, 7, 27]. IL-4-induced activation of STAT6 leads to expression of Th2 master regulator GATA3 which triggers transcription of Il4 gene and other Th2-assotiated cytokines [27]. In addition, IL-2 produced as a result of TCR triggering leads to STAT5-induced IL-4 production; Stat5deficient cells show diminished IL-4 production [8, 28]. Although IL-4 and IL-2 are required for Th2 cell development in vitro, normal in vivo GATA3-dependent Th2 responses were observed in IL-4- and STAT6-deficient mice, suggesting that Th2 cells can differentiate independent of IL-4-STAT6 signaling [8, 29–31]. The cytokine IL-6 is also known to induce early IL-4 production through NFAT activation [32, 33]. TSLP is sufficient to directly facilitate Th2 priming and expansion independent of IL-4 through Stat5-dependent remodeling of IL-4 locus [9]. IL-25 regulates early IL-4 expression during Th2 priming through direct activation of NFATc1 and JunB; in memory Th2 cells, IL-25 signaling helps to maintain GATA3, JunB and c-Maf expression [34]. IL-33 in cooperation with STAT5 activators including IL-2, IL-7 and TSLP induces and maintains GATA3 expression which in turn increases IL-33R expression on resting Th2 cells. IL-33R-expressing Th2 cells produce Th2 cytokines (IL-13 and IL-5) in response IL-33 plus a STAT5 activators in a TCR-independent, NF-κB- and MAPKs- dependent manner [35].
2.3 Transcription factors
STAT6 is the major signal transducer in IL-4 mediated Th2 differentiation [36, 37]. The IL-4R/STAT6 pathway is a positive feedback loop for Th2 development and STAT6 has been shown to be sufficient to induce GATA3 and c-Maf expression. While STAT6 is clearly important for maximal Th2 development [30], STAT6 deficient CD4+ T cells are still capable of minimal Th2 cytokine production which is dependent on the Th2 specific transcriptional factor GATA3 [8, 29]. GATA3 is recognized as the master regulator of Th2 cells and is associated with transcriptional activation of Th2-related genes, interaction with other transcriptional factors and epigenetic modifications. [20]. While Gata3 directly binds to the IL-5 and IL-13 promoters and transactivates these genes in cooperation with STAT5, GATA3 binds to hypersensitive site II (HSII)/IE enhancer of il4 gene and promotes Il4 gene expression [28, 31]. Besides directly acting on the Il4 gene, GATA3 and STAT5 cross-regulate each other [38]; therefore crosstalk and collaboration between IL-4/STAT6/GATA3 and IL-2-STAT5 pathways results in complete Th2 differentiation. Furthermore, GATA3 has been reported to regulate its own expression [39]. GATA3 also requires cooperation with STAT6 for its binding to target sites in Th2 cells [40]. In addition GATA3 is known to inhibit Stat4 and IL-12Rβ expression and interacts with T-bet and Runx3 to inhibit Th1 differentiation [20]. Recently, STAT3 was shown to be important for STAT6 interaction with relevant gene loci in the developing Th2 cells [41]. Another transcription factor IFN regulatory factor-4 (IRF4) also is involved in the Th2 differentiation by upregulating GATA3 and by cooperating with NFATc2 to activate IL-4 expression [42, 43]. c-Maf which was identified as a Th2-specific transcription factor [44] in synergy with NFAT and activating protein (AP)-1 protein JunB, selectively induces IL-4 expression but not IL-5 and IL-13 [9]. Various other factors like Dec2, T cell factor-1 (TCF1) and Growth factor independent-1(gfi-1) have been also identified to influence the Th2 effector gene expression [20].
2.4 Epigenetic regulation
Extensive studies at the molecular and cellular level and in vivo animal models have identified the crucial DNA elements involved in IL-4 regulation which are usually conserved within species (conserved non coding sequences, CNS) and are accessible to DNAase1 (hypersensitive sites, HS) [20, 27]. The IL-4 and IL-13 intergenic region encompasses the hypersensitive sites HSS0-HSS3 (CNS1) which is a well-known GATA responsive element (CGRE) which exerts its effects through the GATA3 transcription factor [20, 45, 46]. The HSI, HSII and HSIII regions are included within the IL-4 gene locus while the HSIV, HSV (CNS2) and HSVA regions are located in the IL-4/Kif3a intergenic region [20, 27]. Apart from the above regions, a Th2 locus control region (LCR), comprising the RHS4, RHS5, RHS6 and RHS7 regions, crucial for the production of all signature Th2 cytokines including IL-4, IL-5 and IL-13 is located in the unrelated rad50gene [20, 27]. The transcription factors GATA-3 and STAT5 bind to the HSII region and increase the accessibility of the IL4 locus and transcription of Il-4 gene[28, 31]. HS V/VA binds to the GATA-3, NFAT1 (NFATc2) and Notch transcription factors and is crucial for IL-4 expression in Th2 and Tfh cells [17, 18, 29, 47]. HSIV is a well-known IL-4 silencer locus and its deletion leads to increase in IL-4 expression in naïve, Th1, Th2 and in macrophages as well. HSIV defective mice show aberrant expression of IFN-c in Th2 cells [48]. The transcription factor Runx3 binds at the HSIV region and inhibits IL-4 expression in Th1 cells and GATA3 interacts with Runx3 to prevents its function in Th2 cells [49, 50]. Apart from the above regions, deletion of the entire Th2 LCR at the3′ end of the unrelated RAD50 locus caused almost complete reduction of all three Th2 cytokines [20]. Deletion of RHS6 region caused a major defect in all three Th2 cytokines while deletion of RHS7 in mice showed defect in IL-4 and IL-13 but not as much in IL-5 [51]. The transcription factor YY1 is known to bind to RHS7 and recruit GATA3 to control Th2 cytokine expression [20, 52]. The role of chromatin remodeling and epigenetic processes like DNA methylation, posttranslational histone modifications (acetylation, methylation, phosphorylation, etc) and cis regulatory elements in regulating IL-4 gene expression during differentiation of naïve T cells to Th2 cells has been extensively investigated and reviewed [53–55]. Upon initiation of Th2 differentiation from naïve T cells, the active histone modifications are increased and stabilized throughout the IL-4 locus in Th2 cells while repressive modifications like H3K27 trimethylation with the association of the H3K27 methyltransferase EZH2 is particularly enriched in the HSS3 and HSIV locus in Th1 cells leading to IL-4 silencing in Th1 cells [9, 27, 54]. DNA methylation is also known to play a crucial role in Th2 cytokine expression including IL-4 expression [56, 57]. Although DNA methylation is not required for initial IL-4 transcription; it is required for its maintenance in polarized Th2 cells[56, 58]. Under Th2 polarizing conditions, TCR signaling along with CD28 costimulation leads to progressive demethylation of the IL-4 promoter and the Th2 cell specific RHS7 locus resulting in IL-4 transcription [55, 59, 60]. The demethylation observed is independent of the Th2 master regulator GATA3. Cells that lack DNMT1 and MBD2 have increased IL-4 production [57, 61].
