ABSTRACT
Avian influenza A H7 viruses have caused multiple outbreaks in domestic poultry throughout North America, resulting in occasional infections of humans in close contact with affected birds. In early 2016, the presence of H7N8 highly pathogenic avian influenza (HPAI) viruses and closely related H7N8 low-pathogenic avian influenza (LPAI) viruses was confirmed in commercial turkey farms in Indiana. These H7N8 viruses represent the first isolation of this subtype in domestic poultry in North America, and their virulence in mammalian hosts and the potential risk for human infection are largely unknown. In this study, we assessed the ability of H7N8 HPAI and LPAI viruses to replicate in vitro in human airway cells and in vivo in mouse and ferret models. Both H7N8 viruses replicated efficiently in vitro and in vivo, but they exhibited substantial differences in disease severity in mammals. In mice, while the H7N8 LPAI virus largely remained avirulent, the H7N8 HPAI virus exhibited greater infectivity, virulence, and lethality. Both H7N8 viruses replicated similarly in ferrets, but only the H7N8 HPAI virus caused moderate weight loss, lethargy, and mortality. The H7N8 LPAI virus displayed limited transmissibility in ferrets placed in direct contact with an inoculated animal, while no transmission of H7N8 HPAI virus was detected. Our results indicate that the H7N8 avian influenza viruses from Indiana are able to replicate in mammals and cause severe disease but with limited transmission. The recent appearance of H7N8 viruses in domestic poultry highlights the need for continued influenza surveillance in wild birds and close monitoring of the potential risk to human health.
IMPORTANCE H7 influenza viruses circulate in wild birds in the United States, but when the virus emerges in domestic poultry populations, the frequency of human exposure and the potential for human infections increases. An H7N8 highly pathogenic avian influenza (HPAI) virus and an H7N8 low-pathogenic avian influenza (LPAI) virus were recently isolated from commercial turkey farms in Indiana. To determine the risk that these influenza viruses pose to humans, we assessed their pathogenesis and transmission in vitro and in mammalian models. We found that the H7N8 HPAI virus exhibited enhanced virulence, and although transmission was only observed with the H7N8 LPAI virus, the ability of this H7 virus to transmit in a mammalian host and quickly evolve to a more virulent strain is cause for concern. Our findings offer important insight into the potential for emerging H7 avian influenza viruses to acquire the ability to cause disease and transmit among mammals.
INTRODUCTION
Avian influenza A viruses, particularly of the H5 and H7 subtypes, continue to pose a global threat to poultry industries and to public health. On 15 January 2016, the U.S. Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed the presence of H7N8 highly pathogenic avian influenza (HPAI) virus following high mortality in a commercial turkey farm in Dubois County, Indiana, necessitating the culling of approximately 60,000 birds. Subsequently, H7N8 low-pathogenic avian influenza (LPAI) viruses sharing high sequence similarity with the H7N8 HPAI virus were isolated from nine nearby turkey farms (1). Through combined containment efforts, including culling, decontamination, and quarantine, avian H7N8 viruses were eventually eradicated from Indiana poultry farms by May 2016, and no new cases of H7N8 virus poultry infection have been reported (2). While H7 influenza viruses with all 9 (N1 to N9) NA subtypes have been detected previously during wild bird influenza surveillance in the United States (3), these H7N8 viruses represent the first detection of this subtype from domestic poultry farms and the first H7 HPAI virus other than H7N3 isolated in North America in recent years. While no human infection with H7N8 viruses has been reported to date, H7 influenza viruses from the North American lineage have caused several laboratory-confirmed human infections during the past decade, including H7N3 HPAI in British Columbia, Canada, in 2004 and Jalisco, Mexico, in 2012 and H7N2 LPAI infection in one person in New York in 2003 (4, 5). Therefore, it is of great importance to assess the potential risk of recently isolated H7 influenza viruses to public health and to better inform the necessary guidelines for disease control and prevention.
H5 or H7 subtype HPAI viruses possess characteristic polybasic residues at the HA cleavage site, the presence of which contributes to systemic spread of virus and high mortality in domestic poultry (6). However, the molecular determinants of avian influenza viruses associated with virulence in mammalian hosts are not fully understood, and there remains a need to employ animal models to predict the risk of avian influenza infection in humans. Both HPAI and LPAI H7 viruses have caused large poultry outbreaks and even fatalities in humans. For example, H7N7 HPAI virus resulted in 89 human cases, including one fatality (7) in The Netherlands in 2003, and the ongoing H7N9 LPAI virus outbreak in China, which first emerged in early 2013, has caused more than 700 confirmed cases of human infection with more than 300 fatalities (8). So far, sustained human-to-human transmission has not been reported for avian influenza viruses. In addition to respiratory symptoms, which are often detected following human infection with H5 influenza virus, conjunctivitis has been observed in a large proportion of humans infected with H7 viruses (9, 10). Mammalian animal models have been developed to reflect the viral pathogenesis, cellular tropism, and potential transmissibility of avian influenza viruses in humans, including H7 subtype viruses (11). In mice, most avian H7 influenza viruses can replicate in the respiratory tract without prior adaption, but only a very few viruses, such as the H7N7 HPAI virus from The Netherlands in 2003, are highly lethal and capable of systemic spread in this model (12, 13). Like H5N1 viruses, most avian H7 viruses replicate throughout the respiratory tract of ferrets, including the lung, though only selected H7N7 and H7N9 viruses have been associated with mortality in ferrets following intranasal inoculation (12, 14). However, unlike wild-type H5 influenza viruses, which typically do not transmit well among ferrets (15), several avian H7 influenza viruses have exhibited a capacity to transmit among cohoused ferrets and, occasionally, by the airborne route as well (14, 16, 17), indicating that certain H7 influenza viruses may be better adapted than H5 influenza viruses to transmit between mammalian hosts.
