Abstract
Recent data have shown that normobaric oxygen (NBO) increases the catalytic and thrombolytic efficiency of recombinant tissue plasminogen activator (rtPA) in vitro, and is as efficient as rtPA at restoring cerebral blood flow in rats subjected to thromboembolic brain ischemia. Therefore, in the present study, we studied the effects of hyperbaric oxygen (HBO) (i) on rtPA-induced thrombolysis in vitro and (ii) in rats subjected to thromboembolic middle cerebral artery occlusion-induced brain ischemia. HBO increases rtPA-induced thrombolysis in vitro to a greater extent than NBO; in addition, HBO treatment of 5-minute duration, but not of 25-minute duration, reduces brain damage and edema in vivo. In line with the facilitating effect of NBO on cerebral blood flow, our findings suggest that 5-minute HBO could have provided neuroprotection by promoting thrombolysis. The lack of effect of HBO exposure of longer duration is discussed.
Keywords: hyperbaric oxygen, thrombolysis, brain ischemia, stroke, brain edema, tissue-plasminogen activator, in vitro, rat
INTRODUCTION
Hyperbaric oxygen (HBO) improves outcome in experimental cerebral ischemia and is therefore emerging as a possible co-treatment for acute stroke in addition of tissue plasminogen activator (tPA), whose recombinant form (rtPA) is considered the best approved treatment for acute brain ischemia to date. Thus, despite controversial results (Lu et al., 2014), numerous studies have shown beneficial effects of HBO on infarct size, neurological outcome, tissue hypoxia, and the decrease in regional glucose metabolism induced by ischemia (Veltkamp et al., 2000; Lou et al., 2007; Sun et al., 2008). In August 2013, the US Food and Drug Administration declared artery occlusion as one of the 13 specific indications for HBO. This provides opportunities to some extent for the further development of HBO in stroke disease, if progress could be made in our understanding of the mechanisms of action of HBO.
Alternatively and interestingly, recent investigations have reported that normobaric oxygen (NBO) increased the catalytic and thrombolytic efficiency of rtPA in vitro, and was as efficient as rtPA at restoring cerebral blood flow in rats subjected to thromboembolic brain ischemia (David et al., 2012). Therefore, here, we investigated the effects of HBO on (i) rtPA induced thrombolysis in vitro, and (ii) on ischemic brain damage in rats subjected to thromboembolic middle cerebral artery occlusion (MCAO)-induced brain ischemia.
MATERIALS AND METHODS
Animals
Forty adult male Sprague-Dawley rats (Janvier, Le Genest Saint-Isle, France) weighing 250–280 g were used. All experimental procedures were in accordance with the framework of the French Law on biomedical research and the European Communities Council Directive issued on 24 November 1986 (86/609/EEC). Before being used, rats were housed at 21 ± 0.5°C in Perspex home cages with free access to food and water. After surgery, they were housed individually. Light was maintained on a light/dark reverse cycle with lights on from 8:00 p.m. to 8:00 a.m.
Gas pharmacology
Oxygen and nitrogen were obtained from Air Liquide Santé (Paris, France). Gas mixtures containing 10% oxygen and 90% nitrogen (10–90/O2–N2), 25% oxygen and 75% nitrogen (25–75/O2–N2) or 22% oxygen and 78% nitrogen (22–78/O2–N2) were obtained using computer-driven oxygen and nitrogen gas mass flowmeters (Aalborg, Orangeburg, NY, USA) of 1% absolute accuracy and an oxygen analyzer.
