Abstract
Normobaric oxygen (NBO) and hyperbaric oxygen (HBO) are emerging as a possible co-treatment of acute ischemic stroke. Both have been shown to reduce infarct volume, to improve neurologic outcome, to promote endogenous tissue plasminogen activator-induced thrombolysis and cerebral blood flow, and to improve tissue oxygenation through oxygen diffusion in the ischemic areas, thereby questioning the interest of HBO compared to NBO. In the present study, in order to investigate and compare the oxygen diffusion effects of NBO and HBO on acute ischemic stroke independently of their effects at the vascular level, we used acute brain slices exposed to oxygen and glucose deprivation, an ex vivo model of brain ischemia that allows investigating the acute effects of NBO (partial pressure of oxygen (pO2) = 1 atmospheres absolute (ATA) = 0.1 MPa) and HBO (pO2 = 2.5 ATA = 0.25 MPa) through tissue oxygenation on ischemia-induced cell injury as measured by the release of lactate dehydrogenase. We found that HBO, but not NBO, reduced oxygen and glucose deprivation-induced cell injury, indicating that passive tissue oxygenation (i.e. without vascular support) of the brain parenchyma requires oxygen partial pressure higher than 1 ATA.
Keywords: hyperbaric oxygen, normobaric oxygen, oxygen diffusion, lactate dehydogenase, cell injury, brain slices, oxygen and glucose deprivation, brain ischemia
INTRODUCTION
Hyperbaric oxygen (HBO) improves outcome in experimental cerebral ischemia and is therefore emerging as a possible co-treatment for acute ischemic stroke in addition of tissue plasminogen activator (tPA), whose recombinant form is considered the best approved treatment for acute brain ischemia to date (Peplow, 2015). Thus, despite controversial results that have shown that HBO enlarges ischemic brain damage by blocking autophagy (Lu et al., 2014) and further produces vasoconstriction (Stirban et al., 2009), a condition thought to be deleterious in stroke disease, numerous investigations have reported beneficial effects of HBO on infarct size and neurological deficits (Veltkamp et al., 2000, 2005; Eschenfelder et al., 2008; Yang et al., 2010; Xu et al., 2016). Although the mechanisms of action of HBO are not well established and are still lively under discussion, HBO has been shown to induce neurogenesis (Lee et al., 2013), to improve the decrease in tissue oxygenation induced by ischemia (Sun et al., 2008), to promote thrombolysis through activation of endogenous tPA (Chazalviel et al., 2016b), and to reduce the decrease in regional glucose metabolism (Lou et al., 2007). Likewise, interestingly, normobaric oxygen (NBO) has also been shown to reduce infarct size (Singhal et al., 2002; Henninger et al., 2007; David et al., 2012), to induce neurogenesis (Wagenfuhr et al., 2016), to promote endogenous tPA-induced thrombolysis (David et al., 2012), to increase cerebral blow flow and to improve the decrease in tissue oxygenation induced by ischemia (Liu et al., 2004, 2006; Shin et al., 2007; Baskerville et al., 2011), thereby questioning the interest of HBO compared to NBO in the treatment of acute brain ischemia.
Therefore, in the present study, we investigated and compared the effects of a post-insult treatment with NBO (partial pressure of oxygen (pO2) = 1 atmospheres absolute (ATA) = 0.1 MPa) or HBO (pO2 = 2.5 ATA = 0.25 MPa) on the release of lactate dehydrogenase (LDH) – used as a marker of cell injury – in acute brain slices exposed to oxygen and glucose deprivation (OGD), an ex vivo model of brain ischemia.
MATERIALS AND METHODS
Materials
Brain slices were drawn from male adult Sprague-Dawley rats (n = 15; Janvier, Le Genest Saint-Isle, France) weighing 250-280 g according to an animal use procedure approved by the University of Caen ethics committee in accordance with the Declaration of Helsinki and the framework of the French legislation for the use of animals in biomedical experimentation.
Rats were sacrificed by decapitation under anesthesia, and the brains were carefully removed and placed in ice-cold freshly prepared artificial cerebrospinal fluid (aCSF) containing 120 mM NaCl, 2 mM KCl, 2 mM CaCl2, 26 mM NaHCO3, 1.19 mM MgSO4, 1.18 mM KH2PO4, 11 mM D-glucose, and 30 mM HEPES (pH = 7.4). Coronal brain slices of 400-μm thickness including the striatum (anteriority: from −1.2 mm to +2 mm from bregma) were cut using a tissue chopper (Mickie Laboratory Engineering Co., Gomshall, Surrey, UK), and allowed to recover at room temperature for 45 minutes.