3. T follicular helper cells as an alternative source of IL-4 production
Apart from Th2 cells, Tfh cells have emerged as a distinct subset of IL-4-secreting T helper cells that are localized in germinal centers (GCs) and provide help to B cells [12, 15]. Tfh cells were identified by their expression of chemokine CXCR5, which allow these cells to migrate to the B cell follicles within GCs [62]. In addition to CXCR5, additional surface Tfh cell markers including ICOS, CD40L, PD-1, and BTLA have been reported [11, 62]. Tfh cell differentiation is regulated by IL-6 and IL-21, possibly via STAT3 factor and B cell lymphoma 6 (Bcl6) which is specifically expressed in Tfh cells and is required for their lineage commitment [63, 64]. While initially it has been acknowledged that Tfh cells only secrete IL-21, that are critical for Tfh development and function [64, 65], recent data suggest that Tfh cells also produce the Th2 signature cytokine IL-4 [15]; however, the regulation and function of Tfh-derived IL-4 is not yet fully understood.
Recently, several groups have analyzed the relationship between IL-4 producing CD4+ T cells and Tfh cells during parasitic infection (Leishmania major, Nippostrongylus brasiliensis, Heligmosomoids polygyrus and Schistosoma mansoni) using IL-4 dual reporter mice (4get/KN2), in which cells that expressed IL-4 are marked by GFP (4get) and IL-4-producing subsets additionally displayed surface huCD2 (KN2) [13, 15, 66]. Analysis of Th differentiation during infection of 4get/KN2 mice confirmed previous reports showing that Tfh cells can express IL-4 [15] and show that most IL-4 producing subset of cells (huCD2+CD4+) express high levels of Tfh markers such as CXCR5, ICOS, PD-1, BTLA, Bcl6 and IL-21 is highly enriched in B cell follicles and germinal centers of the reactive lymph nodes [15]. Within non-lymphoid effector site, huCD2+ IL-4 producing cells do not express the Tfh markers [13], suggesting IL-4 producing cells in the lymph nodes are phenotypically different from peripheral Th2 cells and have canonical characteristics of Tfh cells. Moreover, adoptive transfer of lung and lymph node huCD2+CD4+ T cells from N. brasiliensis infected 4get/KN2 mice into mice deficient in both IL-4 and IL-13 following N. brasiliensis infection shows three-fold higher recruitment of eosinophils to the lung of mice that received lung huCD2+ cells compared to mice that received huCD2+ cells from lymph nodes [67]. Collectively, these studies have shown that IL-4 secreting Tfh cells and Th2 cells are distinct Th subsets with respect to their phenotypical characteristics, localizations and effector functions.
Interestingly, while analysis of μMT/4get mice (B-cell deficient mice) immunized with serum schistosome egg antigen (SEA) revealed the absence of Tfh cells compared to B-cell sufficient 4get mice, the overall percentage of IL-4-competent (IL-4/GFP) CD4+ T cells were similar in both mice, suggesting that Tfh population could be derived from the Th2 lineage in the presence of B cells [13]. Moreover, the finding that transcript level of GATA3, master regulator of Th2 differentiation, was comparable between Tfh cells found in B cell follicles and IL-4-competent cells (PD1-IL-4/GFP+CD4+) from outside the B cells follicles from SEA immunized mice [13], further indicated that Th2 cells localized within lymph nodes have potential to become either Tfh cells within GCs of lymph nodes or migrate to peripheral tissues to perform Th2 effector functions. The relationship between Tfh and Th2 cells was confirmed when Tfh cells developed from CXCR5−PD1−IL4/GFP+CD4+ T cells in a germinal center-dependent manner upon transfer into recipient naïve mice and antigen challenge in vivo [13]. In the view of T cells plasticity, it is not clear whether Th2 to Tfh conversion is a final step in the lineage pathway. It is possible that different signals might regulate the reversible transition between the two Th lineages.
Although generation and accumulation of Tfh cells can accrue in the absence of IL-4, still IL-4 is critical for mature B cell responses [15, 67]. B-T cell conjugates within GCs of lymph nodes from L. major infected mice showed IgG1 switch and germinal transcript by B cells made contact with IL-4-expressing T cells, whereas IgG2a-producing B cells made contact with IFNγ-producing T cells [66], indicating that specific cytokines produced by Tfh cells regulates isotype class switching in conjugated B cells.
Coproduction of IL-4 and IL-21 is coupled in Tfh cells and required for optimal antibody responses [66, 68]. Interestingly, while Tfh cells could persist for up to 60 days after primary antigen activation, memory CD4+ T cells, rather than persistent Tfh cells, are required for Tfh population expansion upon secondary immunization [66]. B cells are necessary for expansion of Tfh cells following secondary immunization; moreover, memory B cells require IL-4 for efficient differentiation to plasmoblasts, indicating a pivotal role for IL-4 in the interplay between Tfh and B cells during secondary immune responses.
4. Critical elements for IL-4 regulation in Tfh cells
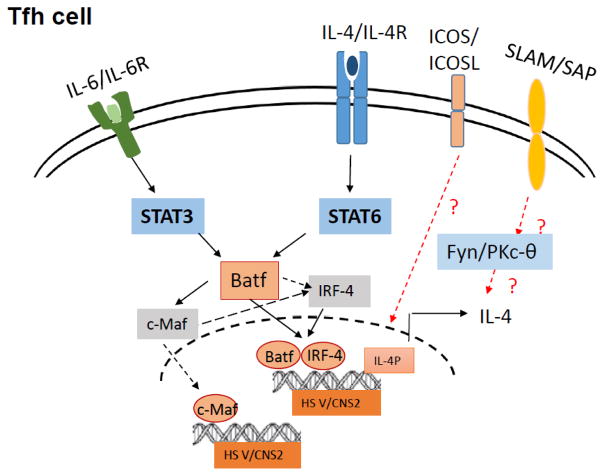
Over the recent past, a lot of interest has been generated in determining the factors leading to IL-4 production by Tfh cell differentiation, since in contrast to Th2 cells regulation of IL-4 expression in Tfh cells exclusively depends on the CNS2 regions in the IL-4 locus (Fig 2). Here, we elaborate on the current findings on cooperative effects of costimulatory molecules, cytokine signaling and epigenetic mechanisms that regulate IL-4 production by Tfh cells.