In vitro thrombolysis studies
The effects of HBO on the thrombolytic efficiency of tPA were assessed as follows. Whole-blood samples of 500 μL volume, drawn from another six male Sprague-Dawley mature rats weighing 600–650 g, were transferred in pre-weighed 1.5-mL sterile tubes and incubated at 37°C for 3 hours. Mature rats were chosen for ethical considerations to limit the number of animals. After clot formation and total serum removal, each tube was weighed to determine the clot weight. Blood clots were selected in the same weight range (0.264 ± 0.033 g) to reduce variability. Each tube was filled with saline solution containing 1 μL rtPA in the form of Actilyse, placed open in a hyperbaric chamber, and pressurized with HBO or 10–90/O2–N2 to 2.5 atmospheres absolute (ATA; 1 ATA = 0.1 MPa) at a compression rate of 2.5 ATA/min. After a 90-minute period of incubation at 37°C at 2.5 ATA, the tubes were decompressed at a decompression rate of 2.5 ATA/min. After decompression, the fluid was removed from the tubes, and the tubes were weighed again to assess the percentage of clot lysis induced by rtPA in the presence of HBO or 10–90/O2–N2. Controls were treated with saline solution containing 1 μL rtPA previously saturated with normobaric medical air containing 25–75/O2–N2 and incubated at 37°C for a 90-minute period. The number of blood clots was n = 25–29 per condition.
In vivo thromboembolic ischemic studies
Male Sprague-Dawley rats (n = 34) weighing 250–275 g were subjected to middle cerebral artery occlusion by administration of an autologous blood clot by the intraluminal method as described previously (Haelewyn et al., 2016). Twenty-four hours before the animals were subjected to brain ischemia, a whole caudal blood sample of 200 μL was withdrawn, allowed to clot at 37°C for 2 hours, extruded from the catheter into a saline-filled petri dish, and stored at 4°C for 22 hours before being used the day after to induce thromboembolic ischemia. On the day of surgery, rats were anesthetized with 2% isoflurane in medical air, intubated, and ventilated artificially. A midline neck incision was performed, and the right common carotid artery was exposed to perform coagulation of the proximal branches of the external carotid artery. A single clot measuring 40 mm in length was injected in a volume of 50 μL saline solution through a polyethylene-10 catheter directed into the internal carotid artery up to 2 mm after the pterygopalatine-internal carotid artery bifurcation. All physiological parameters remained within normal range in all groups during surgery. After a 45-minute period of occlusion during which all rats were given medical air, the catheter was removed from the internal carotid artery to the external carotid artery. Then, the rats were placed immediately in a hyperbaric chamber fitted with a viewing window to allow observation, and pressurized at a compression rate of 0.8 ATA/min to 2.2 ATA HBO. After a steady-state of 5 minutes (HBO-5), 15 minutes (HBO-15), or 25 minutes (HBO-25) at 2.2 ATA, rats were decompressed at a decompression rate of 0.2 ATA/min. The animals were only treated with HBO and were not given rtPA. After decompression, the rats were returned to their home cages with food and water ad libitum before being used for histology. Control animals were treated with normobaric medical air containing 22–78/O2–N2. The number of animals was n = 6–12 per experimental group.
Histological analysis
Twenty-four hours after induction of MCAO, the rats were killed by decapitation under halothane anesthesia. The brain was rapidly removed, frozen in isopentane, and placed at –80°C. Coronal brain sections (20 μm) were cryo-cut, mounted on slides, and stained with thionin. Briefly, slices were immersed in water, stained with thionin, dehydrated with serial alcohol and cleared with xylene, and coverslipped with Eukitt mounting. Brain sections colored with thionin were digitized on a PC. Then, volumes of MCAO-induced brain infarction were analyzed with an image analyzer (ImageJ software; Scion Corporation, Frederick, MD, USA) by a blinded observer. The lesioned areas were delineated by the pallor of staining in the necrotic tissue compared with the surrounding healthy tissue; MCAO-induced brain damages were calculated by integration of the infarcted surfaces over the whole brain, corrected for tissue edema, and expressed in mm3 of infarction volume.
Statistical analysis
Data are expressed as the mean ± SEM, and were analyzed using online software (http://marne.u707.jussieu.fr/biostatgv/). In vitro data were analyzed using the unpaired Student t test with one-tailed testing. In vivo data were analyzed using the Mann-Whitney nonparametric unpaired U test with one-tailed testing. Also, we performed statistical power analysis through an online software (http://www.dssresearch.com/toolkit/sscalc/size_a2.asp) for the sample size used in the present study (n = 7–12 per group) with α error level set at P < 0.05 using previous data that have shown that 100% normobaric oxygen has neuroprotective effects (mean volume of brain damage ± SD for controls = 390 ± 76; mean volume of brain damage ± SD for 100% normobaric oxygen = 111 ± 41) and prothrombolytic properties (normalized mean cerebral blood flow ± SD for controls = 28 ± 32; normalized mean cerebral blood flow ± SD for 100% normobaric oxygen = 246 ± 85). In both cases, power analysis indicated 100% of statistical power.