To better understand the relative risks posed by H7N8 influenza viruses to humans, we examined the pathogenicity and transmissibility of two newly emerged H7N8 viruses (HPAI and LPAI) from Indiana turkey farms in both in vitro and in vivo models. We found that the H7N8 HPAI virus exhibited enhanced virulence in both mouse and ferret models compared to the virulence of both its precursor H7N8 LPAI virus and other previously isolated H7 viruses from North America, including the most recent H7N3 HPAI virus, isolated from Mexico in 2012. We also found that the H7N8 LPAI virus was able to transmit between cohoused ferrets, albeit rarely, while the H7N8 HPAI virus did not transmit between any ferret pairs. The ability of the precursor H7N8 LPAI virus, which rapidly evolved to become more virulent in both poultry and mammals, to initiate limited transmission between ferrets in direct contact underscores the need for continued surveillance and risk assessment of this virus subtype.
MATERIALS AND METHODS
Viruses.
The HPAI A/Turkey/Indiana/1403/2016 (A/Tky/1403 [H7N8]) and LPAI A/Turkey/Indiana/1573-2/2016 (A/Tky/1573 [H7N8]) viruses were first isolated by the National Veterinary Services Laboratory (NVSL), USDA, Ames, IA, and were propagated in the allantoic cavity of 10-day-old embryonated hens' eggs at 37°C for 24 h for HPAI virus or 48 h for LPAI virus. Allantoic fluid pooled from multiple eggs was clarified by centrifugation and stored in aliquots at −80°C. The HA and NA genes were both sequenced to confirm identity with the original virus isolates received from NVSL, and both stocks passed sterility and exclusivity testing to rule out contamination with other influenza virus subtypes. The control viruses HPAI A/Canada/504/04 (Can/504 [H7N3]), HPAI A/Netherlands/219/03 (NL/219 [H7N7]), LPAI A/Goose/Nebraska/17097-4/11 (Gs/NE/11 [H7N9]), A/Anhui/1/2013 (Anhui/1 [H7N9]), and human seasonal A/Panama/2007/99 (Pan/99 [H3N2]) virus were all propagated in 10-day-old embryonated hens' eggs for 24 to 48 h at 33.5 to 37°C as described previously (12, 18). The 50% egg infectious dose (EID50) for each virus stock was calculated by the method of Reed and Muench (19) following serial titration in eggs. All experiments with H7N8 HPAI and LPAI viruses were conducted under biosafety level 3 containment, including enhancements required by the U.S. Department of Agriculture and the Select Agent Program (20).
H7N8 virus replication kinetics in Calu-3 cells.
A human bronchial epithelial cell line (Calu-3) was maintained and cultured in 12-well plates with semipermeable inserts (Costar, Corning, NY) according to previously established methods (18) to allow cells to become polarized. Viral infection of Calu-3 cells was performed as described previously (18). In brief, virus inoculum was added apically to Calu-3 cells at a multiplicity of infection (MOI) of 0.01 EID50 for 1 h at 37°C, after which cell monolayers were washed and viral infection medium containing 0.3% bovine serum albumin (BSA) in minimal essential medium (MEM) was added. Cells were maintained for 72 h postinoculation (p.i.), and supernatants sampled at 2, 24, 48, and 72 h p.i. for viral titration in embryonated hens' eggs.
H7N8 virus infectivity and replication in mice.
All animal experiments were performed under the guidance of the Centers for Disease Control and Prevention's Institutional Animal Care and Use Committee in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facility. Morbidity (measured by weight loss) and mortality in mice were assessed following intranasal (i.n.) inoculation as described previously (21). In brief, groups of eight female BALB/c mice (Charles River Laboratories, Wilmington, MA), 6 to 8 weeks of age, were anesthetized intraperitoneally with 0.2 ml of 2,2,2-tribromoethanol in tert-amyl alcohol (Avertin; Sigma-Aldrich Chemical Co., St. Louis, MO) and inoculated i.n. with 50 μl of serial 10-fold dilutions of virus ranging from 100.0 to 106.0 EID50 prepared in phosphate-buffered saline (PBS). Five mice from each group were monitored daily for clinical signs of disease and weight loss until 14 days p.i.; any mouse with loss of ≥25% of its preinoculation weight was euthanized. The remaining three mice from each group were euthanized on day 3 p.i., and viral replication in the lung was evaluated by titrating tissue homogenates in 10-day-old embryonated hens' eggs. The 50% mouse infectious dose (MID50), based on the presence of virus in lung tissues, and 50% lethal dose (LD50), based on mouse survival, were calculated using the method of Reed and Muench (19). Additionally, to evaluate viral persistence and systemic spread, groups of 3 mice inoculated i.n. with 103.0 or 106.0 EID50 of virus were euthanized on day 6 p.i. to detect infectious virus in lung and brain tissues. The ocular tropism of H7N8 viruses was evaluated by intraocular (i.o.) inoculation with 5 μl of 106.0 EID50 of virus as described previously (22). Briefly, the right eye of each mouse was lightly scarified by three twists of a 2-mm corneal trephine (Katena Products), and then the virus inoculum was dropped onto the corneal surface, followed by gentle massaging with the eyelid. The lung, nose, and eyes were collected from five mice each on days 3 and 6 p.i. for viral titration.