Intervention and total LDH release analysis
After recovery at room temperature, brain slices were incubated individually in a home made 16-vials versatile normobaric-hyperbaric chamber (Blatteau et al., 2014) that was placed in an oven at 36 ± 0.5°C. Temperature was controlled using a temperature probe placed in an empty vial. Each vial contained 1.3 mL of freshly prepared aCSF, saturated, and continuously bubbled with 100% oxygen (25 mL/min per vial). After a 30-minute period of stabilization, aCSF was renewed with oxygenated aCSF, and the slices were then incubated for an additional 1-hour period to allow recording basal LDH levels. Whereas sham slices were incubated for an additional 20-minute period in the same conditions, those corresponding to the ischemic groups were incubated in a glucose-free solution, saturated, and continuously bubbled with 100% nitrogen (OGD slices).
After this 20-minute period of OGD, the medium was replaced in all groups with freshly prepared aCSF, and the slices were treated and continuously bubbled for a 3-hour period with either normobaric medical air composed of 75% nitrogen and 25% oxygen (control slices) or with normobaric 100% oxygen (NBO-treated slices). HBO treated slices were pressurized at a compression rate of 1 ATA/min with 100% oxygen up to 2.5 ATA. After a 3-hour period at 2.5 ATA, during which increased oxygen level was provided to the slices through oxygen diffusion and equilibrium between “air” and saline, decompression was performed at a slow decompression rate of 0.1 ATA/min shown to induce no cell injury (Baskerville et al., 2011; Blatteau et al., 2014). To avoid multiple compression and decompression in the HBO experiment, aCSF was not replaced and served as a pool throughout the 3-hour period of treatment with medical air, NBO or HBO.
OGD-induced neuronal injury was quantified by the amount of LDH released in the incubation solution samples. LDH activity was measured using a spectrophotometer at 340 nm in 50 μL of incubation medium by following the oxidation (decrease in absorbance) of 100 mL of β nicotinamide adenine dinucleotide (NADH) (3 mg in 10 mL of PBS) in 20 μL of sodium pyruvate (6.25 mg in 10 mL of PBS) using a microplate reader. OGD-induced LDH effluxes were expressed as the amount of LDH measured in the incubation solution and as a percentage of pre-OGD control value. The number of animals and the number of slices was respectively n = 4-6 and n = 24-32 per condition.
Statistical analysis
Data are expressed as the mean ± standard error of the mean, and were analyzed using parametric statistics. Between-group comparisons on total LDH release were performed using parametric ANOVA. Following a significant F value, post-hoc analysis was performed using the Tukey's honestly significant difference method for samples of different size (online software: http://statistica.mooo.com/OneWay_Anova_with_TukeyHSD). Level of significance was set up at P < 0.05.
RESULTS
Brain slices were exposed to experimental ischemia in the form of OGD to determine the effect of NBO and HBO on OGD-induced neuronal injury as assessed by the release of LDH. Figure 1 illustrates the effects of a 3-hour treatment with of NBO (pO2 = 1 ATA) or HBO (pO2 = 2.5 ATA) on LDH release induced by OGD. Exposure to OGD led to an increase in LDH release compared with sham slices (Tukey HSD value = 0.0010053; P < 0.01). Post-insult treatment with NBO showed no significant effect on OGD-induced LDH release compared to control slices treated with air (Tukey HSD value = 0.8975409). In contrast, post-insult treatment with HBO led to a significant reduction in LDH release compared to both control slices and NBO-treated slices (Tukey HSD value = 0.0010053; P < 0.01).
Figure 1.
