Abstract
Because inhaled carbon monoxide (CO) provides potent anti-inflammatory and antioxidant effects against ischemia reperfusion injury, we hypothesized that treatment of organ donors with inhaled CO would decrease graft injury after heart transplantation. Hearts were heterotopically transplanted into syngeneic Lewis rats after 8 hours of cold preservation in University of Wisconsin solution. Donor rats were exposed to CO at a concentration of 250 parts per million for 24 hours via a gas-exposure chamber. Severity of myocardial injury was determined by total serum creatine phosphokinase and troponin I levels at three hours after reperfusion. In addition, Affymetrix gene array analysis of mRNA transcripts was performed on the heart graft tissue prior to implantation. Recipients of grafts from CO-exposed donors had lower levels of serum troponin I and creatine phosphokinase; less upregulation of mRNA for interleukin-6, intercellular adhesion molecule-1, and tumor necrosis factor-α; and fewer infiltrating cells. Although donor pretreatment with CO altered the expression of 49 genes expressly represented on the array, we could not obtain meaningful data to explain the mechanisms by which CO potentiated the protective effects. Pretreatment with CO gas before organ procurement effectively protected cardiac grafts from ischemia reperfusion-induced injury in a rat heterotopic cardiac transplant model. A clinical report review indicated that CO-poisoned organ donors may be comparable to non-poisoned donors.
Keywords: ischemia/reperfusion, carbon monoxide, cardiac transplantation, donor, pretreatment, gene array, cold preservation
INTRODUCTION
In critical care medicine, therapeutic gases have received growing attention. Gases have significant advantages, including their low cost, relative abundance, and feasibility of administration, making them ideal potential therapies for critically-ill patients. Trials investigating inhaled carbon monoxide (CO) to prevent rejection and inflammation following solid organ transplant are currently being conducted (Resch et al., 2005; Bathoorn et al., 2007). Preliminary results show that inhaled CO could be adopted for use in the intensive care unit (ICU) in less than 10 years (Robinson et al., 2009).
Several previous studies (Nakao et al., 2003; Akamatsu et al., 2004; Nakao et al., 2006b; Nakao and Toyoda, 2012) have shown the feasibility of CO as a new therapeutic strategy for organ transplantation. CO is well known for its high dose toxicology by binding to hemoglobin and displacing oxygen, leading to tissue hypoxia. However, CO is also known to be physiologically and endogenously generated in mammalian cells via heme catabolism in a rate-limiting step of heme oxygenase systems. CO powerfully protects against cellular injury (Otterbein et al., 1999, 2000; Ryter and Choi, 2010) and reduces inflammatory responses and ischemia/reperfusion (I/R) injury, which is obligate in the surgical procedure for heart transplantation and recognized as a major determinant of primary graft dysfunction. CO relaxes the blood vessels and exerts anti-thrombotic effects by hindering platelet aggregation and depressing fibrinolysis (Fujita et al., 2001). CO also inhibits apoptosis of epithelial and endothelial cells and reduces proliferation of T lymphocytes, fibroblasts, and smooth muscle cells (Nakao et al., 2006b). Therefore, collective evidence supports the idea that CO treatment applied to transplant organs, donors, or recipients can inhibit graft dysfunction from rejection or I/R injury (Nakao et al., 2003, 2006a; Nakao and Toyoda, 2012). Effects of organ protection through molecular biological signal transmission such as mentioned above were already provided, but changes in vivo gene expressions due to external environments are unclear.
We believe that CO may play an important role in the ICU for potential organ donors in the near future. Furthermore, recent data demonstrated that CO-poisoned patients may be acceptable organ donors (Fujisaki et al., 2014), although additional studies, including human clinical trials, are absolutely warranted. Therefore, we hypothesized that prolonged CO pretreatment of a potential organ donor may reduce I/R injury of the cardiac grafts. This study was designed to determine whether organs are suitable and secure for transplantation when donors in the ICU are treated with inhaled CO for a prolonged period.
MATERIALS AND METHODS
Animals
Male Lewis rats (LEW, RT1) weighing 200-250 g were purchased from Japan SLC Inc. (Shizuoka, Japan) and were kept in individual stainless steel cages in a temperature-, humidity-, and light-controlled room (23 ± 3°C, 55 ± 15%; 12-hour light-dark cycle) for 2-5 weeks before the experiments. During this period, all animals were provided standard food (AIN-93G diet; Oriental Kobo Corporation, Tokyo, Japan) and free access to water. All procedures involving rats were conducted in accordance with the guidelines of the Animal Care and Use Committees of the Hyogo College of Medicine and complied with the National Research Council's Guide for the Humane Care and Use of Laboratory Animals.
