Abstract
The reaction of Re(CO)3(H2O)3+ with hen egg lysozyme in aqueous solution results in a single covalent adduct; single crystal X-ray diffraction shows that the rhenium tricarbonyl cation binds to His15 in two significantly populated rotamer conformations.
Graphical Contents Entry
The reaction of Re(CO)3(H2O)3+ with hen egg lysozyme in aqueous solution results in a single covalent adduct; single crystal X-ray diffraction shows that the rhenium tricarbonyl cation binds to His15 in two significantly populated rotamer conformations.
The aqueous chemistry of the organometallic cation Re(CO)3(H2O)3+ has received much attention over the past decade,1,2 due to its relevance to the design of 186/188Re radiopharmaceuticals3,4 as well as its ability to act as a surrogate for the chemistry of 99mTc(CO)3+.5–7 In many cases, these investigations have focused on reactions between biomolecules and Re(CO)3(H2O)3+.4 Although much work has been carried out investigating interactions between Re(CO)3-based compounds and nucleic acids,8–12 amino acids and oligopeptides,13–16 little work has been carried out on reactions between rhenium prodrug or drug model complexes and proteins. Such interactions can be crucial to the biological processing of Tc/Re based imaging agents, since proteins, rather than nucleotides or single amino acids, would be encountered in plasma. Alternatively, protein-Tc/Re adducts could be novel targets for use as imaging or therapeutic agents. In order to probe this chemistry, we began to examine the interactions between Re(CO)3(H2O)3+ and the readily crystallizable protein lysozyme.
We reacted several stoichiometries of Re(CO)3(H2O)3+ with hen egg white lysozyme (MP Biomedicals, 3X crystalized) in pure H2O at 4° C overnight and analyzed these solutions by MALDI mass spectrometry.† Upon exposure to increasing concentrations of Re(CO)3(H2O)3+, a peak with a M/z of 14579 appears, which corresponds to addition of a Re(CO)3+ unit (270 M/z) to the mass of lysozyme (14309 M/z). At a 3:1 stoichiometric ratio of Re:protein, the 14309 M/z:14579 M/z ratio is about 60:40. Optimal protein crystals were obtained by preincubating lysozyme with a stoichiometry of 3:1 [Re(CO)3(H2O)3]Br to protein overnight at 4 °C. Crystals were grown from this solution by the hanging drop method in 0.05 M MES buffer, pH 5.5, 0.8 M NaCl, 50 mg/mL protein. Large tetragonal crystals formed after 48–72 hours. It is important to note that adduct formation was carried out in solution prior to crystal growth; in other cases, metal ligation is achieved only by soaking crystals in reagent solutions.
X-ray diffraction data to 1.6 Å resolution were collected at 1.54 Å (Oxford Diffraction Gemini R) and was integrated and scaled using CrysalisPro (99.6% complete, Rint = 0.091, I/σ(I) = 47.2, redundancy = 22.4). The structure was solved by molecular replacement (Phaser) using PDB 6LYZ and subjected to several cycles of restrained refinement using Refmac5 and rebuilding in Coot.17–20 The final structure (PDB 3KAM, R = 0.181, Rfree = 0.215, rms bonds = 0.015 Å, rms angles = 1.6°) contained clearly identifiable electron density for Re(CO)3(H2O)2+ attached to Nε2 of His15. The occupancy of the Re(CO)3(H2O)2+ fragment in the model is less than 1.0 and was modeled at 0.60, consistent with the MALDI-MS data which indicates partial derivatization of the protein.
The refined Re-O bond distance for water W2 is somewhat long (2.6 Å compared to the expected 2.2 Å as observed for W1), suggesting that there are at least two significantly populated rotamers of the Re(CO)3(H2O)2+ adduct, related by a 90° rotation about the Re-N bond. In the final model, however, only the principal rotamer was modeled. Partial occupancy of a CO ligand at the W2 position results in the Re-O bond having an artificially long bond distance. The observation that the electron density of the Re(CO)3(H2O)2+ fragment is well-ordered, rather than averaged because of free rotation about the Re-N axis, is possibly due to the stabilizing hydrogen bond contributed by Asp87. In the principal rotamer, Asp87 interacts with W1; in the minor rotamer, Asp87 interacts with W2. The Re-N distance of 2.2 Å compares well to related small molecule structures.21 While it is not possible to definitively rule out partial occupancy of chloride ion at position W2 of the rhenium tricarbonyl complex to explain the anomalously long Re-water bond length at this position, we believe the simplest interpretation of the data is the existence of two rotamers with water coordinated at positions W1 and W2.
