Abstract
Traumatic brain injury (TBI) is a serious public health problem in the United States. Survivors of TBI are often left with significant cognitive, behavioral, and communicative disabilities. So far there is no effective treatment/intervention in the daily clinical practice for TBI patients. The protective effects of hyperbaric oxygen therapy (HBOT) have been proved in stroke; however, its efficiency in TBI remains controversial. In this review, we will summarize the results of HBOT in experimental and clinical TBI, elaborate the mechanisms, and bring out our current understanding and opinions for future studies.
Keywords: traumatic brain injury, hyperbaric oxygen therapy, tissue oxygenation, inflammation, apoptosis
INTRODUCTION
Traumatic brain injury (TBI) is an insult or trauma to the brain caused by external mechanical forces. With an estimated 10 million people affected annually by TBI in United States, the burden of mortality and morbidity that this condition imposes on society, makes TBI a pressing public health and medical problem (Hyder et al., 2007). The damage caused by TBI can be focal (confined to one area of the brain) or diffuse (involving more than one area of the brain) (Zhang et al., 2014b). Symptoms of a TBI vary from mild and moderate to severe, depending on the extent of the damage to the brain. Survivors of TBI are often left with significant cognitive, behavioral, and communicative disabilities (Sun et al., 2015; Xiao et al., 2015), and there is no effective treatment/intervention in the daily clinical practice for TBI patients. There are three distinct yet over-lapping pathophysiological states of TBI: acute, subacute and chronic phases. The acute phase usually occurs within 24 hours of injury, the subacute phase takes place in days following TBI, and the chronic phase arises days to weeks after TBI (Algattas and Huang, 2014). The pathophysiology after TBI has a primary and secondary component (Kolias et al., 2013). At the time of impact there is a variable degree of irreversible damage to the brain tissue (primary injury). Following this, a chain of events occurs in which there is ongoing injury to the brain through edema, hypoxia and ischemia secondary to raised intracranial pressure, release of excitotoxic neurotransmitters and impaired calcium homeostasis (secondary injury) (Algattas and Huang, 2014). There is no option to interfere with the primary injury other than prevention of the trauma itself; however, investigations on the secondary injury in TBI are helpful for the identification of therapeutic targets for TBI.
Hyperbaric oxygen therapy (HBOT) is defined as the inhalation of 100% oxygen under the pressure greater than 1 atmosphere absolute (ATA) (1 ATA = 101.3 kPa). HBOT is a current interest in the field of neurological diseases and has been proved to inhibit apoptosis, suppress inflammation, protect the integrity of blood-brain barrier, and promote angiogenesis and neurogenesis (Braswell and Crowe, 2012; Sanchez, 2013). In TBI, the neuroprotection of HBOT has been established in experimental animal models; but remains controversial in clinic. In this review, we will summarize the results of HBOT in experimental and clinical TBI, elaborate the mechanisms, and bring out our current understanding and opinions for future studies.
EXPERIMENTAL STUDIES OF HBOT IN TBI
The application of hyperbaric oxygen (HBO) in treatment of TBI started in 1960s. The first study reporting the neuroprotection of HBO in experimental brain injury in rats was published in 1966 (Coe and Hayes, 1966). At the same year, Dunn and Lawson demonstrated that HBO significantly improved outcomes and reduced mortality in a dog freeze-lesion model of brain injury that simulated a brain contusion (Dunn et al., 1966). In the following years, several experimental studies focusing on the effects of HBO on brain edema, intracranial pressure (ICP) and cerebral blood flow (CBF) appeared (Table 1). Hollin et al. (1968) reported that in dogs using HBOT at 3 ATA for 45 minutes resulted in less brain edema and a significant decrease (> 50%) in mortality compared to non-treated injured animals. In psyllium seed induced brain edema model in dogs, HBOT at 2 ATA for 4 hours reduced cerebrospinal fluid (CSF) pressure and protected brain against ischemia (Sukoff et al., 1968). HBOT at 2 ATA for 4 hours decreased ICP by 30% and CBF by 19% (Miller et al., 1970), and failed to affect the CSF lactate in a dog model of brain injury (Miller and Ledingham, 1971).
Table 1.
Experimental studies of HBOT in TBI
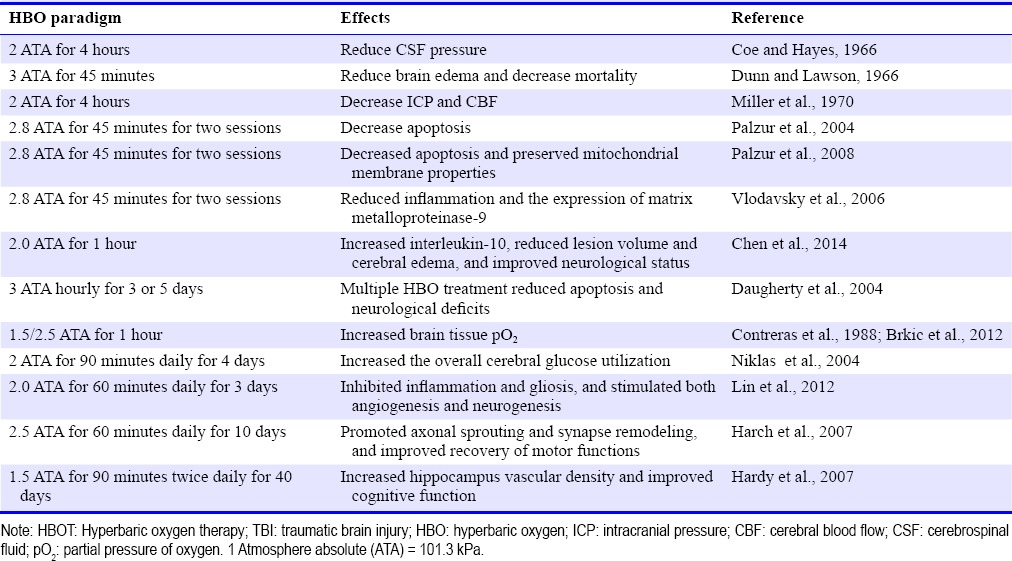
The neuroprotective effects of HBO have been also demonstrated during the acute phase, within 24 hours after TBI. In the rat model of dynamic cortical deformation, HBOT (2.8 ATA for two consecutive sessions of 45 minutes each) starting at 3 hours after injury significantly decreased apoptosis and reduced the severity and extent of secondary brain damage (Palzur et al., 2004). The neuroprotective effects of HBOT were mediated by inhibition of the mitochondrial permeability transition pore (Palzur et al., 2008). In the same model, HBOT also reduced neuroinflammation and the expression of matrix metalloproteinase-9 (Vlodavsky et al., 2006). In the controlled cortical impact model of TBI on mice, HBOT (2.0 ATA for 1 hour) administrated 3hours post-injury increased the level of interleukin-10, resulting in reduced lesion volume, attenuated cerebral edema and improved neurological status (Chen et al., 2014). The therapeutic time window of HBO was further investigated in animal models of TBI. In the early stage of TBI, 3-day HBOT (2.0 ATA for 60 minutes daily, started 3 hours after surgery) inhibited inflammation and gliosis and stimulated both angiogenesis and neurogenesis (Lin et al., 2012). Yang et al. (2014) showed that HBO treatment decreased apoptosis and improved cognitive ability when given 6 hours after TBI in rats. The HBO failed, however, when given 60 days after TBI. Wang et al. (2010) demonstrated that multiple sessions of HBO (3 ATA hourly for 3 or 5 days) are able to extend the therapeutic time window of HBO. Multiple sessions, compared to a single session administrated up to 48 hours post-TBI significantly reduced overall neurological deficit scores and neuronal apoptosis (Wang et al., 2010). It has been also demonstrated that HBOT enhanced recovery of aerobic metabolic function (Daugherty et al., 2004), increased overall cerebral glucose metabolism (Contreras et al., 1988) and cerebral partial pressure of oxygen (pO2) (Niklas et al., 2004). These results of experimental short-term studies during the acute phase of TBI demonstrated that the beneficial effects of HBO, when the treatment started in early stage after TBI.