RESULTS
In vitro thrombolysis studies
First, to examine whether HBO has prothrombolytic effects, we studied the effect of HBO at 2.5 ATA on the thrombolytic efficiency of rtPA in whole-blood samples maintained normothermic at 37°C. As shown in Figure 1, we found that HBO at 2.5 ATA has a facilitating effect on the thrombolytic efficiency of rtPA, increasing by 64% rtPA induced thrombolysis as compared to controls (P < 0.0001). HBO increases rtPA induced thrombolysis to a greater extent than NBO (P < 0.05; data taken however from a previous study (David et al., 2012)). To address the possible contribution of pressure per se in the facilitating effect of HBO on rtPA-induced thrombolysis, we performed additional hyperbaric experiments with 10–90/O2-N2 at 2.5 ATA, conditions that allow maintaining the oxygen partial pressure at the value of 0.25 ATA used in controls. No effect was found, thereby indicating that the facilitating effect of HBO on rtPA-induced thrombolysis is clearly due to the increase in oxygen partial pressure induced by HBO but not to pressure per se.
Figure 1.
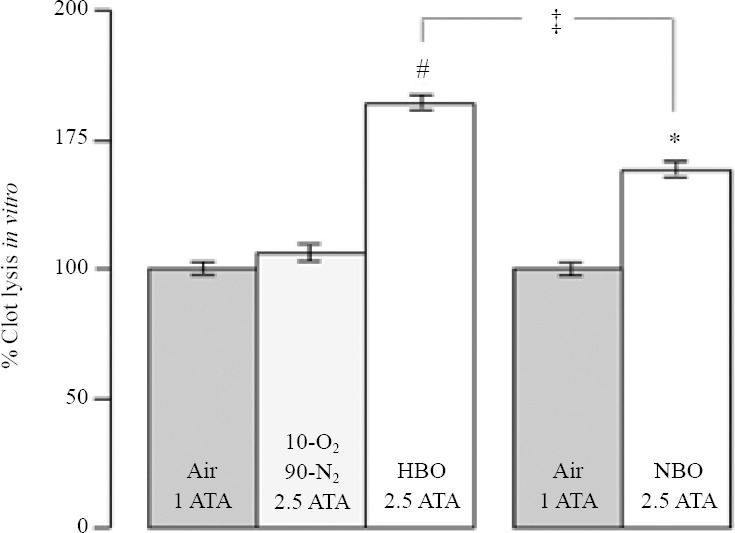
Facilitating effect of hyperbaric oxygen (HBO) on the thrombolytic action of recombinant tissue plasminogen activator (rtPA) in vitro.
Note: HBO at 2.5 atmospheres absolute (ATA; 1 ATA = 0.1 MPa) increases the thrombolytic effect of rtPA by 64% as compared to controls treated with rtPA and air. Also, notably, it should be noted that HBO at 2.5 ATA increases the thrombolytic action of rtPA in a greater manner than normobaric oxygen (NBO), which increases it by 39% as compared to controls. Data with NBO were taken from a previous study (David et al., 2012). Furthermore, additional hyperbaric investigations were performed with 10–90/O2–N2 at 2.5 ATA, conditions that allow maintaining the oxygen partial pressure at the value of 0.25 ATA used in controls. No effect was found indicating that the facilitating of HBO on rtPA-induced thrombolysis in vitro is due to the increase in oxygen partial pressure but not to pressure per se. HBO: #P < 0.0001, vs. its own controls treated with air + rtPA; ‡P < 0.05, vs. NBO; NBO: *P < 0.005, vs. its own controls treated with air + rtPA.