H7N8 virus pathogenesis and transmission in ferrets.
Virus pathogenesis and transmission experiments in ferrets were performed as previously described (21). Briefly, six male Fitch ferrets (Triple F Farms, Sayre, PA), 10 months of age and serologically negative for currently circulating influenza viruses by hemagglutination inhibition (HI), were housed in a Duo-Flo Bioclean unit (Lab Products Incorporated, Seaford, DE) and inoculated i.n. with 106.0 EID50 of LPAI or HPAI H7N8 virus diluted in 1 ml of PBS. Among the six inoculated ferrets, three were housed in individual cages and were observed daily for clinical signs of disease, weight loss, and lethargy, as described previously (23). Nasal wash, ocular wash, and rectal swab samples were collected on days 1, 3, 5, 7, and 9 p.i. for viral titration in eggs. The remaining three ferrets were euthanized on day 3 p.i. to evaluate viral replication, systemic spread, and pathology. Lung tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. Four-micrometer sections of these tissues were stained with hematoxylin and eosin for histopathologic analysis. To evaluate the capacity for viral transmission between ferrets in direct contact, a serologically naive ferret was placed in the same cage as an inoculated ferret 24 h p.i. and nasal wash samples were collected from contact ferrets on days 1, 3, 5, 7, 9, 11, and 13 postcontact (p.c.). Ferrets were euthanized on day 21 p.i./p.c., and convalescent-phase sera were collected and tested for HI titer against homologous H7N8 virus as described previously (24). Hematologic analyses with peripheral blood samples collected on days 3 and 7 p.i. were performed as described previously (25).
RESULTS
H7N8 influenza virus replicates efficiently in Calu-3 cells.
The human lung epithelial cell line Calu-3 has been extensively used to model avian influenza A virus replication in the human respiratory tract. Calu-3 cells express serine proteases, such as TMPRSS2, to cleave HA0 and, therefore, are able to support the replication of most influenza viruses without the addition of exogenous trypsin (26). The ability of H7N8 LPAI and HPAI viruses to replicate in Calu-3 cells was compared with that of several human isolates from the Eurasian lineage (H7N7 HPAI NL/219 virus and H7N9 LPAI Anhui/1 virus), human and avian isolates from the North American lineage (H7N3 HPAI Can/504 virus and H7N9 LPAI Gs/NE/11 virus), and one human seasonal strain (H3N2 Pan/99 virus). Polarized Calu-3 cells grown on Transwell inserts were inoculated with each virus, and apical supernatants were collected at 2, 24, 48, and 72 h p.i. for virus titration. As shown by the results in Fig. 1, at 24 h p.i., all of the viruses except the Gs/NE/11 virus exhibited at least a 3-log increase in viral titer compared to baseline levels, indicating the capacity of most avian H7 viruses to replicate in Calu-3 cells independent of their lineage or virulence. Among all the viruses tested, Anhui/1 virus replicated to the highest titer (109.5 EID50/ml) at 24 h p.i., in accordance with a previous study showing the highly efficient replication of H7N9 viruses in this cell type (14). The HPAI H7N8 and H7N7 (NL/219) viruses reached comparable titers of approximately 108.0 EID50/ml at 24 h p.i., significantly higher than the titers reached by the H7N8 LPAI, H7N9 Gs/NE/11, H7N3 Can/504, and seasonal Pan/99 viruses (P < 0.001). Most of the viral titers peaked at 48 h p.i., with the exception of H7N8 HPAI virus, which reached its peak titer at 24 h p.i. The avian H7N8 viruses replicated to significantly higher titers in Calu-3 cells than did an H7N9 LPAI virus isolated in 2011 (P < 0.001), indicating an enhanced capacity for replication in this cell type not shared by all North American lineage H7 viruses. Taken together, our results show that both LPAI and HPAI H7N8 viruses were able to replicate efficiently in human epithelial cells, with the H7N8 HPAI virus exhibiting high-titer replication kinetics comparable to those seen for H7N7 and H7N9 influenza viruses associated with severe human disease.
FIG 1.