At the chronic stage of TBI, HBOT has been showed to improve behavioral and neurobiological outcomes. Repetitive long-term HBOTs (2.5 ATA for 60 minutes daily for 10 days) improved the recovery of motor functions in rats after suction ablation of the right sensorimotor cortex by intensify neuroplastic responses through promoting axonal sprouting and synapse remodeling (Brkic et al., 2012). Moreover, in a rat model of cortical trauma using the focal cortical weight-drop impact method, a 40-day series of HBOTs (1.5 ATA for 90 minutes twice daily) started at 50 days after the initial brain injury, caused an increase in contused hippocampus vascular density and an improvement in cognitive function (Harch et al., 2007). In clinical trials, HBOT was reported to improve sensorimotor functions as well as neuropsychological disorders in chronic TBI patients (Golden et al., 2006; Hardy et al., 2007). These findings affirmed the role of angiogenesis and neurogenesis in function improvement and provided the perspective for implementation of HBO in clinical strategies for treating TBI even at late chronic stages.
POTENTIAL MECHANISMS OF HBOT
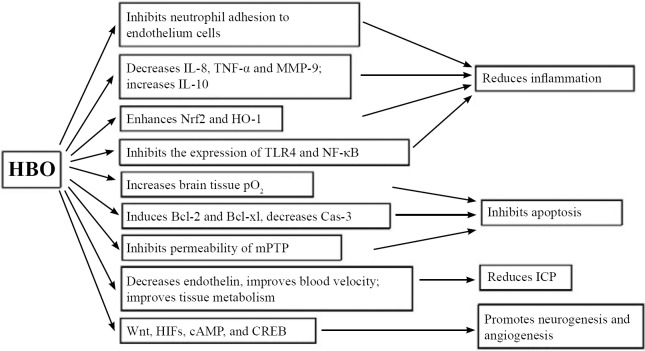
Researches on HBOT in experimental TBI studies have clarified diverse mechanisms leading to neuroprotection. Many of the pathways work parallel, or together, to induce neuroprotection in the brain. These mechanisms include: 1) increasing tissue oxygenation; 2) reducing inflammation; 3) decreasing apoptosis; 4) reducing ICP; 5) promoting neurogenesis and angiogenesis. For the purpose of this review, a brief summary of the recent discoveries in the mechanism of HBOT will be discussed. Table 1 lists most recent exciting discoveries in animal models, and Figure 1 summarizes the potential mechanisms involved in HBOT.
Figure 1.
Potential mechanisms of HBO therapy.
Note: Many of the pathways work parallel, or together, to induce neuroprotection in the brain. These mechanisms include: increasing tissue oxygenation, reducing inflammation, inhibiting apoptosis, reducing ICP, and promoting neurogenesis and angiogenesis. HBO: Hyperbaric oxygen; ICP: intracranial pressure; CBF: cerebral blood flow; CSF: cerebrospinal fluid; pO2: partial pressure of oxygen; IL-8: interleukin-8; IL-10: interleukin-10; TNF-α: tumor necrosis factor-α; MMP-9: matrix metalloproteinase-9; mPTP: mitochondrial permeability transition pore; Cas-3: caspase-3; HIFs: hypoxia-inducible factors; CREB: cAMP response element-binding; Nrf2: nuclear factor (erythroid-derived 2)-like 2; HO-1: heme oxygenase-1; TLR4: Toll-like receptor 4; NF-κB: nuclear factor-kappaB.
HBOT increases tissue oxygenation
Henry's law states that the amount of gas dissolved in a liquid or tissue is proportional to the partial pressure of that gas in contact with the liquid or tissue. This is the basis for increased tissue oxygen tensions with HBO treatment. In physiological condition, most oxygen carried in the blood is bound to hemoglobin, which is 97% saturated at atmospheric pressure. The other oxygen is carried in solution, and this portion is increased with the pressure due to Henry's Law. When breathing normobaric air, arterial oxygen tension is approximately 100 mmHg, and tissue oxygen tension approximately 55 mmHg. However, 100% oxygen at 3 ATA can increase arterial oxygen tensions to 2,000 mmHg, and tissue oxygen tensions to around 500 mmHg (Ratzenhofer-Komenda et al., 2006). The marked increased oxygen tension gradient from the blood to metabolizing cells is a key mechanism by which hyperoxygenation of arterial blood can improve effective cellular oxygenation even at low rates of tissue blood flow (Thom, 2011). And as the oxygen is in solution, it can reach physically obstructed areas where red blood cells cannot pass. HBO (1.5 ATA for 60 minutes) significantly increases brain tissue oxygen pressure (pO2) in both injured and sham-injured rats (Daugherty et al., 2004; Niklas et al., 2004). Results of several studies suggest that increasing brain tissue oxygenation contributed to reduced mortality and improved outcome of TBI patients (Rockswold et al., 2010, 2013).
