Abstract
A fundamental issue in cognitive neuroscience is how the brain encodes others’ actions and intentions. In recent years, a potential advance in our knowledge on this issue is the discovery of mirror neurons in the motor cortex of the nonhuman primate. These neurons fire to both execution and observation of specific types of actions. Researchers use this evidence to fuel investigations of a human mirror system, suggesting a common neural code for perceptual and motor processes. Among the methods used for inferring mirror system activity in humans are changes in a particular frequency band in the electroencephalogram (EEG) called the mu rhythm. Mu frequency appears to decrease in amplitude (reflecting cortical activity) during both action execution and action observation. The current meta-analysis reviewed 85 studies (1,707 participants) of mu that infer human mirror system activity. Results demonstrated significant effect sizes for mu during execution (Cohen’s d = 0.46, N = 701) as well as observation of action (Cohen’s d = 0.31, N = 1,508), confirming a mirroring property in the EEG. A number of moderators were examined to determine the specificity of these effects. We frame these meta-analytic findings within the current discussion about the development and functions of a human mirror system, and conclude that changes in EEG mu activity provide a valid means for the study of human neural mirroring. Suggestions for improving the experimental and methodological approaches in using mu to study the human mirror system are offered.
Keywords: mu rhythm, mirror neurons, EEG, action execution, action observation
A fundamental issue in cognitive neuroscience is how the brain is able to encode others’ actions and intentions. In recent years, a potential advance in our knowledge on how these processes take place is the discovery of mirror neurons. This discovery was made using single-cell recordings in the adult Rhesus macaque ventral premotor cortex and inferior parietal lobe while the monkey observed and executed simple actions (di Pellegrino, Fadiga, Fogassi, Gallese, & Rizzolatti, 1992; Fogassi et al., 2005). It suggested that perceptual and motor processes share a common neural code. Based on their property of firing to both observed and executed actions, it has been hypothesized that a mirror system may likely be present in the human brain, and may play a role in human understanding of others’ actions and intentions by representing actions, at a cortical level, for both motor execution and observation (Fogassi et al., 2005; Gallese, Fadiga, Fogassi, & Rizzolatti, 1996; Rizzolatti, Fadiga, Gallese, & Fogassi, 1996; Rizzolatti, Fogassi, & Gallese, 2001). The activation of the motor system during observation of an action has led some researchers to interpret it not only as evidence of a recognition process but also as a means to repeat the observed action and even understand the intention behind it (Rizzolatti & Sinigaglia, 2010). Thus, a human mirror system has been suggested to represent a neural mechanism underlying action-perception coupling, creating a self–other matching system that both facilitates recognition of others’ actions and provides a means for imitation.
Investigations of the Human Mirror System With fMRI
There has been much interest in translating the discovery of mirror neurons in monkeys to human neurophysiology and human cognition (Gallese, Gernsbacher, Heyes, Hickok, & Iacoboni, 2011). Single-cell recordings identify mirror neurons in the monkey (di Pellegrino et al., 1992; Fogassi et al., 2005); however, the translation from monkey neurophysiology to human brain activity may not be direct. And absent of a consistent ability to directly assess neuronal activity in the human brain, as is done in monkeys (though see Mukamel, Ekstrom, Kaplan, Iacoboni, & Fried, 2010), direct comparisons between the two species are currently not feasible.
That said, many researchers have attempted to identify a mirror system in humans using noninvasive brain imaging methods. There are human homologues of the regions studied in the monkey—ventral premotor cortex, inferior parietal lobe, and also part of the inferior frontal gyrus—which have been examined for mirroring properties in humans using functional neuroimaging (fMRI). Meta-analyses of fMRI studies reveal that these brain regions appear to demonstrate mirroring properties. Across 139 fMRI and PET studies (Caspers, Zilles, Laird, & Eickhoff, 2010), and another 76 fMRI studies (Molenberghs, Cunnington, & Mattingley, 2012), the inferior frontal gyrus, ventral premotor cortex, and inferior parietal lobe were active to both the execution and observation of body actions (including hands, feet, legs, mouth, and face). Though these patterns cannot be attributed to the activation of mirror neurons in the human brain, researchers interpret these regional activation patterns across action-execution and action-observation as support for neural mirroring or a mirror system more generally.
Meta-analyses of fMRI studies also reveal that regions not classically associated with mirror neurons in the nonhuman primate demonstrate mirroring properties in humans, including the dorsal premotor cortex, superior parietal lobe, temporal gyrus, and cerebellum (Molenberghs et al., 2012). The involvement of the dorsal premotor cortex and superior parietal lobe in both action execution and action observation was reported in an additional meta-analysis examining hand movements (Grèzes & Decety, 2001). Further, activations of specific brain regions for observation of action were shown to differ depending on the instructions given to participants: If participants were told to passively observe, there was consistent activation in regions including the inferior parietal lobe, but when participants were told to observe so that they could later imitate the movement, there was no activation in the inferior parietal lobe (although this null result was based on only eight studies; Caspers et al., 2010). Nonetheless, despite the lack of direct translation from monkey to human neurophysiology, fMRI research reveals brain regions in humans (including the ventral premotor cortex and inferior frontal gyrus) that show consistent mirroring properties, suggesting the possibility of a mirror system in humans.
Functional Significance of the Mirror System
The growing number of investigations that focus on a human mirror system is fueled, in part, by its functional significance for cognitive science. Many scholars argue that the potential impact of such a perception-production neural mechanism extends beyond the action domain. For example, a mirror system has been posited as a fundamental building block for understanding others’ actions (Rizzolatti & Fabbri-Destro, 2008). Such functions include supporting several aspects of social perception, such as the ability to predict others’ movements (Csibra, 2007; Kilner, Friston, & Frith, 2007) and the ability to mentally simulate others’ actions (Gallese & Sinigaglia, 2011; Prinz, 1997). More recently, researchers suggest that neural mirroring may play a role in human infants’ ability to map similarities between self and other, and thus may be involved in providing a foundation for imitation and social–cognitive development (Marshall & Meltzoff, 2014).
Researchers have also emphasized ties between the mirror system and empathy (Carr, Iacoboni, Dubeau, Mazziotta, & Lenzi, 2003; Gallese, 2001, 2005; Iacoboni, 2009), and between the mirror system and language (Rizzolatti & Arbib, 1998; Théoret & Pascual-Leone, 2002; Wolf, Gales, Shane, & Shane, 2001). The general notion behind a role for a mirror system in these complex social and communicative abilities rests on the position that when another’s action is perceived, the human mirror system supports internal representation of this perceived action that is linked via a common neural code to one’s own actions, and through that process of direct matching “the mirror neuron system transforms visual information into knowledge” (Rizzolatti & Craighero, 2004, p. 172; see also Rizzolatti et al., 2001). Indeed, recent research has shown that transient disruptions to motor cortex (using transcranial magnetic stimulation; TMS) result in impairments in the ability to recognize and anticipate others’ actions (Michael et al., 2014; Stadler et al., 2012).
More recent views of mirror system function differ somewhat from classic presentations. For example, Kilner and colleagues (2007) criticize classic bottom-up, forward connection models (e.g., Rizzolatti & Craighero, 2004), arguing that the processes by which an observer maps an observed action onto their own motor system and translates the visual information into inferences about intentions and goals are unclear. The notion of inverting such a forward model to infer the cause of an observed action from its elicited internal representation (with matching known cause) is problematic because sensory inputs (visual, proprioceptive, and tactile) are not associated with singular, unique causes. These authors propose a “predictive coding framework,” which outlines alternative processes that draw on Bayesian principles: Backward connections in a hierarchical system give contextual guidance to lower level inputs in a reciprocal process that works to minimize prediction error (Kilner et al., 2007). Csibra (2007) has also criticized the bottom-up, direct matching hypothesis, but unlike Kilner et al. (2007), argues that action mirroring is generated by “emulative action reconstruction” via top-down interpretation processes outside the motor system, and further posits that action understanding may precede rather than follow from action mirroring.
Controversies and Open Questions in Mirror System Research
As already exemplified with the differing views presented in the previous section, there is a good deal of controversy surrounding the proposed origins and functions of a mirror system in both humans and monkeys (e.g., Heyes, 2010; Hickok, 2009; Hickok & Hauser, 2010). Some argue that though neural systems supporting action have been highly investigated, broad inferences about sophisticated social–cognitive functions of these systems reach beyond empirical findings (Dinstein, Thomas, Behrmann, & Heeger, 2008), and strong claims about the function of “action understanding”—an ambiguous term in and of itself—are based on nonfalsifiable logic rather than experimental testing (Steinhorst & Funke, 2014). Indeed, a meta-analysis of fMRI studies investigating action and higher social cognition, such as theory of mind, showed almost no overlap between the regions supporting “mirroring” and those supporting mental-state understanding (Van Overwalle & Baetens, 2009). Even more basic functions such as understanding and recognizing others’ actions have been challenged: Researchers argue that the motor system is unlikely to be responsible for abstract aspects of understanding, including recognizing intentions and goals (Hickok, 2009). And some have argued that the function of a mirror system is to compute (and thereby predict) the motor command for achieving an intention, but not to compute the agent’s intention itself (Jacob, 2008). However, more recent findings challenge these views and seem to support an active role of premotor regions in action understanding (Michael et al., 2014; Urgesi, Candidi, Ionta, & Aglioti, 2007; Urgesi, Moro, Candidi, & Aglioti, 2006).
The fundamental matching properties of a mirror system have also been questioned. Hickok (2009) argues that although most mirror system research shows that neural systems for action execution are indeed correlated with action observation, this work does not clarify the functional significance of this correlation, leaving the strict hypothesis of a single neural code for both action execution and action observation open to alternatives. For example, Heyes (2010, 2014) has argued that mirror neuron activity could reflect associative learning. This associative account suggests that the “self–other matching” properties that are the basis of a mirror system could be formed via contingent execution-observation experiences over time, rather than as the inherent product of an innate system for mirroring. It is also not clear whether a highly flexible mechanism, such as that represented by a mirror system, is sufficiently stable over the course of development to sustain complex cognitive functions. This leaves open the debate about the functional role of a mirror system and its adaptive value.
The role of a mirror system in atypical development and cognition has also been examined. There are proposals that the mirror system may be key to understanding the disordered mind, as in the case of autism spectrum disorders (ASDs). Several researchers posit that the motor, communication, and social–cognitive deficits associated with ASD are due, at least in part, to a dysfunctional mirror system (Oberman, McCleery, Ramachandran, & Pineda, 2007; Perkins, Stokes, McGillivray, & Bittar, 2010; Pineda, Carrasco, Datko, Pillen, & Schalles, 2014; Rizzolatti, Fabbri-Destro, & Cattaneo, 2009; Vivanti & Rogers, 2014; J. H. G. Williams, Whiten, Suddendorf, & Perrett, 2001). However, empirical data provide mixed evidence. There have been several investigations of the human mirror system in individuals with ASD using TMS (Enticott, Kennedy, Bradshaw, Rinehart, & Fitzgerald, 2010; Enticott et al., 2012), neuroimaging (Dapretto et al., 2006; Hadjikhani, Joseph, Snyder, & Tager-Flusberg, 2006; Martineau, Andersson, Barthélémy, Cottier, & Destrieux, 2010; J. G. Williams, Higgins, & Brayne, 2006), and electrophysiology (Bernier, Dawson, Webb, & Murias, 2007; Martineau, Cochin, Magne, & Barthélémy, 2008; Oberman et al., 2005) that report structural abnormalities and diminished recruitment during action-processing tasks in ASD samples compared with typical controls. Yet several studies reveal counterevidence showing that neural systems for action execution and action observation are not distinguishable between ASD patients and controls (Enticott et al., 2013; Fan, Decety, Yang, Liu, & Cheng, 2010; Raymaekers, Wiersema, & Roeyers, 2009; Ruysschaert, Warreyn, Wiersema, Oostra, & Roeyers, 2014). And the general view that a deficit in a mirror system plays a fundamental role in canonical ASD impairments has been challenged (Hamilton, Brindley, & Frith, 2007; Southgate & Hamilton, 2008).
Finally, the function and significance of a mirror system across development is still not understood. Some of the most powerful potential effects of a mirror system should be evident during development (Ferrari, Tramacere, Simpson, & Iriki, 2013). Indeed, the fundamental abilities that a mirror system may underlie—the ability to deploy actions strategically in the service of goals, and the ability to understand the goals of social partners in order to produce adaptive social responses—emerge early in infancy and undergo foundational developments in the first years of life (Woodward & Gerson, 2014). There have been several recent explorations of “neural mirroring” in infancy (see Marshall & Meltzoff, 2011), with their number growing. However, there has been little direct investigation of how mirroring changes with development or how supportive it may be in the emergence of action-perception understanding.
A Need for Evaluation
In order to address the debate over the presence and functions of a human mirror system, the efficacy of current tools for identifying the presence of neural mirroring in humans must be evaluated. fMRI research has laid an important foundation for investigation of the mirror system in humans, and several meta-analyses outlined in the Investigations of the Human Mirror System with fMRI section (Caspers et al., 2010; Molenberghs et al., 2012) demonstrate its utility in identifying neural mirroring in humans. But there are also limits to this brain imaging method. Specifically, fMRI investigations with young children, infants, and impaired subjects are extremely difficult because of the noisy testing environment, the need for the participant to lie still for long periods of time, and the separation between caregiver and participant. Data collection from participants is costly, and in pediatric populations, there is high data loss from motion artifact. Research into a mirror system at these early ages, across development, and in impaired populations is key to investigating the plasticity of the proposed mirror system and its role in higher social cognition. Alternative methods are therefore needed for these critical developmental approaches.
In recent years, there has been an increase in the number of studies examining the mu rhythm within the electroencephalogram (EEG) as a potential index of human neural mirroring. This rhythm has been shown to decrease in amplitude—or desynchronize—when humans both execute and observe action, and, as a result, it has been argued that mu desynchronization is linked to mirror system activity (Cuevas, Cannon, Yoo, & Fox, 2014; Muthukumaraswamy & Johnson, 2004a, 2004b; Pineda, 2005). Examinations of the mu rhythm in infancy are now prevalent, with researchers pursuing questions about the development of neural mirroring and its role in social and cognitive development (Marshall & Meltzoff, 2014). There is a prominent view that mu rhythm suppression reflects activity of a human mirror system. As of yet, however, this view has not been systematically evaluated. Such an evaluation is critical to the field of psychology, as it would allow researchers from multiple areas to understand and evaluate the significance of studies that examine action-perception and action-execution links. The present meta-analysis provides a large-scale, systematic evaluation of the extent to which mu rhythm consistently desynchronizes to both action execution and action observation, thus indexing neural mirroring.
Investigations of the Human Mirror System With EEG Mu Rhythm
EEG methods have many advantages over the use of fMRI. EEG is relatively inexpensive and is relatively easy to use with pediatric and special-needs populations. And EEG offers the unique ability to examine the timing of activation to observation or execution of action. However, unlike single-cell recordings in nonhuman primates, and similar to fMRI, EEG cannot pinpoint the activity of specific neurons.
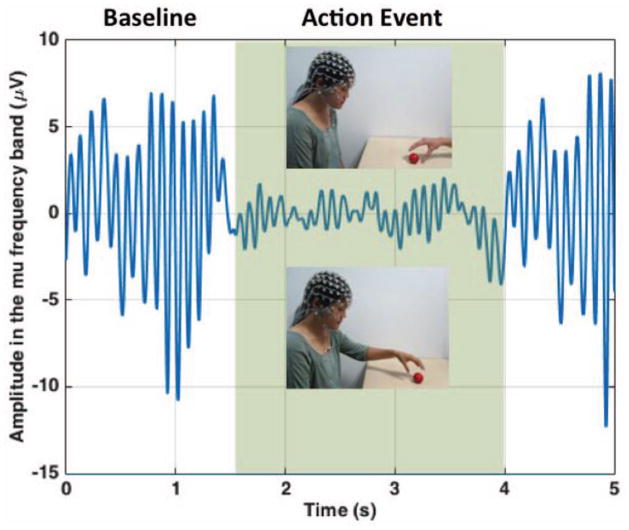
EEG is acquired by placing sensors on a participant’s head and measuring the electricity generated by the brain. These sensors are placed over the scalp in a pattern that roughly corresponds to different areas of the cerebral cortex (left and right frontal, central, temporal, parietal, and occipital areas). First described in 1929 by Berger (Berger, 1929), this electrical activity has two main properties: frequency (oscillations per second) and amplitude (height of the oscillations). Mu rhythm reflects EEG frequency occurring within the standard “alpha” band (i.e., ~8–13 Hz [oscillations per second] in adults; ~6–9 Hz in children) that varies in amplitude as a function of subject action (Lepage & Théoret, 2006; Muthukumaraswamy & Johnson 2004a; Pfurtscheller, Neuper, Andrew, & Edlinger, 1997). For example (see Figure 1), when a subject executes an action (e.g., voluntary hand movement), EEG amplitude in the mu band decreases, often with maximal suppression over central scalp locations (Kuhlman, 1978). Researchers studying mirror activity have examined this decrease in amplitude during observation of action. This decrease in amplitude, which is calculated in reference to a baseline period, is known as desynchronization. The prominence of mu desynchronization recorded from central sites that overlay sensorimotor cortex during action execution suggests it is an index of sensorimotor cortical activation (e.g., Leocani, Toro, Manganotti, Zhuang, & Hallett, 1997; Toro et al., 1994). Though EEG activity at particular electrode locations on the scalp does not necessarily reflect cortical activity directly below these electrodes, there is strong evidence that mu rhythm recorded from central sites is not purely the result of neural activity in occipital regions spreading to central areas (Pineda, 2005). Indeed, a number of studies that estimate cortical source locations underlying mu activity have identified sources primarily clustered around the central sulcus in sensorimotor areas (Hari, Salmelin, Mäkelä, Salenius, & Helle, 1997; Salmelin & Hari, 1994a, 1994b), with some sources observed in parietal areas as well (Salmelin, Hämäläinen, Kajola, & Hari, 1995). Other studies have shown that sources underlying mu rhythm recorded with EEG are also primarily concentrated in central and parietal cortical areas (Manshanden, De Munck, Simon, & Lopes da Silva, 2002; Thorpe, Cannon, & Fox, 2015). Critically, these brain areas are similar to regions that fMRI research has shown activate during both observation and execution of action (Molenberghs et al., 2012). These findings have led researchers to consider mu desynchronization as a candidate for indexing neural mirroring in humans. With the search for a human mirror system, studies have examined the links between mu rhythm desynchronization and observation of others’ actions. Given established links between mu desynchronization and action execution, this rhythm has the potential to demonstrate mirroring properties should a similar pattern of mu desynchronization occur during action observation.