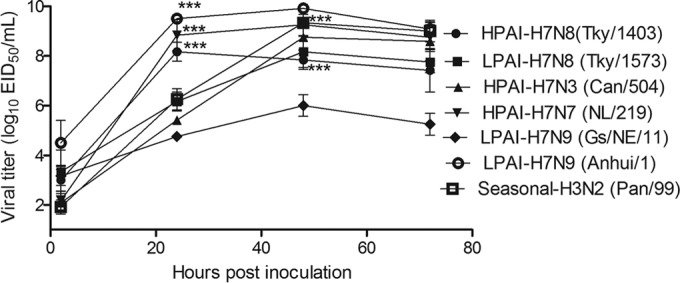
Viral replication kinetics in Calu-3 cells. Polarized Calu-3 cells grown on 12-well Transwell inserts (∼5 × 105 per well) were infected with an MOI of 0.01 EID50 of HPAI Tky/1403 (H7N8), Can/504 (H7N3), NL/219 (H7N7), LPAI Tky/1573 (H7N8), Gs/NE/11, Anhui/1 (H7N9), or human seasonal A/Pan/99 (H3N2) virus. The cell culture supernatants were collected apically at 2, 24, 48, and 72 h p.i., and the viral titers were determined in eggs. Mean viral titers ± SD from triplicate cultures are shown. Statistical analysis was performed using two-way analysis of variance (ANOVA) with GraphPad Prism. Asterisks indicate statistically significant differences between the results for Anhui/1, HPAI H7N8, and NL/219 viruses and the results for the other H7 viruses tested at 24 h and between the results for HPAI and LPAI H7N8 viruses and Gs/NE/11 virus at 48 h. ***, P < 0.001.
H7N8 HPAI virus exhibits enhanced virulence in mice compared to the virulence of H7N8 LPAI virus.
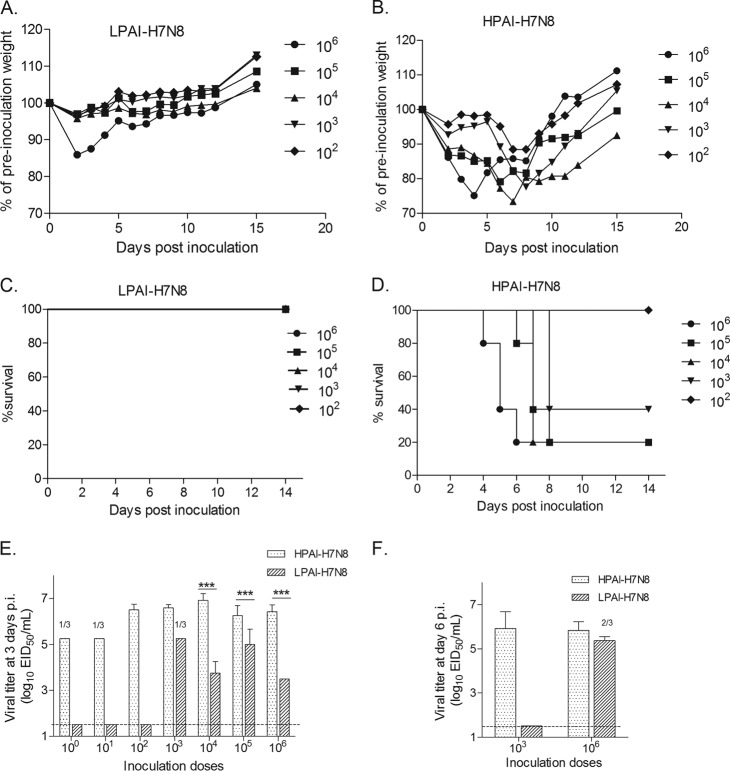
Despite the capacity of most avian influenza viruses to replicate in the murine lung without prior adaption, avian influenza viruses of the H7 subtype have exhibited great heterogeneity in their degrees of replication and pathogenesis in mice following i.n. inoculation (27). In our study, we first assessed the virulence of both HPAI and LPAI H7N8 viruses recently isolated from Indiana turkey farms in groups of BALB/c mice by monitoring morbidity (as measured by weight loss) and mortality after i.n. inoculation with serial 10-fold dilutions of virus. A mean maximum weight loss of approximately 15% at day 2 p.i. was displayed in mice inoculated with 106.0 EID50 of H7N8 LPAI virus, with only minimal weight loss (<5%) observed among mice receiving smaller-viral-inoculum doses; all mice survived the viral challenge (Fig. 2A and C). In contrast, mice inoculated with ≥103.0 EID50 of H7N8 HPAI virus exhibited substantial weight loss and lethality (Fig. 2B and D). The LD50 values for H7N8 HPAI and LPAI viruses were 103.4 and >106.0 EID50, respectively (Table 1), indicating that the HPAI virus possessed enhanced virulence in mice compared to that of the LPAI virus.
FIG 2.
H7N8 virus pathogenesis in mice. Groups of five BALB/c mice were i.n. inoculated with serial 10-fold dilutions (102.0 to 106.0 EID50) of LPAI (A, C) or HPAI (B, D) H7N8 viruses. The infected mice were observed and weighed daily until day 14 p.i., and any mouse with ≥25% weight loss or with severe signs of illness, including neurological symptoms, was euthanized. The mean percentages of preinoculation weight and survival for LPAI or HPAI H7N8 virus-infected mice are shown. (E) Groups of three mice were inoculated with 100.0 to 106.0 EID50 doses of HPAI or LPAI H7N8 virus and were euthanized on day 3 p.i. to determine viral titers in lung tissues. (F) Additional groups of three mice were inoculated i.n. with 103.0 or 106.0 EID50 of either H7N8 virus and were euthanized on day 6 p.i. for viral titration in lung tissues. The mean viral titers + SD in lung tissues on day 3 or day 6 p.i. are expressed as log10 EID50/ml of the clarified tissue homogenate supernatant. The dashed horizontal line indicates the detection limit of 1.5 log10 EID50/ml. If virus was not detected in 100% of mice in any particular group, the number of positive samples out of the total number of mice inoculated is shown. Statistical analysis was performed using two-way ANOVA with GraphPad Prism if all three samples from each group were virus positive. ***, P < 0.001.