HBOT suppresses inflammation
The acute inflammatory response plays an important role in secondary brain damage after TBI, which is characterized by cytokine release, neutrophil activation and microvascular adherence. Reducing inflammation is essential for the treatment of TBI. HBO has been shown to suppress inflammation in many studies (Vlodavsky et al., 2006; Lin et al., 2012; Zhang et al., 2014a; Meng et al., 2016a, b). When healthy humans are exposed to HBO at 2.8 to 3.0 ATA for at least 45 minutes, the ability of circulating neutrophils to adhere to target tissues is temporarily inhibited (Kalns et al., 2002). In a blast-induced traumatic brain injury model in rabbits, HBOT (2 ATA for 60 minutes) administrated at 12 hours after injury reduced the RNA and protein levels of caspase-3, interleukin-8 and tumor necrosis factor-α (Zhang et al., 2014a). In the early stage of TBI in rats, HBOT improved outcomes and reduced inflammation by increasing anti-inflammatory cytokine interleukin-10 (Lin et al., 2012; Chen et al., 2014), attenuating microgliosis and decreasing the level of tumor necrosis factor-α (Lim et al., 2013), and decreasing the expression of matrix metalloproteinase-9 (Vlodavsky et al., 2006). Recently, Meng et al. (2016a) showed HBOT significantly increased the expression of nuclear factor (erythroid-derived 2)-like 2 and heme oxygenase-1, and inhibited the expression of Toll-like receptor 4 and nuclear factor-kappaB in a rat TBI model (Meng et al., 2016b). The inhibitory effect of HBOT on inflammation closely associated with the decreased brain edema, blood-brain barrier leakage, cell apoptosis and improved neurological disorders after TBI.
HBOT decreases apoptosis
Apoptosis occurred in the brain tissues from the first few hours to weeks after TBI (Rink et al., 1995). The growth and progression of TBI lesions depend significantly on the developments in traumatic penumbra area and perilesional region, where neuronal apoptosis occurs. Inhibition of apoptosis becomes a therapeutic strategy to preserve brain tissues and promote functional recovery. In previous studies, HBOT reduced brain infarction and improved neurological deficits by preventing neuron apoptosis in ischemic stroke (Zhou et al., 2000; Yin et al., 2003; Li et al., 2005; Lou et al., 2006; Peng et al., 2009) and hypoxia-ischemia (Calvert et al., 2002, 2003; Liu et al., 2013) animal models. The neuroprotective, anti-apoptotic effects of HBOT in the development of secondary brain damage after TBI have been extensively investigated. Palzur et al. (2004, 2008) proved that HBOT reduced apoptosis in dynamic cortical deformation rats. HBOT suppressed the activation of the mitochondrial mediated apoptotic pathway by inducing the expression of Bcl-2 and preserving mitochondrial integrity (Palzur et al., 2008). The same mechanism was observed that HBOT increased expression of anti-apoptotic proteins (Bcl-2 and Bcl-xl) and decreased apoptosis in rat models of TBI (Vlodavsky et al., 2005; Liu et al., 2006; Xu et al., 2012). These results suggest that the neuroprotective effects of HBO are at least partially mediated by the reduction of apoptosis.
HBOT reduces ICP
Critical elevation of ICP represents the leading cause of morbidity and mortality in patients suffering from severe TBI (Horn et al., 1999). One of the important mechanisms of HBOT for severe brain injury is to lower ICP. Brown et al. (1998) first reported that HBO at 2 ATA for 60 minutes lowered ICP during the first 15 minutes of therapy in head-injured patients, however, rebound elevation were seen during or after therapy (Brown et al., 1988). A definite positive effect of HBOT on ICP was achieved by Rogatsky et al. (2005). HBOT (2.5 ATA for 60 minutes) applied within 24 hours ameliorated the outcome and reduced ICP by decreasing endothelin, improving the blood velocity of middle cerebral artery and decreasing cerebral vascular resistance in severe TBI patients (Ren et al., 2001b). Mechanisms of HBOT reducing ICP can also be related to the subsequent sustained improvement in tissue metabolism of the traumatized brain, as shown by Rockswold et al. (2001). The same conclusion was drawn when applied HBO to rats after severe fluid percussion brain injury. HBOT significantly diminished ICP elevation rate and decreased mortality when HBO was administrated within 2 hours after severe trauma (Rogatsky et al., 2005).
HBOT promotes neurogenesis and angiogenesis
Many studies have reported that multiple HBOT could improve neurological deficits and cognitive impairments at the acute stage (Lin et al., 2012) and at late chronic stages, months to years after TBI (Brown et al., 1988; Contreras et al., 1988; Horn et al., 1999; Ren et al., 2001b; Daugherty et al., 2004; Harch et al., 2007, 2009, 2012; Kernie and Parent, 2010; Brkic et al., 2012; Xu et al., 2012; Boussi-Gross et al., 2013). The therapeutic effects of long-term HBOT may be associated with multifaceted repair, including activation of angiogenesis and triggering of neuroplasticity, and induce proliferation and differentiation of neuronal stem cells. When HBO (2 ATA for 60 minutes twice a day for 3 consecutive days) was given within 3 hours after injury in fluid percussion model of TBI in rats, there was a significant increase in newborn endothelia cells, neurons and glial cells at 4 days after TBI (Lin et al., 2012). Ten exposures of HBO (2.5 ATA for 60 minutes for 10 days) can intensify neuroplastic responses by promoting axonal sprouting and synapse remodeling, which contributes to the recovery of locomotor performances in TBI rats (Brkic et al., 2012); 40-day series of 80 HBOTs (1.5 ATA for 90 minutes each time) caused an increase in contused hippocampus vascular density and an associated improvement in cognitive function (Harch et al., 2007). Activation of several signaling pathways and transcription factors have been suggested to play an important role in HBOT-induced neurogenesis, including Wnt, hypoxia-inducible factors and cAMP response element-binding (Mu et al., 2011).
CLINICAL STUDIES OF HBOT IN TBI PATIENTS
With the sound theoretical underpinning and demonstrated efficacy in experimental studies, intense clinical studies are conducted with the aim of evaluating the efficacy and safety of HBOT in relation to brain injuries and neurological disorders. HBOT in a clinical setting is usually implemented in the form of repetitive sessions over extensive time periods in order to improve neurological outcomes following TBI. Typical treatments involve pressurization to between 1.5 and 3.0 ATA for periods between 60 and 120 minutes, once or more daily. Treatment may range from less than 1 week to several months’ duration, the average being 2 to 4 weeks, depending on the response of the individual patient and the severity of the original problem. The clinical efficiency of HBOT in TBI remains controversial. Since the 1960s, many reports have demonstrated an HBOT-associated reduction in mortalities and/or improvement of neurological functions after TBI (Table 2). However, most are based on case studies or retrospective analyses. Standardized clinical studies reporting HBOT-associated protective effects on TBI mediated brain damage are scarce, and an explicit benefit of HBOT for TBI patients has yet to be established.
Table 2.