Figure 1.
Simulation of mu rhythm desynchronization in the 8- to 13-Hz frequency band. There is a decrease in amplitude in the electroencephalogram from baseline during action observation or execution (action event; highlighted in green). Individual appearing here has consented for her likeness to be published in this article.
Yet the extent to which mu desynchronization may characterize mirror activity in humans is not fully known. Across the now large number of studies that examine mu rhythm activity to action execution and action observation, methods for both eliciting and identifying mu desynchronization vary considerably, especially for action observation (Cuevas et al., 2014). Across individual studies, participants observe a range of different stimuli including, for example, a single hand grasping small objects (e.g., Muthukumaraswamy & Johnson, 2004b), photographs of emotionally expressive eyes (Pineda & Hecht, 2009), whole body movements such as dancing (Orgs, Dombrowski, Heil, & Jansen-Osmann, 2008), and pages of sheet music (Behmer & Jantzen, 2011). There are also variations in how mu desynchronization is calculated across studies in terms of the type of baseline selected as a reference point and the location and number of EEG channels in which activity is examined. These inconsistencies raise questions as to whether mu desynchronization during action observation is consistently present and similar to mu desynchronization during action execution, thus raising the question as to whether mu desynchronization reflects neural mirroring.
Here, we conducted a meta-analysis of 85 EEG studies that all focus on the EEG mu rhythm. We examined the consistency of findings across studies that used EEG to assess responses to action observation and action execution. A clearer understanding of the pattern of results from these studies will help clarify whether mu desynchronization is a valid index of neural mirroring. Given the infant- and child-friendly properties of EEG, meta-analytic results will also provide an important foundation for future research that investigates the role of action and the mirror system in imitation, empathy, and language as these capacities emerge and change across development. Further, investigation of action systems across development provides a platform for testing alternative hypotheses to the origins of a mirror system, such as those that emphasize the role of learned associations through increasing experience (Heyes, 2010). Thus, this meta-analysis marks a first critical step in evaluating the role of mu rhythm in indexing the links between action production and action perception, and more broadly have important implications for the study of a human mirror system.
In addition, beyond fundamental mirroring properties, this meta-analysis can assess the extent to which mu desynchronization may also index properties (i.e., specificity to object-directed or goal-directed action and biological motion) that have been linked to hypothesized functions of the mirror system (e.g., intention-understanding and higher social cognition) and that have been examined in meta-analyses of the human mirror system using fMRI. An examination of these moderators can illuminate the specific contexts to which neural mirroring may be particularly sensitive.
This meta-analysis can also examine the effects of methodological variations in how mu desynchronization is calculated (type of baseline) and how it is elicited during observation (type of stimulus observed). As well, we can examine the extent to which studies demonstrate topographic specificity for central (sensorimotor) scalp locations. Topographic specificity would suggest, though not definitively demonstrate, that mu desynchronization may reflect activation in sensorimotor cortical regions, offering a tighter link to mirroring identified with fMRI in the motor and premotor cortex.
Our meta-analytic approach was as follows. We examined across the now substantial set of human EEG mu rhythm studies: (a) whether there was a significant effect size for mu desynchronization to action execution and to action observation; (b) whether these effects were moderated by key variables that have been shown to moderate neural mirroring activity in the fMRI literature; (c) whether the effect sizes of action execution or action observation were moderated by methodological variation across studies; and (d) whether any such effects exhibit topographic specificity. Critically, the effects and moderators for action execution mu desynchronization are compared with the effects and moderators for action observation mu desynchronization in order to shed light on how well mu rhythm activity demonstrates mirroring properties.
Among the extended set of variables we considered in this study, four deserve brief introduction because of their theoretical importance or their potential effect on mu rhythm activity. These are whether mu rhythm desynchronization is specific to the observation of object-directed action or biological motion, and whether mu desynchronization is influenced by type of baseline and is topographically specific.
Specificity to object-directed action
In monkeys, a classic finding is that neurons that exhibit mirroring properties discharge to object-directed actions but not to non-object-directed actions. Mirror neurons in the macaque were first defined as those that fired when the monkey executed object-directed actions such as grasping, holding, and tearing objects; critically, these neurons also fired when the monkey viewed similar object-directed actions being performed (di Pellegrino et al., 1992). More specifically, Rizzolatti and colleagues (1996) found that mirror neurons in monkeys discharged when the monkey viewed an agent grasping an object, but not when viewing similar hand actions that were not object-directed, or when viewing the object alone. Other studies have supported these early observations (Umiltà et al., 2001).
On the basis of these and other studies, researchers have argued that the selectivity of the monkey mirror neuron system to object-directed action indicates selectivity to goal-directed action more specifically (e.g., Rizzolatti & Fabbri-Destro, 2008; Rizzolatti et al., 1996). Indeed, deliberate and voluntary actions that are directed toward objects are likely to demonstrate the goals behind them: For example, a hand moving toward an object that then pinches the object communicates a clear goal to grasp, whereas it is more difficult to decipher the goal behind a hand moving toward and pinching empty space. Several researchers have attempted to probe the specific function of encoding goals in the monkey mirror neuron system (see Casile, 2013, for recent review), though the extent to which existing research provides evidence for mirror neuron selectivity for goal-directed action (or even object-directed action) per se is currently debated (e.g., Cook, Bird, Catmur, Press, & Heyes, 2014).
Some have argued that although a monkey mirror neuron system may be most responsive to object-directed action, a human mirror system may not be (e.g., Fabbri-Destro & Rizzolatti, 2008). Indeed, if a primary function of the mirror system in monkeys is to encode action goals, then the more cognitively capable human may more easily identify goals in non-object-directed actions and thus demonstrate mirroring activity to viewing actions that are intransitive (e.g., Fadiga, Fogassi, Pavesi, & Rizzolatti, 1995). Individual studies investigating the human mirror system yield inconsistent results: Some studies show activation in purported human mirror regions to object-directed action, whereas others show similar activations to non-object-directed action (see Buccino, Binkofski, & Riggio, 2004, for review). However, considering recent meta-analyses and qualitative reviews of human mirror system investigations with fMRI (Caspers et al., 2010; Morin & Grèzes, 2008; Van Overwalle & Baetens, 2009), there appears to be evidence for regional brain activation during object-directed action. Of those meta-analyses or reviews that systematically examined object- versus non-object-directed actions, there is more consistent activation to object-directed versus non-object-directed actions in brain regions thought to reflect the human mirror system (Brodmann area [BA] 44, lateral premotor cortex BA 6, ventral premotor cortex inferior and superior parietal lobe [Caspers et al., 2010]; ventral premotor and supplementary motor BA 6 [Morin & Grèzes, 2008]; anterior inferior parietal sulcus and premotor cortex [Van Overwalle & Baetens, 2009]). It is possible that studies using human mu rhythm demonstrate similar effects. As such, one goal of this meta-analysis was to examine whether object-directed actions moderate the effect size of mu desynchronization to action observation.
Specificity for biological movement and effectors
A consistent finding in the monkey mirror neuron literature is that activation of these neurons occurs when viewing actions performed by a biological effector (e.g., hand, mouth). Indeed, in the monkey, mirror neurons do not support the encoding of general movement (e.g., a moving car, waving flag)—but rather biological movement specifically. Although there is some evidence demonstrating that mirror neurons fire when a monkey views a tool (nonbiological effector) acting on an object (e.g., Rizzolatti, Fogassi, & Gallese, 2001), these experiments consist of a biological effector (a hand) using a tool and not simply a nonbiological effector acting on its own. Further, only monkeys who have had prior tool use training show mirror neuron activation when viewing experimenters acting on objects with tools (Rochat et al., 2010); monkeys without training do not, even though a prolonged exposure to observing tool actions without having interacted with the tool may activate some mirror neurons within the premotor cortex. Thus, a prominent property of the mirror neuron system in monkeys is its specificity to biological movement and effectors.
It is not clear that a human mirror system is also specific to biological motion. Meta-analyses of fMRI studies yield mixed results. Van Overwalle and Baetens (2009) report that although classic mirror regions (i.e., premotor cortex) were active during observation of biological motion from moving body parts (i.e., the hand), they were not active during observation of whole-body motion, though perhaps this null result was because of the lack of object-directed actions in the whole-body motion cases. Morin and Grèzes (2008) also found lack of specificity to biological motion when actions were not object-directed: The dorsal premotor cortex was activated during observation of both biological and nonbiological non-object-directed actions, suggesting that this region was not specific to biological motion per se but to movement more generally. They also found that the dorsal and ventral premotor cortices were recruited for observing biological actions compared with a static stimulus, but this recruitment was not consistent. Thus, it is not clear that the human mirror system as measured with fMRI shows specificity to biological motion. In the present meta-analysis, we investigate whether such specificity exists for studies using mu desynchronization.
Type of baseline
Mu desynchronization is calculated as the percentage of change in EEG amplitude between an experimental event or executed action and a baseline period. The type of baseline used to compute mu desynchronization will influence the magnitude of the change. If the baseline amplitude in the mu frequency band recorded over central scalp locations is high, then desynchronization of the amplitude in response to a motor action or observation of a motor action will be different than if baseline amplitude is low. In the case of mu desynchronization, certain baseline stimuli may themselves elicit desynchronization of mu amplitude and would decrease the chances of finding significant desynchronization during execution or observation of an action. Tangwiriyasakul, Verhagen, van Putten, and Rutten (2013) investigated the effects of using different types of baseline on mu rhythm desynchronization and found that dynamic (e.g., bouncing balls), rather than static (e.g., picture), baseline stimuli increased the amount of desynchronization for some of their participants. In another study, Puzzo, Cooper, Cantarella, and Russo (2011) examined desynchronization of mu rhythm during observation of actions and found the magnitude of the desynchronization varied based on the type of baseline used (resting EEG or nonbiological movement). Given the variation in type of baseline used across many existing mu rhythm studies, the current meta-analysis investigated type of baseline as a moderator for both action execution and action observation.
Topographic specificity
The mu signal was first identified over central scalp locations. However, there have been few attempts with EEG to localize this signal (but see Thorpe et al., 2015), and fewer still that report the response of amplitude in the mu or alpha frequency band from other scalp locations (Hari et al., 1998; Marshall, Young, & Meltzoff, 2011; van der Helden, Van Schie, & Rombouts, 2010). Research suggests that the mu rhythm is distinct from the occipital alpha rhythm (Formaggio et al., 2008; Hari & Salmelin, 1997; Ritter, Moosmann, & Villringer, 2009; see also Pineda, 2005 for review). Thus, one would expect to find evidence of topographic specificity in central or central-parietal scalp leads during action execution and action observation. On the other hand, lack of topographic specificity might suggest that mu desynchronization may be a result of more general attention processes, or a close coordination of action and attention. Examining topographic specificity across action execution and action observation is a first step in clarifying this important issue and in directing future research.
Summary of Goals of the Meta-Analysis
The current meta-analysis examined the effect sizes for mu desynchronization during execution and observation of action, and contrasted the two to see whether they were significantly different from each other in order to identify neural mirroring. Guided by previous investigations of mirroring with fMRI, two theoretically relevant moderators were identified: (a) object-directed versus non-object-directed action for execution and observation, and (b) biological movement and effector versus nonbiological movement and effector during observation. To better characterize and understand mu rhythm, the current meta-analysis also examined topographic specificity as well as whether the effect sizes of mu desynchronization were moderated by (a) type of baseline, and (b) type of stimulus observed. Significant meta-analytic findings would speak to the consistency across studies of mu desynchronization during action observation and action execution, and would indicate the presence of mirroring should significant effect sizes for desynchronization for both conditions be found. Positive findings on theoretically relevant moderators would highlight the specificity of contexts in which neural mirroring can occur. Findings regarding methodological moderators would highlight the need for tighter experimental control in studies of mu desynchronization.
Method
Literature Search
Studies were identified by searching the electronic databases PubMed, Academic Search Premier, and ProQuest Dissertations and Theses. The keywords “rolandic alpha,” “rolandic mu,” “mu rhythm,” “mu suppression,” “sensorimotor alpha,” “action observation,” “action perception,” “action execution,” “action production,” “action understanding,” and “motor resonance” were used along with each of the secondary keywords “electroencephalography” and “EEG.” Supplementary approaches to identifying relevant studies included searching the references of a review article (Pineda, 2005), word-of-mouth studies, and requesting researchers known for their work on EEG mu rhythm to provide any unpublished data. All studies were cross-referenced to avoid duplicates in the meta-analysis.
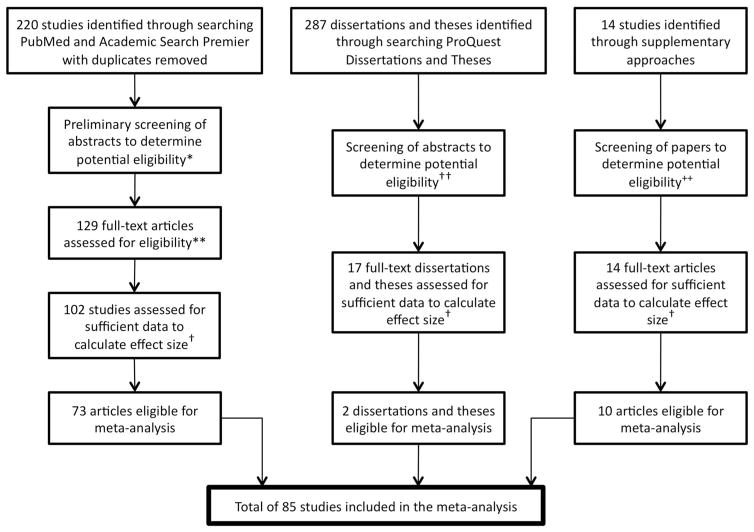
Studies were included in the meta-analysis if they met all of the following criteria: (a) examined mu rhythm activity in nonclinical human subjects, (b) used an experimental paradigm that had an observation condition, execution condition or both, and (c) reported sufficient data to estimate an effect size. For studies that included clinical samples effect sizes were calculated separately for the non-clinical or control participants. Studies published in journals and available online between 1990 and June 2014, as well as unpublished data, were included in the meta-analysis. The application of the criteria resulted in a total of 85 studies included in the meta-analysis (see Figure 2 for a flow diagram of study identification).
Figure 2.
Flow diagram for identifying articles for the meta-analysis. ++ Two ERP studies were excluded; † Studies that did not provide sufficient statistics such as p values, t values, F values to derive effect size estimates were excluded at this step; †† A majority of dissertations and theses were not relevant to the meta-analysis (e.g., examined speech processing); 91 studies were excluded because of their irrelevance; ** 27 studies excluded based on criteria (six BCI and BMI studies, five imagery only studies, three TMS and induced states studies without control groups, six ERP studies, three studies involving clinical group without control groups, four studies did not have observation and or execution conditions). PQTD = ProQuest Dissertations & Theses; BCI = brain-computer interface; BMI = brain-mind interface; TMS = transcranial magnetic stimulation.
Studies were excluded if they involved brain-computer interface (BCI), mind-machine interface (MMI), or brain-machine interface (BMI). Studies were further excluded if they met any of the following criteria: (a) used an experimental paradigm that only included imagery as the stimulus, (b) involved induced states (e.g., TMS, oxytocin) without control groups, or (c) only measured event-related potentials (ERPs). Review articles and chapters in books were excluded and only empirical studies were considered. Finally, although mu desynchronization has been investigated using other brain imaging methods such as magnetoencephalography (Berchicci et al., 2011; Hari et al., 1998; Honaga et al., 2010; Järveläinen, Schürmann, & Hari, 2004), we did not include these studies. The purpose of excluding non-EEG studies was to enhance homogeneity of the set of studies and avoid the “apples and oranges” issue in meta-analysis.