TABLE 1.
Infectivity, morbidity, and mortality observed in mice infected by HPAI or LPAI H7N8 virus
| Virus | Subtype | Pathogenicitya | HA cleavage site sequence | Mean wt loss (%)b | Mean lung titer (log10 EID50/ml) ± SD at dayc: |
MID50d | LD50d | |
|---|---|---|---|---|---|---|---|---|
| 3 | 6 | |||||||
| A/Turkey/Indiana/1403/2016 | H7N8 | HPAI | PENPKKRKTR/G | 24.9 | 6.41 ± 0.29 | 5.83 ± 0.38 | 1.0 | 3.4 |
| A/Turkey/Indiana/1573-02/2016 | H7N8 | LPAI | PENP…..KTR/G | 14.1 | 3.50 | 5.38 ± 0.18 (2/3) | 3.3 | >6.0 |
The pathogenicity phenotype in chickens was determined using the intravenous pathogenicity index (IVPI) (37).
Mean maximum weight loss (%) following i.n. inoculation with 106.0 EID50 of virus (five mice per group).
Mean viral titers in lungs following i.n. inoculation with 106.0 EID50 of virus on day 3 or 6 p.i. among mice with positive virus detection. Titers are reflective of 3 mice per group unless otherwise specified in parentheses.
MID50 and LD50 are expressed as the log10 EID50 required to give a value of 1 for MID50 or LD50.
We next examined the efficiency of viral replication in the mouse respiratory tract for both H7N8 viruses. As shown by the results in Fig. 2E, with inoculation doses of 104.0 EID50 or higher, both viruses could replicate efficiently in the mouse lung on day 3 p.i., reaching titers of at least 103.0 EID50/ml, though the viral loads in the lungs of H7N8 HPAI virus-infected mice at this time point were significantly (P < 0.001) higher (≥1 to 3 log) than those in the H7N8 LPAI-infected mice that received the same inoculation dose. Strikingly, the H7N8 LPAI virus was over 100-fold less infectious than the HPAI virus in mice (Table 1). Additionally, while mice inoculated with a high dose (106.0 EID50) of either the HPAI or LPAI virus exhibited mean lung titers of >105.0 EID50/ml on day 6 p.i., at a low dose (103.0 EID50), only mice inoculated with the HPAI virus had detectable virus in the lung on day 6 p.i. (Fig. 2F). Despite being highly infectious and virulent, the H7N8 HPAI virus failed to spread systemically, as virus was not detected in the brain on day 6 p.i., and no mice displayed neurological symptoms, similar to what was observed for H7N8 LPAI virus (data not shown). Unlike intranasal administration, the H7N8 HPAI and LPAI viruses were not consistently detected in eye, nose, or lung tissues following ocular inoculation. Taken together, these data indicate that the H7N8 HPAI influenza virus exhibits enhanced morbidity, mortality, and replicative ability in mice compared with those of the H7N8 LPAI virus, as demonstrated by greater weight loss, lethality, and viral persistence in the respiratory tract.
H7N8 influenza viruses exhibit limited transmission, despite efficient replication in ferrets.
