Abstract
The occurrence of paroxysmal narcotic episodes including psychotic-like symptoms in divers participating to experimental deep diving programs with various gas mixtures has constituted, beyond the classical symptoms of the high-pressure neurological syndrome, the major limitation for deep diving. With the development of new saturation deep diving programs and experiments by the eastern nations, such as India and China, we believed that it is of interest to examine what could be the ultimate depth that could be reached by saturation human divers. Based on previous data and the critical volume model of inert gas narcosis, we propose that the ultimate depth for saturation diving could be around 1,000 m.
Keywords: deep diving, ultimate depth, psychotic-like disorders, inert gas narcosis, high-pressure neurological syndrome
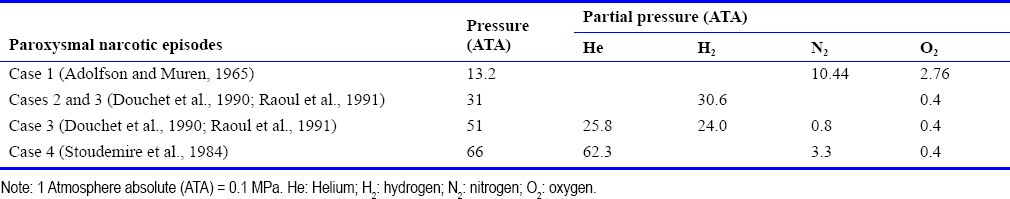
When divers are exposed to depth, i.e. to increased pressure (10 m = 1 atmosphere absolute (ATA); 1 ATA = 0.1 MPa), both the pressure and the partial pressure of each gas in the breathing mixture that dissolve within the divers’ body affect the divers’ organism. The development of saturation diving has removed the constraint of repetitive decompression, leading to a gain of decompression time. As well, the replacement in “air” of nitrogen by helium, which is the inert gas that possesses the lowest narcotic potency, has removed the constraint of nitrogen narcosis (Bennett, 1993). However, at a depth greater than 200 m (21 ATA), breathing helium-oxygen mixtures induces the high-pressure neurological syndrome (HPNS) that is considered to be a function of raised pressure per se and is exacerbated by fast compression rates. This syndrome manifests itself by a general hyperexcitability mainly characterized in man by tremor, myoclonus, electroencephalographic changes, and alterations in cognitive functions. At higher pressure, using faster compression rates than those used in human dives, convulsions occur in animals including primates at around 1,000 m (101 ATA) (Halsey, 1982; Bennett and Rostain, 1993). Strategies used to reduce HPNS mainly include slow compression rates with stages, adaptation with time at depth, and the addition to the basic helium-oxygen breathing mixtures of narcotic gases such as nitrogen and hydrogen. This has allowed human divers to reach depths up to 534.4 m (54.4 ATA) in the open sea as well as pressures up to 71.1 ATA corresponding to simulated depths of up to 701 m in hyperbaric chambers. However, despite such strategies, experimental deep dives using hydrogen-oxygen, helium-hydrogen-nitrogen-oxygen, and helium-nitrogen-oxygen breathing mixtures (Table 1) have had to be stopped because some of the divers experienced psychotic-like disorders (Stoudemire et al., 1984; Douchet et al., 1990; Raoul et al., 1991). Also, interestingly, similar disorders have been reported in pioneer dives with air (Adolfson and Muren, 1965). These symptoms clearly constitute beyond the classical symptoms of HPNS the major limitation to deep diving whatever the gas mixtures used. Among the features shown by the divers were hallucinations, agitation, delirium, and paranoid thoughts. These critical events were demonstrated to be paroxysmal narcotic symptoms that resulted from the sum of the narcotic potency of each gas that composed the diving breathing mixtures (Abraini, 1995a, b). With the development of deep diving successful programs and experiments by eastern nations, such India and China, we believed that is of interest to examine what could be the ultimate depth that could be reached by saturation human divers.
Table 1.