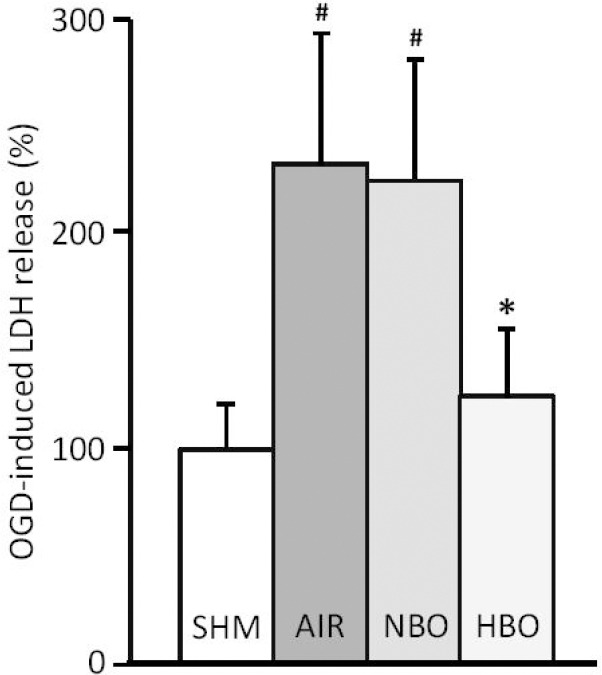
Exposure to oxygen and glucose deprivation (OGD) results in an increase of lactate dehydrogenase (LDH) release compared to sham (SHM) slices taken as a 100% value.
Note: Hyperbaric oxygen (HBO), but not normobaric oxygen (NBO), reduces LDH release in brain slices exposed to OGD compared to control air-treated slices (AIR). #P < 0.01, vs. sham slices; *P < 0.01, vs. control air-treated slices.
DISCUSSION
Before discussing our findings, possible limitations in study design should be examined. First, aCSF was used as a pool for brain slices and was not replaced throughout the experiment, conditions that could have lead to LDH decay or accumulation. However, we used this protocol to avoid multiple compression and decompression in the HBO experiment, conditions that would have led to LDH release induced by decompression stress (Blatteau et al., 2014, 2015) and therefore to experimental bias compared to the control and NBO groups. Second, no hyperbaric experiment was performed with 10% oxygen to investigate the possible effect of pressure per se. However, support for an effect of HBO rather than pressure per se is previous data performed with the same device in our laboratory in in vitro models of thrombolysis (Abraini, 2013; Chazalviel et al., 2016b). In addition, from a clinical perspective, this point is not of major critical importance since such a procedure with 10% oxygen is not current therapeutic practice. Finally, the cerebral slices’ vital activity was not measured. However, we have previously shown using pharmacological and neurochemical approaches measuring carrier-mediated- and KCl-evoked dopamine release that acute brain slices exposed to similar control and OGD conditions that those used in the present report remained functional (David et al., 2008).
That said, both NBO and HBO have been shown to reduce infarct size (Veltkamp et al., 2000, 2005; Singhal et al., 2002; Henninger et al., 2007; Eschenfelder et al., 2008; Yang et al., 2010; David et al., 2012; Xu et al., 2016), to promote endogenous tPA-induced thrombolysis (David et al., 2012; Chazalviel et al., 2016b), to improve ischemia-induced decrease in tissue oxygenation (Liu et al., 2004, 2006; Shin et al., 2007; Sun et al., 2008; Baskerville et al., 2011), and to induce neurogenesis (Lee et al., 2013; Wagenfuhr et al., 2016), thereby questioning the interest of HBO compared to NBO in stroke. In the present study, to investigate this question, we compare the oxygen diffusion effects of NBO and HBO in acute brain slices exposed to OGD, an ex vivo model of brain ischemia that allows investigating the acute effects of NBO and HBO on tissue (parenchyma) oxygenation independently of their facilitating action on cerebral blood flow and thrombolysis at the vascular level and of their long term effects on neurogenesis. We found that HBO, but not NBO, reduced OGD-induced cell injury, thereby indicating that to be fully efficient oxygen diffusion-induced tissue oxygenation of the brain parenchyma requires oxygen partial pressure higher than 1 ATA. Consistent with our findings of a lack of significant effect of NBO through passive-mediated oxygen transport is the fact that both NBO and HBO, administered 1 hour before thrombolysis, have been shown to reduce infarct size in rats subjected to transient thromboembolic brain ischemia, but that only HBO but not NBO has been further demonstrated to decrease infarct volume in permanent thromboembolic middle cerebral artery occlusion-induced ischemia (Sun et al., 2010). The apparent discrepancy between our finding of a lack of effect of NBO at reducing cell injury in brain slices exposed to OGD and the beneficial effect of NBO at reducing infarct size in rats subjected to transient brain ischemia (Sun et al., 2010) could be due to the fact that this latter study was performed in vivo, conditions in which microvasculature could play a major role in oxygen transport (Chazalviel et al., 2016a). Indeed, interestingly, as a possible mechanism for the facilitating action of NBO on cerebral blood flow, NBO has been demonstrated to promote endogenous tPA-induced thrombolysis (David et al., 2012), effect that could maintain the microvasculature of the ischemic areas opened despite ischemia-induced thrombin generation and blood platelet aggregation (Chazalviel et al., 2016a).