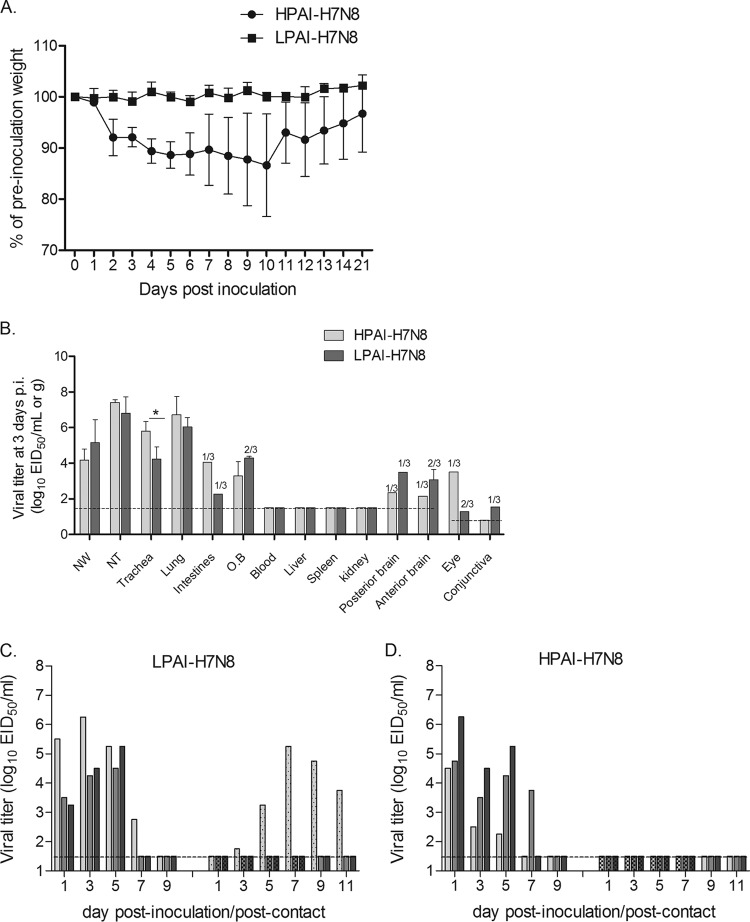
We further evaluated the ability of both H7N8 HPAI and LPAI viruses to cause disease and transmit among ferrets, the gold standard animal model for influenza virus infection in mammals (11). Six ferrets were i.n. inoculated with 106.0 EID50 of H7N8 LPAI or HPAI virus for assessment of clinical signs, lymphopenia, virus dynamics in nasal wash, ocular wash, and rectal swab samples, and viral replication in systemic tissues on day 3 p.i. Similar to the results for other LPAI North American influenza viruses (16), ferrets infected with H7N8 LPAI virus displayed limited weight loss (<2%) and showed no other clinical signs, with the exception of transient fever (1.1°C above baseline) (Fig. 3A; Table 2). In contrast, the H7N8 HPAI virus-infected ferrets exhibited greater weight loss (mean maximum of 15.6%) (Fig. 3A). None of the ferrets infected with H7N8 HPAI virus exhibited overt signs of respiratory or ocular illness, including sneezing or nasal or ocular discharge. Both groups of ferrets exhibited lymphopenia on days 3 and 7 p.i. but with no significant difference between them (data not shown). However, one ferret infected by the HPAI virus exhibited transient diarrhea on day 5 p.i. and was euthanized on day 10 p.i. due to severe weight loss; the postmortem necropsy confirmed the presence of virus in nasal turbinates (102.3 EID50/ml) and in trachea tissues (102.7 EID50/g).
FIG 3.
H7N8 virus pathogenesis and transmission in ferrets. (A) Three ferrets inoculated i.n. with 106.0 EID50 of either HPAI or LPAI H7N8 virus were monitored for weight loss until 21 days p.i. The mean percentages of preinoculation weight of ferrets are shown. (B) Three infected ferrets were euthanized on day 3 p.i., and nasal wash samples (NW) and tissue samples, including nasal turbinates (NT), trachea, lung, intestine, olfactory bulb (OB), blood, liver, spleen, kidney, posterior brain, anterior brain, eye (pooled left and right), and conjunctiva (pooled left and right), were collected for viral titration in eggs. Mean viral titers + SD are expressed as log10 EID50/ml (for NW and NT samples) or log10 EID50/g. If virus was not detected in 100% of any particular tissue samples, the number of positive samples out of the total number is shown on top of the bar graph. (C, D) Three naive contact ferrets were cohoused with LPAI (C) or HPAI (D) H7N8 virus-infected ferrets, and nasal wash samples were collected for viral titration on the indicated days p.i. or p.c. Viral titers in nasal wash samples from each of three individual inoculated ferrets (solid bars, left) and contact ferrets (dotted/checkered bars, right) are expressed as log10 EID50/ml. The dashed horizontal lines indicate the detection limit of 1.5 log10 EID50/ml or log10 EID50/g for NW or most tissue samples, except for eye and conjunctiva samples, which have a detection limit of 0.8 log10 EID50/ml. Statistical analysis was performed using two-way ANOVA with GraphPad Prism if all three samples from same groups were virus positive. *, P < 0.05.
TABLE 2.
Clinical signs, viral replication, and transmission of HPAI and LPAI H7N8 virus among ferrets
| Virus | Result (mean maximum for indicated value or no. of animals with result/no. inoculated or exposed) for indicated parameter in ferrets exposed by: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Inoculation |
Direct contact |
||||||||
| Wt loss (%)a | Temp increase (°C)b | Lethargy score (RII)c | Lethalityd | Respiratory signs | Gastrointestinal signs | Viral titer ± SD (log10 EID50/ml) in nasal wash samples | Virus detected | Seroconversion | |
| A/Turkey/Indiana/1403/2016 (HPAI) | 15.6 | 1.7 | 1.6 | 1/3 (10) | 0/3 | 1/3 | 5.17 ± 0.95 | 0/3 | 0/3 |
| A/Turkey/Indiana/1573-02/2016 (LPAI) | 1.2 | 1.1 | 1.0 | 0/3 | 0/3 | 0/3 | 5.33 ± 0.88 | 1/3 | 1/3 |
Mean maximum weight loss (%) during first 10 days p.i.
Mean maximum temperature increase over baseline during first 10 days p.i.
RII, relative inactivity index (38) during 12 days p.i.
Number of animals that succumbed to infection/total number of animals. The day p.i. that one animal was euthanized due to excessive weight loss is shown in parentheses.