Figure 2. Regulation of IL-4 expression in Tfh cells.
IL-4/STAT6 and IL6/STAT3 signalling trigger Batf expression in Tfh cells, that in turn complex with IRF4 to trigger IL-4 production in Tfh cells by directly binding to and activation of the CNS2 region in the IL-4 gene locus. Additionally, Batf-to-c-Maf signaling is an important determinant of IL-4 expression in Tfh cells. Moreover, ICOS and SLAM signaling contributes to generation of IL-4-producing Tfh cells.
4.1 Costimulation
Signaling lymphocytic activation molecule (SLAM)-associated protein, SAP is expressed in CD4+ T cells and required for GC responses [69]. While absence of SAP had moderate defect in non-CG Tfh cell differentiation, SAP deficient mice have an absence of GC Tfh cell subset [70]. GC center Tfh cells co-expressing CXCR5, PD-1, Bcl6 and GL-7 are a functionally distinct subset of further polarized Tfh cells, with enhanced B cell help capacity and a specialized ability to produce IL-4 in Th2 independent-manner [64, 66]. SAP deficient Tfh cells are defective in IL-4 and IL-21 production. Moreover, SLAM (Slamf1, CD150), a surface receptor that utilizes SAP signaling, is specifically required for IL-4 production by GC Tfh cells [70]. These data reveal a prominent role for SLAM receptor ligation in IL-4 production by GC Tfh cells and GC Tfh differentiation. Further work is required to determine whether downstream components of SAP (Fyn and PKCθ) are required for IL-4 induction in GC Tfh cells.
ICOS is a critical regulator of the generation and function of Tfh cells [71]. ICOSL blockade in L. major infected 4get/KN2 reporter mice led to the reduced GC formation, to fewer hCD2+ IL-4-producing Tfh cells within B cell follicles and GC B cells without affecting IL-4 expressing Th2 cells [67], indicating that ICOSL is not required for Th2 cell development but is essential for IL-4 producing Tfh cells within GCs of reactive lymph nodes.
4.2 Epigenetic control
IL-4 expression is known to be governed by a set of coordinated changes occurring at the IL-4 locus including chromatin remodeling, epigenetic changes and accessibility to various transcription factors [27, 47, 54, 56]. The cell type specificity of gene expression is primarily conferred by distal cis-regulatory elements and the role of various regulatory elements and epigenetic changes in IL-4 regulation in Th2 cells and macrophages has been studied previously and discussed in the earlier section of this review.
The DNase I hypersensitivity site (HS)V overlaps with a highly conserved sequence 2 (CNS2) and has been recently identified as a putative distal enhancer located 3′ of the IL-4 gene [27]. Analyses of either HSV or CNS2 deficient mice demonstrated non-redundant function of conserved cis-element CNS2 in Tfh-specific regulation of IL-4 gene [17, 18]. Deletion of CNS2 resulted in profound reduction in IgG1 and IgE post-immunization, and this defect was mainly due to loss of IL-4 expression by Tfh cells. CNS-GFP mouse model that allowed the visualization of Th cells expressing IL-4 gene demonstrated that GFP+ T cells exhibiting Tfh markers were preferentially localized in B cell follicles and GC areas [17]. Although GFP+ Tfh cells were found to develop normally form naïve T cells post-immunization, CNS2 is an active and specific enhancer for IL-4-producing Tfh cells and crucial for IgG1 and IgE class switching. In contrast to Tfh cells, Th2 cells, basophils and eosinophils are less dependent on HSV and CNS2 for IL-4 production.
Analysis of histone proteins in Tfh cells showed abundance of acetylated H3K9/14 at the HS2, HS5a, and CNS2 sites [17]and H3K4me2 methylation at the CNS2 locus[18]. CNS2 deficiency however did not affect these modifications in other loci except the CNS2 region, suggesting that this region is indeed a critical element that regulates Il4 expression in Tfh cells [17]. In naïve T cells, along with enriched H3K4me2 methylation, the CNS2 region is in a highly demethylated state indicating towards its importance in early IL-4 expression during in vivo immune response. CNS2 deficiency also leads to retained H3k27me3 (silencing marker) recruitment particularly in Tfh cells further suggesting that IL-4 production in Tfh cells is solely CNS2 dependent [18].
CNS2 is known to exert its enhancer effects by the SLAM-SAP pathway and Notch and RBP-J signaling for IL-4 production [19, 70]. Epigenetic marks on CNS2 site were slightly affected in the absence of SLAM and were not altered in the absence of RBP-J, indicating marginal participation of SLAM but not Notch pathway for CNS2 activity in Tfh cells [72]. Our recent study identifies a crucial role of the transcription factor Batf in controlling IL-4 expression in Tfh cells through the CNS2 locus [16]. The recruitment of active histone proteins, AcH3 and H3k4 in the CNS2 region in Tfh cells was Batf-dependent indicating that Batf regulates IL-4 expression in Tfh cells through direct binding and controlling access of the chromatin machinery in the CNS2 region. These data sets collectively indicate that Th2 and Tfh cells utilize unique mechanisms for regulation of IL-4 expression, and further investigation of the molecular pathways for IL-4 expression in Tfh cells is warranted.
4.3 Transcriptional requirement
IL-4 appears to be differentially regulated in Th2 and Tfh cells [18, 67]; however, transcriptional control of IL-4 production in Tfh cells remains mainly unknown. Our recent study identifies a novel role for the transcription factor Batf in regulating IL-4 expression in Tfh cells [16]. Our in vivo results showed a T-cell-intrinsic defect in IL-4 production along with other effector Th2-related cytokines in Batf KO mice. However, contrary to our expectations, analysis of in vitro-differentiated Th2 cells from Batf KO mice did not reveal any defect in the expression of the quintessential Th2 cytokine IL-4, suggesting that Batf could contribute to IL-4 expression in helper subset distinct from Th2. Interestingly, our in vivo studies revealed a novel non-redundant role of Batf in the regulation of IL-4 expression in Tfh cells compared with non Tfh cells.
We also detected that Batf directly binds to and activates CNS2 region in the IL-4 locus [16]. Since Batf lacks the transactivation domain, the transactivation observed at the CNS2 locus could be due to its interaction with endogenous factors. Batf physically interacts withIRF4 leading to transactivation of the CNS2 region in the IL-4 locus. In addition, Batf-IRF4 function towards IL-4 induction in Tfh cells is dependent on both Stat3 and Stat6. Whether Stat3 and Stat6 act alone or in a complex with Batf–IRF4 and therefore contribute to IL-4 expression in Tfh cells awaits further extensive investigation.
