Abstract
Postoperative nausea and vomiting (PONV) is a common complication after general anesthesia. Recent studies suggested that the hippocampus is involved in PONV. Hypothesising that hippocampal dopaminergic neurons are related to PONV, we examined the comprehensive mRNA profile of the hippocampus, using a sevoflurane-treated mouse model to confirm this. This study was conducted after approval from our institutional animal ethics committee, the Animal Research Center of Sapporo Medical University School of Medicine (project number: 12-033). Eight mice were assigned to two groups: a naïve group and a sevoflurane group (Sev group). In the Sev group, four mice were anesthetised with 3.5% sevoflurane for 1 hour. Subsequently, mRNA was isolated from their hippocampal cells and RNA sequencing was performed on an Illumina HiSeq 2500 platform. Mapping of the quality-controlled, filtered paired-end reads to mouse genomes and quantification of the expression levels of each gene were performed using R software. The Rtn4rl2 gene that encodes the Nogo receptor was the most up-regulated gene in the present study. The expression levels of dopamine receptor genes and the tachykinin gene were increased by sevoflurane exposure, while the genes related to serotonin receptors were not altered by sevoflurane exposure. The expression levels of LIM-homeodomain-related genes were highly down-regulated by sevoflurane. These findings suggest that sevoflurane exposure induces dopaminergic stimulation of hippocampal neurons and triggers PONV, while neuronal inflammation caused by LIM-homeodomain-related genes is down-regulated by sevoflurane.
Keywords: transcriptome analysis, gene expression plofiling, postoperative nausea and vomiting, hippocampus, sevoflurane, Nogo receptor, LIM-homeodomain-related gene
INTRODUCTION
Postoperative nausea and vomiting (PONV) is a frequent complication after emergence from general anesthesia. Although sevoflurane anesthesia is known to be a risk factor for PONV (Kanaya et al., 2014), its molecular mechanism has not been fully elucidated. Emerging data from recent research revealed the brain functions underlying the mechanism of PONV development (Carpenter, 1990; Gan, 2007). The occurrence of PONV involves the vomiting center and chemoreceptor trigger zone (i.e., the area postrema and nucleus tractus solitarii); a recent study has suggested that other brain areas, such as the hippocampus, are also related to the pathogenic mechanisms of emesis (Napadow et al., 2013).
Dopamine receptor antagonists, which alter the amount of cyclic adenosine monophosphate within neurons located in the area postrema and nucleus tractus solitarii, play a role in preventing nausea and emesis (Hyde et al., 1996; Sanger and Andrews, 2006). Neurons of the hippocampus are projected through dopaminergic neurons (Mattis et al., 2014; Yu et al,. 2014). The neurotransmitter, dopamine, is known to be the trigger of PONV in the chemoreceptor trigger zone, with the levels of catecholamines in the hippocampus and area postrema varying in conjunction with dopamine levels (Waters et al., 2005). Although the effects of anesthetic agents on electrical activity in the hippocampus have also been studied in detail (Ma and Leung, 2006), changes in the comprehensive mRNA profile of the hippocampus remain elusive. Several animal models have been used to assess the mechanisms of PONV. Although rodents are of little use in studying the neural systems related to the development of PONV because they lack an emetic reflex, they are affected by emetic stimuli, such as radiation and chemotherapy (Yamamoto et al., 2005).
In human studies, surgical operation per se appears to be the one consistent independent risk factor for PONV (Koivuranta et al., 1997; Sinclair et al., 1999). Possible associations between tissue trauma, inflammation, and PONV have been hypothesised in the setting of abdominal surgeries that lead to the release of substance P and serotonin (Horn et al., 2014). This speculation was supported by several studies evaluating whether anti-emetics used to control PONV are also anti-inflammatory in nature (Duffy, 2004; Faerber et al., 2007). Hence, a simple general anesthesia mouse model of PONV is needed to clearly determine the mechanism of PONV, because surgical procedures might bias results.