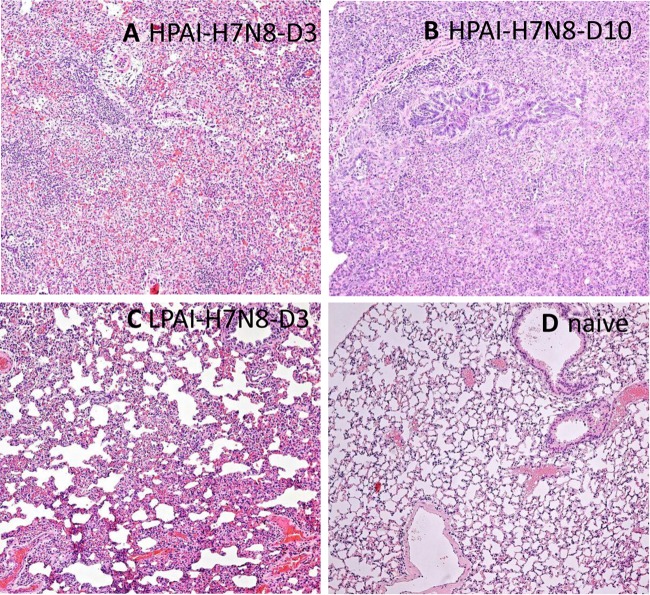
Despite the substantial differences in the amount of weight loss they caused, both H7 viruses replicated efficiently throughout the respiratory tract in ferrets, as demonstrated by high viral titers in nasal wash samples, nasal turbinates, trachea, and lungs. In nasal wash samples, viral titers peaked on day 1 p.i. for H7N8 HPAI virus-infected ferrets, with a mean titer of 105.7 EID50/ml (Fig. 3D), and on day 3 or 5 p.i. for H7N8 LPAI virus-infected ferrets, with a mean titer of 105.0 EID50/ml (Fig. 3C). Virus was cleared in nasal wash samples from all surviving H7N8 virus-infected ferrets by day 9 p.i. On day 3 p.i., the titers of both viruses were >104.0 EID50/g in the trachea, with the H7N8 HPAI virus titers being significantly higher than those of the LPAI virus (P < 0.05) (Fig. 3B). The virus titers in lung tissues of HPAI and LPAI virus-infected ferrets were similar, and both were >106.0 EID50/g. However, histopathologic evaluation of lung tissues indicated a significant difference in the degrees of inflammation. Ferrets infected by H7N8 HPAI virus exhibited prominent intra-alveolar edema, hemorrhage, and moderate to extensive inflammatory infiltrates (Fig. 4A). The H7N8 HPAI virus-infected ferret that was euthanized on day 10 also showed signs of organizing fibrosis (Fig. 4B). In contrast, the majority of the lungs of ferrets infected by LPAI virus were normal, with only focal areas of mild to moderate inflammatory infiltrates (Fig. 4C).
FIG 4.
Histopathological examination of lung tissues from ferrets infected with HPAI or LPAI H7N8 influenza virus. Representative images of hematoxylin and eosin-stained lung tissues from ferrets inoculated with 106.0 EID50 of HPAI H7N8 influenza virus on day 3 p.i. (A) and day 10 p.i. (B), from ferrets inoculated with LPAI H7N8 influenza virus on day 3 p.i. (C), and from naive ferrets (D) are shown under ×100 magnification.
Both HPAI and LPAI viruses were detected sporadically at low titers (≤104.0 EID50/g) in extrapulmonary tissues, including the intestine, olfactory bulb, posterior and anterior brain, eyes, and conjunctiva, on day 3 p.i. Similarly, low titers (≤103.5 EID50/ml) of viruses were also detected sporadically in rectal swab samples on days 1 and 5 p.i. or in eye wash samples on day 3 and 5 p.i. for H7N8 LPAI virus-infected ferrets and in rectal swab samples on days 1 and 7 p.i. for H7N8 HPAI virus-infected ferrets, including the animal euthanized on day 10 p.i. (data not shown). The sporadic presence of H7N8 influenza viruses at low titers in selected extrapulmonary tissues, including the olfactory bulb, brain, and intestine, has also been observed in LPAI A/Anhui/1/2013 (H7N9) and HPAI A/Mexico/InDRE7218/12 (H7N3) virus-infected ferrets (14, 21), but this is in contrast to the increased level of virus replication and systemic spread of HPAI NL/219 (H7N7) virus (12). Our results indicate that the H7N8 influenza viruses isolated from turkeys in Indiana do not yet possess the enhanced virulence that is characteristic of some Eurasian H7N7 HPAI viruses.
H7N8 virus transmissibility was evaluated by cohousing naive ferrets with inoculated ferrets on day 1 p.i.; transmission was assessed by both the presence of virus in nasal wash samples and seroconversion of convalescent-phase sera collected from contact ferrets on day 21 p.c. By these criteria, transmission was not observed in any of the three contact ferrets cohoused with H7N8 HPAI virus-infected ferrets (Fig. 3D). Among the three naive ferrets cohoused with the ferrets infected by H7N8 LPAI virus, one had positive viral titers in nasal wash samples commencing on day 3 p.c., with a peak titer of 105.3 EID50/ml on day 7 p.c. (Fig. 3C). Detectable virus persisted in nasal wash samples from this ferret until at least day 11 p.c. This ferret seroconverted to homologous virus with an HI titer of 40, whereas the two other contact ferrets remained seronegative and all inoculated ferrets possessed HI titers of ≥160 (data not shown). These findings indicate that neither the LPAI nor the HPAI H7N8 virus is capable of efficient transmission among ferrets that are in direct contact. Collectively, we found that despite the finding that both H7N8 influenza viruses replicated efficiently throughout the respiratory tract of ferrets, only the HPAI virus caused moderate morbidity and mortality and only the LPAI virus was capable of limited transmission among cohoused ferrets.