CO exposure
Donor animals were exposed to CO (250 parts per million (ppm)) in air in a stainless steel mixing cylinder and then directed into a 26 cm × 38 cm × 24 cm acrylic board exposure chamber at a flow rate of 1.5 L/min. A CO analyzer (Taiyo, Osaka, Japan) was used to continuously measure CO levels in the chamber to maintain CO concentration at 250 ppm. Animals were maintained in a CO chamber at a concentration of 250 ppm for the duration of the CO exposure period with regular diet and water ad libitum. Carboxyhemoglobin was measured using an OSM3 Hemoximeter (Radiometer Copenhagen, Copenhagen, Denmark). After donor animals were treated with CO, blood carboxyhemoglobin levels significantly increased 22.4% from 1.8% in the sham control groups.
Heterotopic heart transplantation
We performed heterotopic heart transplantation as described previously (Ono and Lindsey, 1969; Nakao et al., 2010). Shortly after anticoagulation with 200 units of heparin, 3–5 mL cold University of Wisconsin (UW) solution (Astellas Pharma Inc., Tokyo, Japan) was infused into the heart through the inferior vena cava to induce cardiac arrest and the heart grafts were excised. Excised grafts were stored in UW solution at 4°C for 8 hours and transplanted into the abdomens of recipient rats. Reanimation times from reperfusion to the start of sinus rhythm in the cardiac graft were monitored. At sacrifice, tissue and blood samples were fixed for histopathological analyses or snap-frozen in liquid nitrogen and kept at -80°C until analyzed.
Experimental groups
Three experimental groups were examined. In the air group, the donors were kept in an air-contained gas exposure chamber for 24 hours (n = 18). In the CO group, the donors were maintained in the gas exposure chamber and exposed to 250 ppm of CO for 24 hours (n = 17). In addition to the transplant groups, naïve animals were used as sham operation rats (n = 6).
Measurement of myocardial injury
Serum total creatine phosphokinase (CPK) was assayed using a Beckman autoanalyzer (Beckman Instruments, Fullerton, CA, USA). We evaluated the gross morphologyof the grafts with their identities masked and assigned a transplant score based on color (1: dead; 2: dark; 3: partially dark; 4: nearly healthy; 5: healthy or normal), contractility (1: dead; 2: not much; 3: moderate function; 4: pretty good; 5: excellent function or normal) and hardness (1: dead; 2:hard; 3: partially hard; 4:nearly soft; 5: soft or normal) at 6 hours post-reperfusion.
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from transplanted heart tissues employing TRIzol® Reagent (Life Technologies, Inc. Waltham, MA, USA). The mRNA levels of interleukin (IL)-6, intercellular adhesion molecule-1 (ICAM-1), tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS), endothelin (ET)1, vascular endothelial growth factor (VEGF), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were quantified using SYBR Green 2-step, RT-PCR in duplicate as previously described (Nakao et al., 2003). Gene expression was normalized to GAPDH mRNA.
Histopathological examination
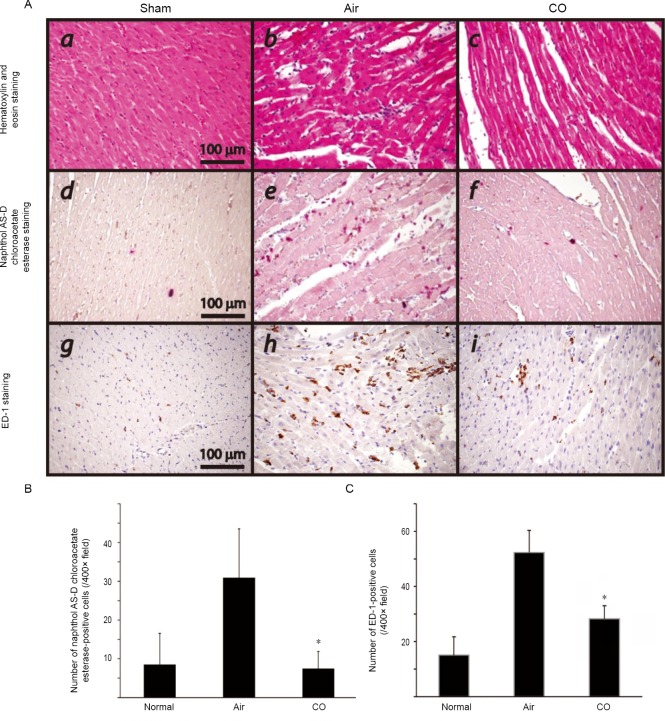
For histopathological examination, heart grafts were harvested at six hours after transplantation and were fixed in 4% neutral buffered paraformaldehyde for 24 hours. The tissue, which was taken from the middle part of the left ventricle and stained with hematoxylin and eosin, was embedded in paraffin and sectioned at 3 μm thickness. Sections were also stained using a naphthol AS-D chloroacetate esterase staining kit (Sigma Diagnostics, St. Louis, MO, USA) for the presence of granulocytes according to the manufacturer's instructions. Bright red-stained neutrophils infiltrated into the cardiac muscle cells and were counted by several people on the research team. Histopathological neutrophil counting analysis was executed with the samples’ identities masked.