Apart from Batf and IRF4, we identified the positive effects of the transcription factor c-Maf in the regulation of IL-4 in Tfh cells [16]. c-Maf is known to be a target of Batf in Tfh cells [73], which is required for regulation of IL-21 expression and consequently for Tfh expansion [71]. c-Maf is also known to control IL-4 expression in cooperation with IRF4 and NFAT in Th2 cells [42]. In our study, we also observed increased binding of c-Maf to the CNS2 locus in Tfh cells as well as c-Maf-induced transactivation of the CNS2 region indicating that c-Maf aids in IL-4 transcription in Tfh cells [16]. Since Batf is essential for c-Maf expression in Tfh cells, Batf-to-c-Maf signaling could additionally contribute to the decreased IL-4 expression in Tfh cells.
We detected high Batf expression only in Tfh and Th2 cells among all T helper subsets suggesting that both subsets could share the similar mechanism for Batf expression [16]. The IL-4/Stat6 pathway is important for Batf expression and hence for IL-4 production in Tfh cells, similar to Th2 cells. Batf expression in Tfh cells is known to be also induced by IL-6 signaling via the transcription factor Stat3. Thus, Batf induction in Tfh cells by IL-6 or IL-4 through Stat3 or Stat6, respectively, helps in the generation of IL-4-expressing Tfh cells. However, the exact kinetics of IL-4/Stat6 and IL-6/Stat3 contribution in Batf-mediated IL-4 expression in Tfh cells needs further extensive investigation.
5. IL-4 function in disease settings
Type 2 immune responses are mainly driven by IL-4, IL-5, IL-9 and IL-13 and are crucial in inducing host protective immunity predominantly during helminth infection, type1 inflammation, wound repair and tissue regeneration following infection or injury [2, 3]. On the other side, type 2 responses can also drive inflammatory responses characterized by eosinophils, mast cells, basophils, IL-4- and/or IL-13-conditioned macrophages and Th2 cells, involved in the pathogenesis of many allergic and fibrotic disorders [2]. During type 2 immune response, IL-4 is mainly secreted by Th2 cells, however various other cell types such as NKT cells, eosinophils, basophils and mast cells also produce IL-4 [74]. In the recent years, Tfh cells have been acknowledged as a major alternative source of IL-4 that is essential during type 2 humoral immunity [12–15]. In the below section, we discuss the current understanding on the biological roles of IL-4 produced by Th2 and Tfh cells (Table 1).
Table 1.
Protective and pathogenic roles of Th2/Tfh cell derived Interleukin-4
| IL-4 in Th2 cells | IL-4 in Tfh cells | |
|---|---|---|
| Protective Functions |
Helminth infection: IgG1 and IgE responses Rheumatoid Arthritis: Inhibits proinflammatory cytokines, promotes differentiation of naive T cells to Th2 cells Autoimmune diabetes: delays onset of diabetes Psoriasis: IL-4 treatment can improve psoriatic symptoms EAE: IL-4 deficient mice display severe autoimmunity. |
Helminth infection: Required for mature IgG1 and IgE responses |
| Pathogenic Functions |
Asthma: Recruitment of eosinophils, basophils, mast cells, allergen specific IgE responses Atopic Dermatitis: High IL-4 in serum/PBMCs |
Asthma: Increase cellular infiltration and Th2 cytokine expression in lung and lung lymphnodes |
5.1 Pathogenic role of IL-4
Hyperreactive and dysregulated type 2 immune responses lead to overproduction of type 2 cytokines including IL-4 thus causing various pathogenic disorders such as allergic asthma, allergic rhinitis, atopic dermatitis, eosinophilic oesophagitis and anaphylaxis, as well as allergies to drugs, toxins and food [2, 75]. It is also instrumental in suppressing the development of protective type 1immunity to various pathogens thus facilitating uncontrolled or persistent infection. Role of IL-4 is also known to promote tumorigenesis through alteration of the tumor suppressing activity of monocytes or macrophages [2, 76, 77].
Over the last 15 years, Th2 cells have emerged as the key players in asthma pathogenesis. Asthma is a chronic inflammatory disease of the airways and is characterized by chronic airway inflammation, increased mucus production, reversible airway obstruction, remodeling of the airways, and airways hyperresponsiveness (AHR) [78]. By releasing a number of typical cytokines (IL-4, IL-5, IL-9 and IL-13), these cells orchestrate a number of inflammatory events that subsequently trigger cascades of other processes ultimately leading to the formation of the disease. These Th2 cytokines are responsible for the recruitment of eosinophils (IL-5), basophils (IL-4) and mast cells (IL-4, IL-9 and IL-13) to the airways and for the allergen-specific development of IgE (IL-4 and IL-13) [79]. The role of IL-4 in the development of allergic airway inflammation (asthma) and its contribution to the production of various inflammatory mediators is well appreciated [80]. High IL-4 levels have been detected in the serum and bronchoalveolar lavage of allergic individuals and peripheral blood mononuclear cells from atopic asthmatics [79, 81–83]. This complex phenotype is believed to arise from manifold interactions of infiltrating immune cells with structural cells of the airways. Studies using both IL-4-deficient mice as well as utilizing neutralizing anti-IL-4 antibodies in mice showed inhibition of the biological activities of IL-4 as well as IL-5 and IL-13 in CD4+ T cells leading to reduction of allergen-specific IgE and eosinophilic infiltration and airway inflammation [84].
Implication of Tfh-derived in allergic airway inflammation is being actively investigated upon. Recent report from our group has also implicated the function of Tfh-derived IL-4 in the pathogenicity of allergic inflammation [16]. Adoptive transfer experiments of WT (CD45.1+,CD4+,CD44+CXCR5hi and PD1hi) Tfh cells from lung and lung lymph nodes of asthmatic mice into congenic mice showed asthmatic symptoms in the recipient mice such as eosinophil and lymphocyte infiltration and production of Th2 cytokines like IL-4, IL-5 and IL-13 in the lungs. These transferred Tfh cells maintained a robust Tfh phenotype including CXCR5 and IL-4 expression thus confirming their involvement in development of the allergic inflammatory responses. These symptoms were found to be dependent on Batf driven IL-4 expression in Tfh cells, since transfer of Batf deficient Tfh cells showed a dramatic decrease in the asthmatic symptoms [16]. Further studies will help in exactly delineating the kinetics of IL-4 expression during allergic disease progression.