Coding System
The coding system is outlined in Table 1 (a detailed version can be requested from the authors). Studies were coded for the following: type of condition (observation, execution, or both), whether a baseline was present (no, yes), type of baseline (static, dynamic; biological, nonbiological), and type of stimulus observed. There were three categories for the type of stimulus observed (each used as a separate moderator): (a) biological or nonbiological, (b) dynamic or static, and (c) live or video. A stimulus was considered “biological” if it included a body (or part of a body) of humans or animals. Studies coded as using a biological stimulus were further subdivided to indicate whether they used a social or nonsocial biological stimulus. For a study to be coded as having a social-biological stimulus, it required meeting one of the following criteria: (a) having two or more agents interacting, or one agent interacting with a participant via live or video feed; (b) agent(s) exhibiting a communicative gesture (e.g., waving hello); or (c) agent(s) exhibiting a facial expression of emotion. Type of stimulus observed that may be considered ambiguous (e.g., Rorschach cards) were coded as nonbiological. Studies were also coded for object-directed actions for observation and execution.
Table 1.
Coding System Used for Studies Included in the Meta-Analysis
| Variable | Coding | Description |
|---|---|---|
| Design | ||
| Type of condition | 1 = Observation only | |
| 2 = Execution only | ||
| 3 = Observation and execution | ||
| Baseline present | 0 = No | |
| 1 = Yes | ||
| Type of baseline (static vs. dynamic) | 1 = Static | If a baseline is present, whether the stimulus is moving or still (e.g., picture of hand versus video of hand movement) |
| 2 = Dynamic | ||
| 5 = No stimulus | ||
| Type of baseline (biological vs. nonbiological) | 1 = Biological | |
| 2 = Nonbiological | ||
| 5 = No stimulus | ||
| Type of stimulus observed (biological vs. nonbiological) | 1 = Biological (Subcode 1 = of the biological, coded as social; Subcode 2 = coded as nonsocial) | Type of stimulus with which the subjects are presented during observation. |
| 2 = Nonbiological | ||
| 3 = Biological and nonbiological | ||
| 5 = Auditory | ||
| Type of stimulus observed (dynamic vs. static) | 1 = Dynamic | Whether the type of stimulus observed is moving or still |
| 2 = Static | ||
| Type of stimulus observed (live vs. video) | 1 = Live | |
| 2 = Video | ||
| Object-directed | 0 = No | |
| 1 = Yes | ||
| Sample characteristics | ||
| Sample size (N) | Numerical value | Sample size for which results are reported |
| Age | 1 = 0–4 years | |
| 2 = 5–18 years | ||
| 3 = >18 years | ||
| Gender | 1 = >70% male | |
| 2 = >70% female | ||
| 3 = mixed | ||
Sample characteristics were coded for (a) the final number of subjects reported in the analyses; (b) the mean age of subjects, subsequently grouped into three categories of 0 to 4 years, 5 to 18 years, and older than 18 years of age; and (c) percentage of males and females, subsequently grouped into three categories of more than 70% male, more than 70% female, and mixed.
The effect size was determined based on the reported statistics, including F, p, and t values. In the absence of statistical information, we estimated the effect size based on the narrative report (as in, e.g., Lepage & Théoret, 2006). In such instances, the estimated effect size may underestimate the actual effect size if the effect is not significant but larger than zero.
Sixty percent of the studies were coded by two individuals in order to determine intercoder reliability. Kappas ranged from 0.81 to 1.00 across all moderator variables (the mean kappa was 0.92). Detailed information regarding studies included in the meta-analysis, sample characteristics, and codes assigned to each moderator for each study are provided in the Appendix and in Table 1 of the online supplemental materials.
Meta-Analytic Methods
The effect size index used for all outcome measures was Cohen’s d, the difference between the means of two conditions (execution and baseline, or observation and baseline) divided by their pooled standard deviation, assuming a within-subject correlation of .80. When the effect was reported to be significant without any further statistic, we assumed p = .05; when the effect was reported to be not significant without any further statistic we assumed p = .50 (one-sided). The Comprehensive Meta-Analysis program (version 3.0; Borenstein, Rothstein, & Cohen, 2005) was used to transform the results of the individual studies into this common metric and to combine effect sizes. Heterogeneity across sets of outcomes was assessed using the Qhomogeneity statistic. Because several data sets were heterogeneous in their effect sizes, and because random effects models are more conservative than fixed effects parameters in such cases, combined effect sizes and confidence intervals (CIs) from random effects models are presented.
We used the “trim and fill” method (Duval & Tweedie, 2000a, 2000b) to calculate the effect of potential data censoring or publication bias on the outcome of the meta-analysis. Using this method, a funnel plot was constructed of each study’s effect size against the sample size or the standard error (usually plotted as 1/SE, or precision). It is expected that this plot has the shape of a funnel, because studies with smaller sample sizes and larger standard errors have increasingly large variation in estimates of their effect size as random variation becomes increasingly influential, whereas studies with larger sample sizes have smaller variation in effect sizes (Duval & Tweedie, 2000b; Sutton, Duval, Tweedie, Abrams, & Jones, 2000). The plots should be shaped like a funnel if no data censoring is present. However, given that smaller non-significant studies are less likely to be published (the “file-drawer” problem; Mullen, 1989), studies in the bottom left-hand corner of the plot are often omitted (Sutton et al., 2000). With the trim-and-fill procedure, the k right-most studies considered to be symmetrically unmatched are trimmed and their missing counterparts are imputed or “filled” as mirror images of the trimmed outcomes. This then allows for the computation of an adjusted overall effect size and CI (Gilbody, Song, Eastwood, & Sutton, 2000). Funnel plots for mu rhythm desynchronization during execution and observation are provided in Figures 3 and 4, respectively. Lastly, we tested the influence of moderators on combined effect sizes with the Qcontrast statistic in a random effects model (Borenstein, Hedges, Higgins, & Rothstein, 2009). A significant Qcontrast value indicates that the difference in effect size between subsets of studies is significant. Effect sizes of sets of studies with (partly) overlapping samples were compared using the 85% CI around the point estimates. Overlapping 85% CIs indicate the absence of a significant difference between the effect sizes (van IJzendoorn, Juffer, & Poelhuis, 2005). As some of the moderator categories’ effect sizes were not independent (i.e., different categories could contain the same subjects), 85% CIs were computed surrounding the calculated average effect size. These 85% CIs were compared as an exploratory test of whether effect sizes significantly differed from one another. An absence of overlap between 85% CIs is considered a statistically significant difference under a random effects model (Astill, Van der Heijden, van IJzendoorn, & Van Someren, 2012; Goldstein & Healy, 1995; van IJzendoorn et al., 2005). Note that we report the conventional 95% CIs in Tables 2 and 3 to test whether the effect sizes within domains are significantly different from zero, whereas in the text and in Figure 5, we also present the 85% CIs in order to compare combined effect sizes of sets of (overlapping) studies.
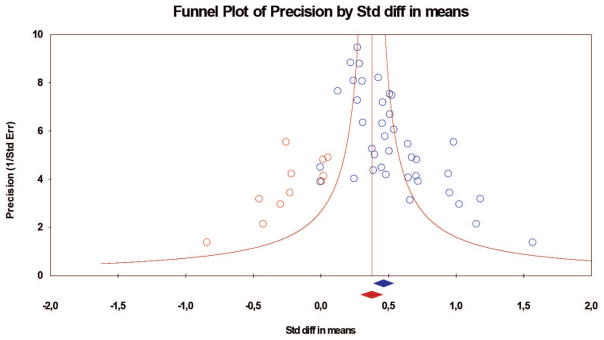
Figure 3.
Funnel plot with trimmed and filled effect sizes for changes in the mu rhythm during execution. Total k = 39; k = 11 studies were trimmed and filled (red dots), resulting in an adjusted combined effect size of d = 0.38 (red diamond; 95% CI [0.29, 0.46]). The blue dots are the effect sizes of the studies included in the meta-analysis, and the blue diamond is the overall effect size of the studies included in the meta-analysis. The red diamond is the estimated overall effect size based on these studies plus the effects added because of the trim and fill technique to address publication bias: It is the smaller overall effect size. CI = confidence interval; Std diff = standardized difference.
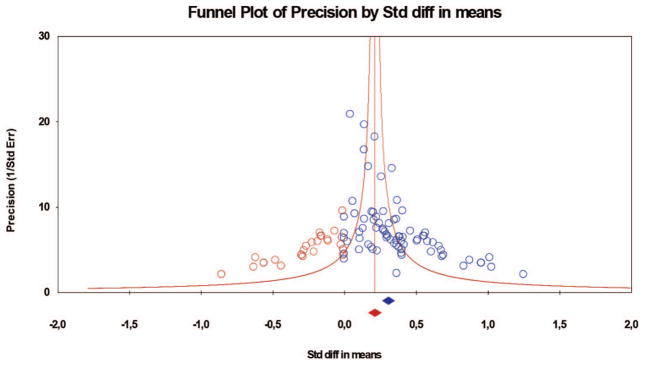
Figure 4.
Funnel plot with trimmed and filled effect sizes for changes in the mu rhythm during observation. Total k = 80; k = 27 studies were trimmed and filled (red dots), resulting in an adjusted combined effect size of d = 0.21 (red diamond; 95% CI [0.16, 0.26]). The blue dots are the effect sizes of the studies included in the meta-analysis, and the blue diamond is the overall effect size of the studies included in the meta-analysis. The red diamond is the estimated overall effect size based on these studies plus the effects added because of the trim and fill technique to address publication bias: It is the smaller overall effect size. CI = confidence interval; Std diff = standardized difference.
Table 2.
Combined Effect Sizes for Mu Desynchronization During Execution
| Moderators | k | N | d | 95% CI | Q homogeneity | Qa contrast |
|---|---|---|---|---|---|---|
| Total for mu desynchronization over central scalp locations | 39 | 701 | .46*** | .39, .54 | 65.88** | |
| Object-directed | .13 | |||||
| No | 16 | 268 | .48*** | .36, .60 | 17.75 | |
| Yes | 23 | 433 | .45*** | .35, .55 | 47.99*** | |
| Type of baseline | 1.59 | |||||
| Static | 11 | 229 | .47*** | .33, .61 | 22.19* | |
| Dynamic | 10 | 200 | .39*** | .25, .54 | 7.48 | |
| No stimulus | 18 | 272 | .51*** | .39, .63 | 33.28** | |
| Type of baseline | .71 | |||||
| Biological | 6 | 143 | .43*** | .24, .61 | 4.46 | |
| Nonbiological | 14 | 253 | .46*** | .33, .58 | 23.22* | |
| No stimulus | 18 | 272 | .51*** | .39, .63 | 33.28** | |
| Age | .07 | |||||
| 0–4 years | 4 | 85 | .43*** | .20, .66 | 3.30 | |
| 5–18 years | 2 | 30 | .61*** | .25, .97 | .34 | |
| >18 years | 33 | 586 | .46*** | .38, .55 | 60.72** | |
| Gender | .19 | |||||
| >70% male | 2 | 30 | .61*** | .27, .95 | .34 | |
| >70% female | 6 | 118 | .46*** | .30, .63 | 19.27** | |
| Mixed | 25 | 488 | .42*** | .34, .50 | 31.50 | |
| Effect size | 15.85*** | |||||
| Reported | 31 | 559 | .52*** | .45, .59 | 43.21 | |
| Estimated | 8 | 142 | .21** | .08, .34 | 3.65 | |
| Noncentral scalp locations | n.a. | |||||
| Frontal | 8 | 119 | .19* | .03, .35 | 12.23 | |
| Parietal | 6 | 117 | .27** | .09, .46 | 10.23 | |
| Temporal | 2 | 26 | .30* | .01, .59 | .01 | |
| Occipital | 9 | 216 | .14* | .01, .27 | 14.83 |
Note. k = number of study outcomes; N = total sample size; d = effect size (Cohen’s d); 95% CI = 95% confidence interval around the point estimate of the effect size; Qhomogeneity = homogeneity statistic; Qcontrast = moderation statistic; n.a. = not applicable.
Subgroups with k < 4 excluded from contrast.
p < .05.
p < .01.
p < .001.
Table 3.
Combined Effect Sizes for Mu Desynchronization During Observation
| Moderators | k | N | d | 95% CI | Q homogeneity | Qa contrast |
|---|---|---|---|---|---|---|
| Total for mu desynchronization for central scalp locations | 80 | 1508 | .31*** | .26, .35 | 161.70*** | |
| Object-directed | .65 | |||||
| No | 32 | 668 | .29*** | .22, .35 | 82.34*** | |
| Yes | 48 | 840 | .32*** | .26, .38 | 73.86** | |
| Type of stimulus observed | 5.74 | |||||
| Biological | 69 | 1320 | .30*** | .26, .35 | 141.24*** | .49 |
| Social | 6 | 109 | .25*** | .10, .41 | 7.07 | |
| Nonsocial | 63 | 1211 | .31*** | .26, .36 | 134.16*** | |
| Nonbiological | 5 | 99 | .51*** | .31, .71 | 1.64 | |
| Biological & nonbiological | 4 | 66 | .23 | −.00, .46 | 2.66 | |
| Auditory | 2 | 23 | .15 | −.03, .34 | .12 | |
| Type of stimulus observed | .72 | |||||
| Dynamic | 68 | 1271 | .31*** | .26, .36 | 140.95*** | |
| Static | 9 | 204 | .37*** | .24, .50 | 9.00 | |
| Type of stimulus observed | .32 | |||||
| Live | 16 | 284 | .33*** | .23, .44 | 20.32 | |
| Video | 54 | 1021 | .30*** | .24, .35 | 119.00*** | |
| Type of baseline | 2.00 | |||||
| Static | 27 | 510 | .33*** | .26, .40 | 32.48 | |
| Dynamic | 31 | 590 | .31*** | .24, .38 | 49.32* | |
| No stimulus | 21 | 396 | .26*** | .18, .33 | 60.90*** | |
| Type of baseline | 1.91 | |||||
| Biological | 12 | 241 | .32*** | .21, .43 | 14.80 | |
| Nonbiological | 46 | 859 | .32*** | .26, .38 | 67.27* | |
| No stimulus | 21 | 396 | .26*** | .18, .33 | 60.90*** | |
| Age | 3.41 | |||||
| 0–4 years | 17 | 360 | .23*** | .14, .32 | 19.93 | |
| 5–18 years | 8 | 112 | .33*** | .18, .48 | 6.38 | |
| >18 years | 55 | 1036 | .33*** | .28, .38 | 131.43*** | |
| Gender | 1.65 | |||||
| >70% male | 9 | 124 | .38*** | .24, .53 | 4.76 | |
| >70% female | 15 | 301 | .27*** | .18, .36 | 20.94 | |
| Mixed | 49 | 950 | .31*** | .25, .36 | 109.31*** | |
| Effect size | 17.64*** | |||||
| Reported | 57 | 1059 | .36*** | .31, .42 | 123.07*** | |
| Estimated | 23 | 449 | .18*** | .11, .25 | 18.84 | |
| Noncentral scalp locations | n.a. | |||||
| Frontal | 14 | 263 | .21*** | .10, .31 | 22.67* | |
| Parietal | 9 | 125 | .39*** | .23, .54 | 10.86 | |
| Temporal | 3 | 36 | .23 | −.00, .47 | .56 | |
| Occipital | 16 | 394 | .28*** | .19, .38 | 31.53** |
Note. k = number of study outcomes; N = total sample size; d = effect size (Cohen’s d); 95% CI = 95% confidence interval around the point estimate of the effect size; Qhomogeneity = homogeneity statistic; Qcontrast = moderation statistic; n.a. = not applicable.
Subgroups with k < 4 excluded from contrast.
p < .05.
p < .01.
p < .001.
Figure 5.
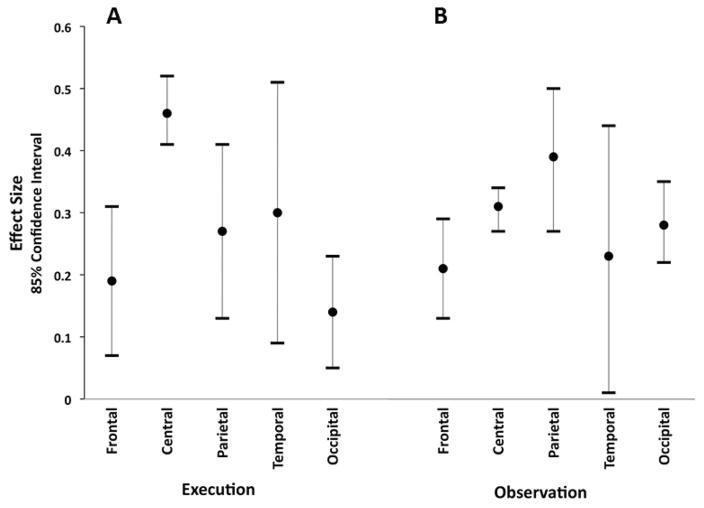
Confidence intervals (85%) by scalp locations for effect size of desynchronization during execution (A) and observation (B).
Results
Mu Rhythm Desynchronization During Execution
Overall effect
For mu desynchronization during execution, the combined effect size of the 39 studies was d = 0.46 (N = 701; 95% CI [0.39, 0.54]; p < .01), in a heterogeneous set of outcomes (Q = 65.88, p < .01; see Table 2). This indicates a moderate effect size according to Cohen’s (1988) criteria. The fail-safe number was 2,646, that is, 2,646 studies with null results would be needed to reduce the overall significant result to nonsignificance. This number is larger than Rosenthal’s (1991) criterion of 5k + 10 (k = the number of studies included in the meta-analysis), indicating that the overall effect size is quite robust assuming a zero effect size. With a criterion of d = 0.10 as a baseline, Orwin’s fail-safe number was 129. More than 100 studies with null results would render the overall result not significantly different from d = 0.10. The trim-and-fill approach showed that 11 studies had to be trimmed and filled, with a resulting adjusted combined effect size of d = 0.38 (95% CI [0.29, 0.46]). Thus, results indicate a significant overall effect size for mu desynchronization during action execution.