Grafted heart tissue was fixed in 4% paraformaldehyde, embedded in paraffin, cut into 3 μm sections, and incubated overnight at room temperature with mouse anti-rat CD68 monoclonal antibody (ED-1, Serotec, Raleigh, NC, USA) to detect macrophages. A secondary antibody conjugated with the Histofine Simple Stain MAX-PO (Nichirei Bioscience, Tokyo, Japan) was applied at room temperature for 30 minutes. Visualization with diaminobenzidine (Liquid DAB+ substrate chromogen system; Dako Japan, Tokyo, Japan) was performed and then counterstained with Mayer's hematoxylin. Slides were visualized with an Olympus DP72 microscope (Olympus, Tokyo, Japan) and images were acquired digitally. Positively-stained cells were counted with the samples’ identities masked. Data are presented as the number of positive cells per 400× high-power field (HPF).
Gene array analysis
The differential gene-expression pattern of the cardiac grafts was studied. Graft tissue was collected for analysis after 24 hours preservation (prior to implantation into the recipient). Changes in gene expression in the grafts taken from the donors exposed to CO for 24 hours were compared to those taken from the donors treated with air. Total RNA from whole transplanted heart specimens was obtained using TRIzol® Reagent according to the manufacturer's recommended protocol. RNA was dissolved with RNAase free water and refined to appropriate RNA concentration. Subsequent Agilent microarray processes were committed to Takara Bio Inc. (Shiga, Japan) on three rat specimens per group (air group and the inhaled CO group). Results of microarray data were thresholded using a false discovery rate (FDR) of 0.1 (Benjamini and Hochberg, 1995).
Statistical analysis
Results are expressed as mean with standard error. All data were analyzed using SPSS Version 12 statistical software (SPSS Inc., Chicago, IL, USA). When analysis of variance (ANOVA) indicated a significant overall effect, differences among individual means were assessed using the Bonferroni post hoc test for multiple comparisons. A P value of < 0.05 was considered statistically significant.
RESULTS
Donor pretreatment with CO attenuated ischemia/reperfusion myocardial damage of the graft
Myocardial injury severity was determined by serum troponin I and CPK levels at 3h after reperfusion. With prolonged preservation for 8 hours, serum CPK levels increased to 4,265 ± 477 from 178.3 ± 33.1 IU/L in normal animals. Donor CO pretreatment significantly reduced CPK levels to 2,132 ± 353 IU/L. Similarly, serum troponin I concentrations of the recipients that received air-treated grafts increased to 154 ± 53.1 IU/L 3 hours after reperfusion, while the troponin I levels of naïve animals were 10.7 ± 6.2 IU/L. On the other hand, serum troponin I levels of the recipients that received CO-treated grafts 3 hours after reperfusion were significantly lower (76.3 ± 14.9 IU/L) compared to those with untreated grafts (Figure 1).
Figure 1.
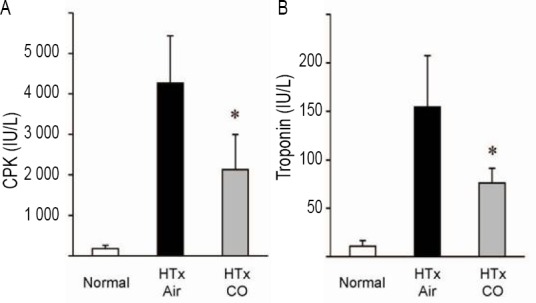
Serum creatinine phosphokinase (CPK) and troponin I levels.
Note: Severity of myocardial damage after reperfusion was determined by serum CPK (A) and troponin I (B) levels 3 hours after reperfusion and 8 hours after cold ischemia. Both serum CPK and troponin I levels were markedly elevated in the grafts of air group; however donor carbon monoxide (CO) treatment significantly reduced these serum markers of myocardial injury in the syngeneic recipients of grafts. Values are expressed as mean ± SD. n = 6-8 for each group, *P < 0.05, vs. air group. HTx: Heart transplantation.