5.2 Protective role of IL-4
In contrast with allergic diseases, IL-4 can act as an anti-inflammatory cytokine in various autoimmune diseases [85]. Th2 derived IL-4 along with its counterpart IL-13 play a critical role in promoting host survival during infection with parasitic helminthes that have tissue-dwelling phases and they can mediate expulsion of intestinal helminthes [86, 87]. Importantly, Th2 responses are associated with development of strong antibody responses, particularly IgG1 and IgE, which in certain helminth infections are implicated in resistance to reinfection [13]. Interestingly, recent mouse studies employing helminth parasite infections showed that IL-4-producing CD4+ T cells are specifically restricted to the B cell follicles and associated with germinal centers [13–15, 66]. Consistent with localization, IL-4 producers express high levels of Tfh markers [13, 15]. Although IL-4 is dispensable for Tfh development, Tfh-derived IL-4 is critical to orchestrate multiple features of a mature B cell responses after helminth infection, particularly regulating isotype class switching in conjugated B cells toward IgG1 and IgE [15, 67]. Further studies designed to understand the origin, development and function of Tfh cells will help address their role in the prevention of the development of severe disease after infection with parasites.
In diseases, such as Rheumatoid arthritis where Th1 cells are the major players, IL-4 derived majorly from Th2 cells, mast cells or basophils can inhibit Th1 cell formation and production of proinflammatory cytokines like TNF-α, IL-1α, IL-1β, IL-6, IL-8, G-CSF, and macrophage inflammatory protein (MIP)-1α, enhances monocyte apoptosis and acts as an autocrine growth factor promoting the differentiation of naive T cells to Th2 cells [85, 88, 89]. Although IL-4 levels are seldom detected in the synovial fluid, the progression of this disease is more aggressive in animals lacking IL-4 and IL-13 than in the wild type counterparts [90, 91]. IL-4 gene therapy can reduce inflammation and ameliorate joint destruction in animal models of rheumatoid arthritis [92, 93]. Mice treated with recombinant IL-4 or constitutively expressing IL-4 are also known to be protected from experimental autoimmune diabetes. Islet Ag-specific T cells displayed a Th2 phenotype and the IL-4 expression in the β-islets of pancreatic cells from transgenic non-obese diabetic (NOD) mice decreased diabetes development. Neutralization of IL-4 along with another Th2 cytokine IL-10 in this system abrogated the ability of NOD-IL-4 splenocytes to delay the onset of diabetes [94]. Other autoimmune diseases driven by Th1 cells like psoriasis can also be improved by IL-4 treatment [95]. The role of IL-4 in regulating inflammation within the CNS has also been demonstrated in the mouse model of MS, experimental autoimmune encephalomyelitis (EAE). IL-4 deficient mice exhibited more severe EAE [96, 97]. IL-4 retroviral-transduced T-cells delivered into the CNS also results in the amelioration of EAE [98]. Furthermore, IL-4 produced by the microglial cells in the central nervous system helps in promoting the alternatively activated macrophages that are important in controlling inflammation caused by EAE [99]. IL-4 is also known to play a protective role in Crohn’s disease [100]. Decreased production of IL-4 in inflammatory bowel disease also contributes to disease pathogenesis. Less IL-4 production modulates lamina propria mononuclear cells (LPMC) reactivity and has defective immunosuppressive and anti-inflammatory mechanisms [100].
6. Conclusion and perspective
Type 2 immune responses have an important role in mediating protective immunity to the host against parasitic infections but also cause allergic diseases such as asthma. Th2 cells and innate immune cells that produce the signature Th2 cytokines interleukin are major players in orchestrating the Th2 responses. Emerging evidence suggest that the Tfh cells, a CD4+T cell subset found in the B cell follicles and germinal centers of lymph nodes, is an alternative source of IL-4 in vivo and the IL-4 produced by these cells is essential for shaping Th2-cell based humoral immunity implicating that Th2 and Tfh cells exhibit overlapping phenotypical and functional characteristics as well as raise the possibility of flexibility between Tfh cells and other T helper effector populations.
In fact, elegant studies by Zaretsky et al show that in response to helminth parasite infections, IL-4 and Gata3 expressing Th2 cells can acquire Tfh cell characteristics including IL-21, CXCR5, and ICOS expression within B cell follicles, suggesting Th2/Tfh plasticity. It still remains unclear whether Tfh cells can generate from other effector T cell types and whether these follicular T cells are able to retain their phenotype beyond the lymph node follicles. Whether signals from the microenvironment dictate the reversible transition Tfh cells toward Th2 or other T helper subsets needs further extensive investigation. Studies designed to increase the understanding of the origin, development and function of Tfh cells should provide insight into the optimal requirements for antibody production in order to meet demands of an appropriate host response during parasitic infection.
Our recent report also shows that IL-4 producing Tfh cells contribute to promotion of allergic airway inflammation as well as retain a stable phenotype during the course of disease. However, while IL-4-producing Th2 cells from asthmatic mice upon adoptive transfer could trigger asthmatic symptoms in the recipient mice, the majority of these cells in lung and lung lymph nodes acquired Tfh phenotype (CXCR5, BTLA and Bcl6), suggesting plasticity of Th2 cells towards Tfh cells upon allergic inflammation and its contribution to asthma pathogenesis. Further studies are necessary to determine the exact nature of relationship between Th2 and Tfh cells during allergic responses and delineate the exact role of IL-4 derived from Tfh cells in type 2 immunity in physiological and disease settings. Furthermore, a complete mechanistic understanding of the molecular details of IL-4 gene regulation and production in Th2 and Tfh cells may facilitate the development of novel approaches to target IL-4 in both Th2 and Tfh cells during allergic inflammation.
Highlights.
IL-4 is produced by two distinct T helper subsets including T helper 2 and T follicular helper cells.
Production of IL-4 is regulated by different transcriptional and epigenetic mechanisms in these T helper subsets.
IL-4 plays beneficial role during parasitic infection or possess a pathogenic role in allergic diseases.
Acknowledgments
This work is supported by NIH research grants (A1R03AI120027 and 1R21AI20012 (RN)), Institutional Research Grant (RN), start-up grant (RN), MD Anderson CIC seed grant (RN).
Abbreviations
- Th2
T helper 2
- Tfh
T follicular helper
- IL
Interleukin
- Ig
Immunoglobulin
- STAT
signal transduction and activator of transcription
- TCR
T cell receptor
- NFAT
nuclear factor of activated T cells
- IRF4
Interferon regulatory factor 4
- ERK
extracellular signal-regulated kinase
- ICOS
Inducible co-stimulator
- CNS
conserved non-coding sequence
- SLAM
signaling lymphocytic activation molecule
- SAP
slam associated protein
- GITR
glucocorticoid-induced tumor necrosis factor receptor
- TSLP
thymic stromal lymphoprotein
- AP
activating protein
- TCF
T cell factor
- Gfi
Growth factor independent
- HS
hypersensitive sites
- LCR
locus control region
- GC
germinal center
- Bcl6
B cell lymphoma 6
- SEA
schistosome egg antigen
- Batf
basic leucine zipper transcription factor ATF
- VCAM
vascular cell adhesion molecule
- MIP
macrophage inflammatory protein
- NOD
non-obese diabetic
Biographies

Anupama Sahoo Anupama received her Ph.D. in Life Sciences (Immunology and Cell biology) from Gwangju Institute of Science and Technology, Gwangju, South Korea in August, 2011. She continued her research in Immunology as a postdoctoral fellow at the Department of Immunology, University of Texas MD Anderson Cancer Center, Houston, Texas, USA (November, 2011 to January, 2015), prior to moving to Sanford Burnham Medical Research Institute, Orlando, FL, USA. Her research interests include exploring the differential expression of novel factors in T lymphocytes and their regulatory functions in infectious and autoimmune diseases and cancer.