The recent progress in genomics enables us to comprehensively describe and analyze cellular modifications at the gene expression level using transcriptome-wide analysis. The DNA microarray technique has uncovered the changes in mRNA expression induced by sevoflurane in several tissues (e.g., the lung, spleen, heart, kidney, whole brain, liver, and blood); however, there is no study regarding the hippocampus by transcriptome-wide association study (Sakamoto et al., 2005). We hypothesised that sevoflurane induces changes in the mRNA profile of neurons in the hippocampus and triggers PONV. The aim of this study was to determine the influence of sevoflurane anesthesia on the comprehensive mRNA expression profile of the mouse hippocampus using transcriptome analysis.
MATERIALS AND METHODS
Animals
With approval from Sapporo Medical University School of Medicine animal ethics committee (project number: 12-033) for this study, male C57/BL6 mice (8 weeks of age, 20–25 g of body weight) were purchased from Japan SLC, Inc. (Hamamatsu, Japan) and housed at 22°C under controlled lighting (12:12-hour light/dark cycle), with food and water provided ad libitum. Eight male mice (8 weeks of age) were assigned to two groups: a naive group (Naive group, n = 4) and an inhalation anesthetic group (Sev group, n = 4). In the Sev group, 3.5% sevoflurane (Maruishi Co., Ltd. Shizuoka, Japan) in 100% oxygen was provided to mice in a plastic chamber for 1 hour.
Tissue and library preparation
Mice were decapitated after being anesthetised with 3.5% sevoflurane. Then, the brain was immediately removed from the skull, frozen at –70°C with 2-methylbutane, and placed into a Petri dish containing ice-cold phosphate-buffered saline. The brain was cut along the longitudinal fissure of the cerebrum and the regions posterior to the lambda were cut off using tissue matrices (Brain Matrices, EM Japan, Tokyo, Japan). Thereafter, the brain was placed with the cortex of the left hemisphere facing down and any non-cortical forebrain tissue was removed. Tissue blocks containing hippocampal cells were obtained using Brain Matrices (EM Japan). Meningeal tissue was removed from the hemisphere according to a previously described method (Beaudoin et al., 2012). Finally, dissected hippocampal cells were homogenised and lysed into six samples for each mouse using the RNeasy® Plus Micro Kit (Qiagen, Hilden, Germany) and QIAcube (Qiagen). Quality control for isolated RNA was performed using the Agilent 2200 TapeStation system (Agilent Technologies, Santa Clara, CA, USA). For samples to pass the initial quality control step, it was necessary to quantify > 1 μg of sample and to have an equivalent RNA integrity number (eRIN) of ≥ 8. Then, isolated RNA was pooled into four samples per group and labeled. The cDNA library preparation was performed using TruSeq® RNA Library Prep Kits (Illumina, Inc., San Diego, CA, USA) according to the manufacturer's instructions. The RNA-seq was performed in the paired-end (100 cycles Χ 2) mode on an Illumina HiSeq 2500 platform (Illumina, Inc.).
Data analysis
Base call (.bcl) files for each cycle of sequencing were generated by Illumina Real Time Analysis software (Illumina, Inc.), and were analyzed primarily and de-multiplexed into a FASTQ (.fastq) file using Illumina's BCL2FASTQ conversion software (ver. 1.8.4, Illumina, Inc.). Raw paired-end RNA-seq reads in FASTQ formats were assessed for base call quality, cycle uniformity, and contamination using FastQC (http://www.bioinformatics.bbsrc.ad.uk/projects/fastqc/). Mapping of the quality control-filtered paired-end reads to mouse genomes and quantification of the expression levels of each gene were performed using R software (ver. 3.1.1 with TCC package) (Robinson et al., 2010; Sun et al., 2013). The quality control-filtered paired-end reads were mapped to public mouse genome data that were published by UCSC (NCBI37/mm9, http://genomes.UCSC.edu/). Differential gene sets were filtered to remove those with fold changes < 1.5 (up- or down-regulated) and with a false discovery rate-corrected P value of > 0.05. Sample size was calculated with the following parameters: power ≥ 0.8, probability level < 0.05, and anticipated effect size = 14.
