Abstract
The NSABP Study of Tamoxifen and Raloxifene (STAR), launched in 1999, compared tamoxifen with raloxifene in a population of healthy postmenopausal women at increased risk for breast cancer to determine the relative effects on the risk of invasive breast cancer. To be eligible for participation, a woman had to be healthy with at least a 5-year predicted breast cancer risk of 1.66% based on the Gail model or a history of lobular carcinoma in situ (LCIS) treated by local excision alone. All participants were at least 35 years of age and postmenopausal. Between July 1999 and November 2004, 19,747 participants were randomized to receive either tamoxifen (20 mg, plus placebo) or raloxifene (60 mg, plus placebo) daily for a 5-year period. The mean age of the participants was 58.5 years; 93% were white and 51.6% had a hysterectomy prior to entering the study. Of the women, 71% had one or more first degree female relatives (mother, sister, daughter) with a history of breast cancer and 9.2% of the women had a personal history of LCIS. A history of atypical hyperplasia of the breast was noted in 22.7% of the participants. The mean predicted 5-year risk of developing breast cancer among the study population was 4.03% (SD, 2.17%) with a lifetime predicted risk of 16%. The mean time of follow-up is 3.9 years (SD, 1.6 years). There was no difference between the effect of tamoxifen and the effect of raloxifene on the incidence of invasive breast cancer; there were 163 cases of invasive breast cancer in the tamoxifen-treated group and 168 cases in those women assigned to raloxifene (incidence 4.30 per 1,000 vs 4.41 per 1,000; RR 1.02; 95% CI, 082–1.28). There were fewer cases of noninvasive breast cancer (LCIS and ductal carcinoma in situ [DCIS1) in the tamoxifen group (57 cases) than in the raloxifene group (80 cases), although the difference is not yet statistically significant (incidence 1.51 vs 2.11 per 1,000; RR, 1.40; 95% CI, 0.98–2.00). There were 36 cases of uterine cancer with tamoxifen and 23 cases with raloxifene (RR, 0.63; 95% CI, 0.35–1.08).
Introduction
The history of medicine demonstrates that often the greatest medical advances are made through disease prevention rather than treatment, a truth that has special currency today with regard to breast cancer. The American Cancer Society has estimated that in 2007 there were 178,480 new cases of invasive breast cancer diagnosed in the United States and more than 1.3 million new cases worldwide (Jemal et al. 2007). Despite advances in both breast cancer screening and treatment, an estimated 465,000 women died as a result of breast cancer last year. Breast cancer is the most common cancer found in women in the United States and the second leading cause of cancer death in women. Finding a breast cancer prevention agent that is effective and acceptable, therefore, is a worthy goal.
In 1998 the National Surgical Adjuvant Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial (P-1) demonstrated that the selective estrogen receptor modulator (SERM) tamoxifen could reduce the incidence of breast cancer by up to 50% in a population of otherwise healthy women at increased risk for the future development of the disease (Fisher et al. 1998; Fisher et al. 2005). However, tamoxifen has several well-documented toxicities, including uterine malignancy, thromboembolic disease, and cataracts. These risks and the perception that tamoxifen was an oncology drug have limited its use for breast cancer prevention.
Raloxifene hydrochloride is also a SERM. In 1998 the United States FDA approved it for the treatment and prevention of osteoporosis; one of the pivotal studies leading to that approval was the Multiple Outcomes of Raloxifene Evaluation (MORE) study, which included 7,705 postmenopausal women (Cummings et al. 1999). The primary endpoint of the MORE study was bone fracture, but breast cancer was a secondary endpoint, and MORE showed that 4 years of raloxifene treatment appeared to reduce the risk of receptor-positive breast cancer by 72%. Like tamoxifen, raloxifene did increase the risk of thromboembolic events, but there was no apparent increase in endometrial cancer. A direct comparison of raloxifene and tamoxifen in a group of women at increased risk for breast cancer was a logical next step.
The Study of Tamoxifen and Raloxifene
The NSABP’s Study of Tamoxifen and Raloxifene (STAR) was a double-blinded, randomized clinical trial (Fig. 1) that began with 19,747 postmenopausal women who were at least 35 years of age and had a history of lobular carcinoma in situ (LCIS) treated by local excision alone or a modified Gail score demonstrating a 5-year risk for invasive breast cancer of at least 1.66% (Vogel et al. 2006; Land et al. 2006). Women in the study were assigned to receive either tamoxifen, 20 mg per day plus a placebo, or raloxifene, 60 mg per day plus a placebo, for a 5-year duration. The primary endpoint of the study was the development of invasive breast cancer. Secondary endpoints included noninvasive breast cancer, uterine malignancy, deep vein thrombosis, pulmonary embolus, transient ischemic attack, cerebral vascular accident, cardiac disease, fractures, cataracts, quality of life, and death. To be eligible, candidates must not have taken tamoxifen, raloxifene, hormone therapy, oral contraceptives, or androgens for at least the previous 3 months. They could not be taking either warfarin or cholestyramine. To minimize the risk of stroke or thromboembolic events, women were not eligible if they had a history of stroke, transient ischemic attack (TEA), pulmonary embolus (PE), deep vein thrombosis (DVT), uncontrolled diabetes, uncontrolled hypertension, or uncontrolled atrial fibrillation.