Shradha Wali Shradha graduated with a Master’s degree from University of Texas at San Antonio, USA. She is a Ph.D. student in the Department of Immunology at the University Of Texas Graduate School Of Biomedical Sciences, Houston, Texas, USA. Her current research is understanding transcriptional regulation in T follicular helper cell differentiation and activation.

Roza Nurieva Dr. Roza Nurieva received her Ph.D. in 2000 from the Gabrichevsky Research Institute of Epidemiology and Microbiology, Moscow, Russia. Her postdoctoral training was with Dr. Chen Dong at the University of Washington, Seattle, focusing on understanding the role of costimulatory molecules in regulating T helper cell activation, differentiation, and function. She is currently an Assistant Professor in the Immunology Departments at MD Anderson Cancer Center. Dr. Nurieva’s main research goal is to understand the molecular and cellular mechanisms that control the development and progress of autoimmunity, inflammation and cancer, with particular emphasis on the signalling and transcriptional mechanisms underlying T helper cell development and T cell mediated immune responses.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oliphant CJ, Barlow JL, McKenzie AN. Insights into the initiation of type 2 immune responses. Immunology. 2011;134:378–85. doi: 10.1111/j.1365-2567.2011.03499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15:271– 82. doi: 10.1038/nri3831. [DOI] [PubMed] [Google Scholar]
- 3.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nature reviews Immunology. 2010;10:225–35. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nature Reviews Immunology. 2013;10:607–14. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ngoc LP, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokines, allergy, and asthma. Current opinion in allergy and clinical immunology. 2005;5:161–6. doi: 10.1097/01.all.0000162309.97480.45. [DOI] [PubMed] [Google Scholar]
- 6.Mowen KA, Glimcher LH. Signaling pathways in Th2 development. Immunological Reviews. 2004;202:203–22. doi: 10.1111/j.0105-2896.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- 7.Okoye IS, Wilson MS. CD4+ T helper 2 cells–microbial triggers, differentiation requirements and effector functions. Immunology. 2011;134:368–77. doi: 10.1111/j.1365-2567.2011.03497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J. Transcriptional regulation of Th2 cell differentiation. Immunology and cell biology. 2010;88:244–9. doi: 10.1038/icb.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annual review of immunology. 2010;28:445. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crotty S. T Follicular Helper Cell Differentiation, Function, and Roles in Disease. Immunity. 2014;41:529–42. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nurieva RI, Chung Y. Understanding the development and function of T follicular helper cells. Cell Mol Immunol. 2010;7:190–7. doi: 10.1038/cmi.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 13.Glatman Zaretsky A, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. 2009;206:991–9. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King C. New insights into the differentiation and function of T follicular helper cells. Nat Rev Immunol. 2009;9:757–66. doi: 10.1038/nri2644. [DOI] [PubMed] [Google Scholar]
- 15.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med. 2009;206:1001–7. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahoo A, Alekseev A, Tanaka K, Obertas L, Lerman B, Haymaker C, et al. Batf is important for IL-4 expression in T follicular helper cells. Nat Commun. 2015;6:7997. doi: 10.1038/ncomms8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada Y, Tanaka S, Motomura Y, Harada Y, Ohno S, Ohno S, et al. The 3′ enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity. 2012;36:188–200. doi: 10.1016/j.immuni.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Vijayanand P, Seumois G, Simpson LJ, Abdul-Wajid S, Baumjohann D, Panduro M, et al. Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity. 2012;36:175–87. doi: 10.1016/j.immuni.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka S, Tsukada J, Suzuki W, Hayashi K, Tanigaki K, Tsuji M, et al. The interleukin-4 enhancer CNS-2 is regulated by Notch signals and controls initial expression in NKT cells and memory-type CD4 T cells. Immunity. 2006;24:689–701. doi: 10.1016/j.immuni.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Lee GR. Transcriptional regulation of T helper type 2 differentiation. Immunology. 2014;141:498–505. doi: 10.1111/imm.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4+ T cells toward distinct T-helper cell subsets. Immunological reviews. 2013;252:12–23. doi: 10.1111/imr.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao X, Constant S, Jorritsma P, Bottomly K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. The Journal of Immunology. 1997;159:5956–63. [PubMed] [Google Scholar]
- 23.Nurieva RI, Duong J, Kishikawa H, Dianzani U, Rojo JM, Ho I-c, et al. Transcriptional regulation of th2 differentiation by inducible costimulator. Immunity. 2003;18:801–11. doi: 10.1016/s1074-7613(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 24.Nurieva RI, Mai XM, Forbush K, Bevan MJ, Dong C. B7h is required for T cell activation, differentiation, and effector function. Proceedings of the National Academy of Sciences. 2003;100:14163–8. doi: 10.1073/pnas.2335041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motta AC, Vissers J, Gras R, Van Esch B, Van Oosterhout A, Nawijn MC. GITR signaling potentiates airway hyperresponsiveness by enhancing Th2 cell activity in a mouse model of asthma. Respir Res. 2009;10:93–100. doi: 10.1186/1465-9921-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polte T, Behrendt A-K, Hansen G. Direct evidence for a critical role of CD30 in the development of allergic asthma. Journal of allergy and clinical immunology. 2006;118:942–8. doi: 10.1016/j.jaci.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–56. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 28.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, et al. Interleukin 2 plays a central role in Th2 differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3880–5. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu J, Guo L, Watson CJ, Hu-Li J, Paul WE. Stat6 is necessary and sufficient for IL-4’s role in Th2 differentiation and cell expansion. The Journal of Immunology. 2001;166:7276–81. doi: 10.4049/jimmunol.166.12.7276. [DOI] [PubMed] [Google Scholar]