Theoretically relevant moderators
Whether the execution of an action was object-directed or not did not moderate the effect size. Note that the moderator biological motion was only examined for observation conditions.
Methodological moderators and sample characteristics
Type of baseline did not moderate the effect size, nor did any sample characteristics (age, gender). The only significant moderator of the combined effect size for mu desynchronization during execution was whether the effect size was specified in the study or conservatively estimated on the basis of its being significant or not significant (reported vs. estimated). Studies reporting exact effect sizes showed stronger effects (d = 0.52) than studies reporting that mu desynchronization was significant or not significant without further specification (d = 0.21), Q(1) = 15.85, p < .001.
Topographic specificity
Noncentral scalp locations (frontal, parietal, temporal, and occipital) showed overlapping 85% CIs (85% CI for frontal [0.07, 0.31]; parietal [0.13, 0.41]; temporal [0.09, 0.51]; occipital [0.05, 0.23]), indicating no differences in effect size between these four noncentral scalp locations (see Table 2 in the online supplemental material for studies that report statistics on noncentral scalp locations). However, the 85% CIs of the effect sizes for the frontal and occipital scalp locations were not overlapping with the 85% CI of the effect size for the central scalp locations (85% CI [0.41, 0.52]), indicating that the effects for these two noncentral scalp locations were significantly smaller than the effects for the central scalp locations (see Figure 5a). Thus, there is evidence for topographic specificity for mu desynchronization during action execution. Specifically, it was strongest for central scalp locations compared with frontal and occipital scalp locations.
Mu Rhythm Desynchronization During Observation
Overall effect
The combined effect size of the 80 studies on mu desynchronization during observation was d = 0.31 (N = 1,508; 95% CI [0.26, 0.35]; p < .01) in a heterogeneous set of outcomes (Q = 161.70, p < .01; see Table 3). This indicates a small to moderate effect size according to Cohen’s (1988) criteria. The fail-safe number was 7,949, that is, 7,949 studies with null results would be needed to reduce the overall significant result to nonsignificance. Again, this number is larger than Rosenthal’s (1991) criterion, indicating that the overall effect size is quite robust. With a criterion of d = 0.10 as a baseline, Orwin’s fail-safe number was 122, indicating that 122 additional studies with null results would render the overall effect not significantly different from d = 0.10. The trim-and-fill approach showed that 27 studies had to be trimmed and filled, with a resulting adjusted combined effect size of d = 0.21 (95% CI [0.16, 0.26]). Thus, as with mu desynchronization during execution, there was a significant effect size for mu during observation of action.
Theoretically relevant moderators
Neither of the theoretically relevant moderators (i.e., object-directed action or biological motion) was significant. If anything, observing biological stimuli tended to be associated with smaller effect sizes (d = 0.30) compared with observing nonbiological stimuli (d = 0.51).
Methodological moderators and sample characteristics
Type of baseline did not moderate the effect size. Gender was a significant moderator of the effect size. Although the overall Q statistic for the difference between the three groups (>70% male, >70% female, mixed) was not significant (see Table 3), the contrast between samples with a majority of females versus a majority of males indicated that studies with predominantly male samples (d = 0.38) showed significantly more mu desynchronization to observation of action than studies with predominantly female samples (d = 0.27), Q (1) = 4.24, p = .04. Age was not a significant moderator. As with action execution, whether the effect size was specified in the study or estimated (reported vs. estimated) on the basis of it being significant or not significant was a significant moderator. Studies reporting exact effect sizes showed stronger effects (d = 0.36) than studies reporting that mu desynchronization was significant or not significant without further specification (d = 0.20), Q(1) = 17.645, p < .001.
Topographic specificity
Noncentral scalp locations (frontal, parietal, temporal, and occipital) showed no differences in effect size; the 85% CIs (85% CI for frontal [0.13, 0.29]; parietal [0.27, 0.50]; temporal [0.01, 0.44]; occipital [0.22, 0.35]) were overlapping for all four noncentral scalp locations, and each of them was also overlapping with the 85% CI of the combined effect size based on the central scalp locations (85% CI [0.27, 0.34]; see Figure 5b). Note that for these analyses, the subsets of studies with assessments for different scalp locations, especially the temporal scalp locations (k = 3), were small (see Table 2 in online supplemental materials for studies that report statistics on noncentral scalp locations). However, it appears that mu desynchronization to action observation does not show topographic specificity to central scalp locations.
Comparing Mu Desynchronization During Action Execution to Mu Desynchronization During Action Observation
A final analysis compared the effect sizes of the two conditions. The 85% CI of the combined effect size for mu desynchronization during execution (85% CI [0.41, 0.52]) did not overlap with that of the combined effect size for mu desynchronization during observation (85% CI [0.27, 0.34]), showing that execution was associated with more mu desynchronization than observation.
Power Analysis and Publication Bias
Mu desynchronization studies using EEG often involve small sample sizes, and studies in this area may be underpowered, which might add to the risk of publication bias against smaller studies with nonsignificant effect sizes. We performed a power analysis with G*Power 3.1 program (Faul, Erdfelder, Lang, & Buchner, 2007) to compute the minimum sample size required for an individual study to reach the combined effect size that we found in the current meta-analysis (i.e., the assumed population effect size) with a power of 0.80 and a one-sided significance level of 0.05. We assume t test type of statistics for a within-subject or matched-pairs design. The power analysis indicated that a sample size of N = 31 would be required for an individual study to detect the combined effect size of d = 0.46 with a power of 0.80 for execution, and a sample size of N = 66 to detect the combined effect size of d = 0.31 for observation. We also calculated the actual power values of the smallest and largest study within our set of studies to estimate the range of power of the included studies to detect the combined effect size. The power values of the included studies to detect the combined effect size for execution ranged from 0.14 for the study with the smallest sample size (N = 3; Pfurtscheller & Neuper, 1992) to 0.88 for the study with the largest sample size (N = 39; Woodruff, Martin, & Bilyk, 2011). For observation, the power values ranged from 0.16 for the study with the smallest sample size (N = 6; Holmes, Collins, & Calmels, 2006) to 0.61 for the study with the largest sample size (N = 40; Cochin, Barthélémy, Lejeune, Roux, & Martineau, 1998). Certainly for EEG studies on observation, larger sample sizes are needed to detect the expected modest effect sizes and to create a cumulative science (Hulley, Cummings, Browner, Grady, & Newman, 2013). Finally, the risk for finding null effects is rather high in this area because of the small Ns of studies using EEG and the rather modest population effect sizes, and thus the chance of publication bias is increased. Indeed, highly significant Begg and Mazumdar rank correlation, and Egger’s regression intercept, supported the suggestion that considerable publication bias was present (Borenstein, 2005) during both action execution and action observation. Although a publication bias of modest size was found, fail-safe, funnel plot, and trim-and-fill approaches also showed that key findings of this meta-analysis remain significant and theoretically meaningful with the inclusion of all relevant studies. Our findings are therefore in line with the second category of Borenstein, Hedges, Higgins, and Rothstein’s (2007, p. 286) publication bias categorization: The impact of publication bias is modest but, critically, the main findings are still valid.
Discussion
At the broadest level, the results of this meta-analysis support the notion that there is consistent EEG mu desynchronization during action execution and action observation. The effect sizes for mu desynchronization during execution (d = 0.46) and observation (d = 0.31) are both significant. Further, these effects occur across diverse experimental conditions that vary in methodology (e.g., type of stimulus observed) and across a variety of actions (e.g., object-directed and non-object-directed). For action execution, results demonstrated topographic specificity for central scalp locations (i.e., greater desynchronization for central compared with frontal and occipital), but this topographic specificity was not found for action observation (although few studies in general reported activity from multiple scalp locations). Additionally, the effect size during execution was significantly greater than the effect size during observation. Note that for both execution and observation, studies that reported the exact effect size (vs. estimated) showed stronger effects; studies that did not report exact effect sizes were assumed to have p values of .05, and thus analyses potentially underestimated the combined effect size for action observation and action execution. Even so, and despite potential differences in topographic specificity, results of the meta-analysis revealed significant overall effects for both execution and observation of action. Thus, data presented here provide robust evidence that EEG mu rhythm is a valid index of neural mirroring.
Examining Neural Mirroring: EEG Mu Versus fMRI
In line with our results, meta-analyses using fMRI to investigate the mirror system also demonstrate mirroring properties in the brain. Specifically, fMRI meta-analyses show that several brain regions are active during both execution and observation of action (Caspers et al., 2010; Molenberghs et al., 2012), suggesting that neural mirroring can be indexed with fMRI. As further comparison with fMRI investigations, two moderators of mu desynchronization were examined in the present meta-analysis, given their theoretical importance and their examination in studies of neural mirroring using fMRI methods: (a) object-directed versus non-object-directed actions, and (b) biological versus nonbiological motions. In both cases, we did not find these to be significant moderators of the effect sizes for mu desynchronization during either execution or observation of action. Lack of power for moderator tests might be an explanation, but the current set of studies is not exceptionally small compared with other meta-analyses (Bakermans-Kranenburg & van IJzendoorn, 2013; van IJzendoorn et al., 2005), and the number of moderators is restricted.
The lack of moderating effects here for both object-directed action and biological motion is somewhat congruent with fMRI studies. For object-directed action, meta-analyses of fMRI data suggest that there is greater activation for object-directed actions compared with non-object-directed actions in human mirror regions. However, across fMRI studies, the particular brain regions activated vary. For example, Van Overwalle and Baetens (2009) report that object-directed action increases the activation of the anterior intraparietal sulcus, whereas for the premotor cortex, it does not. Caspers and colleagues (2010), on the other hand, report activation differences between observation of object-related and non-object-related hand actions in the frontoparietal and temporo-occipital areas. Further, some fMRI studies have shown that non-object-directed communicative gestures activate these regions to a similar degree (Montgomery, Isenberg, & Haxby, 2007).
The lack of moderation of the EEG mu rhythm by whether the type of stimulus observed had biological motion or not also appears to be consistent with human brain imaging studies of neural mirroring. In their review of fMRI studies, Morin and Grèzes (2008) report mixed findings for premotor activity during observation of biological and nonbiological motion—specifically, there is no consistent activation of the same premotor area (whether BA 6 or BA 44) across studies that compare observation of biological and nonbiological motion. In a separate meta-analysis of fMRI studies, observation of biological movement (e.g., whole-body movements such as walking) did not recruit the human mirror areas (Van Overwalle & Baetens, 2009).
The lack of specificity for both object-directed action and biological motion in the current meta-analysis, and the inconsistent findings in meta-analyses of fMRI studies, calls into question exactly what types of stimuli or contexts will elicit mirroring activity in humans. Indeed, the literature on mirror neuron research in nonhuman primates debates the importance of goal-directed actions in eliciting mirror neuron activity (e.g., Cook & Bird, 2013; Cook et al., 2014; Fabbri-Destro & Rizzolatti, 2008). It may be that goal-directed action (vs. simply object-directed) is a more effective moderator of human mirroring activity. Unfortunately, this distinction is rarely examined in the fMRI or EEG mu mirroring literatures.
The lack of a moderating effect for biological motion in both mu rhythm and fMRI data may be a result of the manner in which human subjects perceive or interpret nonbiological motion. For example, what may begin as a “nonbiological” stimulus may change across the duration of the study, as humans have the ability to anthropomorphize even nonbiological objects. Similarly, “non-biological” stimuli may imply the presence of an agent versus presenting as truly inanimate objects.
Two moderators that were not examined in the present meta-analysis but were examined in fMRI meta-analyses are type of instructions given to participants and type of body part observed. Both of these moderators affect the pattern of activation in fMRI studies (Caspers et al., 2010). Within the mu literature, most studies examined observation of hand movements. Very few examined movements of other parts of the body (e.g., foot movement, although, see Saby, Meltzoff, & Marshall, 2013). With regard to type of instructions, Caspers et al. (2010) found that differences in instruction—asking subjects to either passively observe or have the intent to imitate—moderated activity in mirror regions. Information about type of instructions was unavailable across the majority of studies included in the current meta-analysis.
Methodological Issues and Approaches to Move Ahead
The results of this meta-analysis provide an opportunity to identify methodological issues to the study of mirroring and the use of EEG mu desynchronization that could improve the quality of the data and potentially clarify some of the issues raised with respect to the assessment of a human mirror system.
Experimental design
There are several design issues that should be addressed in future research. First, as uncovered in the current meta-analysis, the majority of EEG studies do not include examination of mu desynchronization during both action observation and action execution. Such a within-subject design would allow identification of mu desynchronization to the same stimulus during execution and observation. With this design, one could identify overlap and similarity (i.e., the effects of moderators, topography, and spectral characteristics) between execute and observe conditions in the same set of participants, and thus provide a more precise assessment of mirroring in the EEG.
Control conditions and the confound of attention
In addition to having conditions in which an action is executed and in which an action is observed, studies should have a third condition in which no action is executed or observed but participants are required to engage in the same attention shifts across the scene or view the same stimuli acted upon in the action condition. EEG during these control conditions could be subtracted from the EEG in each of the two action conditions to examine amplitude and suppression during action more precisely. Such controls are critical given that EEG recorded off the scalp is the sum of signals from multiple areas of the cortex. In addition, the mu signal recorded from electrodes over sensorimotor areas is at the same frequency as the alpha signal (8–13 Hz in adults)—a signal exquisitely sensitive to changes in attention state. Control conditions would help account for the effects of and differences in attention to type of stimulus observed.
The fact that the mu signal recorded from electrodes over sensorimotor areas peaks at frequencies in the same band as the posterior alpha rhythm (8–13 Hz in adults) complicates the task of distinguishing between patterns of desynchronization attributable to the two rhythms, especially given mu and alpha can exist simultaneously in overlapping cortical areas (Andrew & Pfurtscheller, 1997). Recent work has established that patterns of alpha activity are highly dependent on various aspects of attentional state. Indeed, it has been well established that deployment of spatial attention to the left or right side of visual space in anticipation of a target results in lateralization of alpha-band EEG amplitude over posterior scalp areas, such that alpha amplitude is enhanced over the hemisphere ipsilateral to the attended hemifield (Kelly, Lalor, Reilly, & Foxe, 2006; Rihs, Michel, & Thut, 2007; Worden, Foxe, Wang, & Simpson, 2000), or else suppressed over the contralateral hemifield (Sauseng et al., 2005; Thut, Nietzel, Brandt, & Pascual-Leone, 2006; Yamagishi, Goda, Callan, Anderson, & Kawato, 2005). These complementary effects have been interpreted two ways. On the one hand, alpha desynchronization has been proposed to enhance the efficacy of the response to the stimulus at the attended location. On the other, alpha synchronization has been proposed to suppress processing of information at the unattended location. In support of the notion that alpha oscillations might serve as a mechanism for active suppression of unwanted information processing in general, Snyder and Foxe (2010) have shown alpha amplitude is also modulated by shifts in featural attention, such that alpha amplitude is enhanced in areas thought to be actively processing the unattended feature. In line with this, work by Romei, Gross, and Thut (2010) has shown that alpha oscillations induced by rhythmic TMS diminish subjects’ ability to detect visual targets in the visual hemifield contralateral to the stimulated hemisphere. Such findings obviate the importance of considering attentional confounds in studies analyzing the modulation of alpha-band EEG, which, critically, includes mu rhythm. To begin to parse the role of attention in investigations of mu desynchronization during action observation, eye-tracking methods—which offer an index of attention by measuring shifts in gaze and gaze duration—may be particularly useful. Additionally, different frequency bands (e.g., beta) should be examined for mirroring properties. Frequencies outside of the alpha range may be less sensitive to attentional confounds, potentially circumventing issues noted in this section. Further research suggests that examination of desynchronization in the beta frequency (e.g., 15–25 Hz) may be more sensitive to action observation (Hari et al., 1998).
Chronometry of mu desynchronization
Few studies have taken advantage of the ability to use EEG to assess the chronometry of mu desynchronization (Avanzini et al., 2012; Brown, Wiersema, Pourtois, & Brüne, 2013; Heimann, Umiltà, & Gallese, 2013; Muthukumaraswamy & Johnson, 2004b; Nyström, 2008; Orgs et al., 2008). There were too few studies to systematically examine timing effects on mu desynchronization in the present meta-analysis. Changes in mu desynchronization may take place before, during, or after observation of an action. Identifying the time course of mu during both action observation and action execution would provide further evidence of mirroring effects in the brain.