RESULTS
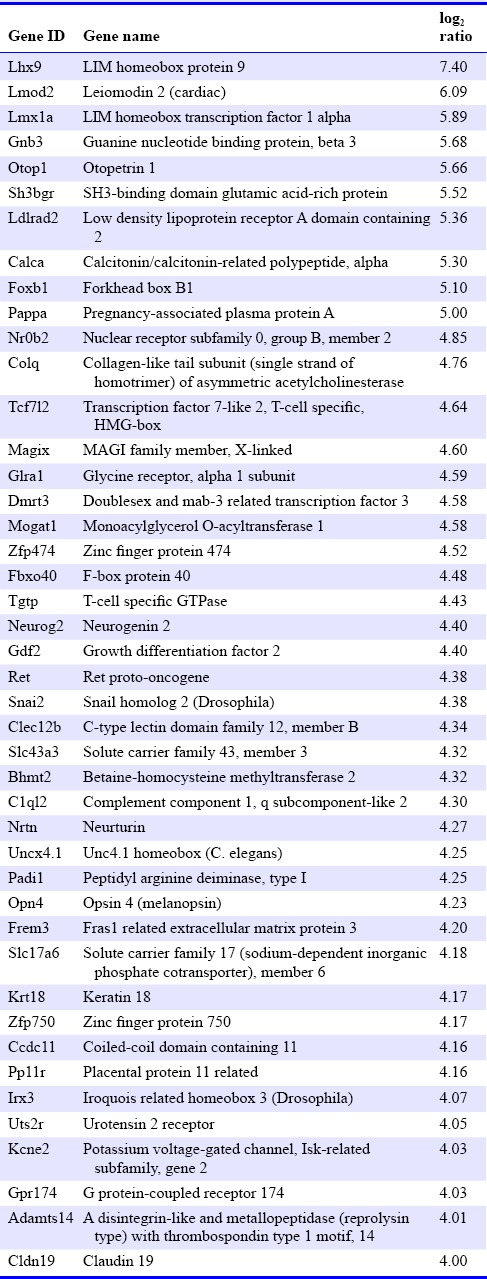
All total RNA samples had a quantity ≥ 1 μg and eRIN value ≥ 8. The average called bases after primary filtration were 41,778,219 base pairs, and the average of mean quality score (Phred quality score) was 36.7. We investigated changes in expression levels of a total of 37,681 genes. Ten thousand, two hundred and fifty-two genes were filtered because they showed little changes in expression levels. Microarray plotting presented a total of 5,459 genes that were expressed differentially after sevoflurane exposure (Figure 1). Three hundred and forty-five genes showed changes of log2 ratio with expression levels ≥ 3 (i.e., highly up- or down-regulated, supplementary Table 1 online (1.3MB, pdf) ). The Rtn4rl2 gene was the most up-regulated gene (Table 1). This gene is a member of the Nogo receptor family and may be involved in regulating axonal regeneration and plasticity in the adult central nervous system (Lauren et al., 2003). Notably, Chrm1, Chrm5, Tac1, Drd1a, and Drd2 genes showed expression levels of log2 ratio ≥ 3 (i.e., highly up-regulated). In contrast, serotonin-related genes, such as the 5htr3a gene, which are considered to be critically involved in PONV, showed no significant up- or down-regulation. The Lhx9 gene was the most down-regulated gene in the present study (Table 2). This gene encodes a member of the LIM homeobox gene family of developmentally expressed transcription factors. The Lmx1a gene, which is also a member of the LIM-homeobox gene family, was highly down-regulated in the present study.
Figure 1.
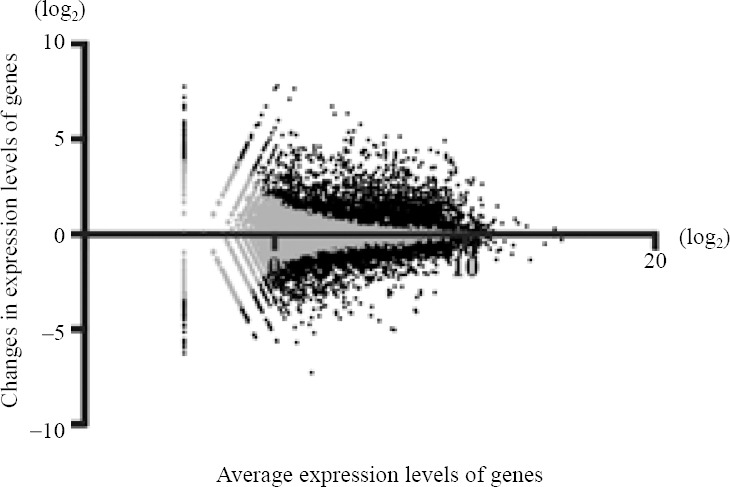
Changes in the expression levels of genes using microarray plotting.