Fig. 1.
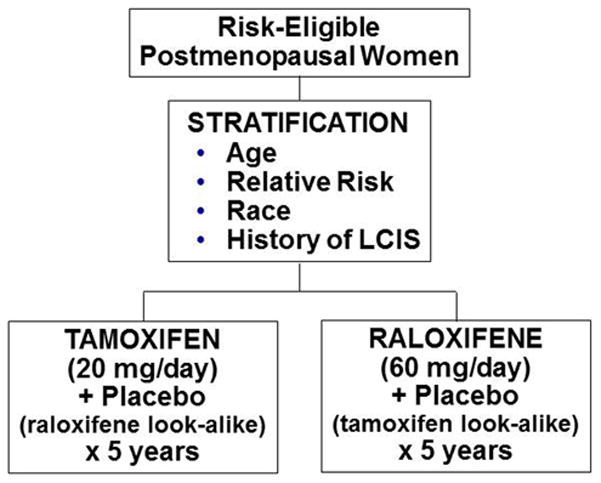
Schema for the NSABP STAR trial.
A detailed description of the participant population has been published (Vogel et al. 2006). At the time of randomization, the mean age of this postmenopausal population of women was 58.5 years. The mean predicted 5-year risk of developing breast cancer in the study population was 4.03%, and their projected lifetime risk to 80 years of age was 16%. Over 70% of the women entering the trial had one or more first degree female relatives with a history of breast cancer. More than 9% reported a personal history of LCIS treated by local excision prior to enrollment in the study, and 22.7% had a breast biopsy prior to enrollment that demonstrated either atypical ductal or atypical lobular hyperplasia. More than half the participants reported having undergone a hysterectomy before randomization.
RESULTS
Invasive Breast Cancer
With a mean follow-up time of 3.9 years, 163 of the women assigned to tamoxifen and 168 of those assigned to raloxifene had developed invasive breast cancer, demonstrating that there was no difference between the effect of tamoxifen and the effect of raloxifene on the incidence of invasive breast cancer. The rate per 1,000 was 4.30 in the tamoxifen group and 4.41 in the raloxifene group [risk ratio (RR), 1.02; 95% confidence interval (CI), 0.82–1.28). There was no placebo-alone group in this trial. However, using the Gail model scores of the women who entered the trial, we can estimate the number of invasive breast cancers that would have occurred in an untreated group (Fig. 2) and demonstrate that there was about a 47% reduction in incidence from treatment in the trial (Costantino et al. 1999). The cumulative incidence of invasive breast cancer through 72 months for the two treatment groups was 25.1 for the tamoxifen group and 24.8 for the raloxifene group (p=0.83). When the treatment groups were compared by baseline characteristics of age, history of LCIS, or atypical hyperplasia, Gail score, and number of first degree female relatives with breast cancer, the pattern of no differential effect by treatment assignment remained consistent, and none of the RRs in these subsets were statistically significant. The characteristics of the invasive breast tumors, which were obtained from submitted pathology and laboratory reports, showed no significant differences between the treatment groups with regard to distribution by tumor size, nodal status, or estrogen receptor level. A central pathology review of the tumors has not been performed.
Fig. 2.
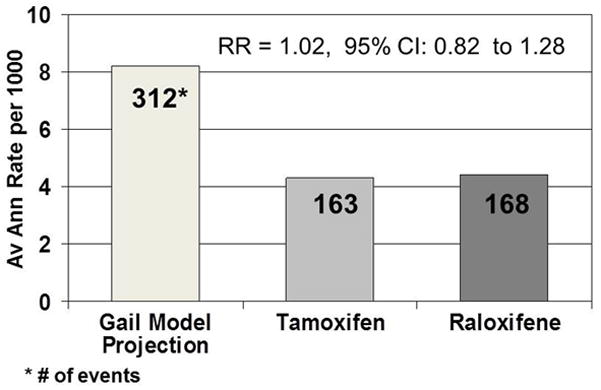
Average annual rate and number of invasive breast cancers, including cancers that would have occurred in an untreated group in the STAR trial.