Topographic specificity and source localization
Although EEG traditionally has not been seen as a metric that can provide information regarding the source of its signal, there are recent improvements in methods for source localization that would help identify the brain areas that are activated during execution or observation of an action. A recent study by Thorpe and colleagues (2015) conducted a source localization of mu during execution and identified brain areas quite similar to those identified using fMRI methods. Other neuroimaging techniques with good spatial resolution (e.g., fMRI) may also help inform the cortical specificity of the mu rhythm and its relation to human mirror activity. Research that examines connections between mu desynchronization and fMRI Blood-oxygen-level dependent (BOLD) signal could help further evaluation of mu as a tool for inferring mirror system activity. As an example, studies of sequential EEG and fMRI (Ball et al., 1999; Braadbaart, Williams, & Waiter, 2013) and simultaneous EEG and fMRI (Arnstein, Cui, Keysers, Maurits, & Gazzola, 2011) demonstrated links between these two brain signals.
Additional moderators
Two additional moderators warrant attention in future research. First, meta-analytic results indicated that the effect size for mu desynchronization was significantly greater for studies with predominately male participants compared with studies with predominately female participants, in particular for observation of action. This result was unexpected and warrants further investigation. Second, the meta-analysis found no significant age differences in the effect sizes for mu desynchronization during observation and execution. When examining mu rhythm desynchronization, studies with infants and young children use different mu frequency bands compared with studies with adults, as the alpha band changes with age (Marshall, Bar-Haim, & Fox, 2002). Future research investigating the development of mu desynchronization during execution and observation of action may identify changes in the mirror system with experience or age.
Conclusion
There has been a great deal of interest and excitement in multiple areas of psychology about the possible existence of a mirror system in the human brain. This system—thought of in some instances as a core neural mechanism underlying links between action and perception—has great intuitive appeal, and the existence of mirror neurons in the nonhuman primate brain spurred on the interest in human neuroscience and psychology for a neural mirroring mechanism. This interest extends to the field of developmental psychology, in which research on the emergence of social cognition, including imitation, empathy, and theory of mind, could potentially benefit from identification of a neural mirroring system.
The present meta-analysis reveals significant effect sizes for mu desynchronization for both execution and observation of action, thus suggesting that measuring the mu rhythm during action execution and action observation is a valid tool for indexing neural mirroring. Although the meta-analysis provides clear evidence for mirroring using EEG, additional findings warrant attention to clarify the specificity of EEG mu as an index for a mirror system. We did not find evidence that mu desynchronization was specific to either object-directed action or biological motion, nor was there evidence for topographic specificity of mu desynchronization for action observation. Future work in this area must attend to experimental details including design, use of appropriate controls for attention confounds, examination of multiple scalp locations, and additional frequency bands. The current results should be placed within the context of the greater body of work from multiple brain imaging methods that have identified a mirror system in the human brain. Critically, results of the current meta-analysis bolster the general pattern of results across other imaging modalities and provide clear evidence that mu desynchronization can index neural mirroring.
Acknowledgments
Nathan A. Fox, Kathryn H. Yoo, Lindsay C. Bowman, Erin N. Cannon, Ross E. Vanderwert and Pier F. Ferrari were supported by a grant from the National Institutes of Health (P01 HD 064653). Marian J. Bakermans-Kranenburg and Marinus H. van IJzendoorn were supported by awards from The Netherlands Organization for Scientific Research (SPINOZA and VICI, respectively) and by the Gravitation Program of the Dutch Ministry of Education, Culture, and Science and the Netherlands Organization for Scientific Research (NWO grant number 024.001.003). We thank Samuel Thorpe for his assistance with a MATLAB script for the simulated mu desynchronization provided in Figure 1.
Appendix. List of Studies Included in the Meta-Analysis and Their Effect Size Estimates (95% CI)
| Study | Subsamples | Age group | Gender | N | Execution d [95% CI] | Observation d [95% CI] |
|---|---|---|---|---|---|---|
| Andelin (2011) | >18 years | Mixed | 30 | 0.53 [0.27, 0.79] | 0.21 [–0.03, 0.44] | |
| Avanzini et al. (2012) | >18 years | Mixed | 11 | 0.68 [0.21, 1.15] | ||
| Babiloni et al. (2008) | >18 years | Mixed | 12 | 0.52 [0.22, 0.81] | ||
| Behmer & Jantzen (2011) | Musicians | >18 years | Mixed | 12 | 0.69 [0.24, 1.14] | |
| Nonmusicians | >18 years | Mixed | 12 | 0.69 [0.24, 1.14] | ||
| Bernier et al. (2007) | Nonclinical | >18 years | >70% male | 15 | 0.95 [0.48, 1.41] | 1.01 [0.53, 1.50] |
| Bernier et al. (2013) | 5–18 years | >70% male | 14 | 0.37 [0.01, 0.72] | ||
| Bilyk (2012) | 18–28 years | >70% female | 37 | 0.19 [–0.03, 0.40] | ||
| Braadbaart et al. (2013) | >18 years | >70% male | 15 | 0.19 [–0.18, 0.56] | ||
| Brown et al. (2013) | >18 years | >70% female | 17 | 0.33 [0.01, 0.65] | ||
| Cannon et al. (2014) | Novices | >18 years | >70% female | 12 | 0.27 [0.01, 0.54] | |
| Observers | >18 years | >70% male | 10 | 0.62 [0.28, 0.96] | ||
| Performers | >18 years | >70% female | 11 | 0.88 [0.35, 1.40] | ||
| Cochin et al. (1998) | >18 years | Mixed | 40 | 0.26 [0.11, 0.40] | ||
| Cochin et al. (1999) | >18 years | Mixed | 20 | 0.46 [0.15, 0.77] | 0.58 [0.25, 0.91] | |
| Cochin et al. (2001) | 0–4 years | N/Rb | 30 | 0.21 [0.11, 0.32] | ||
| Colombi (2008) | Nonclinical | 5–18 years | >70% male | 9 | 0.40 [–0.05, 0.86] | |
| Experiment | 5–18 years | >70% male | 29 | 0.04 [–0.05, 0.14] | ||
| 2/nonclinical | ||||||
| Experiment | 5–18 years | >70% male | 19 | 0.00 [–0.28, 0.28] | ||
| 3/nonclinical | ||||||
| Cooper et al. (2013) | >18 years | Mixed | 20 | 0.11 [–0.17, 0.39] | ||
| Cuellar et al. (2012) | Experiment 1 | >18 years | >70% female | 10 | 0.17 [0.04, 0.30] | |
| Experiment 2 | >18 years | >70% female | 13 | 0.14 [0.04, 0.24] | ||
| de Klerk et al. (2015)a | 0–4 years | Mixed | 31 | 0.00 [–0.22, 0.22] | ||
| Derambure et al. (1997) | Nonclinical | >18 years | N/Rb | 10 | 0.65 [0.17, 1.14] | |
| Désy & Lepage (2013) | >18 years | Mixed | 18 | 0.65 [0.29, 1.01] | 0.66 [0.30, 1.02] | |
| Fargier et al. (2012) | >18 years | Mixed | 16 | 0.11 [–0.20, 0.42] | ||
| Francuz & Zapała (2011) | >18 years | Mixed | 10 | 0.96 [0.38, 1.53] | ||
| Frenkel-Toledo et al. (2013) | >18 years | Mixed | 27 | 1.16 [0.23, 2.08] | 1.25 [0.31, 2.19] | |
| Giromini et al. (2010) | >18 years | Mixed | 19 | 0.51 [0.18, 0.84] | ||
| Gutsell & Inzlicht (2010) | Mixed | 30 | 0.51 [0.25, 0.77] | 0.14 [−0.09, 0.37] | ||
| Heimann et al. (2013) | >18 years | Mixed | 15 | 0.68 [0.28, 1.08] | 0.68 [0.28, 1.08] | |
| Holmes et al. (2006) | >18 years | >70% female | 6 | 0.00 [–0.51, 0.51] | 0.00 [–0.51, 0.51] | |
| Kranczioch et al. (2008) | >18 years | >70% female | 13 | 0.71 [0.23, 1.19] | ||
| Kumar et al. (2013) | N/Rb | >70% female | 17 | 0.36 [0.14, 0.59] | ||
| Leocani et al. (1997) | N/Rb | Mixed | 9 | 0.39 [–0.06, 0.84] | ||
| Lepage & Théoret (2006) | 5–18 years | Mixed | 15 | 0.17 [–0.18, 0.52] | ||
| Li et al. (2011) | >18 years | Mixed | 14 | 0.51 [0.13, 0.89] | ||
| Marshall et al. (2009) | N/Rb | Mixed | 20 | 0.39 [0.09, 0.69] | ||
| Marshall et al. (2011) | 0–4 years | Mixed | 30 | 0.31 [0.07, 0.55] | 0.31 [0.07, 0.56] | |
| Martineau & Cochin (2003) | Nonclinical | 0–4 years | Mixed | 14 | 0.07 [–0.14, 0.29] | |
| Martineau et al. (2008) | 5–18 years | Mixed | 14 | 0.02 [–0.31, 0.35] | ||
| McCormick et al. (2012) | >18 years | >70% male | 16 | 0.33 [0.20, 0.47] | ||
| McGarry et al. (2012) | >18 years | >70% female | 33 | 0.22 [0.00, 0.45] | 0.22 [0.00, 0.45] | |
| Meyer et al. (2011) | 0–4 years | >70% male | 7 | 0.83 [0.19, 1.48] | ||
| Milston et al. (2013) | >18 years | >70% female | 26 | 0.46 [0.19, 0.74] | 0.46 [0.19, 0.74] | |
| Moore et al. (2008) | >18 years | Mixed | 34 | 0.29 [0.07, 0.51] | ||
| Moore et al. (2012) | N/Rb | Mixed | 11 | 0.37 [0.19, 0.55] | ||
| Müller-Putz et al. (2007) | Nonclinical | >18 years | >70% male | 9 | 0.49 [0.02, 0.96] | |
| Muthukumaraswamy & Johnson (2004a) | >18 years | Mixed | 13 | 0.38 [0.01, 0.76] | ||
| Muthukumaraswamy & Johnson (2004b) | >18 years | Mixed | 8 | 0.00 [–0.44, 0.44] | 0.00 [–0.44, 0.44] | |
| Muthukumaraswamy et al. (2004) | >18 years | Mixed | 12 | 0.40 [0.01, 0.79] | 0.40 [0.01, 0.79] | |
| Nyström (2008) | Adults | >18 years | N/Rb | 15 | 0.35 [0.01, 0.69] | |
| Infants | 0–4 years | N/Rb | 19 | 0.31 [0.00, 0.61] | ||
| Nyström et al. (2011) | 0–4 years | N/Rb | 23 | 0.27 [0.00, 0.54] | ||
| Oberman et al. (2007) | Experiment 1 | >18 years | >70% female | 17 | 0.36 [0.04, 0.69] | |
| Experiment 2 | >18 years | >70% female | 20 | 0.51 [0.19, 0.83] | ||
| Oberman et al. (2008) | Nonclinical | 5–18 years | >70% male | 13 | 0.61 [0.19, 1.02] | |
| Oberman et al. (2005) | Nonclinical | 5–18 years | >70% male | 10 | 0.72 [0.22, 1.23] | 0.40 [–0.03, 0.83] |
| Orgs et al. (2008) | Dancers | >18 years | Mixed | 10 | 0.96 [0.38, 1.53] | |
| Nondancers | >18 years | Mixed | 10 | 0.23 [–0.18, 0.64] | ||
| Perry & Bentin (2009) | >18 years | Mixed | 24 | 0.23 [–0.04, 0.49] | ||
| Perry & Bentin (2010) | >18 years | Mixed | 24 | 0.13 [–0.13, 0.39] | ||
| Perry et al. (2011) | >18 years | Mixed | 28 | 0.25 [0.00, 0.49] | 0.25 [0.00, 0.49] | |
| Perry et al. (2010) | >18 years | Mixed | 24 | 0.55 [0.26, 0.85] | ||
| Pfurtscheller & Neuper (1992) | N/Rb | N/Rb | 3 | 1.57 [0.13, 3.02] | ||
| Pineda & Oberman (2006) | >18 years | Mixed | 18 | 0.32 [0.01, 0.62] | 0.20 [–0.01, 0.41] | |
| Pineda et al. (2011) | >18 years | Mixed | 24 | 0.56 [0.26, 0.86] | ||
| Porcelli et al. (2013) | >18 years | >70% female | 24 | 0.37 [–0.53, 1.26] | ||
| Puzzo et al. (2010) | >18 years | Mixed | 20 | 0.38 [0.08, 0.68] | ||
| Raymaekers et al. (2009) | Nonclinical | 5–18 years | >70% male | 20 | 0.54 [0.22, 0.87] | 0.41 [0.11, 0.72] |
| Reid et al. (2011) | 0–4 years | Mixed | 10 | 0.67 [0.18, 1.16] | 0.11 [−0.29, 0.50] | |
| Ruysschaert et al. (2013) | Live setting group | 0–4 years | Mixed | 18 | 0.30 [–0.01, 0.60] | |
| Video setting group | 0–4 years | Mixed | 16 | 0.00 [–0.31, 0.31) | ||
| Schuch et al. (2010) | >18 years | >70% female | 27 | 0.99 [0.63, 1.34] | 0.57 [0.28, 0.85] | |
| Serrien & Brown (2003) | N/Rb | N/Rb | 6 | 0.66 [0.03, 1.29] | ||
| Silas et al. (2010) | >18 years | Mixed | 33 | 0.43 [0.19, 0.67] | 0.35 [0.12, 0.58] | |
| Southgate & Begus (2013) | 0–4 years | Mixed | 33 | 0.20 [–0.19, 0.59] | ||
| Southgate et al. (2010) | 0–4 years | Mixed | 22 | 0.29 [0.01, 0.56] | ||
| Southgate et al. (2009) | 0–4 years | Mixed | 15 | 0.71 [0.30, 1.12] | 0.42 [0.07, 0.77] | |
| Stancák & Pfurtscheller (1996a) | >18 years | Mixed | 10 | 0.45 [0.01, 0.89] | ||
| Stancák et al. (1997) | >18 years | Mixed | 13 | 0.38 [0.01, 0.76] | ||
| Stancák & Pfurtscheller (1996b) | >18 years | Mixed | 23 | 0.27 [0.00, 0.54] | ||
| Streltsova et al. (2010) | >18 years | Mixed | 11 | 1.18 [0.56, 1.81] | 0.41 [0.20, 0.61] | |
| Ulloa & Pineda (2007) | >18 years | >70% female | 20 | 0.30 [0.00, 0.59] | ||
| Upshaw et al. (2014)a | 0–4 years | N/Rb | 23 | 0.25 [–0.24, 0.74] | 0.06 [–0.12, 0.24] | |
| van Elk et al. (2008) | 0–4 years | Mixed | 12 | 0.40 [0.01, 0.79] | ||
| Virji-Babul et al. (2012)c | 0–4 years | Mixed | 10 | |||
| Warreyn et al. (2012) | 0–4 years | Mixed | 17 | 0.48 [0.14, 0.82] | 0.40 [0.07, 0.73] | |
| Woodruff et al. (2011) | N/Rb | Mixed | 39 | 0.28 [0.07, 0.48] | 0.28 [0.07, 0.48] | |
| Wriesnegger et al. (2013) | >18 years | >70% female | 17 | 0.14 [0.02, 0.26] | ||
| Yang et al. (2009) | >18 years | >70% male | 16 | 0.13 [–0.13, 0.39] | ||
| Yuan et al. (2010) | >18 years | Mixed | 10 | 0.96 [0.38, 1.53] |
Note. CI - confidence interval.
Unpublished at the time of data request.
Specific age/gender distribution of participants was not reported (N/R); thus, we could not assign a code.
95% CI for observation was calculated for parietal (0.41[–0.02, 0.85]) and temporal (0.11[–0.28, 0.51]) areas.
Footnotes
Supplemental materials: http://dx.doi.org/10.1037/bul0000031.supp
Contributor Information
Nathan A. Fox, University of Maryland
Marian J. Bakermans-Kranenburg, Leiden University
Kathryn H. Yoo, University of Maryland
Lindsay C. Bowman, University of Maryland
Erin N. Cannon, University of Maryland
Ross E. Vanderwert, Cardiff University
Pier F. Ferrari, University of Parma
Marinus H. van IJzendoorn, Leiden University
References
References marked with an asterisk indicate studies included in the meta-analysis.