Environmental conditions of absolute pressure and partial pressure of each inert gas during the experimental dives in which paroxysmal narcotic episodes occurred
Diving gases at raised pressure has narcotic effects which relative potencies are highly correlated with lipid solubility (Smith and Paton, 1976; Dodson et al., 1985; Bennett, 1993; Abraini, 1995a, b). Although their structural mechanisms of action-thought to be similar to that of the noble and general anesthetic gases xenon and nitrous oxide (Colloc’h et al., 2007; Abraini et al., 2014; Sauguet et al., 2016)-are still under discussion (Dodson et al., 1985; Abraini et al., 1998; David et al., 2001; Abraini et al., 2003), the critical volume model (or some extension of it) (Miller et al., 1973; Halsey et al., 1978; Abraini, 1995a, b) has allowed predictive studies in both humans (Abraini, 1995a, b, 1997) and experimental animals (Dodson et al., 1985). This model states that, for a similar pharmacological effect, narcosis occurs when the volume of a hydrophobic cell region is caused to expand beyond a certain critical volume by the absorption of an inert substance. The fractional expansion E that occurs when a gas at a partial pressure Pi dissolves in the hydrophobic site is given by:
E = Vi . Xi . Pi/Vm
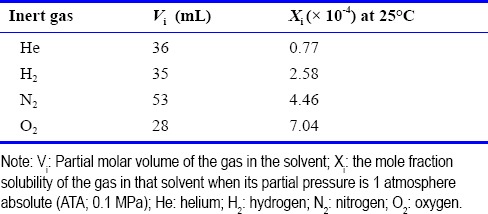
Where Vi is the partial molar volume of the gas in the solvent (or some model of it such as olive oil or benzene) of molar volume Vm and Xi is the mole fraction solubility of the gas in that solvent when its partial pressure is 1 ATA. For a mixture of gases, the net effect is given by the sum of the individual terms if each gas. Table 2 indicates the values for Vi and Xi for the range of gases that have been used for deep diving; the value of Vm is estimated to be 640 mL (Dodson et al., 1985).
Table 2.
Partial molar volume and mole fraction solubility of the gases used during the experimental dives in which paroxysmal narcotic episodes occurred using benzene as a model of the gases’ hydrophobic site of action
Given that the psychotic-like episodes occurring at raised pressure have been shown to result from the sum of the narcotic potency of each gas used to compose the breathing mixture, the advantage of adding the narcotic gases nitrogen or hydrogen to the basic helium-oxygen breathing mixture does not appear readily apparent inasmuch the physical strategies used to reduce HPNS, such as slow compression rates with stages and adaptation with time at depth, have allowed divers breathing helium-oxygen mixtures to reach depths up to 610 (62 ATA) as early as the 1970s (Bennett and Rostain, 1993). As shown in Figure 1, calculations with the critical volume model have allowed establishing that the mean expansion of the diving gas hydrophobic site of action necessary for the expression of psychotic-like narcotic episodes is about 0.0453 ± 0.0032% (Abraini, 1995a, b). As also illustrated in Figure 1, taking into account this expansion value, the onset depth for the occurrence of psychotic-like disorders with the basic helium-oxygen mixture may be estimated between 930 m and 1,080 m (94–109 ATA) (mean depth: 1,005 m, i.e., 101.5 ATA).
Figure 1.
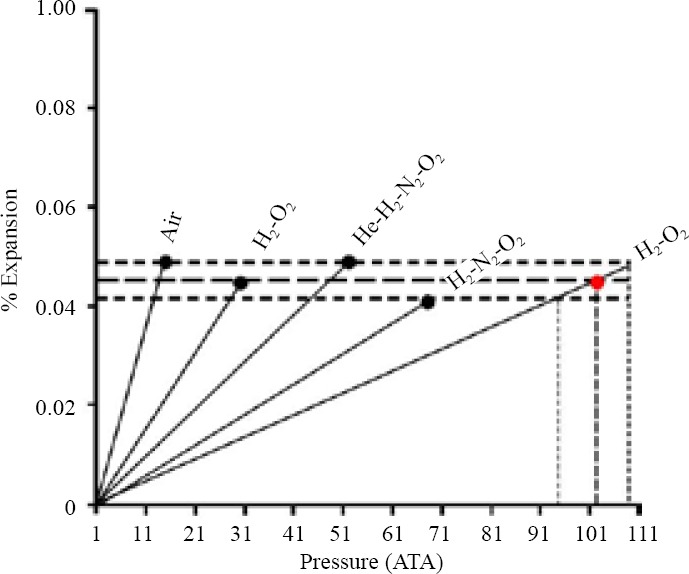
Full squares represent the net theoretical expansion of the gases’ hydrophobic site of action, using benzene as a model solvent, at the time paroxysmal narcotic episodes occurred using air, and hydrogen (H2)-oxygen (O2), helium (He)-H2-nitrogen (N2)-O2, or H2-N2-O2 breathing mixtures.