CO improved gross appearance of the cardiac grafts
To assess cardiac graft function, we evaluated the gross morphology of the grafts with their identities masked. Graft preservation in UW solution for 8 hours and reperfusion was associated with deterioration of the gross appearance of the cardiac grafts, resulting in prolonged reanimation time, darker color, weaker heart beat, and harder cardiac grafts. However, cardiac grafts taken from the donors pretreated with CO demonstrated quicker reanimation time and improved gross structural appearance, indicating earlier functional recovery (Figure 2).
Figure 2.
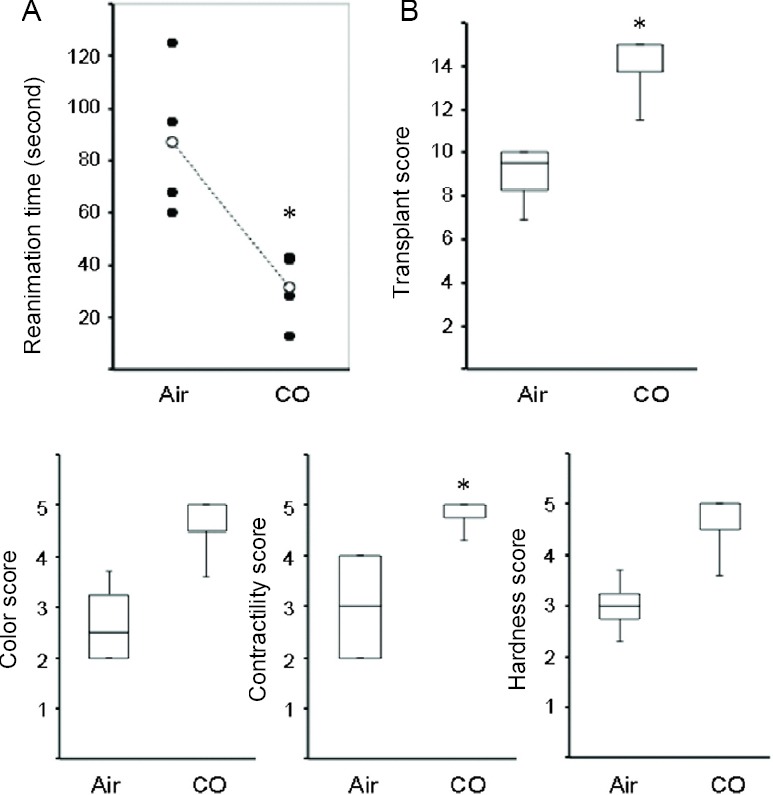
Macroscopic observations of cardiac grafts.
Note: (A) Time to reanimation after reperfusion. (B) Transplant score means total score added each following parameters, color, contractility and hardness. Each parameter scored on a scale of 1 to 4. n = 4 for each group, *P < 0.05, vs. air group. CO: Carbon monoxide.
Pretreatment with co attenuated graft inflammatory response
I/R injury leads to early activation of inflammatory mediators such as proinflammatory cytokines and adhesion molecules. Intragraft mRNAs levels for TNF-α, IL-6, iNOS, ICAM-1, ET-1, and VEGF were significantly increased after eight hours of cold storage and 3 hours of reperfusion. Pretreatment with CO inhibited the upregulation of TNF-α, IL-6, iNOS, and ICAM-1 mRNA levels. CO treatment did not affect the mRNA levels of ET-1. Interestingly, mRNA expression of VEGF significantly increased in the CO-pretreated grafts three hours after reperfusion compared to that in the air-pretreated grafts (Figure 3).
Figure 3.