- *.Andelin A. Unpublished master’s thesis. Northern Arizona University; Flagstaff, Arizona: 2011. Manipulation of hand movement observation and execution on mu suppression measured by electroencephalography. [Google Scholar]
- Andrew C, Pfurtscheller G. On the existence of different alpha band rhythms in the hand area of man. Neuroscience Letters. 1997;222:103–106. doi: 10.1016/s0304-3940(97)13358-4. http://dx.doi.org/10.1016/S0304-3940(97)13358-4. [DOI] [PubMed] [Google Scholar]
- Arnstein D, Cui F, Keysers C, Maurits NM, Gazzola V. μ-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. The Journal of Neuroscience. 2011;31:14243–14249. doi: 10.1523/JNEUROSCI.0963-11.2011. http://dx.doi.org/10.1523/JNEUROSCI.0963-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astill RG, Van der Heijden KB, van IJzendoorn MH, Van Someren EJW. Sleep, cognition, and behavioral problems in school-age children: A century of research meta-analyzed. Psychological Bulletin. 2012;138:1109–1138. doi: 10.1037/a0028204. http://dx.doi.org/10.1037/a0028204. [DOI] [PubMed] [Google Scholar]
- *.Avanzini P, Fabbri-Destro M, Dalla Volta R, Daprati E, Rizzolatti G, Cantalupo G. The dynamics of sensorimotor cortical oscillations during the observation of hand movements: An EEG study. PLoS ONE. 2012;7(5):e37534. doi: 10.1371/journal.pone.0037534. http://dx.doi.org/10.1371/journal.pone.0037534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Babiloni C, Del Percio C, Iacoboni M, Infarinato F, Lizio R, Marzano N, … Eusebi F. Golf putt outcomes are predicted by sensorimotor cerebral EEG rhythms. The Journal of Physiology. 2008;586:131–139. doi: 10.1113/jphysiol.2007.141630. http://dx.doi.org/10.1113/jphysiol.2007.141630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Sniffing around oxytocin: Review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Translational Psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. http://dx.doi.org/10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T, Schreiber A, Feige B, Wagner M, Lücking CH, Kristeva-Feige R. The role of higher-order motor areas in voluntary movement as revealed by high-resolution EEG and fMRI. NeuroImage. 1999;10:682–694. doi: 10.1006/nimg.1999.0507. http://dx.doi.org/10.1006/nimg.1999.0507. [DOI] [PubMed] [Google Scholar]
- *.Behmer LP, Jr, Jantzen KJ. Reading sheet music facilitates sensorimotor mu-desynchronization in musicians. Clinical Neurophysiology. 2011;122:1342–1347. doi: 10.1016/j.clinph.2010.12.035. http://dx.doi.org/10.1016/j.clinph.2010.12.035. [DOI] [PubMed] [Google Scholar]
- Berchicci M, Zhang T, Romero L, Peters A, Annett R, Teuscher U, … Comani S. Development of mu rhythm in infants and preschool children. Developmental Neuroscience. 2011;33:130–143. doi: 10.1159/000329095. http://dx.doi.org/10.1159/000329095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Über das Elektrenkephalogramm des Menschen [On the electroencephalogram in humans] Archiv für Psychiatrie und Nervenkrankheiten. 1929;87:527–570. http://dx.doi.org/10.1007/BF01797193. [Google Scholar]
- *.Bernier R, Aaronson B, McPartland J. The role of imitation in the observed heterogeneity in EEG mu rhythm in autism and typical development. Brain and Cognition. 2013;82:69–75. doi: 10.1016/j.bandc.2013.02.008. http://dx.doi.org/10.1016/j.bandc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- *.Bernier R, Dawson G, Webb S, Murias M. EEG mu rhythm and imitation impairments in individuals with autism spectrum disorder. Brain and Cognition. 2007;64:228–237. doi: 10.1016/j.bandc.2007.03.004. http://dx.doi.org/10.1016/j.bandc.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bilyk N. Unpublished master’s thesis. Northern Arizona University; Flagstaff, Arizona: 2012. An investigation of the relationship between mirror neurons and empathy. [Google Scholar]
- Borenstein M. Software for publication bias. In: Rothstein HR, Sutton AJ, Borenstein M, editors. Publication bias in meta-analysis: Prevention, assessment and adjustments. Chichester, UK: Wiley; 2005. pp. 193–220. http://dx.doi.org/10.1002/0470870168.ch11. [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to meta-analysis. Chichester, UK: Wiley; 2007. http://dx.doi.org/10.1002/9780470743386. [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to Meta-Analysis. Chichester, UK: Wiley; 2009. http://dx.doi.org/10.1002/9780470743386.ch13. [Google Scholar]
- Borenstein M, Rothstein D, Cohen J. Comprehensive meta-analysis: A computer program for research synthesis. Englewood, NJ: Biostat; 2005. [Google Scholar]
- *.Braadbaart L, Williams JHG, Waiter GD. Do mirror neuron areas mediate mu rhythm suppression during imitation and action observation? International Journal of Psychophysiology. 2013;89:99–105. doi: 10.1016/j.ijpsycho.2013.05.019. [DOI] [PubMed] [Google Scholar]
- *.Brown EC, Wiersema JR, Pourtois G, Brüne M. Modulation of motor cortex activity when observing rewarding and punishing actions. Neuropsychologia. 2013;51:52–58. doi: 10.1016/j.neuropsychologia.2012.11.005. http://dx.doi.org/10.1016/j.neuropsychologia.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Riggio L. The mirror neuron system and action recognition. Brain and Language. 2004;89:370–376. doi: 10.1016/S0093-934X(03)00356-0. http://dx.doi.org/10.1016/S0093-934X(03)00356-0. [DOI] [PubMed] [Google Scholar]
- *.Cannon EN, Yoo KH, Vanderwert RE, Ferrari PF, Woodward AL, Fox NA. Action experience, more than observation, influences mu rhythm desynchronization. PLoS ONE. 2014;9(3):e92002. doi: 10.1371/journal.pone.0092002. http://dx.doi.org/10.1371/journal.pone.0092002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. http://dx.doi.org/10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casile A. Mirror neurons (and beyond) in the macaque brain: An overview of 20 years of research. Neuroscience Letters. 2013;540:3–14. doi: 10.1016/j.neulet.2012.11.003. http://dx.doi.org/10.1016/j.neulet.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. NeuroImage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. http://dx.doi.org/10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cheng Y, Lee P-L, Yang C-Y, Lin C-P, Hung D, Decety J. Gender differences in the mu rhythm of the human mirror-neuron system. PLoS ONE. 2008;3(5):e2113. doi: 10.1371/journal.pone.0002113. http://dx.doi.org/10.1371/journal.pone.0002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cochin S, Barthélémy C, Lejeune B, Roux S, Martineau J. Perception of motion and qEEG activity in human adults. Electroencephalography & Clinical. Neurophysiology. 1998;107:287–295. doi: 10.1016/s0013-4694(98)00071-6. http://dx.doi.org/10.1016/S0013-4694(98)00071-6. [DOI] [PubMed] [Google Scholar]
- *.Cochin S, Barthélémy C, Roux S, Martineau J. Observation and execution of movement: Similarities demonstrated by quantified electroencephalography. The European Journal of Neuroscience. 1999;11:1839–1842. doi: 10.1046/j.1460-9568.1999.00598.x. http://dx.doi.org/10.1046/j.1460-9568.1999.00598.x. [DOI] [PubMed] [Google Scholar]
- *.Cochin S, Barthélémy C, Roux S, Martineau J. Electroencephalographic activity during perception of motion in childhood. European Journal of Neuroscience. 2001;13:1791–1796. doi: 10.1046/j.0953-816x.2001.01544.x. http://dx.doi.org/10.1046/j.0953-816x.2001.01544.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. London, UK: Routledge; 1988. [Google Scholar]
- *.Colombi C. Unpublished doctoral dissertation. University of California; Davis, California: 2008. Mirror neuron system activation in autism in response to transitive and intransitive actions. [Google Scholar]
- Cook R, Bird G. Do mirror neurons really mirror and do they really code for action goals? Cortex: A Journal Devoted to the Study of the Nervous System and Behavior. 2013;49:2944–2945. doi: 10.1016/j.cortex.2013.05.006. http://dx.doi.org/10.1016/j.cortex.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Cook R, Bird G, Catmur C, Press C, Heyes C. Mirror neurons: From origin to function. Behavioral and Brain Sciences. 2014;37:177–192. doi: 10.1017/S0140525X13000903. http://dx.doi.org/10.1017/S0140525X13000903. [DOI] [PubMed] [Google Scholar]
- *.Cooper NR, Puzzo I, Pawley AD, Bowes-Mulligan RA, Kirkpatrick EV, Antoniou PA, Kennett S. Bridging a yawning chasm: EEG investigations into the debate concerning the role of the human mirror neuron system in contagious yawning. Cognitive, Affective & Behavioral Neuroscience. 2012;12:393–405. doi: 10.3758/s13415-011-0081-7. http://dx.doi.org/10.3758/s13415-011-0081-7. [DOI] [PubMed] [Google Scholar]
- *.Cooper NR, Simpson A, Till A, Simmons K, Puzzo I. Beta event-related desynchronization as an index of individual differences in processing human facial expression: Further investigations of autistic traits in typically developing adults. Frontiers in Human Neuroscience. 2013;7:159. doi: 10.3389/fnhum.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibra G. Action mirroring and action understanding: An alternative account. In: Haggard P, Rossetti Y, Kawato M, editors. Sensorimotor foundations of higher cognition attention and performance. Attention and performance. Vol. 22. New York, NY: Oxford University Press; 2007. pp. 435–459. [Google Scholar]
- *.Cuellar M, Bowers A, Harkrider AW, Wilson M, Saltuklaroglu T. Mu suppression as an index of sensorimotor contributions to speech processing: Evidence from continuous EEG signals. International Journal of Psychophysiology. 2012;85:242–248. doi: 10.1016/j.ijpsycho.2012.04.003. http://dx.doi.org/10.1016/j.ijpsycho.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Cuevas K, Cannon EN, Yoo K, Fox NA. The infant EEG mu rhythm: Methodological considerations and best practices. Developmental Review. 2014;34:26–43. doi: 10.1016/j.dr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. http://dx.doi.org/10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.de Klerk CCJM, Johnson MH, Heyes CM, Southgate V. Baby steps: Investigating the development of perceptual-motor couplings in infancy. Developmental Science. 2015;18:270–280. doi: 10.1111/desc.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Derambure P, Bourriez JL, Defebvre L, Cassim F, Josien E, Duhamel A, … Guieu JD. Abnormal cortical activation during planning of voluntary movement in patients with epilepsy with focal motor seizures: Event-related desynchronization study of electroencephalographic mu rhythm. Epilepsia. 1997;38:655–662. doi: 10.1111/j.1528-1157.1997.tb01234.x. [DOI] [PubMed] [Google Scholar]
- *.Désy MC, Lepage JF. Skin color has no impact on motor resonance: Evidence from mu rhythm suppression and imitation. Neuroscience Research. 2013;77:58–63. doi: 10.1016/j.neures.2013.08.003. http://dx.doi.org/10.1016/j.neures.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Thomas C, Behrmann M, Heeger DJ. A mirror up to nature. Current Biology. 2008;18:R13–R18. doi: 10.1016/j.cub.2007.11.004. http://dx.doi.org/10.1016/j.cub.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: A neurophysiological study. Experimental Brain Research. 1992;91:176–180. doi: 10.1007/BF00230027. http://dx.doi.org/10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. A nonparametric “trim and fill” method for accounting for publication bias in meta-analysis. Journal of the American Statistical Association. 2000a;95:89–98. [Google Scholar]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000b;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. http://dx.doi.org/10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Kennedy HA, Bradshaw JL, Rinehart NJ, Fitzgerald PB. Understanding mirror neurons: Evidence for enhanced corticospinal excitability during the observation of transitive but not intransitive hand gestures. Neuropsychologia. 2010;48:2675–2680. doi: 10.1016/j.neuropsychologia.2010.05.014. http://dx.doi.org/10.1016/j.neuropsychologia.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Kennedy HA, Rinehart NJ, Bradshaw JL, Tonge BJ, Daskalakis ZJ, Fitzgerald PB. Interpersonal motor resonance in autism spectrum disorder: Evidence against a global “mirror system” deficit. Frontiers in Human Neuroscience. 2013;7:218. doi: 10.3389/fnhum.2013.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott PG, Kennedy HA, Rinehart NJ, Tonge BJ, Bradshaw JL, Taffe JR, … Fitzgerald PB. Mirror neuron activity associated with social impairments but not age in autism spectrum disorder. Biological Psychiatry. 2012;71:427–433. doi: 10.1016/j.biopsych.2011.09.001. http://dx.doi.org/10.1016/j.biopsych.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Fabbri-Destro M, Rizzolatti G. Mirror neurons and mirror systems in monkeys and humans. Physiology (Bethesda, MD) 2008;23:171–179. doi: 10.1152/physiol.00004.2008. http://dx.doi.org/10.1152/physiol.00004.2008. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: A magnetic stimulation study. Journal of Neurophysiology. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Fan YT, Decety J, Yang CY, Liu JL, Cheng Y. Unbroken mirror neurons in autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2010;51:981–988. doi: 10.1111/j.1469-7610.2010.02269.x. http://dx.doi.org/10.1111/j.1469-7610.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- *.Fargier R, Paulignan Y, Boulenger V, Monaghan P, Reboul A, Nazir TA. Learning to associate novel words with motor actions: Language-induced motor activity following short training. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior. 2012;48:888–899. doi: 10.1016/j.cortex.2011.07.003. http://dx.doi.org/10.1016/j.cortex.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. http://dx.doi.org/10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Tramacere A, Simpson EA, Iriki A. Mirror neurons through the lens of epigenetics. Trends in Cognitive Sciences. 2013;17:450–457. doi: 10.1016/j.tics.2013.07.003. http://dx.doi.org/10.1016/j.tics.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: From action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. http://dx.doi.org/10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Formaggio E, Storti SF, Avesani M, Cerini R, Milanese F, Gasparini A, … Manganotti P. EEG and FMRI coregistration to investigate the cortical oscillatory activities during finger movement. Brain Topography. 2008;21:100–111. doi: 10.1007/s10548-008-0058-1. http://dx.doi.org/10.1007/s10548-008-0058-1. [DOI] [PubMed] [Google Scholar]
- *.Francuz P, Zapała D. The suppression of the μ rhythm during the creation of imagery representation of movement. Neuroscience Letters. 2011;495:39–43. doi: 10.1016/j.neulet.2011.03.031. http://dx.doi.org/10.1016/j.neulet.2011.03.031. [DOI] [PubMed] [Google Scholar]
- *.Frenkel-Toledo S, Bentin S, Perry A, Liebermann DG, Soroker N. Dynamics of the EEG power in the frequency and spatial domains during observation and execution of manual movements. Brain Research. 2013;1509:43–57. doi: 10.1016/j.brainres.2013.03.004. http://dx.doi.org/10.1016/j.brainres.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Gallese V. The “shared manifold” hypothesis: From mirror neurons to empathy. Journal of Consciousness Studies. 2001;8:33–50. [Google Scholar]
- Gallese V. Embodied simulation: From neurons to phenomenal experience. Phenomenology and the Cognitive Sciences. 2005;4:23–48. http://dx.doi.org/10.1007/s11097-005-4737-z. [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain: A Journal of Neurology. 1996;119:593–609. doi: 10.1093/brain/119.2.593. http://dx.doi.org/10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gallese V, Gernsbacher MA, Heyes C, Hickok G, Iacoboni M. Mirror Neuron Forum. Perspectives on Psychological Science. 2011;6:369–407. doi: 10.1177/1745691611413392. http://dx.doi.org/10.1177/1745691611413392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Sinigaglia C. What is so special about embodied simulation? Trends in Cognitive Sciences. 2011;15:512–519. doi: 10.1016/j.tics.2011.09.003. http://dx.doi.org/10.1016/j.tics.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Gilbody SM, Song F, Eastwood AJ, Sutton A. The causes, consequences and detection of publication bias in psychiatry. Acta Psychiatrica Scandinavica. 2000;102:241–249. doi: 10.1034/j.1600-0447.2000.102004241.x. http://dx.doi.org/10.1034/j.1600-0447.2000.102004241.x. [DOI] [PubMed] [Google Scholar]
- *.Giromini L, Porcelli P, Viglione DJ, Parolin L, Pineda JA. The feeling of movement: EEG evidence for mirroring activity during the observations of static, ambiguous stimuli in the Rorschach cards. Biological Psychology. 2010;85:233–241. doi: 10.1016/j.biopsycho.2010.07.008. http://dx.doi.org/10.1016/j.biopsycho.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Goldstein H, Healy M. On the graphical presentation of a collection of means. Journal of the Royal Statistical Society Series A (Statistics in Society) 1995;158:175–177. http://dx.doi.org/10.2307/2983411. [Google Scholar]