Clinical studies of HBOT in TBI patients
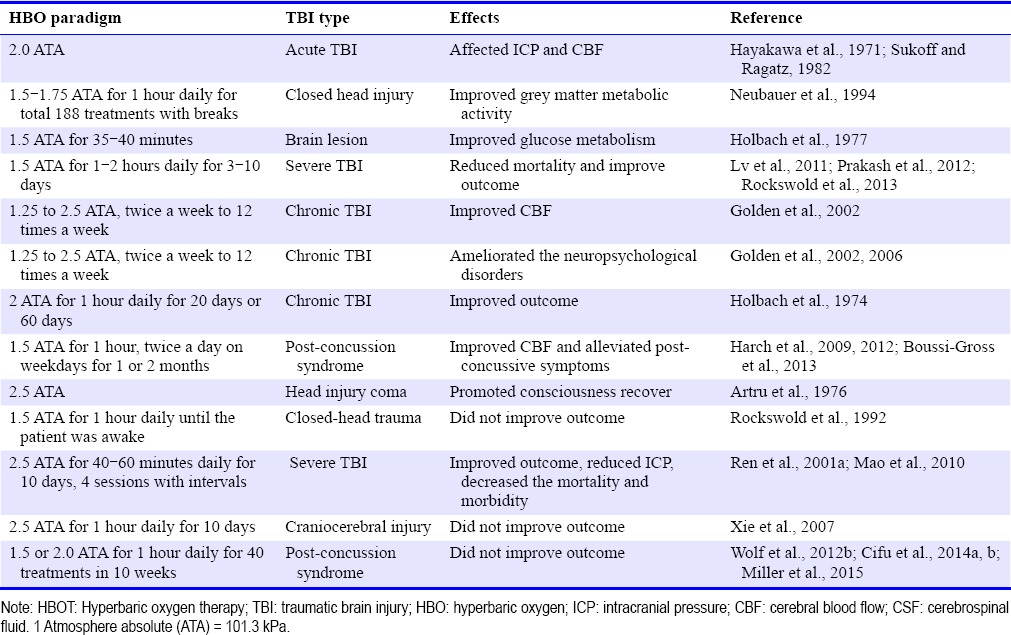
The first clinical observation that presented a therapeutic effect of HBOT in TBI patients was carried out by Fasano et al. (1964), in which HBO improved the outcome following brain trauma. HBOT has been shown to change ICP (Hayakawa et al., 1971) and reduce CSF pressure in patients with acute cerebral damage (Hayakawa et al., 1971; Sukoff and Ragatz, 1982), improved grey matter metabolic activity on single-photon emission computerized tomography scan in closed head injury (Neubauer et al., 1994), and improved glucose metabolism after brain injury (Holbach et al., 1977). In severe TBI, HBOT has decreased mortality and improved functional outcome (Lv et al., 2011; Prakash et al., 2012; Rockswold et al., 2013). In chronic brain injury, HBOT improved CBF (Golden et al., 2002), ameliorated the neuropsychological disorders (Golden et al., 2002, 2006), and enhanced neuropsychological and electrophysiological improvements (Holbach et al., 1974). HBOT has also been reported to show positive effects by improving the quality of life in patients with post concussion syndrome or mild TBI at late chronic stage (Harch et al., 2009, 2012; Boussi-Gross et al., 2013). These cases and studies showed the successful use of intensive HBO as a therapeutic modality in various TBI patients.
However, it should also be noted that there were conflicting results of HBOT in TBI patients. Bennett et al. (2012) reviewed the trials with “HBOT and acute TBI” that published between 1974 and 2010, and analyzed 7 studies involving 571 people. In the seven papers in this review (Holbach et al., 1974; Artru et al., 1976; Rockswold et al., 1992, 2010; Ren et al., 2001a; Xie et al., 2007; Mao et al., 2010), the authors performed Meta-analysis with proportion of participants with an unfavorable functional outcome, mortality, intracranial pressure and improvements in Glasgow Outcome Score, pulmonary effects of HBOT and neurological oxygen toxicity with HBOT. The authors concluded that HBOT showed no evidence in improving life quality after TBI, although the evidence did suggest an improvement in survival (Bennett et al., 2012). In other four studies on HBOT effect on mild TBI or post-concussion syndrome, there was no evidence that supported for routine application of HBOT to mild TBI patients (Wolf et al., 2012b; Cifu et al., 2014a, b; Miller et al., 2015). In these four prospective, randomized studies from Wolf et al. (2012b), Cifu et al. (2014a, b), and Miller et al. (2015), the subjects were U.S. military service members who received 30 to 40 sessions of either a sham or HBO in the treatment of post-concussion syndrome. The results of these studies failed to prove the therapeutic effects of HBO in post concussion syndrome after mild TBI and the authors didn’t recommend HBO for the treatment of post concussion syndrome.
ISSUES AFFECT THE EFFICIENCY OF HBOT IN TBI
Currently, the results of HBOT in clinical TBI trials are controversial and the efficiency of HBOT in TBI has not been well established. Here we will discuss the issues that affect the efficiency of HBOT in TBI patients.
First, the optimal time window for HBO administration is one of the crucial facts that determine its efficacy in TBI. The neuroprotective effects of HBO have all been achieved when intervention was administered during the acute phase, within hours after TBI (Palzur et al., 2004, 2008; Vlodavsky et al., 2006; Lin et al., 2012; Rockswold et al., 2013). The prolonged therapeutic time window of HBO was investigated in animal models of TBI. HBO administrated within 6 hours after TBI decreased apoptosis and improved cognitive ability (Yang et al., 2014), and multiple sessions of HBO (3 ATA hourly for 3 or 5 days) could extend the time window up to 48 hours post-TBI (Wang et al., 2010). The data of these pre-clinic and clinic studies indicated that HBO is beneficial when it is applied in the early stage. Application of HBOT within a therapeutic time window established in preclinical study is an important requirement the efficiency.
Second, objective and precise assessment methods are another challenge in the evaluation of the efficacy of HBOT in TBI patients. Cognitive, emotional, behavioral, and physical impairments are common sequelae of TBI. In most clinical studies, the outcome was evaluated by neuropsychological tests, such as the Rivermead Post-Concussion Symptoms Questionnaire (RPQ), Neurobehavioral Symptom Inventory and Automated Neuropsychological Assessment Metrics. All the assessments are well established, however, they are all subjective performance evaluations. It is well known that RPQ displays several flaws in its implementation and in its ability to accurately reflect test-taker experience (Potter et al., 2006). Interpretation and accuracy of the RPQ can vary widely due to self-administration and the various confounding variables involved, because it is sensitive to subjective patient memory, social desirability, stress, and other covariates such as personality factors and willingness to reveal problems, as are the two other methods. Relying totally on the self-administration assessments is a weakness of these studies. More objective assessment methods, such as brain single photon emission computed tomography imaging or electrophysiological measurement may be needed to provide authentic evidences for HBOT or control interventions and to allow a greater refinement of HBOT in TBI.
Third, heterogeneity of the patients and HBO paradigms (pressure, frequency, length of treatment course) partly affect or determine the outcome. There were variations in age of patients, and in severity and nature of the injury in the studies. There is significant preference that HBO showed more efficiency in younger age (He et al., 2005; Lund et al., 2005). And it is possible that HBOT has a positive effect in a subgroup of patients with moderate injury but not in those with extensive cerebral injury. Repetitive long-term HBO treatment following TBI would have more pronounced effects and might modulate different cerebral functions than short-term treatment during the acute phase of TBI only (Kraitsy et al., 2014).