The presence of Re(CO)3+ adducts in these single crystals was confirmed by IR spectroscopy. Figure 2 shows the spectra of native hen egg lysozyme before exposure to Re(CO)3(H2O)3+ and of lysozyme crystals grown from the 3:1 reaction described above. In the 1800–2200 cm−1 region, unmodified lysozyme is transparent, but the Re(CO)3+ modified crystals show ν(CO) stretching bands corresponding to e and a1 modes (1890, 2001 and 2013 cm−1) resulting from the pseudo-C3v symmetry of the facial Re(CO)3+ unit. These bands do not correspond to those of the Re(CO)3(H2O)3 species, which shows an a1 band at 2036 cm−1.22 The appearance of two a1 bands is consistent with the presence of multiple rotamers of the Re(CO)3+ adduct as described above.
Fig. 2.
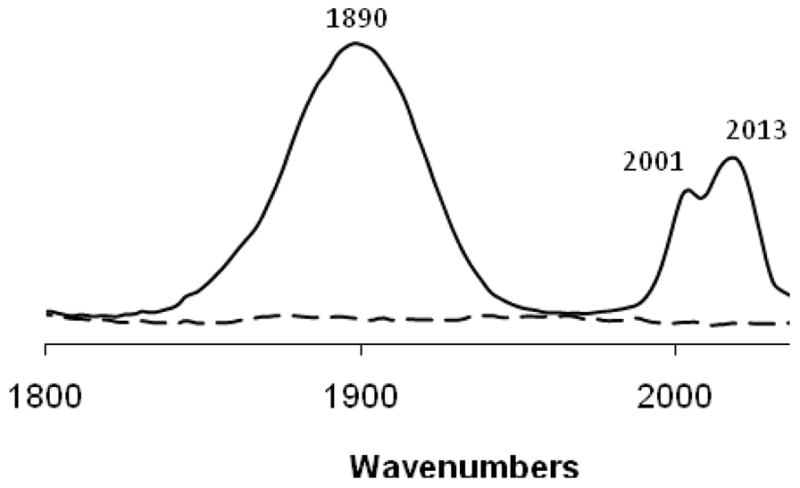
IR spectra of the ν(CO) stretching region of native hen egg white lysozyme (dashed line) and Re(CO)3+ modified lysozyme (solid line).†
To the best of our knowledge, the above described work is the first example of a crystallographically characterized Re(CO)3(H2O)2+-protein conjugate. There is a significant body of work on the ligation of non-natural metal ions to the periphery of lysozyme, such as the binding of cisplatin to the His15 site.23 More recently, organometallic complexes have been attached to this and other proteins, including Fischer-type metallocarbenes,24 Mn(CO)3(H2O)2+,25 half-sandwich Ru(II) species26 and most notably Re(diimine)(CO)3+ adducts which were used for long distance electron transfer studies and also have been crystallographically characterized.27 In addition to the medicinal relevance of our work, the peripheral modification with Re(CO)3+ could be used to further the fundamental characterization of proteins. With regard to X-ray structural elucidation, Re(CO)3(H2O)3+ could be used as a heavy atom reagent to assist crystal phasing, since it meets all the essential requirements of phasing agents.28 The cation contains a heavy atom, is water soluble, doesn’t hydrolyze, is relatively non-toxic, has selectivity since it can readily form a covalent bond with a surface histidine but is not expected to react with other common groups such as amines and thioethers, and binds to the protein in a way that does not alter the crystal lattice of the protein. Alternatively, Re(CO)3(H2O)3+ could be used to generate the aforementioned Re(diimine)(CO)3+ type adducts. The ligation of Re(diimine)(CO)3X or Re(diimine)(CO)3(H2O)+ to the periphery of proteins can require more than three weeks of reaction time at 37 °C.27 In the current work, we observed that Re(CO)3(H2O)3+ reacts with the protein at 4 °C in as little as twelve hours. Subsequent reaction of the conjugate with a diimine would result in the desired electron transfer conjugate. We are continuing our work in the area of protein-Re(CO)3(H2O)2+ interactions.