- Grèzes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta-analysis. Human Brain Mapping. 2001;12:1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. http://dx.doi.org/10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Gutsell JN, Inzlicht M. Empathy constrained: Prejudice predicts reduced mental simulation of actions during observation of outgroups. Journal of Experimental Social Psychology. 2010;46:841–845. http://dx.doi.org/10.1016/j.jesp.2010.03.011. [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cerebral Cortex. 2006;16:1276–1282. doi: 10.1093/cercor/bhj069. http://dx.doi.org/10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Brindley RM, Frith U. Imitation and action understanding in autistic spectrum disorders: How valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia. 2007;45:1859–1868. doi: 10.1016/j.neuropsychologia.2006.11.022. http://dx.doi.org/10.1016/j.neuropsychologia.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G. Activation of human primary motor cortex during action observation: A neuromagnetic study. PNAS Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15061–15065. doi: 10.1073/pnas.95.25.15061. http://dx.doi.org/10.1073/pnas.95.25.15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: A neuromagnetic view through the skull. Trends in Neurosciences. 1997;20:44–49. doi: 10.1016/S0166-2236(96)10065-5. http://dx.doi.org/10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- Hari R, Salmelin R, Mäkelä JP, Salenius S, Helle M. Magnetoencephalographic cortical rhythms. International Journal of Psychophysiology. 1997;26:51–62. doi: 10.1016/s0167-8760(97)00755-1. http://dx.doi.org/10.1016/S0167-8760(97)00755-1. [DOI] [PubMed] [Google Scholar]
- *.Heimann K, Umiltà MA, Gallese V. How the motor-cortex distinguishes among letters, unknown symbols and scribbles: A high density EEG study. Neuropsychologia. 2013;51:2833–2840. doi: 10.1016/j.neuropsychologia.2013.07.014. http://dx.doi.org/10.1016/j.neuropsychologia.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Heyes C. Where do mirror neurons come from? Neuroscience and Biobehavioral Reviews. 2010;34:575–583. doi: 10.1016/j.neubiorev.2009.11.007. http://dx.doi.org/10.1016/j.neubiorev.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Heyes C. Tinbergen on mirror neurons. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2014;369:20130180. doi: 10.1098/rstb.2013.0180. http://dx.doi.org/10.1098/rstb.2013.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. Journal of Cognitive Neuroscience. 2009;21:1229–1243. doi: 10.1162/jocn.2009.21189. http://dx.doi.org/10.1162/jocn.2009.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Hauser M. (Mis)understanding mirror neurons. Current Biology. 2010;20:R593–R594. doi: 10.1016/j.cub.2010.05.047. http://dx.doi.org/10.1016/j.cub.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Holmes P, Collins D, Calmels C. Electroencephalographic functional equivalence during observation of action. Journal of Sports Sciences. 2006;24:605–616. doi: 10.1080/02640410500244507. http://dx.doi.org/10.1080/02640410500244507. [DOI] [PubMed] [Google Scholar]
- Honaga E, Ishii R, Kurimoto R, Canuet L, Ikezawa K, Takahashi H, … Takeda M. Post-movement beta rebound abnormality as indicator of mirror neuron system dysfunction in autistic spectrum disorder: An MEG study. Neuroscience Letters. 2010;478:141–145. doi: 10.1016/j.neulet.2010.05.004. http://dx.doi.org/10.1016/j.neulet.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Hulley S, Cummings S, Browner W, Grady D, Newman T. Designing clinical research. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- Iacoboni M. Imitation, empathy, and mirror neurons. Annual Review of Psychology. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. http://dx.doi.org/10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- Jacob P. What do mirror neurons contribute to human social cognition? Mind & Language. 2008;23:190–223. http://dx.doi.org/10.1111/j.1468-0017.2007.00337.x. [Google Scholar]
- Järveläinen J, Schürmann M, Hari R. Activation of the human primary motor cortex during observation of tool use. NeuroImage. 2004;23:187–192. doi: 10.1016/j.neuroimage.2004.06.010. http://dx.doi.org/10.1016/j.neuroimage.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. Journal of Neurophysiology. 2006;95:3844–3851. doi: 10.1152/jn.01234.2005. http://dx.doi.org/10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD. Predictive coding: An account of the mirror neuron system. Cognitive Processing. 2007;8:159–166. doi: 10.1007/s10339-007-0170-2. http://dx.doi.org/10.1007/s10339-007-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kranczioch C, Athanassiou S, Shen S, Gao G, Sterr A. Short-term learning of a visually guided power-grip task is associated with dynamic changes in EEG oscillatory activity. Clinical Neurophysiology. 2008;119:1419–1430. doi: 10.1016/j.clinph.2008.02.011. http://dx.doi.org/10.1016/j.clinph.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Kuhlman WN. Functional topography of the human mu rhythm. Electroencephalography & Clinical Neurophysiology. 1978;44:83–93. doi: 10.1016/0013-4694(78)90107-4. http://dx.doi.org/10.1016/0013-4694(78)90107-4. [DOI] [PubMed] [Google Scholar]
- *.Kumar S, Riddoch MJ, Humphreys G. Mu rhythm desynchronization reveals motoric influences of hand action on object recognition. Frontiers in Human Neuroscience. 2013;7:66. doi: 10.3389/fnhum.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Leocani L, Toro C, Manganotti P, Zhuang P, Hallett M. Event-related coherence and event-related desynchronization/ synchronization in the 10 Hz and 20 Hz EEG during self-paced movements. Electroencephalography and Clinical Neurophysiology. 1997;104:199–206. doi: 10.1016/s0168-5597(96)96051-7. http://dx.doi.org/10.1016/S0168-5597(96)96051-7. [DOI] [PubMed] [Google Scholar]
- *.Lepage JF, Théoret H. EEG evidence for the presence of an action observation-execution matching system in children. European Journal of Neuroscience. 2006;23:2505–2510. doi: 10.1111/j.1460-9568.2006.04769.x. http://dx.doi.org/10.1111/j.1460-9568.2006.04769.x. [DOI] [PubMed] [Google Scholar]
- *.Li S, Hong B, Gao X, Wang Y, Gao S. Event-related spectral perturbation induced by action-related sound. Neuroscience Letters. 2011;491:165–167. doi: 10.1016/j.neulet.2011.01.016. http://dx.doi.org/10.1016/j.neulet.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Manshanden I, De Munck JC, Simon NR, Lopes da Silva FH. Source localization of MEG sleep spindles and the relation to sources of alpha band rhythms. Clinical Neurophysiology. 2002;113:1937–1947. doi: 10.1016/s1388-2457(02)00304-8. http://dx.doi.org/10.1016/S1388-2457(02)00304-8. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. http://dx.doi.org/10.1016/S1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- *.Marshall PJ, Bouquet CA, Shipley TF, Young T. Effects of brief imitative experience on EEG desynchronization during action observation. Neuropsychologia. 2009;47:2100–2106. doi: 10.1016/j.neuropsychologia.2009.03.022. http://dx.doi.org/10.1016/j.neuropsychologia.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Meltzoff AN. Neural mirroring systems: Exploring the EEG μ rhythm in human infancy. Developmental Cognitive Neuroscience. 2011;1:110–123. doi: 10.1016/j.dcn.2010.09.001. http://dx.doi.org/10.1016/j.dcn.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Meltzoff AN. Neural mirroring mechanisms and imitation in human infants. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2014;369:20130620. doi: 10.1098/rstb.2013.0620. http://dx.doi.org/10.1098/rstb.2013.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Marshall PJ, Young T, Meltzoff AN. Neural correlates of action observation and execution in 14-month-old infants: An event-related EEG desynchronization study. Developmental Science. 2011;14:474–480. doi: 10.1111/j.1467-7687.2010.00991.x. http://dx.doi.org/10.1111/j.1467-7687.2010.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau J, Andersson F, Barthélémy C, Cottier JP, Destrieux C. Atypical activation of the mirror neuron system during perception of hand motion in autism. Brain Research. 2010;1320:168–175. doi: 10.1016/j.brainres.2010.01.035. http://dx.doi.org/10.1016/j.brainres.2010.01.035. [DOI] [PubMed] [Google Scholar]
- *.Martineau J, Cochin S. Visual perception in children: Human, animal and virtual movement activates different cortical areas. International Journal of Psychophysiology. 2003;51:37–44. doi: 10.1016/s0167-8760(03)00151-x. http://dx.doi.org/10.1016/S0167-8760(03)00151-X. [DOI] [PubMed] [Google Scholar]
- *.Martineau J, Cochin S, Magne R, Barthélémy C. Impaired cortical activation in autistic children: Is the mirror neuron system involved? International Journal of Psychophysiology. 2008;68:35–40. doi: 10.1016/j.ijpsycho.2008.01.002. http://dx.doi.org/10.1016/j.ijpsycho.2008.01.002. [DOI] [PubMed] [Google Scholar]
- *.McCormick LM, Brumm MC, Beadle JN, Paradiso S, Yamada T, Andreasen N. Mirror neuron function, psychosis, and empathy in schizophrenia. Psychiatry Research: Neuroimaging. 2012;201:233–239. doi: 10.1016/j.pscychresns.2012.01.004. http://dx.doi.org/10.1016/j.pscychresns.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.McGarry LM, Russo FA, Schalles MD, Pineda JA. Audio-visual facilitation of the mu rhythm. Experimental Brain Research. 2012;218:527–538. doi: 10.1007/s00221-012-3046-3. http://dx.doi.org/10.1007/s00221-012-3046-3. [DOI] [PubMed] [Google Scholar]
- *.Meyer M, Hunnius S, van Elk M, van Ede F, Bekkering H. Joint action modulates motor system involvement during action observation in 3-year-olds. Experimental Brain Research. 2011;211:581–592. doi: 10.1007/s00221-011-2658-3. http://dx.doi.org/10.1007/s00221-011-2658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael J, Sandberg K, Skewes J, Wolf T, Blicher J, Overgaard M, Frith CD. Continuous theta-burst stimulation demonstrates a causal role of premotor homunculus in action understanding. Psychological Science. 2014;25:963–972. doi: 10.1177/0956797613520608. http://dx.doi.org/10.1177/0956797613520608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Milston SI, Vanman EJ, Cunnington R. Cognitive empathy and motor activity during observed actions. Neuropsychologia. 2013;51:1103–1108. doi: 10.1016/j.neuropsychologia.2013.02.020. http://dx.doi.org/10.1016/j.neuropsychologia.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neuroscience and Biobehavioral Reviews. 2012;36:341–349. doi: 10.1016/j.neubiorev.2011.07.004. http://dx.doi.org/10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Montgomery KJ, Isenberg N, Haxby JV. Communicative hand gestures and object-directed hand movements activated the mirror neuron system. Social Cognitive and Affective Neuroscience. 2007;2:114–122. doi: 10.1093/scan/nsm004. http://dx.doi.org/10.1093/scan/nsm004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Moore A, Gorodnitsky I, Pineda J. EEG mu component responses to viewing emotional faces. Behavioural Brain Research. 2012;226:309–316. doi: 10.1016/j.bbr.2011.07.048. http://dx.doi.org/10.1016/j.bbr.2011.07.048. [DOI] [PubMed] [Google Scholar]
- *.Moore RA, Gale A, Morris PH, Forrester D. Alpha power and coherence primarily reflect neural activity related to stages of motor response during a continuous monitoring task. International Journal of Psychophysiology. 2008;69:79–89. doi: 10.1016/j.ijpsycho.2008.03.003. http://dx.doi.org/10.1016/j.ijpsycho.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Morin O, Grèzes J. What is “mirror” in the premotor cortex? A review. Clinical Neurophysiology. 2008;38:189–195. doi: 10.1016/j.neucli.2008.02.005. http://dx.doi.org/10.1016/j.neucli.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-neuron responses in humans during execution and observation of actions. Current Biology. 2010;20:750–756. doi: 10.1016/j.cub.2010.02.045. http://dx.doi.org/10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen B. Advanced BASIC meta-analysis. Hillsdale, NJ: Erlbaum; 1989. [Google Scholar]
- *.Müller-Putz GR, Zimmermann D, Graimann B, Nestinger K, Korisek G, Pfurtscheller G. Event-related beta EEG-changes during passive and attempted foot movements in paraplegic patients. Brain Research. 2007;1137:84–91. doi: 10.1016/j.brainres.2006.12.052. http://dx.doi.org/10.1016/j.brainres.2006.12.052. [DOI] [PubMed] [Google Scholar]
- *.Muthukumaraswamy SD, Johnson BW. Changes in rolandic mu rhythm during observation of a precision grip. Psychophysiology. 2004a;41:152–156. doi: 10.1046/j.1469-8986.2003.00129.x. http://dx.doi.org/10.1046/j.1469-8986.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- *.Muthukumaraswamy SD, Johnson BW. Primary motor cortex activation during action observation revealed by wavelet analysis of the EEG. Clinical Neurophysiology. 2004b;115:1760–1766. doi: 10.1016/j.clinph.2004.03.004. http://dx.doi.org/10.1016/j.clinph.2004.03.004. [DOI] [PubMed] [Google Scholar]
- *.Muthukumaraswamy SD, Johnson BW, McNair NA. Mu rhythm modulation during observation of an object-directed grasp. Cognitive Brain Research. 2004;19:195–201. doi: 10.1016/j.cogbrainres.2003.12.001. http://dx.doi.org/10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- *.Nyström P. The infant mirror neuron system studied with high density EEG. Social Neuroscience. 2008;3:334–347. doi: 10.1080/17470910701563665. http://dx.doi.org/10.1080/17470910701563665. [DOI] [PubMed] [Google Scholar]
- *.Nyström P, Ljunghammar T, Rosander K, Von Hofsten C. Using mu rhythm desynchronization to measure mirror neuron activity in infants. Developmental Science. 2010;14:327–335. doi: 10.1111/j.1467-7687.2010.00979.x. [DOI] [PubMed] [Google Scholar]
- *.Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cognitive Brain Research. 2005;24:190–198. doi: 10.1016/j.cogbrainres.2005.01.014. http://dx.doi.org/10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- *.Oberman LM, McCleery J, Ramachandran V, Pineda J. EEG evidence for mirror neuron activity during the observation of human and robot actions: Toward an analysis of the human qualities of interactive robots. Neurocomputing: An International Journal. 2007;70:2194–2203. http://dx.doi.org/10.1016/j.neucom.2006.02.024. [Google Scholar]
- *.Oberman LM, Ramachandran VS, Pineda JA. Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: The mirror neuron hypothesis. Neuropsychologia. 2008;46:1558–1565. doi: 10.1016/j.neuropsychologia.2008.01.010. http://dx.doi.org/10.1016/j.neuropsychologia.2008.01.010. [DOI] [PubMed] [Google Scholar]
- *.Orgs G, Dombrowski JH, Heil M, Jansen-Osmann P. Expertise in dance modulates alpha/beta event-related desynchronization during action observation. European Journal of Neuroscience. 2008;27:3380–3384. doi: 10.1111/j.1460-9568.2008.06271.x. http://dx.doi.org/10.1111/j.1460-9568.2008.06271.x. [DOI] [PubMed] [Google Scholar]
- Perkins T, Stokes M, McGillivray J, Bittar R. Mirror neuron dysfunction in autism spectrum disorders. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia. 2010;17:1239–1243. doi: 10.1016/j.jocn.2010.01.026. http://dx.doi.org/10.1016/j.jocn.2010.01.026. [DOI] [PubMed] [Google Scholar]
- *.Perry A, Bentin S. Mirror activity in the human brain while observing hand movements: A comparison between EEG desynchronization in the mu-range and previous fMRI results. Brain Research. 2009;1282:126–132. doi: 10.1016/j.brainres.2009.05.059. http://dx.doi.org/10.1016/j.brainres.2009.05.059. [DOI] [PubMed] [Google Scholar]
- *.Perry A, Bentin S. Does focusing on hand-grasping intentions modulate electroencephalogram μ and α suppressions? Neuroreport: For Rapid Communication of Neuroscience Research. 2010;21:1050–1054. doi: 10.1097/WNR.0b013e32833fcb71. http://dx.doi.org/10.1097/WNR.0b013e32833fcb71. [DOI] [PubMed] [Google Scholar]
- *.Perry A, Stein L, Bentin S. Motor and attentional mechanisms involved in social interaction—Evidence from mu and alpha EEG suppression. NeuroImage. 2011;58:895–904. doi: 10.1016/j.neuroimage.2011.06.060. http://dx.doi.org/10.1016/j.neuroimage.2011.06.060. [DOI] [PubMed] [Google Scholar]
- *.Perry A, Troje NF, Bentin S. Exploring motor system contributions to the perception of social information: Evidence from EEG activity in the mu/alpha frequency range. Social Neuroscience. 2010;5:272–284. doi: 10.1080/17470910903395767. http://dx.doi.org/10.1080/17470910903395767. [DOI] [PubMed] [Google Scholar]
- *.Pfurtscheller G, Neuper C. Simultaneous EEG 10 Hz desynchronization and 40 Hz synchronization during finger movements. Neuroreport. 1992;3:1057–1060. doi: 10.1097/00001756-199212000-00006. http://dx.doi.org/10.1097/00001756-199212000-00006. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger G. Foot and hand area mu rhythms. International Journal of Psychophysiology. 1997;26:121–135. doi: 10.1016/s0167-8760(97)00760-5. http://dx.doi.org/10.1016/S0167-8760(97)00760-5. [DOI] [PubMed] [Google Scholar]
- Pineda JA. The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing. Brain Research Reviews. 2005;50:57–68. doi: 10.1016/j.brainresrev.2005.04.005. http://dx.doi.org/10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Pineda JA, Carrasco K, Datko M, Pillen S, Schalles M. Neurofeedback training produces normalization in behavioural and electrophysiological measures of high-functioning autism. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2014;369:20130183. doi: 10.1098/rstb.2013.0183. http://dx.doi.org/10.1098/rstb.2013.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Pineda JA, Giromini L, Porcelli P, Parolin L, Viglione DJ. Mu suppression and human movement responses to the Rorschach test. NeuroReport: For Rapid Communication of Neuroscience Research. 2011;22:223–226. doi: 10.1097/WNR.0b013e328344f45c. http://dx.doi.org/10.1097/WNR.0b013e328344f45c. [DOI] [PubMed] [Google Scholar]
- Pineda JA, Hecht E. Mirroring and mu rhythm involvement in social cognition: Are there dissociable subcomponents of theory of mind? Biological Psychology. 2009;80:306–314. doi: 10.1016/j.biopsycho.2008.11.003. http://dx.doi.org/10.1016/j.biopsycho.2008.11.003. [DOI] [PubMed] [Google Scholar]