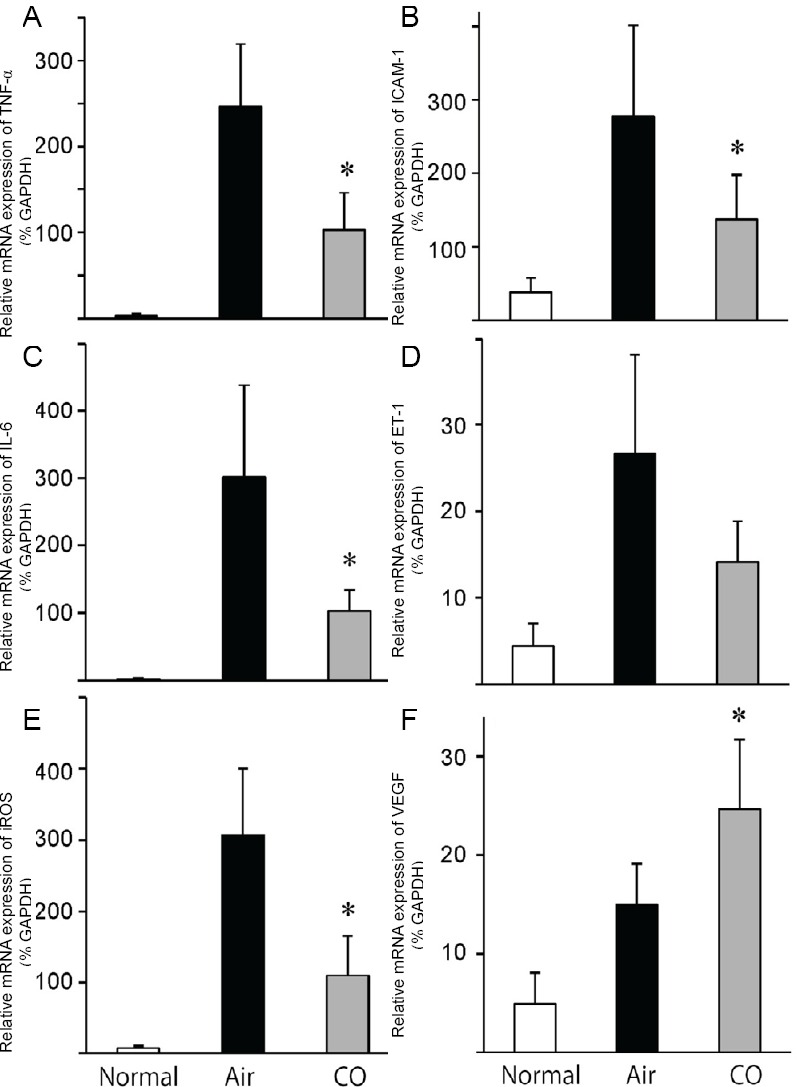
Real-time reverse-transcription polymerase chain reaction for inflammatory mediators in the cardiac graft tissue 3 hours after reperfusion.
Note: (A-F) Relative mRNA expression of TNF-α, ICAM-1, IL-6, ET-1, iNOS, and VEGF Values are expressed as mean ± SD. n = 5-6 for each group. *P < 0.05, vs. air group. CO: Carbon monoxide; TNF-α: tumor necrosis factor-α; ICAM-1: intercellular adhesion molecule-1; IL-6: interleukin-6; ET-1: endothelin1; iNOS: inducible nitric oxide synthase; VEGF: vascular endothelial growth factor; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
Pretreatment with CO decreased the infiltration of inflammatory cells
Routine histopathology of the graft samples 6 hours after reperfusion revealed variable levels of cellular infiltration. Grafted heart exposed to 250 ppm CO for 24 hours attenuated neutrophil infiltration into cardiac myocytes. On the other hand, neutrophil count increased with statistical significance, especially in the air group compared to the sham group and CO group (Figure 4A).
Figure 4.
Effect of carbon monoxide (CO) pretreatment on the infiltration of inflammatory cells in the cardiac graft tissue 6 hours after reperfusion.
Note: (A) A number of infiltrating naphthol-positive neutrophils and ED-1-positive macrophages were noted in the grafts taken from the air-control animals. There were fewer infiltrating cells in the grafts from the CO-pretreated donors. (B) Number of naphthol AS-D chloroacetate esterase-positive cells. (C) Number of ED-1-positive cells. Values are expressed as the mean ± SD. *P < 0.05, vs. air group. ET-1: Endothelin1.
Macrophages play important roles in cardiac I/R injury pathogenesis. Infiltrating macrophages (indicated by ED-1-positive cells) were observed more often in the air-control group than in the CO-treated group 6 hours after reperfusion, indicating that CO pretreatment inhibited the infiltration of macrophages (Figure 4).