- 31.Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–48. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- 32.Diehl S, Chow C-W, Weiss L, Palmetshofer A, Twardzik T, Rounds L, et al. Induction of NFATc2 expression by interleukin 6 promotes T helper type 2 differentiation. The Journal of experimental medicine. 2002;196:39–49. doi: 10.1084/jem.20020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Molecular immunology. 2002;39:531–6. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y-H, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC–activated Th2 memory cells. The Journal of experimental medicine. 2007;204:1837–47. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louten J, Rankin AL, Li Y, Murphy EE, Beaumont M, Moon C, et al. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation. International immunology. 2011:dxr006. doi: 10.1093/intimm/dxr006. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–9. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 37.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, et al. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–30. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 38.Liao W, Schones DE, Oh J, Cui Y, Cui K, Roh T-Y, et al. Priming for T helper type 2 differentiation by interleukin 2–mediated induction of interleukin 4 receptor α-chain expression. Nature immunology. 2008;9:1288–96. doi: 10.1038/ni.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouyang W, Löhning M, Gao Z, Assenmacher M, Ranganath S, Radbruch A, et al. Stat6-Independent GATA-3 Autoactivation Directs IL-4-Independent Th2 Development and Commitment. Immunity. 2000;12:27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 40.Horiuchi S, Onodera A, Hosokawa H, Watanabe Y, Tanaka T, Sugano S, et al. Genome-wide analysis reveals unique regulation of transcription of Th2-specific genes by GATA3. The Journal of Immunology. 2011;186:6378–89. doi: 10.4049/jimmunol.1100179. [DOI] [PubMed] [Google Scholar]
- 41.Stritesky GL, Muthukrishnan R, Sehra S, Goswami R, Pham D, Travers J, et al. The transcription factor STAT3 is required for T helper 2 cell development. Immunity. 2011;34:39–49. doi: 10.1016/j.immuni.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rengarajan J, Mowen KA, McBride KD, Smith ED, Singh H, Glimcher LH. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J Exp Med. 2002;195:1003–12. doi: 10.1084/jem.20011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rengarajan J, Tang B, Glimcher LH. NFATc2 and NFATc3 regulate T(H)2 differentiation and modulate TCR-responsiveness of naive T(H)cells. Nat Immunol. 2002;3:48–54. doi: 10.1038/ni744. [DOI] [PubMed] [Google Scholar]
- 44.Ho IC, Hodge MR, Rooney JW, Glimcher LH. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973–83. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- 45.Mohrs M, Blankespoor CM, Wang Z-E, Loots GG, Afzal V, Hadeiba H, et al. Deletion of a coordinate regulator of type 2 cytokine expression in mice. Nature immunology. 2001;2:842–7. doi: 10.1038/ni0901-842. [DOI] [PubMed] [Google Scholar]
- 46.Takemoto N, Arai K-i, Miyatake S. Cutting edge: the differential involvement of the N-finger of GATA-3 in chromatin remodeling and transactivation during Th2 development. The Journal of Immunology. 2002;169:4103–7. doi: 10.4049/jimmunol.169.8.4103. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal S, Avni O, Rao A. Cell-type-restricted binding of the transcription factor NFAT to a distal IL-4 enhancer in vivo. Immunity. 2000;12:643–52. doi: 10.1016/s1074-7613(00)80215-0. [DOI] [PubMed] [Google Scholar]
- 48.Ansel KM, Greenwald RJ, Agarwal S, Bassing CH, Monticelli S, Interlandi J, et al. Deletion of a conserved Il4 silencer impairs T helper type 1–mediated immunity. Nature immunology. 2004;5:1251–9. doi: 10.1038/ni1135. [DOI] [PubMed] [Google Scholar]
- 49.Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nature immunology. 2007;8:145–53. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 50.Yagi R, Junttila IS, Wei G, Urban JF, Zhao K, Paul WE, et al. The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-γ. Immunity. 2010;32:507–17. doi: 10.1016/j.immuni.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee GR, Spilianakis CG, Flavell RA. Hypersensitive site 7 of the TH2 locus control region is essential for expressing TH2 cytokine genes and for long-range intrachromosomal interactions. Nature immunology. 2005;6:42–8. doi: 10.1038/ni1148. [DOI] [PubMed] [Google Scholar]
- 52.Hwang SS, Kim YU, Lee S, Jang SW, Kim MK, Koh BH, et al. Transcription factor YY1 is essential for regulation of the Th2 cytokine locus and for Th2 cell differentiation. Proceedings of the National Academy of Sciences. 2013;110:276–81. doi: 10.1073/pnas.1214682110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–56. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 54.Ansel KM, Lee DU, Rao A. An epigenetic view of helper T cell differentiation. Nature immunology. 2003;4:616–23. doi: 10.1038/ni0703-616. [DOI] [PubMed] [Google Scholar]
- 55.Zeng W-p. ‘All things considered’: transcriptional regulation of T helper type 2 cell differentiation from precursor to effector activation. Immunology. 2013;140:31–8. doi: 10.1111/imm.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee DU, Agarwal S, Rao A. Th2 lineage commitment and efficient IL-4 production involves extended demethylation of the IL-4 gene. Immunity. 2002;16:649–60. doi: 10.1016/s1074-7613(02)00314-x. [DOI] [PubMed] [Google Scholar]
- 57.Makar KW, Pérez-Melgosa M, Shnyreva M, Weaver WM, Fitzpatrick DR, Wilson CB. Active recruitment of DNA methyltransferases regulates interleukin 4 in thymocytes and T cells. Nature immunology. 2003;4:1183–90. doi: 10.1038/ni1004. [DOI] [PubMed] [Google Scholar]
- 58.Tykocinski L-O, Hajkova P, Chang H-D, Stamm T, SÖzeri O, LÖhning M, et al. A critical control element for interleukin-4 memory expression in T helper lymphocytes. Journal of Biological Chemistry. 2005;280:28177–85. doi: 10.1074/jbc.M502038200. [DOI] [PubMed] [Google Scholar]
- 59.Fields PE, Lee GR, Kim ST, Bartsevich VV, Flavell RA. Th2-specific chromatin remodeling and enhancer activity in the Th2 cytokine locus control region. Immunity. 2004;21:865–76. doi: 10.1016/j.immuni.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 60.Kim ST, Fields PE, Flavell RA. Demethylation of a specific hypersensitive site in the Th2 locus control region. Proceedings of the National Academy of Sciences. 2007;104:17052–7. doi: 10.1073/pnas.0708293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, et al. A Critical Role for Dnmt1 and DNA Methylation in T Cell Development, Function, and Survival. Immunity. 2001;15:763–74. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 62.Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012;209:1241–53. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–5. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–49. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–63. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fairfax KC, Everts B, Amiel E, Smith AM, Schramm G, Haas H, et al. IL-4-secreting secondary T follicular helper (Tfh) cells arise from memory T cells, not persisting Tfh cells, through a B cell-dependent mechanism. J Immunol. 2015;194:2999–3010. doi: 10.4049/jimmunol.1401225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–93. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McGuire HM, Vogelzang A, Warren J, Loetsch C, Natividad KD, Chan TD, et al. IL-21 and IL-4 Collaborate To Shape T-Dependent Antibody Responses. The Journal of Immunology. 2015;195:5123–35. doi: 10.4049/jimmunol.1501463. [DOI] [PubMed] [Google Scholar]