Note: Data show that whatever the gas mixture used the fractional expansion was remarkably similar (mean value: 0.0453 ± 0.0032%) at the time the dives have had to be stopped because of the occurrence of paroxysmal narcotic episodes including psychotic-like symptoms. Taking into account this expansion value, the onset depth for the occurrence of paroxysmal narcotic episodes in helium-oxygen mixture may be thus estimated between 930 m and 1,080 m (94–109 ATA). 1 ATA = 0.1 MPa. ATA: Atmosphere absolute.
Support for an onset depth between 930 m and 1,080 m for the occurrence of psychotic-like disorders with the basic helium-oxygen mixture is the fact that no electroencephalographic epileptic patterns have been recorded in human divers at depths up to 701 m (71.1 ATA) and that convulsions in primates, while using much faster compression rates than those used in human divers, only occurred at around 1,000 m (101 ATA) (Bennett and Rostain, 1993). From this onset depth, if one considers (1) approximately 50 % of the divers participating to the dives in which psychotic-like narcotic episodes occurred were concerned by such symptoms, (2) it is only necessary to increase the minimal anesthetic concentration of common inhalational anesthetics (that is the concentration that produces anesthesia in 50% of subjects) by 10–15% to narcotize the vast majority of subjects (de Jong and Eger, 1975), it can be estimated that no human dives would be possible beyond 1,030–1,200 m (104–121 ATA) even in divers showing a low sensitivity to helium-oxygen narcosis.
In conclusion, we suggest that improvement of the physical strategies used to reduce HPNS, such as slow compression rates with stages and adaptation with time at depth, may be the key for successfully going deeper, beyond the current world record human dives of 534 m (54.4 ATA) in the open sea and of 701 m (71.1 ATA) in hyperbaric chambers.
Footnotes
Conflicts of interest
The authors declared no competing interest.
REFERENCES
- Abraini JH. Evidence for inert gas narcosis mechanisms in the occurrence of psychotic-like episodes at pressure environment. Neuroreport. 1995a;6:2435–2439. doi: 10.1097/00001756-199511270-00036. [DOI] [PubMed] [Google Scholar]
- Abraini JH. Some considerations regarding the narcotic potency of helium and oxygen in humans. In: Macdonald AG, Marquis RE, Rostain JC, editors. Basic and Applied High Pressure Biology IV. Marseille, France: Medsubhyp; 1995b. pp. 77–82. [Google Scholar]
- Abraini JH. Inert gas and raised pressure: evidence that motor decrements are due to pressure per se and cognitive decrements due to narcotic action. Pflügers Arch. 1997;433:788–791. doi: 10.1007/s004240050346. [DOI] [PubMed] [Google Scholar]
- Abraini JH, Rostain JC, Kriem B. Sigmoidal compression rate-dependence of inert gas narcotic potency in rats: implication for lipid vs. protein theories of inert gas action in the central nervous system. Brain Res. 1998;808:300–304. doi: 10.1016/s0006-8993(98)00760-4. [DOI] [PubMed] [Google Scholar]
- Abraini JH, Kriem B, Balon N, Rostain JC, Risso JJ. Gamma-aminobutyric acid neuropharmacological investigations on narcosis produced by nitrogen, argon, or nitrous oxide. Anesth Analg. 2003;96:746–749. doi: 10.1213/01.ANE.0000050282.14291.38. table of contents. [DOI] [PubMed] [Google Scholar]