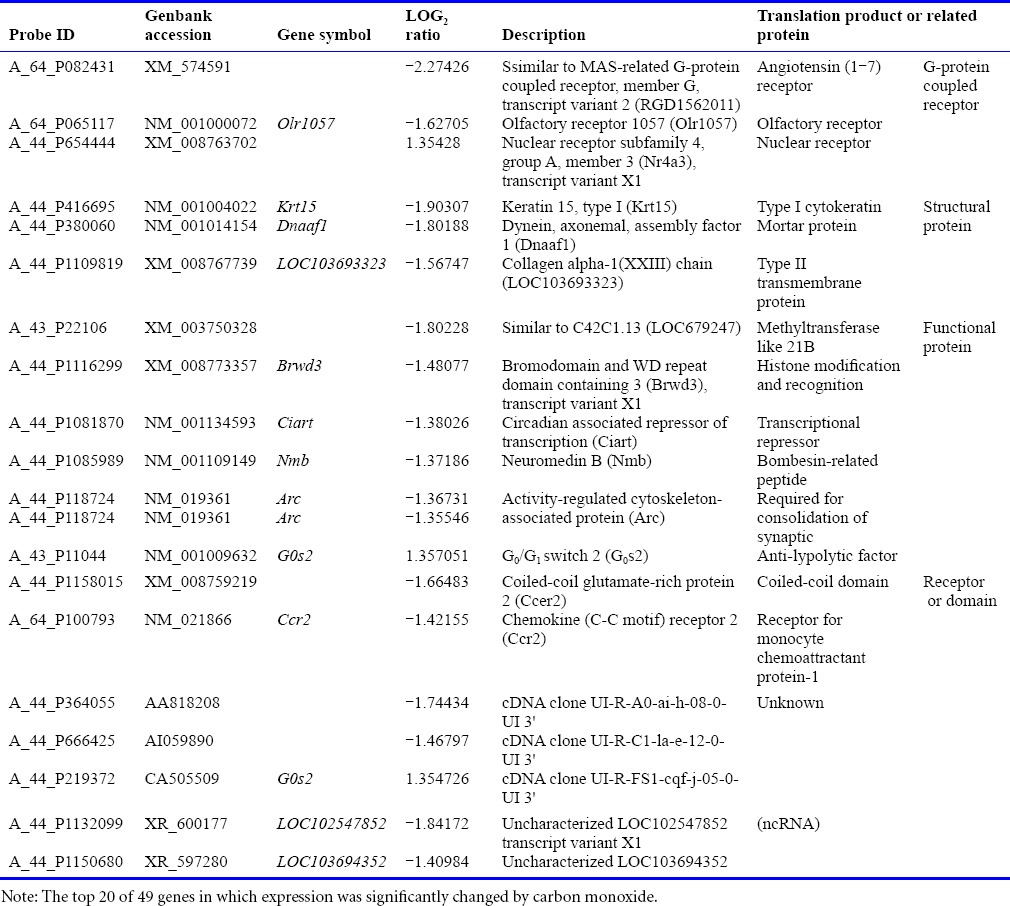
Gene array
To compare and analyze gene expression in the heart tissue, we extracted genes and examined mean signal value and whether it significantly differed between the two groups (P < 0.05, q < 0.10). After filtering by signal value, 49 genes were isolated; large variance in particular revealed log2 ratio > 1 or < –1 (Table 1). An undeniable gene associated with the anti-inflammatory response system and apoptosis pathway to prevent heart graft from I/R injury mechanism was not ascertained.
Table 1.
Result of gene array in the graft heart tissue
DISCUSSION
Our results provide additional evidence that pretreatment of donors with low concentrations of CO confers cardioprotective effects in the clinically relevant setting of heart transplantation. These beneficial effects were associated with the suppression of I/R-induced inflammatory responses.
Even though donor hearts are chosen carefully based on established and optimized donor selection criteria, primary graft dysfunction can still occur in patients receiving hearts from ideal donors. Heart grafts are exposed to numerous injuries before harvest, and many factors, including brain death, infection, shock, etc., may predispose them to primary graft dysfunction in the donor's body. However, the likelihood of primary graft dysfunction is still difficult to predict, complicating recipient care. The I/R injury process is a complex sequelae concluding in pathophysiological features of persistent intrarenal vasoconstriction, injury of microvascular endothelial cells and tubular epithelial cells, and activation of inflammatory cascades. It is launched by lack of oxygen during cold preservation and ATP depletion, followed by alteration in concentrations intracellular sodium and calcium and activation of cytotoxic enzymes (e.g., phospholipases, proteases) (Lim et al., 2013). Ensuing warm reperfusion of grafts initiates a rapid esclalation in the generation of reactive oxygen species, which further encourages cell damage and activates inflammatory cascades (Hoetzel et al., 2009).