Supplementary Material
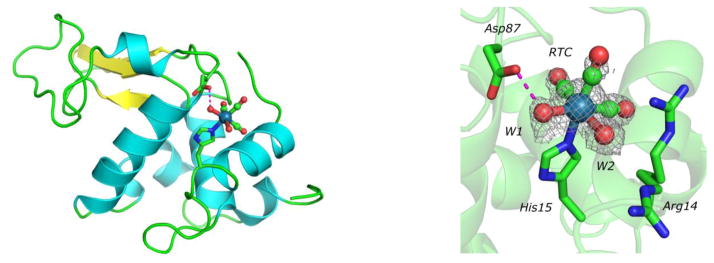
Fig 1.
Left: Ribbon diagram of hen egg white lysozyme with Re(CO)3(H2O)2+ bound to its unique site at Nε2 of His15. Hydrogen bond between Asp87 and water W1 of Re(CO)3(H2O)2+ is depicted as a magenta dashed line. Right: Fo-Fc omit map of lysozyme-Re(H2O)2(CO)3+ (RTC) adduct, contoured at 2.5σ. Arg14 has weak electron density and has alternate conformations, and does not appear to interact with Re(H2O)2(CO)3+.
Acknowledgments
C.J.Z. would like to acknowledge a grant from the National Institutes of Health (R15 GM 083322) for support of this work. R.S.H. thanks the Research Corporation (CC6663/6616) for research support. This work was also supported in part by a grant from the National Science Foundation to R.S.R. (CHE-0819686)
Footnotes
Electronic supplementary information (ESI) available: MALDI mass spectra of native hen egg lysozyme reacted in aqueous solution with various stiochiometries of Re(H2O)3(CO)3+ and full spectra of the data shown in Figure 2 see DOI: 10.1039/b000000x
Contributor Information
Christopher J. Ziegler, Email: ziegler@uakron.edu.
Richard S. Herrick, Email: rherrick@holycross.edu.
Roger S. Rowlett, Email: rrowlett@colgate.edu.
Notes and references
- 1.Alberto R, Schibli R, Waibel R, Abram U, Schubiger AP. Coord Chem Rev. 1999;190–192:901. [Google Scholar]
- 2.Herrick RS. New Developments in Organometallic Research. Nova Science Publishers, Inc; New York: 2006. pp. 115–149. [Google Scholar]
- 3.Schibli R, Schubiger PA. Eur J Nucl Med Mol Imaging. 2002;29:1529. doi: 10.1007/s00259-002-0900-8. [DOI] [PubMed] [Google Scholar]
- 4.Schibli R, Schwarzbach R, Alberto R, Ortner K, Schmalle H, Dumas C, Egli A, Schubiger PA. Bioconj Chem. 2002;13:750. doi: 10.1021/bc015568r. [DOI] [PubMed] [Google Scholar]
- 5.Alberto R, Ortner K, Wheatley N, Schibli R, Schubiger AP. J Am Chem Soc. 2001;123:3135. doi: 10.1021/ja003932b. [DOI] [PubMed] [Google Scholar]
- 6.Alberto R, Egli A, Abram U, Hegetschweiler K, Gramlich V, Schubiger PA. J Chem Soc, Dalton Trans. 1994:2815. [Google Scholar]
- 7.Liu S, Edwards DS. Chem Rev. 1999;99:2235. doi: 10.1021/cr980436l. [DOI] [PubMed] [Google Scholar]
- 8.Adams KM, Marzilli PA, Marzilli LG. Inorg Chem. 2007;46:9172. doi: 10.1021/ic701038f. [DOI] [PubMed] [Google Scholar]
- 9.Zobi F, Blacque O, Sigel RKO, Alberto R. Inorg Chem. 2007;46:10458. doi: 10.1021/ic701647m. [DOI] [PubMed] [Google Scholar]
- 10.Zobi F, Spingler B, Alberto R. ChemBioChem. 2005;6:1397. doi: 10.1002/cbic.200400453. [DOI] [PubMed] [Google Scholar]
- 11.Zobi F, Blacque O, Schmalle HW, Spingler B, Alberto R. Inorg Chem. 2004;43:2087. doi: 10.1021/ic035012a. [DOI] [PubMed] [Google Scholar]