- Abraini JH, Marassio G, David HN, Vallone B, Prange T, Colloc’h N. Crystallographic studies with xenon and nitrous oxide provide evidence for protein-dependent processes in the mechanisms of general anesthesia. Anesthesiology. 2014;121:1018–1027. doi: 10.1097/ALN.0000000000000435. [DOI] [PubMed] [Google Scholar]
- Adolfson J, Muren A. Air breathing at 13 atmospheres. Psychological and physiological observations. Forsvarsmedicin. 1965;1:31–37. [PubMed] [Google Scholar]
- Bennett PB. Inert gas narcosis. In: Bennett PB, Elliott DH, editors. The Physiology and Medicine of Diving. London, UK: Saunders; 1993. pp. 170–193. [Google Scholar]
- Bennett PB, Rostain JC. The high-pressure nervous syndrome. In: Bennett PB, Elliott DH, editors. The Physiology and Medicine of Diving. London, UK: Saunders; 1993. pp. 194–237. [Google Scholar]
- Colloc’h N, Sopkova-de Oliveira Santos J, Retailleau P, Vivares D, Bonnete F, Langlois d’Estainto B, Gallois B, Brisson A, Risso JJ, Lemaire M, Prange T, Abraini JH. Protein crystallography under xenon and nitrous oxide pressure: comparison with in vivo pharmacology studies and implications for the mechanism of inhaled anesthetic action. Biophys J. 2007;92:217–224. doi: 10.1529/biophysj.106.093807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David HN, Balon N, Rostain JC, Abraini JH. Nitrogen at raised pressure interacts with the GABA(A) receptor to produce its narcotic pharmacological effect in the rat. Anesthesiology. 2001;95:921–927. doi: 10.1097/00000542-200110000-00021. [DOI] [PubMed] [Google Scholar]
- de Jong RH, Eger EI., 2nd MAC expanded: AD50 and AD95 values of common inhalation anesthetics in man. Anesthesiology. 1975;42:384–389. [PubMed] [Google Scholar]
- Dodson BA, Furmaniuk ZW, Jr, Miller KW. The physiological effects of hydrostatic pressure are not equivalent to those of helium pressure on Rana pipiens. J Physiol. 1985;362:233–244. doi: 10.1113/jphysiol.1985.sp015673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douchet JC, Raoul Y, Trividic A, Gillard J, Cosson P. Psycho-activité de l’hydrogène en pression. DRET report n° 89-1305. Plongée humaine en mélange hydrogéne-oxygéne dite Hydra IX. CERB/IMNSSA, Toulon Naval, France. 1990 [Google Scholar]
- Halsey MJ. Effects of high pressure on the central nervous system. Physiol Rev. 1982;62:1341–1377. doi: 10.1152/physrev.1982.62.4.1341. [DOI] [PubMed] [Google Scholar]
- Halsey MJ, Wardley-Smith B, Green CJ. Pressure reversal of general anaesthesia--a multi-site expansion hypothesis. Br J Anesth. 1978;50:1091–1097. doi: 10.1093/bja/50.11.1091. [DOI] [PubMed] [Google Scholar]
- Miller KW, Paton WD, Smith RA, Smith EB. The pressure reversal of general anesthesia and the critical volume hypothesis. Mol Pharmacol. 1973;9:131–143. [PubMed] [Google Scholar]
- Raoul Y, Douchet JC, Trividic A, Gillard J, Cosson P. Psycho-action of pressurized hydrogen. Apropos of 3 cases. Ann Med Psychol (Paris) 1991;149:309–322. [PubMed] [Google Scholar]
- Sauguet L, Fourati Z, Prange T, Delarue M, Colloc’h N. Structural basis for xenon inhibition in a cationic pentameric ligand-gated ion channel. PLoS One. 2016;11:e0149795. doi: 10.1371/journal.pone.0149795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoudemire A, Miller J, Schmitt F, Logue P, Shelton D, Latson G, Bennett P. Development of an organic affective syndrome during a hyperbaric diving experiment. Am J Psychiatry. 1984;141:1251–1254. doi: 10.1176/ajp.141.10.1251. [DOI] [PubMed] [Google Scholar]