Since CO acts as a cytoprotective molecule, research studies exploiting its therapeutic effects in organ transplantation are increasing. Several studies show that donor CO pretreatment improves graft function in animals. Akamatsu et al. (2004) demonstrated in a syngeneic rat cardiac transplantation model that exposure of the donor graft to 400 ppm of CO and incubation of the heart in 1% CO in air during ischemia prevented I/R injury. Importantly, administration of CO to donors/grafts was sufficient to afford significant protectionfrom I/R injury. In an islet allograft model, pretreatment of donors with CO blocked upregulation of toll-like receptor-4, reducing the inflammatory response and cytokine-induced apoptosis, which protected the graft from rejection (Wang et al., 2005). Donors receiving inhaled CO or suffering cold ischemia with CO perfusion have shown improved graft function, which was associated with lower apoptosis and higher viability of cardiomyocytes and endothelial cells (Akamatsu et al., 2004). Induction of CO in the donor via oral methylene chloride prevented chronic rejection of rat renal allografts (Martins et al., 2006). Thus, donor treatment with CO prior to organ harvesting was advantageous for the survival of several types of organ grafts in animal models. In addition, clinical reports may suggest that we can anticipate similar efficacies of CO in grafts procured from CO-poisoned human donors (Fujisaki et al., 2014).
The mechanisms responsible for the protection conferred by CO donor pretreatment against I/R injury remain largely unknown. A possible explanation is the ability of CO to induce VEGF, a potent growth factor that promotes differentiation and proliferation of endothelial cells and mediates endothelium-dependent vasodilatation. VEGF is also known to promote injured vessel repair by stimulating neovascularization and re-establishing vascular integrity (Miyamoto et al., 2004), which is consistent with our previous study(Faleo et al., 2008). Our data demonstrated that CO pretreatment resulted in upregulation of VEGF mRNA levels 3 hours after reperfusion, which may contribute to the protective mechanisms in our model.
We conducted a gene array analysis to determine other possible mechanisms of CO's protection by detecting previously unknown molecular changes that may underlie the beneficial effects of donor treatment with inhaled CO. The gene array showed several upregulated genes as well as downregulated genes. Although we carefully examined the expression of several of the upregulated/downregulated genes related to stress-response molecules or ATP-synthesis-related molecules, anti-apoptosis molecules, antioxidant molecules, and the transcription factors regulating their expression in our experimental setting, we could not obtain meaningful data to explain the mechanisms by which CO potentiated the protective effects. We assume that CO preloaded in the grafts may function after reperfusion in the recipient circulation, which still remains elusive. Alternatively, CO might function effectively by binding to crucial heme proteins in excised grafts, as in our previous study (Nakao et al., 2008).
CO inhalation has been successfully demonstrated as a potential therapeutic in a number of animal models in the past decades and has now been tested in several phase I and/or II clinical trials, including those involving sepsis-induced acute respiratory syndrome, aortic valve surgery, and pulmonary artery hypertension (Nakahira and Choi, 2015). Thus, accumulating evidence shows that CO can be used for acute phase critically-ill patients.
In conclusion, this study shows that donor inhalation of low doses of CO effectively mitigates the I/R injury of cardiac grafts through downregulation of proinflammatory mediators. Inhaled low-dose CO represents a novel and appealing therapy to prevent I/R injury for organ donor candidates. We believe that CO treatment of potential organ donors can maintain the current pool and increase the future pool of organs and tissues suitable for transplantation, which could have a substantial clinical impact.
Footnotes
Funding: This work was supported by JSPS KAKENHI, No. 15K20354.
Conflicts of interest
None.
REFERENCES
- Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graca-Souza AV, Ollinger R, Czismadia E, May GA, Ifedigbo E, Otterbein LE, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J. 2004;18:771–772. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- Bathoorn E, Slebos DJ, Postma DS, Koeter GH, van Oosterhout AJ, van der Toorn M, Boezen HM, Kerstjens HA. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur Respir J. 2007;30:1131–1137. doi: 10.1183/09031936.00163206. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Faleo G, Neto JS, Kohmoto J, Tomiyama K, Shimizu H, Takahashi T, Wang Y, Sugimoto R, Choi AM, Stolz DB, Carrieri G, McCurry KR, Murase N, Nakao A. Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation. 2008;85:1833–1840. doi: 10.1097/TP.0b013e31817c6f63. [DOI] [PubMed] [Google Scholar]