The vertical axis represents the tendency of gene expression in the Sevoflurane group compared to the Naive group. The horizontal axis represents log ratios of the average expression in both groups. Grey closed circles indicate non-differentially expressed genes (i.e., false discovery rate ≥ 0.05), and black closed circles indicate differentially expressed genes
Table 1.
Genes highly up-regulated by sevoflurane exposure
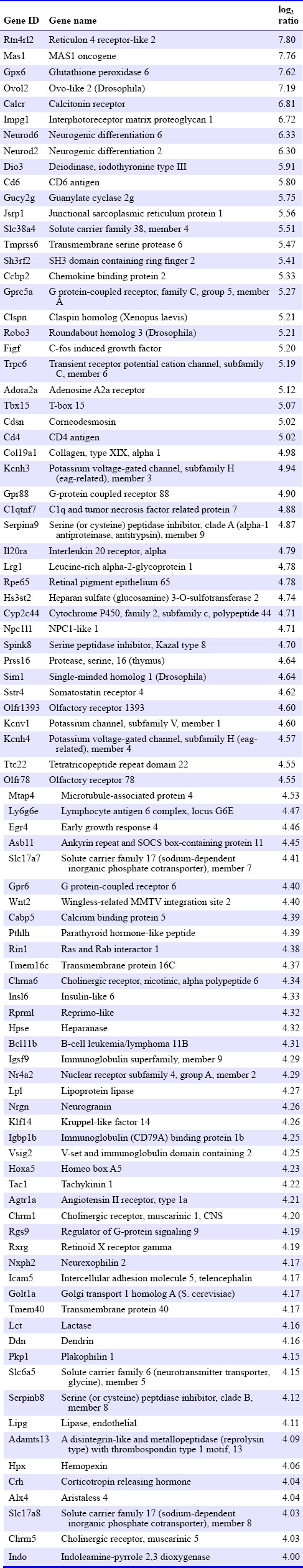
Table 2.
Genes highly down-regulated by sevoflurane exposure
Changes in the expression levels of genes induced by sevoflurane
DISCUSSION
We initially confirmed the quality of isolated RNA samples to confirm that all samples passed the primary quality control using the TapeStation system (i.e., total quantity > 1 μg and eRIN ≥ 8). The eRIN determined by 2500 Bioanalyzer Instruments (Agilent Technologies) has been reported to provide accurate information (Eger et al., 1965). Next, we demonstrated the changes in the comprehensive mRNA expression profile induced by sevoflurane exposure, and found that large numbers of genes in the hippocampus were up- or down-regulated by sevoflurane treatment for 1 hour. Notably, over 5,000 genes were significantly up- or down-regulated by sevoflurane. As seen in clinical situations, volatile anesthetics show minimal inter-individual differences in efficacy. This effect in the clinical setting might be due to the fact that volatile anaesthetics act via multimodal cell signalling (Schroeder et al., 2006). Our data showed that the Rtn4rl2 gene was the most up-regulated gene after sevoflurane exposure. The Nogo receptor, which is encoded by the Rtn4rl2 gene, is reportedly involved in the adhesion of dendritic cells to myelin in the central nervous system (McDonald et al., 2011). This data might reinforce the fact that general anesthesia induces neuronal inflammation in rodents, and the current concerns about the harm caused by general anesthesia to the developing brain (Shen et al., 2013). Further studies that uncover the molecular basis for this are needed to confirm the neuronal inflammation induced by the Nogo receptor family.