Fourth, HBOT is not a completely benign process and there are concerns about its safety aspects. Possible complications during HBOT include barotraumatic lesions (middle ear, nasal sinuses, inner ear, lung, teeth), oxygen toxicity (central nervous system, lung), confinement anxiety, and ocular effects (myopia, cataract growth) (Camporesi, 2014). The predominant complication is represented by pressure equalization problems within the middle ear, and can be prevented or minimized by teaching autoinflation techniques. Serious complications such as seizures rarely occur and can be controlled by reducing oxygen pressure (Hadanny et al., 2016). Other adverse events such as pulmonary barotrauma and edema derive from oxygen toxicity occur rarely in specific patient cohorts (Wolf et al., 2012a). In conclusion, if safety guidelines are strictly followed, HBOT is a modality with an acceptable rate of complications.
CONCLUSIONS
HBOT has been demonstrated to have neuroprotective effects without increased oxygen toxicity in experimental TBI models when administered at pressures less than 3 ATA (Table 1). The improved tissue oxygenation and cellular metabolism, anti-inflammation, anti-apoptosis and promoting neurogenesis and angiogenesis may constitute the multiple and complementary mechanisms underlying HBOT-induced neuroprotection (Figure 1). Due to the heterogeneity of human TBI, the efficacy of clinical HBOT remains controversial (Table 2). Delayed treatment time, subjective methods for outcome measurement, and inappropriate HBOT paradigms could contribute to misinterpretation of results and prevent a positive recommendation of HBO in TBI patients. These key factors should be considered in the future clinical studies of HBO in TBI and other neurological diseases.
Footnotes
Conflicts of interest
The authors declare that there is no completing interest regarding the publication of this paper.
REFERENCES
- Algattas H, Huang JH. Traumatic brain injury pathophysiology and treatments: early, intermediate, and late phases post-injury. Int J Mol Sci. 2014;15:309–341. doi: 10.3390/ijms15010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artru F, Chacornac R, Deleuze R. Hyperbaric oxygenation for severe head injuries. Preliminary results of a controlled study. Eur Neurol. 1976;14:310–318. doi: 10.1159/000114753. [DOI] [PubMed] [Google Scholar]
- Bennett MH, Trytko B, Jonker B. Hyperbaric oxygen therapy for the adjunctive treatment of traumatic brain injury. Cochrane Database Syst Rev. 2012;12:CD004609. doi: 10.1002/14651858.CD004609.pub3. [DOI] [PubMed] [Google Scholar]
- Boussi-Gross R, Golan H, Fishlev G, Bechor Y, Volkov O, Bergan J, Friedman M, Hoofien D, Shlamkovitch N, Ben-Jacob E, Efrati S. Hyperbaric oxygen therapy can improve post concussion syndrome years after mild traumatic brain injury-randomized prospective trial. PLoS One. 2013;8:e79995. doi: 10.1371/journal.pone.0079995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braswell C, Crowe DT. Hyperbaric oxygen therapy. Compend Contin Educ Vet. 2012;34:E1–5. quiz E6. [PubMed] [Google Scholar]
- Brkic P, Stojiljkovic M, Jovanovic T, Dacic S, Lavrnja I, Savic D, Parabucki A, Bjelobaba I, Rakic L, Pekovic S. Hyperbaric oxygenation improves locomotor ability by enhancing neuroplastic responses after cortical ablation in rats. Brain Inj. 2012;26:1273–1284. doi: 10.3109/02699052.2012.667593. [DOI] [PubMed] [Google Scholar]
- Brown JA, Preul MC, Taha A. Hyperbaric oxygen in the treatment of elevated intracranial pressure after head injury. Pediatr Neurosci. 1988;14:286–290. doi: 10.1159/000120406. [DOI] [PubMed] [Google Scholar]
- Calvert JW, Zhou C, Nanda A, Zhang JH. Effect of hyperbaric oxygen on apoptosis in neonatal hypoxia-ischemia rat model. J Appl Physiol 1985. 2003;95:2072–2080. doi: 10.1152/japplphysiol.00630.2003. [DOI] [PubMed] [Google Scholar]
- Calvert JW, Yin W, Patel M, Badr A, Mychaskiw G, Parent AD, Zhang JH. Hyperbaric oxygenation prevented brain injury induced by hypoxia-ischemia in a neonatal rat model. Brain Res. 2002;951:1–8. doi: 10.1016/s0006-8993(02)03094-9. [DOI] [PubMed] [Google Scholar]
- Camporesi EM. Side effects of hyperbaric oxygen therapy. Undersea Hyperb Med. 2014;41:253–257. [PubMed] [Google Scholar]
- Chen X, Duan XS, Xu LJ, Zhao JJ, She ZF, Chen WW, Zheng ZJ, Jiang GD. Interleukin-10 mediates the neuroprotection of hyperbaric oxygen therapy against traumatic brain injury in mice. Neuroscience. 2014;266:235–243. doi: 10.1016/j.neuroscience.2013.11.036. [DOI] [PubMed] [Google Scholar]
- Cifu DX, Hart BB, West SL, Walker W, Carne W. The effect of hyperbaric oxygen on persistent postconcussion symptoms. J Head Trauma Rehabil. 2014a;29:11–20. doi: 10.1097/HTR.0b013e3182a6aaf0. [DOI] [PubMed] [Google Scholar]
- Cifu DX, Walker WC, West SL, Hart BB, Franke LM, Sima A, Graham CW, Carne W. Hyperbaric oxygen for blast-related postconcussion syndrome: three-month outcomes. Ann Neurol. 2014b;75:277–286. doi: 10.1002/ana.24067. [DOI] [PubMed] [Google Scholar]
- Coe JE, Hayes TM. Treatment of experimental brain injury by hyperbaric oxygenation. Preliminary report. Am Surg. 1966;32:493–495. [PubMed] [Google Scholar]
- Contreras FL, Kadekaro M, Eisenberg HM. The effect of hyperbaric oxygen on glucose utilization in a freeze-traumatized rat brain. J Neurosurg. 1988;68:137–141. doi: 10.3171/jns.1988.68.1.0137. [DOI] [PubMed] [Google Scholar]
- Daugherty WP, Levasseur JE, Sun D, Rockswold GL, Bullock MR. Effects of hyperbaric oxygen therapy on cerebral oxygenation and mitochondrial function following moderate lateral fluid-percussion injury in rats. J Neurosurg. 2004;101:499–504. doi: 10.3171/jns.2004.101.3.0499. [DOI] [PubMed] [Google Scholar]
- Dunn JE, Lawson DD. Effects of Hypobaric and Hyperbaric Oxygen on Experimental brain injury. In Origins of Hyperbaric Medicine. National Research Council. 1966:447–454. [Google Scholar]