- 69.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–7. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 70.Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, et al. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) J Immunol. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–75. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li W, Sofi MH, Rietdijk S, Wang N, Terhorst C, Chang CH. The SLAM-associated protein signaling pathway is required for development of CD4+ T cells selected by homotypic thymocyte interaction. Immunity. 2007;27:763–74. doi: 10.1016/j.immuni.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat Immunol. 2011;12:536–43. doi: 10.1038/ni.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koyasu S, Moro K. Type 2 innate immune responses and the natural helper cell. Immunology. 2011;132:475–81. doi: 10.1111/j.1365-2567.2011.03413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–72. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, et al. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–17. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 77.Gocheva V, Wang H-W, Gadea BB, Shree T, Hunter KE, Garfall AL, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes & development. 2010;24:241–55. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murdoch JR, Lloyd CM. Chronic inflammation and asthma. Mutation Research. 2010;690:24–39. doi: 10.1016/j.mrfmmm.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steinke JW, Borish L. Th2 cytokines and asthma—Interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respiratory research. 2001;2:66. doi: 10.1186/rr40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chatila TA. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends in Molecular Medicine. 2004;10:493–9. doi: 10.1016/j.molmed.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Daher S, Santos L, Sole D, De Lima M, Naspitz C, Musatti C. Interleukin-4 and soluble CD23 serum levels in asthmatic atopic children. Journal of investigational allergology & clinical immunology. 1994;5:251–4. [PubMed] [Google Scholar]
- 82.Leonard C, Tormey V, Burke C, Poulter L. Allergen-induced cytokine production in atopic disease and its relationship to disease severity. American journal of respiratory cell and molecular biology. 1997;17:368–75. doi: 10.1165/ajrcmb.17.3.2797. [DOI] [PubMed] [Google Scholar]
- 83.Walker C, Bauer W, Braun RK, Menz G, Braun P, Schwarz F, et al. Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. American journal of respiratory and critical care medicine. 1994;150:1038–48. doi: 10.1164/ajrccm.150.4.7921434. [DOI] [PubMed] [Google Scholar]
- 84.Hansbro PM, Kaiko GE, Foster PS. Cytokine/anti-cytokine therapy – novel treatments for asthma? British Journal of Pharmacology. 2011;163:81–95. doi: 10.1111/j.1476-5381.2011.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zamorano J, Rivas M, Perez G. Interleukin-4: A multifunctional cytokine. Immunologia. 2003;22:215–24. [Google Scholar]
- 86.Anthony RM, Urban JF, Alem F, Hamed HA, Rozo CT, Boucher J-L, et al. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nature medicine. 2006;12:955–60. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–5. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- 88.Hart PH, Hunt EK, Bonder CS, Watson CJ, Finlay-Jones JJ. Regulation of surface and soluble TNF receptor expression on human monocytes and synovial fluid macrophages by IL-4 and IL-10. The Journal of Immunology. 1996;157:3672–80. [PubMed] [Google Scholar]
- 89.Mosmann T, Coffman R. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual review of immunology. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 90.Finnegan A, Grusby MJ, Kaplan CD, O’Neill SK, Eibel H, Koreny T, et al. IL-4 and IL-12 regulate proteoglycan-induced arthritis through Stat-dependent mechanisms. The Journal of Immunology. 2002;169:3345–52. doi: 10.4049/jimmunol.169.6.3345. [DOI] [PubMed] [Google Scholar]
- 91.Miossec P, Naviliat M, Dupuy dAA, Sany J, Banchereau J. Low levels of interleukin-4 and high levels of transforming growth factor beta in rheumatoid synovitis. Arthritis and rheumatism. 1990;33:1180–7. doi: 10.1002/art.1780330819. [DOI] [PubMed] [Google Scholar]
- 92.Morita Y, Yang J, Gupta R, Shimizu K, Shelden EA, Endres J, et al. Dendritic cells genetically engineered to express IL-4 inhibit murine collagen-induced arthritis. The Journal of clinical investigation. 2001;107:1275–84. doi: 10.1172/JCI11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Woods JM, Katschke KJ, Volin MV, Ruth JH, Woodruff DC, Amin MA, et al. IL-4 adenoviral gene therapy reduces inflammation, proinflammatory cytokines, vascularization, and bony destruction in rat adjuvant-induced arthritis. The Journal of Immunology. 2001;166:1214–22. doi: 10.4049/jimmunol.166.2.1214. [DOI] [PubMed] [Google Scholar]
- 94.Gallichan WS, Balasa B, Davies JD, Sarvetnick N. Pancreatic IL-4 expression results in islet-reactive Th2 cells that inhibit diabetogenic lymphocytes in the nonobese diabetic mouse. The Journal of Immunology. 1999;163:1696–703. [PubMed] [Google Scholar]
- 95.Ghoreschi K, Thomas P, Breit S, Dugas M, Mailhammer R, van Eden W, et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nature medicine. 2003;9:40–6. doi: 10.1038/nm804. [DOI] [PubMed] [Google Scholar]
- 96.Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10-and IL-4-deficient and transgenic mice. The Journal of Immunology. 1998;161:3299–306. [PubMed] [Google Scholar]
- 97.Falcone M, Rajan AJ, Bloom BR, Brosnan CF. A critical role for IL-4 in regulating disease severity in experimental allergic encephalomyelitis as demonstrated in IL-4-deficient C57BL/6 mice and BALB/c mice. The Journal of Immunology. 1998;160:4822–30. [PubMed] [Google Scholar]
- 98.Shaw MK, Lorens JB, Dhawan A, DalCanto R, Harley YT, Tran AB, et al. Local delivery of interleukin 4 by retrovirus-transduced T lymphocytes ameliorates experimental autoimmune encephalomyelitis. The Journal of experimental medicine. 1997;185:1711–4. doi: 10.1084/jem.185.9.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ponomarev ED, Maresz K, Tan Y, Dittel BN. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. The Journal of neuroscience. 2007;27:10714–21. doi: 10.1523/JNEUROSCI.1922-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.West G, Matsuura T, Levine A, Klein J, Fiocchi C. Interleukin 4 in inflammatory bowel disease and mucosal immune reactivity. Gastroenterology. 1996;110:1683–95. doi: 10.1053/gast.1996.v110.pm8964392. [DOI] [PubMed] [Google Scholar]