In vivo thromboembolic ischemic studies
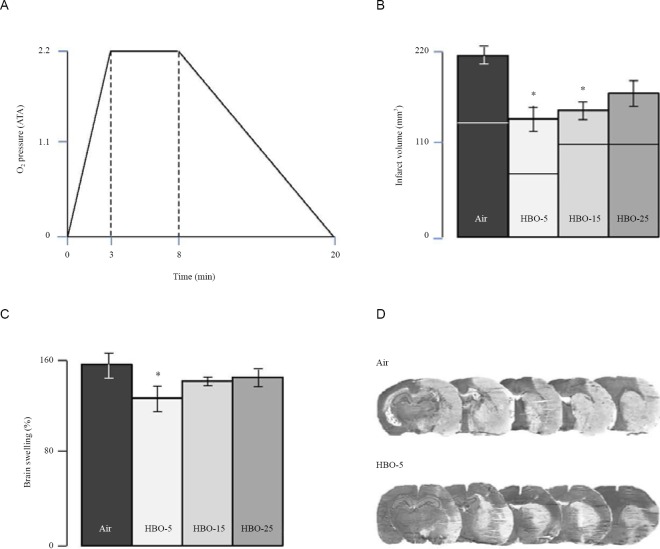
Next, to investigate whether HBO could have prothrombolytic effects and thereby induce subsequent reduction of brain damage and edema, we studied the effects of HBO in male adult Sprague-Dawley rats subjected to thromboembolic brain ischemia. As illustrated in Figure 2, control rats treated with saline solution and medical air had total brain damage of 214 ± 19 mm3, cortical brain damage of 137 ± 20 mm3, striatal brain damage of 77 ± 16 mm3, and brain swelling of 55 ± 13%. Compared to control animals, rats treated with HBO-5 had reduced total brain damage of 142 ± 31 mm3 (P < 0.05), reduced cortical brain damage of 74 ± 25 mm3 (P < 0.05), and reduced brain swelling of 29 ± 12% (P < 0.05), but showed no reduction in striatal brain damage with 68 ± 16 mm3 of infarct volume (P > 0.05). Rats treated with HBO-15 had reduced total brain damage of 148 ± 20 mm3 (P < 0.05) and reduced striatal brain damage of 38 ± 7 mm3 (P < 0.05), but showed reduction neither in cortical brain damage, with 110 ± 20 mm3 of infarct volume, nor in brain swelling (39 ± 6%) (P > 0.05, vs. control animals). Rats treated with HBO-25 showed reduction neither in total, cortical, and striatal brain damage, with 173 ± 29 mm3, 112 ± 34 mm3, and 57 ± 9 mm3 of infarct volume, respectively, nor in brain swelling (43 ± 8%) (P > 0.05, vs. control animals). These data, taken together, show that the reduction in total brain damage and swelling induced by HBO decreased as the duration of exposure increased.
Figure 2.
Neuroprotective properties of short-term treatment with HBO of 5-min duration (HBO-5), 15-min duration (HBO-15) or 25-min duration (HBO-25).
Note: (A) Diving profile of HBO treatment. Whatever the duration of treatment, the compression time and decompression time were similar. Steady state at pressure was 5 min (as illustrated) or 15 or 25 min. 1 ATA = 0.1 MPa. (B) Effects of HBO-5, HBO-15 and HBO-25 on total brain damage, cortical brain damage (the lower part of the histograms) and striatal brain damage (upper part of the histograms). HBO-5 and HBO-15, but not HBO-25, provide neuroprotection. Suprinsingly, HBO-15 and HBO-25 did not increase neuroprotection compared to HBO-5. (C) Effects of HBO-5, HBO-15, and HBO-25 on brain swelling. HBO-5, but not HBO-15 and HBO-25, decreases brain swelling. (D) typical examples of brain damage in rats treated with Air or HBO-5. *P < 0.05, vs. control animals treated with saline and air. ATA: Atmospheres absolute; HBO: hyperbaric oxygen; min: minute(s).