Although our data indicated that the Tac1, Drd1a, and Drd2 genes were highly up-regulated by exposure to sevoflurane, serotonin receptor families were not affected. This data supported our speculation that the hippocampus is related to PONV via the dopaminergic system. As mentioned above, the hippocampal neurons are projected through dopaminergic neurons (DiGruccio et al., 2015); our data also indicated that the hippocampal dopaminergic neurons might be susceptible to dopamine stimulation via sevoflurane exposure. Moreover, neurokinin1, which is encoded by the Tac1 gene, is also known to trigger PONV (Diemunsch et al., 2009). According to our data, neurokinin1 receptor antagonists and dopamine receptor antagonists might be useful for the treatment of PONV. Although our data did not provide evidence of the efficacy of serotonin receptor antagonists for the treatment of PONV, serotonin receptor antagonists are frequently used and have been recognized for their usefulness in clinical settings (Candiotti et al., 2014). We opined that this discordance is caused by the mechanism of serotonin-induced PONV. Surgical procedures directly induce serotonin secretion by enterochromaffin cells, and activate vagal afferent nerves connected to the nucleus tractus solitarii and area postrema (Bunce and Tyers, 1992; Fukui et al., 1992; Minami et al., 1996). We simply exposed the mice to sevoflurane for 1 hour in the present study in order to confirm the changes in the comprehensive mRNA expression profile induced by sevoflurane. We did not perform any surgical procedures on the mice to avoid surgical effects on the regulation of serotonin receptor families. A precise surgical mouse model is needed to confirm the relationship between serotonin receptor families and surgical procedures.
Chrm1 and Chrm5 genes were also highly up-regulated in the present study. Chrm1 and Chrm5 genes encode muscarinic cholinergic receptors 1 and 5, respectively. The muscarinic cholinergic receptor is known to be involved in the emetic pathway (Herrstedt et al., 1993), and recent human genome-wide association studies have indicated that the Chrm3 gene, which encodes the muscarinic cholinergic receptor 3, is the gene that is most associated with PONV (Janicki et al., 2011). Our results might support the fact that sevoflurane also potentiates PONV via the cholinergic pathway. Emerging data regarding the association between memory impairment and aging have shown that cholinergic fibers in the hippocampus are related to cognitive function or learning (He et al., 2014). The selective control of neural stem cell differentiation is expected to have therapeutic potential in cases with impaired memory or cognitive dysfunction (Gu et al., 2015). Our results might reflect the neuronal inflammation induced by sevoflurane and the effect on the repair mechanism. Therefore, further comprehensive mRNA expression profile studies in other nuclei, such as the nucleus tractus solitarii and area postrema, are needed to confirm our speculations, and the influence of duration of sevoflurane exposure on the mRNA expression profile also needs to be determined.
Regarding down-regulated genes, Lhx9 gene was the most down-regulated gene in the present study. The Lhx9 gene encodes a LIM-homeodomain factor, which is essential for the development of gonads, spinal cord interneurons, and thalamic neurons (Retaux et al., 1999; Birk et al., 2000; Failli et al., 2000). A recent study reported that the thalamocortical network shows hyperexcitability after exposure to general anesthesia during brain development (Todorovic; DiGruccio et al., 2015). Sevoflurane might suppress brain development via LIM-homeodomain factors, or compensate for the hyperexcitability of the thalamocortical network by suppressing LIM-homeodomain factors. Although we could not determine whether sevoflurane is harmful for the developing brain, sevoflurane might not improve neuronal inflammation, as indicated by the up-regulation of the Rtn4rl2 gene, the down-regulation of the Lhx9 gene, and previously reported neuronal inflammation pathways (Koivuranta et al., 1997). The Lmx1a gene was also highly down-regulated after sevoflurane exposure. This gene also encodes a LIM-homeodomain factor and is related to cell differentiation, especially of dopaminergic neurons (Fathi et al., 2015). Our data suggested that sevoflurane suppresses the differentiation of stem cells into dopaminergic neurons in the hippocampus. The difference between the risk factors of PONV in adults and children might be potentially related to the direct effect of general anesthesia on the differentiation of stem cells in the central nervous system (Eberhart et al., 2004).