Alternatively and in contrast with these beneficial effects, there is a growing number of evidence highlighting potential harmful effect of hyperoxia in acute ischemic events such as stroke and cardiac arrest (Austin et al., 2016; Sepehrvand and Ezekowitz, 2016). These effects are suggested to be gauged by the increased production of reactive oxygen species and the related oxidative stress resulting from hyperoxia-induced vasoconstriction in the cerebral, coronary, and systemic vasculature. As a consequence, targeting oxidative stress and inflammation in addition of excitotoxicity has been suggested as a promising strategy (Chamorro et al., 2016). However, providing hyperoxia through NBO or HBO during ischemia (Veltkamp et al., 2000, 2005; Singhal et al., 2002; Henninger et al., 2007; Eschenfelder et al., 2008; Yang et al., 2010; David et al., 2012; Xu et al., 2016), but not during reperfusion (Mickel et al., 1987; Aronowski et al., 1997; Haelewyn et al., 2011), has been repeatedly shown to be a safe and effective therapy in animal models of acute brain ischemia. Consistent with these data, recent findings have shown that very brief exposure to HBO of 5-minute duration reduced ischemic brain damage probably by promoting thrombolysis, while in contrast longer exposure to HBO of 25-minute duration increases brain damage (Chazalviel et al., 2016b). Therefore, it is likely that hyperoxia could have dual effects: on one hand, inducing benefits when administered during ischemia by promoting thrombolysis (David et al., 2012) thereby avoiding blood platelet aggregation and coagulation (Chazalviel et al., 2016a) and increasing oxygen tension in the ischemic penumbra (Liu et al., 2004, 2006; Shin et al., 2007; Sun et al., 2008; Baskerville et al., 2011) through vascular oxygen transport (Shin et al., 2007; Baskerville et al., 2011; Chazalviel et al., 2016a) and so far HBO is concerned passive-mediated oxygen transport as shown in the present study, and on the other hand by inducing adverse responses through oxidative stress and free radical formation that would overturn the benefits of hyperoxia and particularly HBO when administered in a prolonged fashion after ischemia (Mickel et al., 1987; Aronowski et al., 1997; Austin et al., 2016; Chamorro et al., 2016; Sepehrvand and Ezekowitz, 2016).
In conclusion, this study provides evidence that HBO, but not NBO, can induce passive-mediated oxygen diffusion (i.e. without vascular support) of the brain parenchyma. This indicates that tissue oxygenation through oxygen diffusion requires oxygen partial pressures higher than 1 ATA. This highlights one of the mechanisms by which HBO, in addition of other multiple processes, seems to be efficient at reducing brain damage in acute stroke models.
Footnotes
Conflicts of interest
The authors declared no competing interest.