- Fasano VA, Nunno T, Urciolo R, Lombard G. First observation on the use of oxygen under high pressure for the treatment of traumatic coma. In: Boerema I, Brummelkamp WH, Meijne NG, editors. Clinical application of Hyperbaric Oxygen. Amsterdam: Elsevier; 1964. [Google Scholar]
- Golden Z, Golden CJ, Neubauer RA. Improving neuropsychological function after chronic brain injury with hyperbaric oxygen. Disabil Rehabil. 2006;28:1379–1386. doi: 10.1080/09638280600638364. [DOI] [PubMed] [Google Scholar]
- Golden ZL, Neubauer R, Golden CJ, Greene L, Marsh J, Mleko A. Improvement in cerebral metabolism in chronic brain injury after hyperbaric oxygen therapy. Int J Neurosci. 2002;112:119–131. doi: 10.1080/00207450212027. [DOI] [PubMed] [Google Scholar]
- Hadanny A, Meir O, Bechor Y, Fishlev G, Bergan J, Efrati S. Seizures during hyperbaric oxygen therapy: retrospective analysis of 62, 614 treatment sessions. Undersea Hyperb Med. 2016;43:21–28. [PubMed] [Google Scholar]
- Harch PG, Kriedt C, Van Meter KW, Sutherland RJ. Hyperbaric oxygen therapy improves spatial learning and memory in a rat model of chronic traumatic brain injury. Brain Res. 2007;1174:120–129. doi: 10.1016/j.brainres.2007.06.105. [DOI] [PubMed] [Google Scholar]
- Harch PG, Fogarty EF, Staab PK, Van Meter K. Low pressure hyperbaric oxygen therapy and SPECT brain imaging in the treatment of blast-induced chronic traumatic brain injury (post-concussion syndrome) and post traumatic stress disorder: a case report. Cases J. 2009;2:6538. doi: 10.1186/1757-1626-0002-0000006538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harch PG, Andrews SR, Fogarty EF, Amen D, Pezzullo JC, Lucarini J, Aubrey C, Taylor DV, Staab PK, Van Meter KW. A phase I study of low-pressure hyperbaric oxygen therapy for blast-induced post-concussion syndrome and post-traumatic stress disorder. J Neurotrauma. 2012;29:168–185. doi: 10.1089/neu.2011.1895. [DOI] [PubMed] [Google Scholar]
- Hardy P, Johnston KM, De Beaumont L, Montgomery DL, Lecomte JM, Soucy JP, Bourbonnais D, Lassonde M. Pilot case study of the therapeutic potential of hyperbaric oxygen therapy on chronic brain injury. J Neurol Sci. 2007;253:94–105. doi: 10.1016/j.jns.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Kanai N, Kuroda R, Yamada R, Mogami H. Response of cereborspinal fluid pressure to hyperbaric oxygenation. J Neurol Neurosurg Psychiatry. 1971;34:580–586. doi: 10.1136/jnnp.34.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Crook JE, Meschia JF, Brott TG, Dickson DW, McKinney M. Aging blunts ischemic-preconditioning-induced neuroprotection following transient global ischemia in rats. Curr Neurovasc Res. 2005;2:365–374. doi: 10.2174/156720205774962674. [DOI] [PubMed] [Google Scholar]
- Holbach KH, Wassmann H, Kolberg T. Improved reversibility of the traumatic midbrain syndrome using hyperbaric oxygen. Acta Neurochir (Wien) 1974;30:247–256. doi: 10.1007/BF01405583. [DOI] [PubMed] [Google Scholar]
- Holbach KH, Caroli A, Wassmann H. Cerebral energy metabolism in patients with brain lesions of normo- and hyperbaric oxygen pressures. J Neurol. 1977;217:17–30. doi: 10.1007/BF00316313. [DOI] [PubMed] [Google Scholar]
- Hollin SA, Sukoff MH, Jacobson JH., 2nd The protective effect of hyperbaric oxygenation in experimentally produced cerebral edema and compression. Prog Brain Res. 1968;30:479–489. doi: 10.1016/S0079-6123(08)61501-0. [DOI] [PubMed] [Google Scholar]
- Horn P, Munch E, Vajkoczy P, Herrmann P, Quintel M, Schilling L, Schmiedek P, Schurer L. Hypertonic saline solution for control of elevated intracranial pressure in patients with exhausted response to mannitol and barbiturates. Neurol Res. 1999;21:758–764. doi: 10.1080/01616412.1999.11741010. [DOI] [PubMed] [Google Scholar]
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22:341–353. [PubMed] [Google Scholar]
- Kalns J, Lane J, Delgado A, Scruggs J, Ayala E, Gutierrez E, Warren D, Niemeyer D, George Wolf E, Bowden RA. Hyperbaric oxygen exposure temporarily reduces Mac-1 mediated functions of human neutrophils. Immunol Lett. 2002;83:125–131. doi: 10.1016/s0165-2478(02)00068-8. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Parent JM. Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiol Dis. 2010;37:267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolias AG, Guilfoyle MR, Helmy A, Allanson J, Hutchinson PJ. Traumatic brain injury in adults. Pract Neurol. 2013;13:228–235. doi: 10.1136/practneurol-2012-000268. [DOI] [PubMed] [Google Scholar]
- Kraitsy K, Uecal M, Grossauer S, Bruckmann L, Pfleger F, Ropele S, Fazekas F, Gruenbacher G, Patz S, Absenger M, Porubsky C, Smolle-Juettner F, Tezer I, Molcanyi M, Fasching U, Schaefer U. Repetitive long-term hyperbaric oxygen treatment (HBOT) administered after experimental traumatic brain injury in rats induces significant remyelination and a recovery of sensorimotor function. PLoS One. 2014;9:e97750. doi: 10.1371/journal.pone.0097750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhou C, Calvert JW, Colohan AR, Zhang JH. Multiple effects of hyperbaric oxygen on the expression of HIF-1 alpha and apoptotic genes in a global ischemia-hypotension rat model. Exp Neurol. 2005;191:198–210. doi: 10.1016/j.expneurol.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Lim SW, Wang CC, Wang YH, Chio CC, Niu KC, Kuo JR. Microglial activation induced by traumatic brain injury is suppressed by postinjury treatment with hyperbaric oxygen therapy. J Surg Res. 2013;184:1076–1084. doi: 10.1016/j.jss.2013.04.070. [DOI] [PubMed] [Google Scholar]
- Lin KC, Niu KC, Tsai KJ, Kuo JR, Wang LC, Chio CC, Chang CP. Attenuating inflammation but stimulating both angiogenesis and neurogenesis using hyperbaric oxygen in rats with traumatic brain injury. J Trauma Acute Care Surg. 2012;72:650–659. doi: 10.1097/TA.0b013e31823c575f. [DOI] [PubMed] [Google Scholar]