- Fujisaki N, Nakao A, Osako T, Nishimura T, Yamada T, Kohama K, Sakata H, Ishikawa-Aoyama M, Kotani J. Can carbon monoxide-poisoned victims be organ donors? Med Gas Res. 2014;4:13. doi: 10.1186/2045-9912-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- Hoetzel A, Schmidt R, Vallbracht S, Goebel U, Dolinay T, Kim HP, Ifedigbo E, Ryter SW, Choi AM. Carbon monoxide prevents ventilator-induced lung injury via caveolin-1. Crit Care Med. 2009;37:1708–1715. doi: 10.1097/CCM.0b013e31819efa31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SH, Lee S, Noda K, Kawamura T, Tanaka Y, Shigemura N, Nakao A, Toyoda Y. Adenosine injection prior to cardioplegia enhances preservation of senescent hearts in rat heterotopic heart transplantation. Eur J Cardiothorac Surg. 2013;43:1202–1208. doi: 10.1093/ejcts/ezs509. [DOI] [PubMed] [Google Scholar]
- Martins PN, Reutzel-Selke A, Jurisch A, Denecke C, Attrot K, Pascher A, Kotsch K, Pratschke J, Neuhaus P, Volk HD, Tullius SG. Induction of carbon monoxide in donor animals prior to organ procurement reduces graft immunogenicity and inhibits chronic allograft dysfunction. Transplantation. 2006;82:938–944. doi: 10.1097/01.tp.0000232716.91887.c5. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Kitamoto Y, Tokunaga H, Takeya M, Ezaki T, Imamura T, Tomita K. Protective effect of vascular endothelial growth factor/vascular permeability factor 165 and 121 on glomerular endothelial cell injury in the rat. Lab Invest. 2004;84:1126–1136. doi: 10.1038/labinvest.3700134. [DOI] [PubMed] [Google Scholar]
- Nakahira K, Choi AM. Carbon monoxide in the treatment of sepsis. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1387–1393. doi: 10.1152/ajplung.00311.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, Toyoda Y. Application of carbon monoxide for transplantation. Curr Pharm Biotechnol. 2012;13:827–836. doi: 10.2174/138920112800399266. [DOI] [PubMed] [Google Scholar]
- Nakao A, Choi AM, Murase N. Protective effect of carbon monoxide in transplantation. J Cell Mol Med. 2006a;10:650–671. doi: 10.1111/j.1582-4934.2006.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, Toyokawa H, Abe M, Kiyomoto T, Nakahira K, Choi AM, Nalesnik MA, Thomson AW, Murase N. Heart allograft protection with low-dose carbon monoxide inhalation: effects on inflammatory mediators and alloreactive T-cell responses. Transplantation. 2006b;81:220–230. doi: 10.1097/01.tp.0000188637.80695.7f. [DOI] [PubMed] [Google Scholar]
- Nakao A, Faleo G, Shimizu H, Nakahira K, Kohmoto J, Sugimoto R, Choi AM, McCurry KR, Takahashi T, Murase N. Ex vivo carbon monoxide prevents cytochrome P450 degradation and ischemia/reperfusion injury of kidney grafts. Kidney Int. 2008;74:1009–1016. doi: 10.1038/ki.2008.342. [DOI] [PubMed] [Google Scholar]
- Nakao A, Kimizuka K, Stolz DB, Neto JS, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Nalesnik MA, Otterbein LE, Murase N. Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury. Am J Pathol. 2003;163:1587–1598. doi: 10.1016/S0002-9440(10)63515-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, Kaczorowski DJ, Wang Y, Cardinal JS, Buchholz BM, Sugimoto R, Tobita K, Lee S, Toyoda Y, Billiar TR, McCurry KR. Amelioration of rat cardiac cold ischemia/reperfusion injury with inhaled hydrogen or carbon monoxide, or both. J Heart Lung Transplant. 2010;29:544–553. doi: 10.1016/j.healun.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969;57:225–229. [PubMed] [Google Scholar]
- Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest. 1999;103:1047–1054. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Resch H, Zawinka C, Weigert G, Schmetterer L, Garhofer G. Inhaled carbon monoxide increases retinal and choroidal blood flow in healthy humans. Invest Ophthalmol Vis Sci. 2005;46:4275–4280. doi: 10.1167/iovs.05-0417. [DOI] [PubMed] [Google Scholar]
- Robinson BR, Athota KP, Branson RD. Inhalational therapies for the ICU. Curr Opin Crit Care. 2009;15:1–9. doi: 10.1097/MCC.0b013e3283220e34. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Choi AM. Heme oxygenase-1/carbon monoxide: novel therapeutic strategies in critical care medicine. Curr Drug Targets. 2010;11:1485–1494. doi: 10.2174/1389450111009011485. [DOI] [PubMed] [Google Scholar]
- Wang H, Lee SS, Gao W, Czismadia E, McDaid J, Ollinger R, Soares MP, Yamashita K, Bach FH. Donor treatment with carbon monoxide can yield islet allograft survival and tolerance. Diabetes. 2005;54:1400–1406. doi: 10.2337/diabetes.54.5.1400. [DOI] [PubMed] [Google Scholar]