REFERENCES
- Abraini JH. Oxygen for the ischemic organ: much more than an oxygen provider. Undersea Hyperb Med. 2013;40:211–212. [PubMed] [Google Scholar]
- Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Austin V, Crack PJ, Bozinovski S, Miller AA, Vlahos R. COPD and stroke: are systemic inflammation and oxidative stress the missing links? Clin Sci (Lond) 2016;130:1039–1050. doi: 10.1042/CS20160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville TA, Deuchar GA, McCabe C, Robertson CA, Holmes WM, Santosh C, Macrae IM. Influence of 100% and 40% oxygen on penumbral blood flow, oxygen level, and T2*-weighted MRI in a rat stroke model. J Cereb Blood Flow Metab. 2011;31:1799–1806. doi: 10.1038/jcbfm.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatteau JE, David HN, Vallee N, Meckler C, Demaistre S, Risso JJ, Abraini JH. Cost-efficient method and device for the study of stationary tissular gas bubble formation in the mechanisms of decompression sickness. J Neurosci Methods. 2014;236:40–43. doi: 10.1016/j.jneumeth.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Blatteau JE, David HN, Vallée N, Demaistre S, Lambrechts K, Risso JJ, Abraini JH. Xenon blocks neuronal injury induced by tissular gas bubble formation in decompression sickness. Sci Rep. 2015;5:15093. doi: 10.1038/srep15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- Chazalviel L, David HN, Haelewyn B, Blatteau JE, Vallee N, Risso JJ, Besnard S, Abraini JH. The underestimated effect of normobaric hyperoxia on cerebral blood flow and its relationship to neuroprotection. Brain. 2016a doi: 10.1093/brain/aww178. doi: 10.1093/brain/aww.178. [DOI] [PubMed] [Google Scholar]
- Chazalviel L, Haelewyn B, Degoulet M, Blatteau JE, Vallée N, Risso JJ, Besnard S, Abraini JH. Hyperbaric oxygen increases tissue-plasminogen activator-induced thrombolysis in vitro, and reduces ischemic brain damage and edema in rats subjected to thromboembolic brain ischemia. Med Gas Res. 2016b;6:64–69. doi: 10.4103/2045-9912.184713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David HN, Haelewyn B, Degoulet M, Colomb DG, Jr, Risso JJ, Abraini JH. Prothrombolytic action of normobaric oxygen given alone or in combination with recombinant tissue-plasminogen activator in a rat model of thromboembolic stroke. J Appl Physiol (1985) 2012;112:2068–2076. doi: 10.1152/japplphysiol.00092.2012. [DOI] [PubMed] [Google Scholar]
- David HN, Haelewyn B, Rouillon C, Lecoq M, Chazalviel L, Apiou G, Risso JJ, Lemaire M, Abraini JH. Neuroprotective effects of xenon: a therapeutic window of opportunity in rats subjected to transient cerebral ischemia. FASEB J. 2008;22:1275–1286. doi: 10.1096/fj.07-9420com. [DOI] [PubMed] [Google Scholar]
- Eschenfelder CC, Krug R, Yusofi AF, Meyne JK, Herdegen T, Koch A, Zhao Y, Carl UM, Deuschl G. Neuroprotection by oxygen in acute transient focal cerebral ischemia is dose dependent and shows superiority of hyperbaric oxygenation. Cerebrovasc Dis. 2008;25:193–201. doi: 10.1159/000113856. [DOI] [PubMed] [Google Scholar]
- Haelewyn B, Chazalviel L, Nicole O, Lecocq M, Risso JJ, Abraini JH. Moderately delayed post-insult treatment with normobaric hyperoxia reduces excitotoxin-induced neuronal degeneration but increases ischemia-induced brain damage. Med Gas Res. 2011;1:2. doi: 10.1186/2045-9912-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninger N, Bouley J, Nelligan JM, Sicard KM, Fisher M. Normobaric hyperoxia delays perfusion/diffusion mismatch evolution, reduces infarct volume, and differentially affects neuronal cell death pathways after suture middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2007;27:1632–1642. doi: 10.1038/sj.jcbfm.9600463. [DOI] [PubMed] [Google Scholar]
- Lee YS, Chio CC, Chang CP, Wang LC, Chiang PM, Niu KC, Tsai KJ. Long course hyperbaric oxygen stimulates neurogenesis and attenuates inflammation after ischemic stroke. Mediators Inflamm 2013. 2013 doi: 10.1155/2013/512978. 512978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Shi H, Liu W, Furuichi T, Timmins GS, Liu KJ. Interstitial pO 2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24:343–349. doi: 10.1097/01.WCB.0000110047.43905.01. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:1274–1284. doi: 10.1038/sj.jcbfm.9600277. [DOI] [PubMed] [Google Scholar]