Noninvasive Breast Cancer
Raloxifene did not appear to be as effective as tamoxifen in reducing the incidence of noninvasive breast cancer (LCIS or ductal carcinoma in situ [DCIS]), although the difference did not reach statistical significance. There were 57 cases of noninvasive breast cancer among the women assigned to tamoxifen and 80 among women who took raloxifene [1.51 per 1,000 women assigned to tamoxifen and 2.11 per 1,000 women assigned to raloxifene (RR, 1.40; 95% CI, 0.98–2.00) (Fig. 3)]. The cumulative incidence through 6 years was 8.1 per 1,000 in the tamoxifen group and 11.6 per 1,000 in the raloxifene group (p= .052).
Fig. 3.
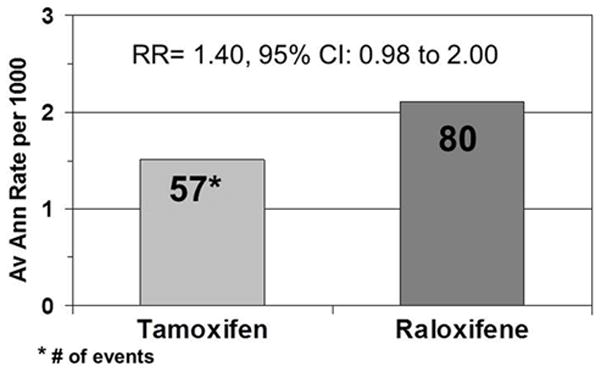
Average annual rate and number of noninvasive (in situ) cancers in the STAR trial.
Other Secondary Endpoints
More uterine malignancies occurred in the tamoxifen-treated women than in those treated with raloxifene, but the difference was not statistically significant. There were 36 cases in the tamoxifen group and 23 cases in the raloxifene group, with an annual incidence rate of 1.99 per 1,000 for tamoxifen and 1.25 per 1,000 for raloxifene (RR, 0.63; 95% CI, 0.35–1.08). Uterine hyperplasia (with and without atypia) was less common in the raloxifene-treated group (14 cases raloxifene; 84 cases tamoxifen [RR, 0.16; 95% CI, 0.09–0.29]). There were significantly fewer hysterectomies performed due to nonmalignant indications in the raloxifene group (221 tamoxifen; 87 raloxifene [RR, 0.39; 95% CI, 0.30–0.50]). There were no statistically significant differences between the treatment groups in regard to other malignancies.
No statistically significant differences were noted between the two treatment groups relative to the incidence of ischemic heart disease, TIA, stroke, or fractures (osteoporotic fractures or total fracture). Significantly fewer thromboembolic events (DVT or PE) occurred in the raloxifene group, 141 in the tamoxifen group, sand 100 in the raloxifene group, demonstrating a 30% reduction in favor of the raloxifene-treated women (RR, 0.70; 95% CI, 0.54–0.91). Fewer women on raloxifene developed cataracts during treatment, and fewer underwent surgical removal of their cataracts. After 6 years, the cumulative incidence of cataracts occurring during treatment was 77.9 per 1,000 in the tamoxifen group and 56.3 per 1,000 in the raloxifene group (p= .002); 260 in the tamoxifen group and 215 in the raloxifene group underwent cataract surgery (RR, 0.82; 95% CI, 0.68–0.99).
Mortality in the two groups was similar, with 101 deaths in those assigned to tamoxifen and 96 in those assigned to raloxifene, resulting in a rate of 2.64 per 1,000 and 2.49 per 1,000, respectively (RR, 0.94; 95% CI, 0.71–1.26). The distribution by cause of death did not differ by treatment.
Discussion
The results of the STAR trial demonstrate that raloxifene is an effective alternative to tamoxifen for reducing the risk of invasive breast cancer in healthy postmenopausal women at increased risk for the disease. Raloxifene is also an attractive choice for these women because it has fewer serious side effects. Although the difference in endometrial cancer has not yet reached statistical significance, the tamoxifen-treated women did have a significant increase in endometrial hyperplasia, a known risk factor for endometrial cancer. There were also more than twice as many hysterectomies for benign disease in the tamoxifen group. Participants in both groups continue to be followed.
Raloxifene does not appear to be as effective as tamoxifen in preventing the development of noninvasive breast cancer, DCIS, or LCIS. However, there is no suggestion that raloxifene is increasing the risk of noninvasive disease compared to tamoxifen; the difference in the average annual rate of noninvasive disease is only 0.6 per 1,000. In the NSABP P-1 prevention trial that compared tamoxifen to placebo, tamoxifen reduced noninvasive disease by 50%. That trial included both pre- and postmenopausal women, but the reduction in noninvasive disease was apparent regardless of menopausal status. The mechanism to explain the differing effect between these two SERMs is not clear. It is interesting to note that women who entered STAR with a previous breast biopsy that demonstrated either atypical hyperplasia or LCIS benefited equally from tamoxifen and raloxifene in the reduction of risk of invasive breast cancer (Fig. 4). This suggests that raloxifene may actually be as effective as tamoxifen in blocking the progression of premalignant or noninvasive disease to invasive breast cancer.