Although we assessed the mRNA expression profile in the mouse hippocampus after sevoflurane exposure for 1 hour, this period of exposure corresponds to a relatively long surgery in humans. We did not examine the role of duration of sevoflurane exposure on the mRNA expression profile in the present study, and we could not determine whether the changes in the mRNA expression levels of individual genes were caused by sevoflurane per se or other pathways. However, our data indicated that the there was high variation in the mRNA expression profile after sevoflurane exposure. Although the molecular mechanisms of PONV after sevoflurane exposure were predicted in the present study, further experiments based on the regulation of individual genes are needed to confirm our speculations. Furthermore, we did not examine the behaviors of the animals that might suggest a feeling of nausea, because, although rodents are susceptible to emetic stimuli such as chemotherapy, mice lack an emetic response. While our data cannot be directly extrapolated to humans, they might provide clues for the molecular mechanism of PONV. In addition, the sample size was small in this study, despite having been determined to obtain a power of ≥ 0.8, and we overlooked changes in the expression of genes that were expressed at low levels. Further studies containing greater numbers of samples are needed to confirm the changes in genes that are expressed at low levels.
In conclusion, the expression of dopamine receptor and tachykinin genes was highly up-regulated in the hippocampus after exposure of mice to sevoflurane for 1 hour, suggesting that sevoflurane stimulates hippocampal dopaminergic neurons; these findings may be useful for exploring the molecular mechanisms of PONV. We found that sevoflurane regulated the genes involved in neuronal stem cell differentiation, which may be useful for exploring the molecular mechanisms of neuronal inflammation after general anesthesia.
Acknowledgements
This work was supported by a Grant-in-Aid for Young Scientists (B) (No. 24791606, 2012–2014, to TH) from the Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan.
Footnotes
Conflicts of interest
The authors declare that they have no competing interests.
Supplementary data
Supplementary information is available at https://dx.doi.org/10.6084/m9.figshare.3115486.v1
REFERENCES
- Beaudoin GM, 3rd, Lee SH, Singh D, Yuan Y, Ng YG, Reichardt LF, Arikkath J. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat Protoc. 2012;7:1741–1754. doi: 10.1038/nprot.2012.099. [DOI] [PubMed] [Google Scholar]
- Birk OS, Casiano DE, Wassif CA, Cogliati T, Zhao L, Zhao Y, Grinberg A, Huang S, Kreidberg JA, Parker KL, Porter FD, Westphal H. The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature. 2000;403:909–913. doi: 10.1038/35002622. [DOI] [PubMed] [Google Scholar]
- Bunce KT, Tyers MB. The role of 5-HT in postoperative nausea and vomiting. Br J Anaesth. 1992;69:60s–62s. doi: 10.1093/bja/69.supplement_1.60s. [DOI] [PubMed] [Google Scholar]
- Candiotti KA, Ahmed SR, Cox D, Gan TJ. Palonosetron versus ondansetron as rescue medication for postoperative nausea and vomiting: a randomized, multicenter, open-label study. BMC Pharmacol Toxicol. 2014;15:45. doi: 10.1186/2050-6511-15-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter DO. Neural mechanisms of emesis. Can J Physiol Pharmacol. 1990;68:230–236. doi: 10.1139/y90-036. [DOI] [PubMed] [Google Scholar]
- Diemunsch P, Joshi GP, Brichant JF. Neurokinin-1 receptor antagonists in the prevention of postoperative nausea and vomiting. Br J Anaesth. 2009;103:7–13. doi: 10.1093/bja/aep125. [DOI] [PubMed] [Google Scholar]