- 12.Zobi F, Spingler B, Fox T, Alberto R. Inorg Chem. 2003;42:2818. doi: 10.1021/ic030028m. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson KA, Zubieta J, Banerjee SR, Levadala MK, Taggart L, Ryan L, McFarlane N, Boreham DR, Maresca KP, Babich JW, Valliant JF. Bioconjugate Chem. 2004;15:128. doi: 10.1021/bc034128s. [DOI] [PubMed] [Google Scholar]
- 14.Stephenson KA, Banerjee SR, Sogbein OO, Levadala MK, McFarlane N, Boreham DR, Maresca KP, Babich JW, Zubieta J, Valliant JF. Bioconjugate Chem. 2005;16:1189. doi: 10.1021/bc0500591. [DOI] [PubMed] [Google Scholar]
- 15.He H, Lipowska M, Xu X, Taylor AT, Carlone M, Marzilli LG. Inorg Chem. 2005;44:5437. doi: 10.1021/ic0501869. [DOI] [PubMed] [Google Scholar]
- 16.He H, Lipowska M, Xu X, Taylor AT, Marzilli LG. Inorg Chem. 2007;46:3385. doi: 10.1021/ic0619299. [DOI] [PubMed] [Google Scholar]
- 17.Murshudov GN, Vagin AA, Dodson EJ. Acta Crystallogr D Biol Crystallogr. 1997;53:240. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 18.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. J Appl Crystallogr. 2007;40:658. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emsley P, Cowtan K. Acta Crystallogr D Biol Crystallogr. 2004;60:2126. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 20.Oxford Diffraction. Oxford Diffraction Ltd, Xcalibur CCD system, CrysAlisPro Software system, Version 1.171.33.48. 2007. [Google Scholar]
- 21.Franklin BR, Herrick RS, Ziegler CJ, Çetin A, Barone N, Condon LR. Inorg Chem. 2008;47:5902. doi: 10.1021/ic800363w. [DOI] [PubMed] [Google Scholar]
- 22.Herrick RS, Ziegler CJ, Çetin A, Franklin BR. Eur J Inorg Chem. 2007:1632. [Google Scholar]
- 23.Casini A, Mastrobuoni G, Temperini C, Gabbiani C, Francese S, Moneti G, Supuran CT, Scozzafava A, Messori L. Chem Commun. 2007:156. doi: 10.1039/b611122j. [DOI] [PubMed] [Google Scholar]
- 24.Salmain M, Blais J-C, Tran-Huy H, Compain C, Jaouen G. Eur J Biochem. 2001;268:5479. doi: 10.1046/j.0014-2956.2001.02489.x. [DOI] [PubMed] [Google Scholar]
- 25.Razavet M, Artero V, Cavazza C, Oudart Y, Lebrun C, Fontecilla-Camps JC, Fontecave M. Chem Commun. 2007:2805. doi: 10.1039/b703887a. [DOI] [PubMed] [Google Scholar]
- 26.McNae IW, Fishburne K, Habtemariam A, Hunter TM, Melchart M, Wang F, Walkinshaw MD, Sadler PJ. Chem Commun. 2004:1786. doi: 10.1039/b408141b. [DOI] [PubMed] [Google Scholar]
- 27.Blanco-Rodriguez AM, Busby M, Gradinaru C, Crane BR, Di Bilio AJ, Matousek P, Towrie M, Leigh BS, Richards A, Vlcek JH, Jr, Gray HB. J Am Chem Soc. 2006;128:4365. doi: 10.1021/ja057451+. [DOI] [PubMed] [Google Scholar]
- 28.Kostic NM. Comments Inorg Chem. 1988;8:137. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.