Alternatively, as it can be seen in the video link attached to the present report (supplementary Video 1 online), rats treated with HBO had a rapid and sudden recovery from thromboembolic brain ischemia compared to room-air treated rats. Though this cannot be proven definitively since cerebral blood flow was not measured because of technical limitations in our hyperbaric device, the rats’ recovery from ischemia was more likely to result from the breaking of the blood clot previously injected in the rat's internal carotid artery rather than from an oxygen diffusion induced by HBO because of its sudden and rapid occurrence. In the video, the rat at the left is a control, room air-treated, rat and the rat at the right is a rat treated with HBO at 2.2 ATA. Both were subjected to thromboembolic brain ischemia before treatment. The duration of treatment as it appears in the video is expressed in minutes, calculated from the beginning of treatment, and is the same for both animals. The corresponding pressure of HBO for the HBO-treated rat is expressed in ATA. Note that the control animal at the left is cowering in a corner and has a fast breathing rate; in contrast, the HBO-treated rat at the right has normal breathing rate and rapidly shows exploring, rearing and grooming normal behavioral activities.
DISCUSSION
We found that HBO increased rtPA-induced thrombolysis in vitro, and that exposure to HBO as short as 5 minutes (HBO-5) duration reduced ischemic brain damage in vivo. Taken together with the video attached to the present report that demonstrate a rapid and sudden recovery of the rats exposed to HBO-5, these data suggest that HBO could act by promoting thrombolysis in vivo through facilitation of endogenous tPA. This would confirm and extend previous data that have shown that NBO increased the catalytic efficiency and thrombolytic properties of rtPA in vitro, and further achieved clot lysis, and reduces infarct size, brain hemorrhages, and disruption of the blood-brain barrier in rats subjected to thromboembolic brain ischemia (David et al., 2012). In line with these findings, NBO and HBO administered 1 hour before rtPA have been shown to reduce infarct volume, brain hemorrhages, and blood-brain barrier damage in rats subjected to thromboembolic brain ischemia, compared to rtPA alone, thereby suggesting that both NBO and HBO could have yet produced recanalyzation or partial recanalyzation before rtPA administration (Sun et al., 2010). In contrast with its beneficial effects when administered alone, NBO given in combination with rtPA has been shown to increase rtPA-induced brain hemorrhages and disruption of th blood brain barrier (David et al., 2012). This questions what could be the effects of combined HBO and rtPA in rats subjected to thromboembolic brain ischemia, conditions that could not have been investigated unfortunately in the present study because of technical limitations in our hyperbaric device.
Alternatively, with a reduction of brain damage of 214 mm3 to 142 mm3, corresponding to a 34% decrease of infarct size, the efficiency of HBO-5 at reducing ischemic brain damage in the present study is in excellent agreement with the mean neuroprotective effect of HBO that is 32% (Xu et al., 2016). However, surprisingly, longer exposures to 2.2 ATA HBO (HBO-15 or HBO-25) did not increase neuroprotection, and even exibited lack of neuroprotection (HBO-25). If one assumes that HBO-5 is enough to break the clot, then it could be possible that HBO exposure of longer durations might lead to an excess of oxygen and thereby to reperfusion injury. Also, it is worth noting that previous studies have reported that moderately delayed NBO treatment (1 hour post-insult) reduced NMDA-induced calcium influxes in vitro and NMDA-induced neuronal degeneration in vivo (Haelewyn et al., 2011) - results in line with the inhibiting effect of HBO on glutamate release (Badr et al., 2001a; Yang et al., 2010) - but increased oxygen and glucose deprivation-induced cell injury in vitro and ischemia-induced infarct volume in vivo (Haelewyn et al., 2011). As well, other investigations have shown that delayed HBO treatment (3 hours post-ischemic) enlarges infarct volume by blocking autophagy through reactive oxygen species-induced activation of extracellular signal-regulated kinase 1/2 (Lu et al., 2014). Nevertheless, in contrast with the latter, other data have reported that HBO did not promote brain damage inflicted by reactive oxygen species in experimental stroke (Sun et al., 2014). In addition, numerous studies using higher HBO pressures and/or longer durations of exposure than those used in the present report have shown beneficial effects of HBO on infarct size, brain edema, neurological outcome, tissue hypoxia, and the decrease in regional glucose metabolism induced by ischemia (Mink and Dutka, 1995; Veltkamp et al., 2000, 2005; Lou et al., 2007; Sun et al., 2008; Michalski et al., 2011). It is therefore possible that longer exposure to HBO in the rat model of thromboembolic stroke used in the present study could have allow restoring neuroprotection through other mechanisms than thrombolysis, thereby indicating if such a possible biphasic neuroprotective effect of HBO. Alternatively and interestingly, it should be noted that beneficial effects of long course HBO treatment have been reported on neurogenesis in the rat (Lee et al., 2013) and on memory and brain metabolism in humans (Boussi-Gross et al., 2015). All of these data, taken together with other studies that have shown that neuroprotection induced by HBO is dose-dependent (Eschenfelder et al., 2008) and has a limited time window (Badr et al., 2001b), clearly question the way by which HBO should be applied in terms of pressure, duration of treatment, and therapeutic window.