- Lou M, Zhang H, Wang J, Wen SQ, Tang ZQ, Chen YZ, Yan WQ, Ding MP. Hyperbaric oxygen treatment attenuated the decrease in regional glucose metabolism of rats subjected to focal cerebral ischemia: a high resolution positron emission tomography study. Neuroscience. 2007;146:555–561. doi: 10.1016/j.neuroscience.2007.01.046. [DOI] [PubMed] [Google Scholar]
- Lu Y, Kang J, Bai Y, Zhang Y, Li H, Yang X, Xiang X, Wang X, Huang Y, Su J, Chen Y, Li B, Sun L. Hyperbaric oxygen enlarges the area of brain damage in MCAO rats by blocking autophagy via ERK1/2 activation. Eur J Pharmacol. 2014;728:93–99. doi: 10.1016/j.ejphar.2014.01.066. [DOI] [PubMed] [Google Scholar]
- Mickel HS, Vaishnav YN, Kempski O, von Lubitz D, Weiss JF, Feuerstein G. Breathing 100% oxygen after global brain ischemia in Mongolian Gerbils results in increased lipid peroxidation and increased mortality. Stroke. 1987;18:426–430. doi: 10.1161/01.str.18.2.426. [DOI] [PubMed] [Google Scholar]
- Peplow PV. Neuroimmunomodulatory effects of transcranial laser therapy combined with intravenous tPA administration for acute cerebral ischemic injury. Neural Regen Res. 2015;10:1186–1190. doi: 10.4103/1673-5374.162687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehrvand N, Ezekowitz JA. Oxygen therapy in patients with acute heart failure: friend or foe? JACC Heart Fail. 2016 doi: 10.1016/j.jchf.2016.03.026. doi: 10.1016/j.jchf.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Shin HK, Dunn AK, Jones PB, Boas DA, Lo EH, Moskowitz MA, Ayata C. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain. 2007;130:1631–1642. doi: 10.1093/brain/awm071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal AB, Dijkhuizen RM, Rosen BR, Lo EH. Normobaric hyperoxia reduces MRI diffusion abnormalities and infarct size in experimental stroke. Neurology. 2002;58:945–952. doi: 10.1212/wnl.58.6.945. [DOI] [PubMed] [Google Scholar]
- Stirban A, Lentrodt S, Nandrean S, Pop A, Tschoepe D, Scherbaum WA. Functional changes in microcirculation during hyperbaric and normobaric oxygen therapy. Undersea Hyperb Med. 2009;36:381–390. [PubMed] [Google Scholar]
- Sun L, Marti HH, Veltkamp R. Hyperbaric oxygen reduces tissue hypoxia and hypoxia-inducible factor-1 alpha expression in focal cerebral ischemia. Stroke. 2008;39:1000–1006. doi: 10.1161/STROKEAHA.107.490599. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhou W, Mueller C, Sommer C, Heiland S, Bauer AT, Marti HH, Veltkamp R. Oxygen therapy reduces secondary hemorrhage after thrombolysis in thromboembolic cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1651–1660. doi: 10.1038/jcbfm.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltkamp R, Warner DS, Domoki F, Brinkhous AD, Toole JF, Busija DW. Hyperbaric oxygen decreases infarct size and behavioral deficit after transient focal cerebral ischemia in rats. Brain Res. 2000;853:68–73. doi: 10.1016/s0006-8993(99)02250-7. [DOI] [PubMed] [Google Scholar]
- Veltkamp R, Siebing DA, Sun L, Heiland S, Bieber K, Marti HH, Nagel S, Schwab S, Schwaninger M. Hyperbaric oxygen reduces blood-brain barrier damage and edema after transient focal cerebral ischemia. Stroke. 2005;36:1679–1683. doi: 10.1161/01.STR.0000173408.94728.79. [DOI] [PubMed] [Google Scholar]
- Wagenfuhr L, Meyer AK, Marrone L, Storch A. Oxygen tension within the neurogenic niche regulates dopaminergic neurogenesis in the developing midbrain. Stem Cells Dev. 2016;25:227–238. doi: 10.1089/scd.2015.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Ji R, Wei R, Yin B, He F, Luo B. The efficacy of hyperbaric oxygen therapy on middle cerebral artery occlusion in animal studies: a meta-analysis. PLoS One. 2016;11:e0148324. doi: 10.1371/journal.pone.0148324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, Xie Y, Bosco GM, Chen C, Camporesi EM. Hyperbaric oxygenation alleviates MCAO-induced brain injury and reduces hydroxyl radical formation and glutamate release. Eur J Appl Physiol. 2010;108:513–522. doi: 10.1007/s00421-009-1229-9. [DOI] [PubMed] [Google Scholar]