Fig. 4.
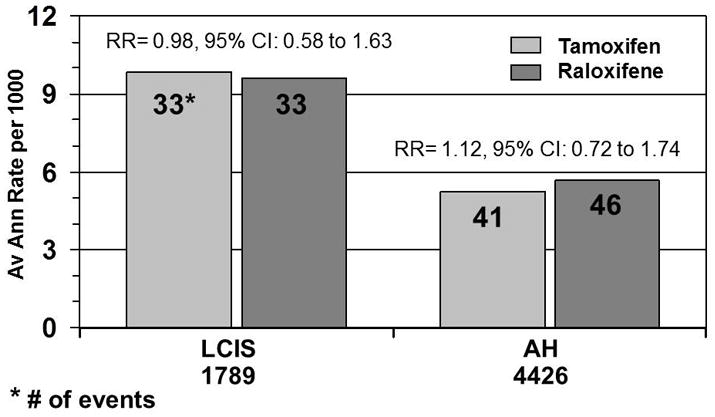
Average annual rate and number of invasive breast cancers by atypical hyperplasia (AH) and lobular carcinoma in situ (LCIS).
Whether the data on noninvasive disease prove to be a barrier to the use of raloxifene remains to be seen. The postmenopausal woman who decides to take a SERM to reduce her risk of breast cancer could select tamoxifen and avoid this concern about noninvasive disease. Unfortunately, at the present time, there are no risk profiles or other methods to identify who is at greater or lesser risk of developing noninvasive disease. In the STAR study, the incidence of invasive breast cancer, despite the reduction achieved with either SERM, was more than twice the rate of noninvasive disease in the raloxifene group. The benefits of fewer endometrial cancers, fewer deep vein thromboses and pulmonary emboli, and fewer cataracts in the raloxifene-treated women may balance if not outweigh the noninvasive disease benefit currently known to be achievable with tamoxifen.
Follow-up recommendations for either raloxifene or tamoxifen are the same. Almost all of the noninvasive breast cancers in the STAR trial were identified as microcalcifications seen on annual mammograms. As a result, the tumors were small and most women had the option of breast-conserving procedures.
In summary, women at risk for breast cancer have the option of taking tamoxifen or raloxifene. However, tamoxifen, approved for this purpose in 1998, has been underutilized. Tamoxifen was well known to oncologists who had used it extensively to treat breast cancer patients with receptor-positive disease, but it was relatively unknown to primary care physicians who are the key providers of preventive healthcare. Tamoxifen was viewed as a “cancer drug,” and media reports highlighting its toxicities proved to be a barrier to its use.
Raloxifene, on the other hand, has been utilized for more than a decade for the treatment and prevention of osteoporosis. Over 500,000 women in the United States are currently taking this drug for its benefits in bone, and on average, these women are older and have a lower breast cancer risk than do the women in the STAR trial. Most raloxifene prescriptions have been written by primary care providers. Thus, because these physicians are already familiar with this drug, barriers relative to its use for breast cancer chemoprevention may be lessened.
The ideal chemopreventive agent may still lie somewhere in the future, and significant work remains to be done before we arrive at that point. Tamoxifen and raloxifene reduce the risk of invasive breast cancer by 50%, an impressive benefit but one that leaves substantial room for improvement. The cancers that are prevented by SERMs are estrogen receptor positive. While these estrogen receptor-positive tumors make up the majority of breast cancers that occur, estrogen receptor-negative cancer is not rare. Although tamoxifen is approved for premenopausal women, raloxifene is not. Efforts already underway in the laboratory and the clinic should help us address these gaps. However, at this point in time, raloxifene may offer the best chance for breast cancer prevention for many women.
References
- Costantino JP, Gail MH, Pee D, Anderson S, Redmond CK, Benichou J, Wieand HS. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91:1541–1548. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan JC. The effect of raloxifene on risk of breast cancer in postmenopausal women: Results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation (Erratum in JAMA 1999; 282:2124) JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino J, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG, Wolmark N. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 2005;97:1652–166. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Land SR, Wickerham DL, Costantino JP, Ritter MW, Vogel VG, Lee M, Pajon ER, Wade JL, 3rd, Dakhil S, Lockhart JB, Jr, Wolmark N, Ganz PA. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial (erratum in JAMA 2007; 298:973) JAMA. 2006;295:2742–2751. doi: 10.1001/jama.295.23.joc60075. [DOI] [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Jr, Wade JL, 3rd, Robidoux A, Margolese R, James J, Lippman SM, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial (errata in JAMA 2006; 296:2962; JAMA 2007; 398:973) JAMA. 2006;295:2727–274. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]