- Liu XH, Yan H, Xu M, Zhao YL, Li LM, Zhou XH, Wang MX, Ma L. Hyperbaric oxygenation reduces long-term brain injury and ameliorates behavioral function by suppression of apoptosis in a rat model of neonatal hypoxia-ischemia. Neurochem Int. 2013;62:922–930. doi: 10.1016/j.neuint.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Liu Z, Jiao QF, You C, Che YJ, Su FZ. Effect of hyperbaric oxygen on cytochrome C, Bcl-2 and Bax expression after experimental traumatic brain injury in rats. Chin J Traumatol. 2006;9:168–174. [PubMed] [Google Scholar]
- Lou M, Ding MP, Wen SQ. Effect of hyperbaric oxygenation treatment on the apoptotic cell death pathway after transient focal cerebral ischemia. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2006;22:1–5. [PubMed] [Google Scholar]
- Lund VE, Kentala E, Scheinin H, Lertola K, Klossner J, Aitasalo K, Sariola-Heinonen K, Jalonen J. Effect of age and repeated hyperbaric oxygen treatments on vagal tone. Undersea Hyperb Med. 2005;32:111–119. [PubMed] [Google Scholar]
- Lv LQ, Hou LJ, Yu MK, Ding XH, Qi XQ, Lu YC. Hyperbaric oxygen therapy in the management of paroxysmal sympathetic hyperactivity after severe traumatic brain injury: a report of 6 cases. Arch Phys Med Rehabil. 2011;92:1515–1518. doi: 10.1016/j.apmr.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Mao JH, Sun ZS, Xiang Y. Observation of curative effects of hyperbaric oxygen for treatment on severe craniocerebral injury. J Clin Neurol. 2010;23:386–388. [Google Scholar]
- Meng XE, Zhang Y, Li N, Fan DF, Yang C, Li H, Guo DZ, Pan SY. Effects of hyperbaric oxygen on the Nrf2 signaling pathway in secondary injury following traumatic brain injury. Genet Mol Res. 2016a doi: 10.4238/gmr.15016933. doi:10.4238/gmr.15016933. [DOI] [PubMed] [Google Scholar]
- Meng XE, Zhang Y, Li N, Fan DF, Yang C, Li H, Guo DZ, Pan SY. Hyperbaric oxygen alleviates secondary brain injury after trauma through inhibition of TLR4/NF-kappaB signaling pathway. Med Sci Monit. 2016b;22:284–288. doi: 10.12659/MSM.894148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Ledingham IM. Reduction of increased intracranial pressure. Comparison between hyperbaric oxygen and hyperventilation. Arch Neurol. 1971;24:210–216. doi: 10.1001/archneur.1971.00480330038003. [DOI] [PubMed] [Google Scholar]
- Miller JD, Fitch W, Ledingham IM, Jennett WB. The effect of hyperbaric oxygen on experimentally increased intracranial pressure. J Neurosurg. 1970;33:287–296. doi: 10.3171/jns.1970.33.3.0287. [DOI] [PubMed] [Google Scholar]
- Miller RS, Weaver LK, Bahraini N, Churchill S, Price RC, Skiba V, Caviness J, Mooney S, Hetzell B, Liu J, Deru K, Ricciardi R, Fracisco S, Close NC, Surrett GW, Bartos C, Ryan M, Brenner LA. Effects of hyperbaric oxygen on symptoms and quality of life among service members with persistent postconcussion symptoms: a randomized clinical trial. JAMA Intern Med. 2015;175:43–52. doi: 10.1001/jamainternmed.2014.5479. [DOI] [PubMed] [Google Scholar]
- Mu J, Krafft PR, Zhang JH. Hyperbaric oxygen therapy promotes neurogenesis: where do we stand. Med Gas Res. 2011;1:14. doi: 10.1186/2045-9912-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer RA, Gottlieb SF, Pevsner NH. Hyperbaric oxygen for treatment of closed head injury. South Med J. 1994;87:933–936. doi: 10.1097/00007611-199409000-00015. [DOI] [PubMed] [Google Scholar]
- Niklas A, Brock D, Schober R, Schulz A, Schneider D. Continuous measurements of cerebral tissue oxygen pressure during hyperbaric oxygenation--HBO effects on brain edema and necrosis after severe brain trauma in rabbits. J Neurol Sci. 2004;219:77–82. doi: 10.1016/j.jns.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Palzur E, Zaaroor M, Vlodavsky E, Milman F, Soustiel JF. Neuroprotective effect of hyperbaric oxygen therapy in brain injury is mediated by preservation of mitochondrial membrane properties. Brain Res. 2008;1221:126–133. doi: 10.1016/j.brainres.2008.04.078. [DOI] [PubMed] [Google Scholar]
- Palzur E, Vlodavsky E, Mulla H, Arieli R, Feinsod M, Soustiel JF. Hyperbaric oxygen therapy for reduction of secondary brain damage in head injury: an animal model of brain contusion. J Neurotrauma. 2004;21:41–48. doi: 10.1089/089771504772695931. [DOI] [PubMed] [Google Scholar]
- Peng Z, Xiao P, Guo H, Liu Q. Effect of early hyperbaric oxygen on neuronal apoptosis and learning and memory of cerebral ischemia-reperfusion injury in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34:468–475. [PubMed] [Google Scholar]
- Potter S, Leigh E, Wade D, Fleminger S. The Rivermead Post Concussion Symptoms Questionnaire: a confirmatory factor analysis. J Neurol. 2006;253:1603–1614. doi: 10.1007/s00415-006-0275-z. [DOI] [PubMed] [Google Scholar]
- Prakash A, Parelkar SV, Oak SN, Gupta RK, Sanghvi BV, Bachani M, Patil R. Role of hyperbaric oxygen therapy in severe head injury in children. J Pediatr Neurosci. 2012;7:4–8. doi: 10.4103/1817-1745.97610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzenhofer-Komenda B, Favory R, Weslau W, Smolle-Jüttner FM, Mathieu D. Handbook on Hyperbaric Medicine. Netherlands: Springer; 2006. [Google Scholar]
- Ren H, Wang W, Ge Z. Glasgow Coma Scale, brain electric activity mapping and Glasgow Outcome Scale after hyperbaric oxygen treatment of severe brain injury. Chin J Traumatol. 2001a;4:239–241. [PubMed] [Google Scholar]
- Ren H, Wang W, Ge Z, Zhang J. Clinical, brain electric earth map, endothelin and transcranial ultrasonic Doppler findings after hyperbaric oxygen treatment for severe brain injury. Chin Med J (Engl) 2001b;114:387–390. [PubMed] [Google Scholar]
- Rink A, Fung KM, Trojanowski JQ, Lee VM, Neugebauer E, McIntosh TK. Evidence of apoptotic cell death after experimental traumatic brain injury in the rat. Am J Pathol. 1995;147:1575–1583. [PMC free article] [PubMed] [Google Scholar]