- DiGruccio MR, Joksimovic S, Joksovic PM, Lunardi N, Salajegheh R, Jevtovic-Todorovic V, Beenhakker MP, Goodkin HP, Todorovic SM. Hyperexcitability of rat thalamocortical networks after exposure to general anesthesia during brain development. J Neurosci. 2015;35:1481–1492. doi: 10.1523/JNEUROSCI.4883-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy RA. Potential therapeutic targets for neurokinin-1 receptor antagonists. Expert Opin Emerg Drugs. 2004;9:9–21. doi: 10.1517/eoed.9.1.9.32956. [DOI] [PubMed] [Google Scholar]
- Eberhart LH, Morin AM, Guber D, Kretz FJ, Schauffelen A, Treiber H, Wulf H, Geldner G. Applicability of risk scores for postoperative nausea and vomiting in adults to paediatric patients. Br J Anaesth. 2004;93:386–392. doi: 10.1093/bja/aeh221. [DOI] [PubMed] [Google Scholar]
- Eger EI, 2nd, Saidman LJ, Brandstater B. Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology. 1965;26:756–763. doi: 10.1097/00000542-196511000-00010. [DOI] [PubMed] [Google Scholar]
- Faerber L, Drechsler S, Ladenburger S, Gschaidmeier H, Fischer W. The neuronal 5-HT3 receptor network after 20 years of research--evolving concepts in management of pain and inflammation. Eur J Pharmacol. 2007;560:1–8. doi: 10.1016/j.ejphar.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Failli V, Rogard M, Mattei MG, Vernier P, Retaux S. Lhx9 and Lhx9alpha LIM-homeodomain factors: genomic structure, expression patterns, chromosomal localization, and phylogenetic analysis. Genomics. 2000;64:307–317. doi: 10.1006/geno.2000.6123. [DOI] [PubMed] [Google Scholar]
- Fathi A, Rasouli H, Yeganeh M, Salekdeh GH, Baharvand H. Efficient differentiation of human embryonic stem cells toward dopaminergic neurons using recombinant LMX1A factor. Mol Biotechnol. 2015;57:184–194. doi: 10.1007/s12033-014-9814-5. [DOI] [PubMed] [Google Scholar]
- Fukui H, Yamamoto M, Sato S. Vagal afferent fibers and peripheral 5-HT3 receptors mediate cisplatin-induced emesis in dogs. Jpn J Pharmacol. 1992;59:221–226. doi: 10.1254/jjp.59.221. [DOI] [PubMed] [Google Scholar]
- Gan TJ. Mechanisms underlying postoperative nausea and vomiting and neurotransmitter receptor antagonist-based pharmacotherapy. CNS drugs. 2007;21:813–833. doi: 10.2165/00023210-200721100-00003. [DOI] [PubMed] [Google Scholar]
- Gu G, Zhang W, Li M, Ni J, Wang P. Transplantation of NSC-derived cholinergic neuron-like cells improves cognitive function in APP/PS1 transgenic mice. Neuroscience. 2015;291:81–92. doi: 10.1016/j.neuroscience.2015.01.073. [DOI] [PubMed] [Google Scholar]
- He Y, Zhu J, Huang F, Qin L, Fan W, He H. Age-dependent loss of cholinergic neurons in learning and memory-related brain regions and impaired learning in SAMP8 mice with trigeminal nerve damage. Neural Regen Res. 2014;9:1985–1994. doi: 10.4103/1673-5374.145380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrstedt J, Hyttel J, Pedersen J. Interaction of the antiemetic metopimazine and anticancer agents with brain dopamine D2, 5-hydroxytryptamine3, histamine H1, muscarine cholinergic and alpha 1-adrenergic receptors. Cancer Chemother Pharmacol. 1993;33:53–56. doi: 10.1007/BF00686023. [DOI] [PubMed] [Google Scholar]
- Horn CC, Wallisch WJ, Homanics GE, Williams JP. Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. Eur J Pharmacol. 2014;722:55–66. doi: 10.1016/j.ejphar.2013.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde TM, Knable MB, Murray AM. Distribution of dopamine D1-D4 receptor subtypes in human dorsal vagal complex. Synapse (New York, NY) 1996;24:224–232. doi: 10.1002/(SICI)1098-2396(199611)24:3<224::AID-SYN4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Janicki PK, Vealey R, Liu J, Escajeda J, Postula M, Welker K. Genome-wide Association study using pooled DNA to identify candidate markers mediating susceptibility to postoperative nausea and vomiting. Anesthesiology. 2011;115:54–64. doi: 10.1097/ALN.0b013e31821810c7. [DOI] [PubMed] [Google Scholar]