- *.Pineda JA, Oberman LM. What goads cigarette smokers to smoke? Neural adaptation and the mirror neuron system. Brain Research. 2006;1121:128–135. doi: 10.1016/j.brainres.2006.08.128. http://dx.doi.org/10.1016/j.brainres.2006.08.128. [DOI] [PubMed] [Google Scholar]
- *.Porcelli P, Giromini L, Parolin L, Pineda JA, Viglione DJ. Mirroring activity in the brain and movement determinant in the Rorschach test. Journal of Personality Assessment. 2013;95:444–456. doi: 10.1080/00223891.2013.775136. http://dx.doi.org/10.1080/00223891.2013.775136. [DOI] [PubMed] [Google Scholar]
- Prinz W. Perception and action planning. European Journal of Cognitive Psychology. 1997;9:129–154. [Google Scholar]
- Puzzo I, Cooper NR, Cantarella S, Russo R. Measuring the effects of manipulating stimulus presentation time on sensorimotor alpha and low beta reactivity during hand movement observation. NeuroImage. 2011;57:1358–1363. doi: 10.1016/j.neuroimage.2011.05.071. http://dx.doi.org/10.1016/j.neuroimage.2011.05.071. [DOI] [PubMed] [Google Scholar]
- *.Puzzo I, Cooper NR, Vetter P, Russo R. EEG activation differences in the pre-motor cortex and supplementary motor area between normal individuals with high and low traits of autism. Brain Research. 2010;1342:104–110. doi: 10.1016/j.brainres.2010.04.060. [DOI] [PubMed] [Google Scholar]
- *.Raymaekers R, Wiersema JR, Roeyers H. EEG study of the mirror neuron system in children with high functioning autism. Brain Research. 2009;1304:113–121. doi: 10.1016/j.brainres.2009.09.068. http://dx.doi.org/10.1016/j.brainres.2009.09.068. [DOI] [PubMed] [Google Scholar]
- *.Reid VM, Striano T, Iacoboni M. Neural correlates of dyadic interaction during infancy. Developmental Cognitive Neuroscience. 2011;1:124–130. doi: 10.1016/j.dcn.2011.01.001. http://dx.doi.org/10.1016/j.dcn.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G. Mechanisms of selective inhibition in visual spatial attention are indexed by α-band EEG synchronization. European Journal of Neuroscience. 2007;25:603–610. doi: 10.1111/j.1460-9568.2007.05278.x. http://dx.doi.org/10.1111/j.1460-9568.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- Ritter P, Moosmann M, Villringer A. Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Human Brain Mapping. 2009;30:1168–1187. doi: 10.1002/hbm.20585. http://dx.doi.org/10.1002/hbm.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA. Language within our grasp. Trends in Neurosciences. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. http://dx.doi.org/10.1016/S0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. http://dx.doi.org/10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M. The mirror system and its role in social cognition. Current Opinion in Neurobiology. 2008;18:179–184. doi: 10.1016/j.conb.2008.08.001. http://dx.doi.org/10.1016/j.conb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M, Cattaneo L. Mirror neurons and their clinical relevance. Nature Clinical Practice Neurology. 2009;5:24–34. doi: 10.1038/ncpneuro0990. http://dx.doi.org/10.1038/ncpneuro0990. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. http://dx.doi.org/10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews Neuroscience. 2001;2:661–670. doi: 10.1038/35090060. http://dx.doi.org/10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. The functional role of the parietofrontal mirror circuit: Interpretations and misinterpretations. Nature Reviews Neuroscience. 2010;11:264–274. doi: 10.1038/nrn2805. http://dx.doi.org/10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Rochat MJ, Caruana F, Jezzini A, Escola L, Intskirveli I, Grammont F, … Umiltà MA. Responses of mirror neurons in area F5 to hand and tool grasping observation. Experimental Brain Research. 2010;204:605–616. doi: 10.1007/s00221-010-2329-9. http://dx.doi.org/10.1007/s00221-010-2329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Gross J, Thut G. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: Correlation or causation? The Journal of Neuroscience. 2010;30:8692–8697. doi: 10.1523/JNEUROSCI.0160-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.0160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research. Newbury Park, CA: Sage; 1991. http://dx.doi.org/10.4135/9781412984997. [Google Scholar]
- *.Ruysschaert L, Warreyn P, Wiersema JR, Metin B, Roeyers H. Neural mirroring during the observation of live and video actions in infants. Clinical Neurophysiology. 2013;124:1765–1770. doi: 10.1016/j.clinph.2013.04.007. http://dx.doi.org/10.1016/j.clinph.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Ruysschaert L, Warreyn P, Wiersema JR, Oostra A, Roeyers H. Exploring the role of neural mirroring in children with autism spectrum disorder. Autism Research. 2014;7:197–206. doi: 10.1002/aur.1339. http://dx.doi.org/10.1002/aur.1339. [DOI] [PubMed] [Google Scholar]
- Saby JN, Meltzoff AN, Marshall PJ. Infants’ somatotopic neural responses to seeing human actions: I’ve got you under my skin. PLoS ONE. 2013;8(10):e77905. doi: 10.1371/journal.pone.0077905. http://dx.doi.org/10.1371/journal.pone.0077905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R, Hämäläinen M, Kajola M, Hari R. Functional segregation of movement-related rhythmic activity in the human brain. NeuroImage. 1995;2:237–243. doi: 10.1006/nimg.1995.1031. http://dx.doi.org/10.1006/nimg.1995.1031. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience. 1994a;60:537–550. doi: 10.1016/0306-4522(94)90263-1. http://dx.doi.org/10.1016/0306-4522(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R. Characterization of spontaneous MEG rhythms in healthy adults. Electroencephalography and Clinical Neurophysiology. 1994b;91:237–248. doi: 10.1016/0013-4694(94)90187-2. http://dx.doi.org/10.1016/0013-4694(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, … Birbaumer N. A shift of visual spatial attention is selectively associated with human EEG alpha activity. European Journal of Neuroscience. 2005;22:2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. http://dx.doi.org/10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- *.Schuch S, Bayliss AP, Klein C, Tipper SP. Attention modulates motor system activation during action observation: Evidence for inhibitory rebound. Experimental Brain Research. 2010;205:235–249. doi: 10.1007/s00221-010-2358-4. http://dx.doi.org/10.1007/s00221-010-2358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Serrien DJ, Brown P. The integration of cortical and behavioural dynamics during initial learning of a motor task. European Journal of Neuroscience. 2003;17:1098–1104. doi: 10.1046/j.1460-9568.2003.02534.x. http://dx.doi.org/10.1046/j.1460-9568.2003.02534.x. [DOI] [PubMed] [Google Scholar]
- *.Silas J, Levy JP, Nielsen MK, Slade L, Holmes A. Sex and individual differences in induced and evoked EEG measures of action observation. Neuropsychologia. 2010;48:2417–2426. doi: 10.1016/j.neuropsychologia.2010.03.004. http://dx.doi.org/10.1016/j.neuropsychologia.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Snyder AC, Foxe JJ. Anticipatory attentional suppression of visual features indexed by oscillatory alpha-band power increases: A high-density electrical mapping study. The Journal of Neuroscience. 2010;30:4024–4032. doi: 10.1523/JNEUROSCI.5684-09.2010. http://dx.doi.org/10.1523/JNEUROSCI.5684-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Southgate V, Begus K. Motor activation during the prediction of nonexecutable actions in infants. Psychological Science. 2013;24:828–835. doi: 10.1177/0956797612459766. http://dx.doi.org/10.1177/0956797612459766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V, Hamilton AF. Unbroken mirrors: Challenging a theory of autism. Trends in Cognitive Sciences. 2008;12:225–229. doi: 10.1016/j.tics.2008.03.005. http://dx.doi.org/10.1016/j.tics.2008.03.005. [DOI] [PubMed] [Google Scholar]
- *.Southgate V, Johnson MH, El Karoui I, Csibra G. Motor system activation reveals infants’ on-line prediction of others’ goals. Psychological Science. 2010;21:355–359. doi: 10.1177/0956797610362058. http://dx.doi.org/10.1177/0956797610362058. [DOI] [PubMed] [Google Scholar]
- *.Southgate V, Johnson MH, Osborne T, Csibra G. Predictive motor activation during action observation in human infants. Biology Letters. 2009;5:769–772. doi: 10.1098/rsbl.2009.0474. http://dx.doi.org/10.1098/rsbl.2009.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler W, Ott DVM, Springer A, Schubotz RI, Schütz-Bosbach S, Prinz W. Repetitive TMS suggests a role of the human dorsal premotor cortex in action prediction. Frontiers in Human Neuroscience. 2012;6:20. doi: 10.3389/fnhum.2012.00020. http://dx.doi.org/10.3389/fnhum.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Stancák A, Jr, Pfurtscheller G. Murhythm changes in brisk and slow self-paced finger movements. Neuroreport. 1996a;7:1161–1164. doi: 10.1097/00001756-199604260-00013. http://dx.doi.org/10.1097/00001756-199604260-00013. [DOI] [PubMed] [Google Scholar]
- *.Stancák A, Jr, Pfurtscheller G. The effects of handedness and type of movement on the contralateral preponderance of murhythm desynchronisation. Electroencephalography & Clinical Neurophysiology. 1996b;99:174–182. doi: 10.1016/0013-4694(96)95701-6. http://dx.doi.org/10.1016/0013-4694(96)95701-6. [DOI] [PubMed] [Google Scholar]
- *.Stancák A, Jr, Riml A, Pfurtscheller G. The effects of external load on movement-related changes of the sensorimotor EEG rhythms. Electroencephalography and Clinical Neurophysiology. 1997;102:495–504. doi: 10.1016/s0013-4694(96)96623-0. http://dx.doi.org/10.1016/S0013-4694(96)96623-0. [DOI] [PubMed] [Google Scholar]
- Steinhorst A, Funke J. Mirror neuron activity is no proof for action understanding. Frontiers in Human Neuroscience. 2014;8:333. doi: 10.3389/fnhum.2014.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Streltsova A, Berchio C, Gallese V, Umilta’ MA. Time course and specificity of sensorymotor alpha modulation during the observation of hand motor acts and gestures: A high density EEG study. Experimental Brain Research. 2010;205:363–373. doi: 10.1007/s00221-010-2371-7. http://dx.doi.org/10.1007/s00221-010-2371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. British Medical Journal. 2000;320:1574–1577. doi: 10.1136/bmj.320.7249.1574. http://dx.doi.org/10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangwiriyasakul C, Verhagen R, van Putten MJAM, Rutten WLC. Importance of baseline in event-related desynchronization during a combination task of motor imagery and motor observation. Journal of Neural Engineering. 2013;10:026009. doi: 10.1088/1741-2560/10/2/026009. http://dx.doi.org/10.1088/1741-2560/10/2/026009. [DOI] [PubMed] [Google Scholar]
- Théoret H, Pascual-Leone A. Language acquisition: Do as you hear. Current Biology. 2002;12:R736–R737. doi: 10.1016/s0960-9822(02)01251-4. http://dx.doi.org/10.1016/S0960-9822(02)01251-4. [DOI] [PubMed] [Google Scholar]
- Thorpe SG, Cannon EN, Fox NA. Spectral and source structural development of mu and alpha rhythms from infancy through adulthood. Clinical Neurophysiology. 2015 doi: 10.1016/j.clinph.2015.03.004. Advance online publication. http://dx.doi.org/10.1016/j.clinph.2015.03.004. [DOI] [PMC free article] [PubMed]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. The Journal of Neuroscience. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. http://dx.doi.org/10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro C, Deuschl G, Thatcher R, Sato S, Kufta C, Hallett M. Event-related desynchronization and movement-related cortical potentials on the ECoG and EEG. Electroencephalography and Clinical Neurophysiology. 1994;93:380–389. doi: 10.1016/0168-5597(94)90126-0. http://dx.doi.org/10.1016/0168-5597(94)90126-0. [DOI] [PubMed] [Google Scholar]
- *.Ulloa ER, Pineda JA. Recognition of point-light biological motion: Mu rhythms and mirror neuron activity. Behavioural Brain Research. 2007;183:188–194. doi: 10.1016/j.bbr.2007.06.007. http://dx.doi.org/10.1016/j.bbr.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Umiltà MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G. I know what you are doing. a neurophysiological study. Neuron. 2001;31:155–165. doi: 10.1016/s0896-6273(01)00337-3. http://dx.doi.org/10.1016/S0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- *.Upshaw K, Bernier R, Sommerville J. EEG mu rhythm in infants. Department of Psychology, University of Washington; 2014. Unpublished manuscript. [Google Scholar]
- Urgesi C, Candidi M, Ionta S, Aglioti SM. Representation of body identity and body actions in extrastriate body area and ventral premotor cortex. Nature Neuroscience. 2007;10:30–31. doi: 10.1038/nn1815. http://dx.doi.org/10.1038/nn1815. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Moro V, Candidi M, Aglioti SM. Mapping implied body actions in the human motor system. The Journal of Neuroscience. 2006;26:7942–7949. doi: 10.1523/JNEUROSCI.1289-06.2006. http://dx.doi.org/10.1523/JNEUROSCI.1289-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helden J, van Schie HT, Rombouts C. Observational learning of new movement sequences is reflected in frontoparietal coherence. PLoS ONE. 2010;5:e14482. doi: 10.1371/journal.pone.0014482. http://dx.doi.org/10.1371/journal.pone.0014482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.van Elk M, van Schie HT, Hunnius S, Vesper C, Bekkering H. You’ll never crawl alone: Neurophysiological evidence for experience-dependent motor resonance in infancy. NeuroImage. 2008;43:808–814. doi: 10.1016/j.neuroimage.2008.07.057. http://dx.doi.org/10.1016/j.neuroimage.2008.07.057. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH, Juffer F, Poelhuis CWK. Adoption and cognitive development: A meta-analytic comparison of adopted and nonadopted children’s IQ and school performance. Psychological Bulletin. 2005;131:301–316. doi: 10.1037/0033-2909.131.2.301. http://dx.doi.org/10.1037/0033-2909.131.2.301. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: A meta-analysis. NeuroImage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. http://dx.doi.org/10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- *.Virji-Babul N, Rose A, Moiseeva N, Makan N. Neural correlates of action understanding in infants: Influence of motor experience. Brain and Behavior. 2012;2:237–242. doi: 10.1002/brb3.50. http://dx.doi.org/10.1002/brb3.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanti G, Rogers SJ. Autism and the mirror neuron system: Insights from learning and teaching. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2014;369:20130184. doi: 10.1098/rstb.2013.0184. http://dx.doi.org/10.1098/rstb.2013.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Warreyn P, Ruysschaert L, Wiersema JR, Handl A, Pattyn G, Roeyers H. Infants’ mu suppression during the observation of real and mimicked goal-directed actions. Developmental Science. 2013;16:173–185. doi: 10.1111/desc.12014. http://dx.doi.org/10.1111/desc.12014. [DOI] [PubMed] [Google Scholar]
- Williams JG, Higgins JPT, Brayne CEG. Systematic review of prevalence studies of autism spectrum disorders. Archives of Disease in Childhood. 2006;91:8–15. doi: 10.1136/adc.2004.062083. http://dx.doi.org/10.1136/adc.2004.062083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JHG, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neuroscience and Biobehavioral Reviews. 2001;25:287–295. doi: 10.1016/s0149-7634(01)00014-8. http://dx.doi.org/10.1016/S0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Wolf N, Gales M, Shane E, Shane M. The developmental trajectory from amodal perception to empathy and communication: The role of mirror neurons in this process. Psychoanalytic Inquiry. 2001;21:94–112. http://dx.doi.org/10.1080/07351692109348925. [Google Scholar]
- *.Woodruff CC, Martin T, Bilyk N. Differences in self- and other-induced Mu suppression are correlated with empathic abilities. Brain Research. 2011;1405:69–76. doi: 10.1016/j.brainres.2011.05.046. http://dx.doi.org/10.1016/j.brainres.2011.05.046. [DOI] [PubMed] [Google Scholar]
- Woodward AL, Gerson SA. Mirroring and the development of action understanding. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2014;369:20130181. doi: 10.1098/rstb.2013.0181. http://dx.doi.org/10.1098/rstb.2013.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. The Journal of Neuroscience. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wriessnegger SC, Leeb R, Kaiser V, Neuper C, Müller-Putz GR. Watching object related movements modulates mirror-like activity in parietal brain regions. Clinical Neurophysiology. 2013;124:1596–1604. doi: 10.1016/j.clinph.2013.02.019. http://dx.doi.org/10.1016/j.clinph.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Yamagishi N, Goda N, Callan DE, Anderson SJ, Kawato M. Attentional shifts towards an expected visual target alter the level of alpha-band oscillatory activity in the human calcarine cortex. Cognitive Brain Research. 2005;25:799–809. doi: 10.1016/j.cogbrainres.2005.09.006. http://dx.doi.org/10.1016/j.cogbrainres.2005.09.006. [DOI] [PubMed] [Google Scholar]
- *.Yang CY, Decety J, Lee S, Chen C, Cheng Y. Gender differences in the mu rhythm during empathy for pain: An electroencephalographic study. Brain Research. 2009;1251:176–184. doi: 10.1016/j.brainres.2008.11.062. http://dx.doi.org/10.1016/j.brainres.2008.11.062. [DOI] [PubMed] [Google Scholar]
- *.Yuan H, Perdoni C, He B. Relationship between speed and EEG activity during imagined and executed hand movements. Journal of Neural Engineering. 2010;7:026001. doi: 10.1088/1741-2560/7/2/026001. http://dx.doi.org/10.1088/1741-2560/7/2/026001. [DOI] [PMC free article] [PubMed] [Google Scholar]