In conclusion, this study shows that HBO facilitates rtPA-induced thrombolysis in vitro and further shows that short exposure to HBO provides fast neuroprotection in vivo, suggesting that HBO could act in vivo by promoting thrombolysis through activation of endogenous tPA. Further experiments are needed to study thoroughly the prothrombolytic properties of HBO, when given alone or in combination with rtPA, on cerebral blood flow in rats subjected to thromboembolic brain ischemia and its subsequent effects on neuroprotection, neurologic outcome, and therapeutic window.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
Supplementary data
Supplement information is available at https://figshare.com/articles/Video_HBO-vs-Air_mpg/3439892.
REFERENCES
- Badr AE, Yin W, Mychaskiw G, Zhang JH. Effect of hyperbaric oxygen on striatal metabolites: a microdialysis study in awake freely moving rats after MCA occlusion. Brain Res. 2001a;916:85–90. doi: 10.1016/s0006-8993(01)02867-0. [DOI] [PubMed] [Google Scholar]
- Badr AE, Yin W, Mychaskiw G, Zhang JH. Dual effect of HBO on cerebral infarction in MCAO rats. Am J Physiol Regul Integr Comp Physiol. 2001b;280:R766–770. doi: 10.1152/ajpregu.2001.280.3.R766. [DOI] [PubMed] [Google Scholar]
- Boussi-Gross R, Golan H, Volkov O, Bechor Y, Hoofien D, Beeri MS, Ben-Jacob E, Efrati S. Improvement of memory impairments in poststroke patients by hyperbaric oxygen therapy. Neuropsychology. 2015;29:610–621. doi: 10.1037/neu0000149. [DOI] [PubMed] [Google Scholar]
- David HN, Haelewyn B, Degoulet M, Colomb DG, Jr, Risso JJ, Abraini JH. Prothrombolytic action of normobaric oxygen given alone or in combination with recombinant tissue-plasminogen activator in a rat model of thromboembolic stroke. J Appl Physiol. 2012;112:2068–2076. doi: 10.1152/japplphysiol.00092.2012. [DOI] [PubMed] [Google Scholar]
- Eschenfelder CC, Krug R, Yusofi AF, Meyne JK, Herdegen T, Koch A, Zhao Y, Carl UM, Deuschl G. Neuroprotection by oxygen in acute transient focal cerebral ischemia is dose dependent and shows superiority of hyperbaric oxygenation. Cerebrovasc Dis. 2008;25:193–201. doi: 10.1159/000113856. [DOI] [PubMed] [Google Scholar]
- Haelewyn B, Chazalviel L, Nicole O, Lecocq M, Risso JJ, Abraini JH. Moderately delayed post-insult treatment with normobaric hyperoxia reduces excitotoxin-induced neuronal degeneration but increases ischemia-induced brain damage. Med Gas Res. 2011;1:2. doi: 10.1186/2045-9912-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haelewyn B, David HN, Blatteau JE, Vallee N, Meckler C, Risso JJ, Abraini JH. Modulation by the noble gas helium of tissue plasminogen activator: effects in a rat model of thromboembolic stroke. Crit Care Med. 2016;44:e383–389. doi: 10.1097/CCM.0000000000001424. [DOI] [PubMed] [Google Scholar]
- Lee YS, Chio CC, Chang CP, Wang LC, Chiang PM, Niu KC, Tsai KJ. Long course hyperbaric oxygen stimulates neurogenesis and attenuates inflammation after ischemic stroke. Mediators Inflamm 2013. 2013:512978. doi: 10.1155/2013/512978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou M, Zhang H, Wang J, Wen SQ, Tang ZQ, Chen YZ, Yan WQ, Ding MP. Hyperbaric oxygen treatment attenuated the decrease in regional glucose metabolism of rats subjected to focal cerebral ischemia: a high resolution positron emission tomography study. Neuroscience. 2007;146:555–561. doi: 10.1016/j.neuroscience.2007.01.046. [DOI] [PubMed] [Google Scholar]