- Rockswold GL, Ford SE, Anderson DC, Bergman TA, Sherman RE. Results of a prospective randomized trial for treatment of severely brain-injured patients with hyperbaric oxygen. J Neurosurg. 1992;76:929–934. doi: 10.3171/jns.1992.76.6.0929. [DOI] [PubMed] [Google Scholar]
- Rockswold SB, Rockswold GL, Zaun DA, Liu J. A prospective, randomized Phase II clinical trial to evaluate the effect of combined hyperbaric and normobaric hyperoxia on cerebral metabolism, intracranial pressure, oxygen toxicity, and clinical outcome in severe traumatic brain injury. J Neurosurg. 2013;118:1317–1328. doi: 10.3171/2013.2.JNS121468. [DOI] [PubMed] [Google Scholar]
- Rockswold SB, Rockswold GL, Vargo JM, Erickson CA, Sutton RL, Bergman TA, Biros MH. Effects of hyperbaric oxygenation therapy on cerebral metabolism and intracranial pressure in severely brain injured patients. J Neurosurg. 2001;94:403–411. doi: 10.3171/jns.2001.94.3.0403. [DOI] [PubMed] [Google Scholar]
- Rockswold SB, Rockswold GL, Zaun DA, Zhang X, Cerra CE, Bergman TA, Liu J. A prospective, randomized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injury. J Neurosurg. 2010;112:1080–1094. doi: 10.3171/2009.7.JNS09363. [DOI] [PubMed] [Google Scholar]
- Rogatsky GG, Kamenir Y, Mayevsky A. Effect of hyperbaric oxygenation on intracranial pressure elevation rate in rats during the early phase of severe traumatic brain injury. Brain Res. 2005;1047:131–136. doi: 10.1016/j.brainres.2005.02.049. [DOI] [PubMed] [Google Scholar]
- Sanchez EC. Mechanisms of action of hyperbaric oxygenation in stroke: a review. Crit Care Nurs Q. 2013;36:290–298. doi: 10.1097/CNQ.0b013e318294e9e3. [DOI] [PubMed] [Google Scholar]
- Sukoff MH, Ragatz RE. Hyperbaric oxygenation for the treatment of acute cerebral edema. Neurosurgery. 1982;10:29–38. [PubMed] [Google Scholar]
- Sukoff MH, Hollin SA, Espinosa OE, Jacobson JH., 2nd The protective effect of hyperbaric oxygenation in experimental cerebral edema. J Neurosurg. 1968;29:236–241. doi: 10.3171/jns.1968.29.3.0236. [DOI] [PubMed] [Google Scholar]
- Sun HY, Li Q, Chen XP, Tao LY. Mismatch negativity, social cognition, and functional outcomes in patients after traumatic brain injury. Neural Regen Res. 2015;10:618–623. doi: 10.4103/1673-5374.155437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(Suppl 1):131s–141s. doi: 10.1097/PRS.0b013e3181fbe2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky E, Palzur E, Feinsod M, Soustiel JF. Evaluation of the apoptosis-related proteins of the BCL-2 family in the traumatic penumbra area of the rat model of cerebral contusion, treated by hyperbaric oxygen therapy: a quantitative immunohistochemical study. Acta Neuropathol. 2005;110:120–126. doi: 10.1007/s00401-004-0946-8. [DOI] [PubMed] [Google Scholar]
- Vlodavsky E, Palzur E, Soustiel JF. Hyperbaric oxygen therapy reduces neuroinflammation and expression of matrix metalloproteinase-9 in the rat model of traumatic brain injury. Neuropathol Appl Neurobiol. 2006;32:40–50. doi: 10.1111/j.1365-2990.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- Wang GH, Zhang XG, Jiang ZL, Li X, Peng LL, Li YC, Wang Y. Neuroprotective effects of hyperbaric oxygen treatment on traumatic brain injury in the rat. J Neurotrauma. 2010;27:1733–1743. doi: 10.1089/neu.2009.1175. [DOI] [PubMed] [Google Scholar]
- Wolf EG, Prye J, Michaelson R, Brower G, Profenna L, Boneta O. Hyperbaric side effects in a traumatic brain injury randomized clinical trial. Undersea Hyperb Med. 2012a;39:1075–1082. [PubMed] [Google Scholar]
- Wolf G, Cifu D, Baugh L, Carne W, Profenna L. The effect of hyperbaric oxygen on symptoms after mild traumatic brain injury. J Neurotrauma. 2012b;29:2606–2612. doi: 10.1089/neu.2012.2549. [DOI] [PubMed] [Google Scholar]
- Xiao H, Yang Y, Xi JH, Chen ZQ. Structural and functional connectivity in traumatic brain injury. Neural Regen Res. 2015;10:2062–2071. doi: 10.4103/1673-5374.172328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Zhuang M, Lin L, Xu H, Chen L, Hu L. Changes of plasma C-reactive protein in patients with craniocerebral injury before and after hyperbaric oxygenation: a randomly controlled study. Neural Regen Res. 2007;2:314–317. [Google Scholar]
- Xu S, Liu J, Zhang Y, Wang C, Wang J, Yang Y, Huo J, Sun W. Apoptosis-related protein expression in rabbits with blast brain injury following early hyperbaric oxygen therapy. Neural Regen Res. 2012;7:1318–1324. doi: 10.3969/j.issn.1673-5374.2012.17.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang YG, Lin GA, Xie HQ, Pan HT, Huang BQ, Liu JD, Liu H, Zhang N, Li L, Chen JH. The effects of different hyperbaric oxygen manipulations in rats after traumatic brain injury. Neurosci Lett. 2014;563:38–43. doi: 10.1016/j.neulet.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Yin D, Zhou C, Kusaka I, Calvert JW, Parent AD, Nanda A, Zhang JH. Inhibition of apoptosis by hyperbaric oxygen in a rat focal cerebral ischemic model. J Cereb Blood Flow Metab. 2003;23:855–864. doi: 10.1097/01.WCB.0000073946.29308.55. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang Y, Tang H, Sun W, Xiong X, Smerin D, Liu J. Hyperbaric oxygen therapy ameliorates local brain metabolism, brain edema and inflammatory response in a blast-induced traumatic brain injury model in rabbits. Neurochem Res. 2014a;39:950–960. doi: 10.1007/s11064-014-1292-4. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Cai J, Shields LB, Liu N, Xu XM, Shields CB. Traumatic brain injury using mouse models. Transl Stroke Res. 2014b;5:454–471. doi: 10.1007/s12975-014-0327-0. [DOI] [PubMed] [Google Scholar]
- Zhou JG, Liu JC, Fang YQ. Effect of hyperbaric oxygen on the expression of proteins Bcl-2 and Bax in the gerbil hippocampus CA1 following forebrain ischemia reperfusion. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2000;16:298–301. [PubMed] [Google Scholar]