- Kanaya A, Kuratani N, Satoh D, Kurosawa S. Lower incidence of emergence agitation in children after propofol anesthesia compared with sevoflurane: a meta-analysis of randomized controlled trials. J Anesth. 2014;28:4–11. doi: 10.1007/s00540-013-1656-y. [DOI] [PubMed] [Google Scholar]
- Koivuranta M, Laara E, Snare L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia. 1997;52:443–449. doi: 10.1111/j.1365-2044.1997.117-az0113.x. [DOI] [PubMed] [Google Scholar]
- Lauren J, Airaksinen MS, Saarma M, Timmusk T. Two novel mammalian Nogo receptor homologs differentially expressed in the central and peripheral nervous systems. Mol Cell Neurosci. 2003;24:581–594. doi: 10.1016/s1044-7431(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Ma J, Leung LS. Limbic system participates in mediating the effects of general anesthetics. Neuropsychopharmacology. 2006;31:1177–1192. doi: 10.1038/sj.npp.1300909. [DOI] [PubMed] [Google Scholar]
- Mattis J, Brill J, Evans S, Lerner TN, Davidson TJ, Hyun M, Ramakrishnan C, Deisseroth K, Huguenard JR. Frequency-dependent, cell type-divergent signaling in the hippocamposeptal projection. J Neurosci. 2014;34:11769–11780. doi: 10.1523/JNEUROSCI.5188-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CL, Steinbach K, Kern F, Schweigreiter R, Martin R, Bandtlow CE, Reindl M. Nogo receptor is involved in the adhesion of dendritic cells to myelin. J Neuroinflammation. 2011;8:113. doi: 10.1186/1742-2094-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M, Endo T, Hirafuji M. [Role of serotonin in emesis] Nihon Yakurigaku Zasshi. 1996;108:233–242. doi: 10.1254/fpj.108.233. [DOI] [PubMed] [Google Scholar]
- Napadow V, Sheehan JD, Kim J, Lacount LT, Park K, Kaptchuk TJ, Rosen BR, Kuo B. The brain circuitry underlying the temporal evolution of nausea in humans. Cereb Cortex (New York, NY: 1991) 2013;23:806–813. doi: 10.1093/cercor/bhs073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retaux S, Rogard M, Bach I, Failli V, Besson MJ. Lhx9: a novel LIM-homeodomain gene expressed in the developing forebrain. J Neurosci. 1999;19:783–793. doi: 10.1523/JNEUROSCI.19-02-00783.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics (Oxford, England) 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A, Imai J, Nishikawa A, Honma R, Ito E, Yanagisawa Y, Kawamura M, Ogawa R, Watanabe S. Influence of inhalation anesthesia assessed by comprehensive gene expression profiling. Gene. 2005;356:39–48. doi: 10.1016/j.gene.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Andrews PL. Treatment of nausea and vomiting: gaps in our knowledge. Auton Neurosci. 2006;129:3–16. doi: 10.1016/j.autneu.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118:502–515. doi: 10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair DR, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted. Anesthesiology. 1999;91:109–118. doi: 10.1097/00000542-199907000-00018. [DOI] [PubMed] [Google Scholar]
- Waters RP, Emerson AJ, Watt MJ, Forster GL, Swallow JG, Summers CH. Stress induces rapid changes in central catecholaminergic activity in Anolis carolinensis: restraint and forced physical activity. Brain Res Bull. 2005;67:210–218. doi: 10.1016/j.brainresbull.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Nohara K, Furuya T, Yamatodani A. Ondansetron, dexamethasone and an NK1 antagonist block radiation sickness in mice. Pharmacol Biochem Behav. 2005;82:24–29. doi: 10.1016/j.pbb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Yu Y, Zeng C, Shu S, Liu X, Li C. Similar effects of substance P on learning and memory function between hippocampus and striatal marginal division. Neural Regen Res. 2014;9:857–863. doi: 10.4103/1673-5374.131603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Changes in the expression levels of genes induced by sevoflurane