- Lu Y, Kang J, Bai Y, Zhang Y, Li H, Yang X, Xiang X, Wang X, Huang Y, Su J, Chen Y, Li B, Sun L. Hyperbaric oxygen enlarges the area of brain damage in MCAO rats by blocking autophagy via ERK1/2 activation. Eur J Pharmacol. 2014;728:93–99. doi: 10.1016/j.ejphar.2014.01.066. [DOI] [PubMed] [Google Scholar]
- Michalski D, Pelz J, Weise C, Kacza J, Boltze J, Grosche J, Kamprad M, Schneider D, Hobohm C, Hartig W. Early outcome and blood-brain barrier integrity after co-administered thrombolysis and hyperbaric oxygenation in experimental stroke. Exp Transl Stroke Med. 2011;3:5. doi: 10.1186/2040-7378-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink RB, Dutka AJ. Hyperbaric oxygen after global cerebral ischemia in rabbits reduces brain vascular permeability and blood flow. Stroke. 1995;26:2307–2312. doi: 10.1161/01.str.26.12.2307. [DOI] [PubMed] [Google Scholar]
- Sun L, Marti HH, Veltkamp R. Hyperbaric oxygen reduces tissue hypoxia and hypoxia-inducible factor-1 alpha expression in focal cerebral ischemia. Stroke. 2008;39:1000–1006. doi: 10.1161/STROKEAHA.107.490599. [DOI] [PubMed] [Google Scholar]
- Sun L, Wolferts G, Veltkamp R. Oxygen therapy does not increase production and damage induced by reactive oxygen species in focal cerebral ischemia. Neurosci Lett. 2014;577:1–5. doi: 10.1016/j.neulet.2014.05.060. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhou W, Mueller C, Sommer C, Heiland S, Bauer AT, Marti HH, Veltkamp R. Oxygen therapy reduces secondary hemorrhage after thrombolysis in thromboembolic cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1651–1660. doi: 10.1038/jcbfm.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltkamp R, Warner DS, Domoki F, Brinkhous AD, Toole JF, Busija DW. Hyperbaric oxygen decreases infarct size and behavioral deficit after transient focal cerebral ischemia in rats. Brain Res. 2000;853:68–73. doi: 10.1016/s0006-8993(99)02250-7. [DOI] [PubMed] [Google Scholar]
- Veltkamp R, Siebing DA, Sun L, Heiland S, Bieber K, Marti HH, Nagel S, Schwab S, Schwaninger M. Hyperbaric oxygen reduces blood-brain barrier damage and edema after transient focal cerebral ischemia. Stroke. 2005;36:1679–1683. doi: 10.1161/01.STR.0000173408.94728.79. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ji R, Wei R, Yin B, He F, Luo B. The efficacy of hyperbaric oxygen therapy on middle cerebral artery occlusion in animal studies: a meta-analysis. PLoS One. 2016;11:e0148324. doi: 10.1371/journal.pone.0148324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, Xie Y, Bosco GM, Chen C, Camporesi EM. Hyperbaric oxygenation alleviates MCAO-induced brain injury and reduces hydroxyl radical formation and glutamate release. Eur J Appl Physiol. 2010;108:513–522. doi: 10.1007/s00421-009-1229-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.