DISCUSSION
As natural hosts for avian influenza A viruses, wild birds typically display no symptoms upon influenza infection but can nonetheless disseminate and spread virus to domestic poultry (28). The avian influenza H7N8 viruses that recently emerged in Indiana share high genetic similarity to avian influenza viruses from wild birds in North America, indicative of their wild bird origin (29). The H7N8 HPAI Tky/1403 virus, which was highly virulent in turkeys, most likely evolved from the H7N8 LPAI Tky/1573 virus or similar viruses during their circulation in domestic poultry (29). In this study, we assessed the pathogenicity of two H7N8 viruses (HPAI and LPAI) from Indiana in both mice and ferrets and found that the HPAI (Tky/1403) virus exhibited enhanced virulence compared to its precursor LPAI (Tky/1573) virus. In mice, H7N8 LPAI virus was largely avirulent, only causing transient weight loss at a high inoculation dose, while H7N8 HPAI virus was more infectious and caused severe weight loss even at low inoculation doses. Moreover, H7N8 HPAI virus exhibited more robust replication in the murine lung than did H7N8 LPAI virus. Similarly, H7N8 HPAI virus caused greater lethargy and more substantial weight loss in ferrets, including one lethal outcome, which is in contrast to the minimal weight loss observed in ferrets inoculated with H7N8 LPAI virus. The Indiana H7N8 influenza viruses differ from each other by only 8 amino acids (including a change from asparagine in HPAI virus to aspartic acid in LPAI virus at position 740 in polymerase protein PB2 [PB2-N740D], PA-S218G, PA-G347D, HA-L468M, HA-N511K [H7 numbering starting at the first Met], NA-L357V, M1-I203M, and NP-N377S), in addition to an insertion of three basic residues (-KRK-) at the HA cleavage site in the H7N8 HPAI virus. The presence of polybasic residues at the HA cleavage site of H5 or H7 subtype HPAI viruses directly correlates with the enhanced viral replication and systemic spread during viral infection in chickens due to the intracellular HA cleavage by ubiquitous furinlike proteases (6). In contrast, the cleavage of LPAI with a single basic residue at the HA cleavage site is mediated by extracellular trypsinlike proteases that are present in the respiratory and intestinal tracts of chickens; therefore, viral infection by LPAI has a limited tissue tropism in this species (6). Although multibasic cleavage sites in the HAs of H5 and H7 influenza viruses act as an important molecular marker for high lethality in chickens, their contribution in viral pathogenesis in mammals is less straightforward. For example, the H7 HPAI virus A/Netherlands/230/2003 virus did not cause lethal infection in mice, whereas H7 LPAI viruses lacking a multibasic cleavage site, such as A/Anhui/1/2013 (H7N9) or A/Rhea/North Carolina/93 (H7N1) virus, were lethal in mice (14, 27), demonstrating that avian influenza virus pathogenicity in mammals is a multifactorial trait. However, since none of the 8 amino acid substitutions associated with these two Indiana H7N8 viruses are located at positions previously linked to influenza virulence in mammals, we postulate that the three-basic-residue insertion (-KRK-) at the HA cleavage site plays a major role in the enhanced virulence of the H7N8 HPAI virus. Similar effects of basic residue insertions on viral pathogenicity in mammals have been reported for other H7 viruses, such as a laboratory variant of influenza A/Seal/Massachusetts/1/80 (H7N7) virus, in which a three-arginine-residue insertion in HA conferred a lethal phenotype to the original avirulent wild-type virus in mice (30). Furthermore, despite comparable replication dynamics in the lower respiratory tract of ferrets, more severe histopathologic changes were observed in the lungs of those infected by HPAI virus. Therefore, further identification of the molecular determinants of virulence associated with H7 viruses and how these virulence markers may interact in a synergistic manner in mammals is needed.
Several mammalian adaption markers in avian influenza viruses have been associated with enhanced viral attachment and replication in mammals; among the best characterized are mutations within the HA receptor-binding domain, mainly at positions 226 and 228 (H3 numbering) for H7 viruses, and the E627K and D701N mutations in PB2 (31). Selected H7 viruses, like the H7N9 LPAI virus, with such mammalian adaption markers have emerged and exhibited increased virulence in animal models (14). Moreover, the PB2 627K mutation present in the H7N7 HPAI NL/219 virus was also found to be responsible for its lethal phenotypes in mammals (32). Sequence analysis of two recently emerged H7N8 viruses reveals no such substitutions, indicating that H7N8 viruses have not become well adapted to infect mammals. However, avian influenza viruses can evolve rapidly in domestic poultry and mammalian adaption markers can emerge during virus circulation in poultry (33). Although the H7N8 influenza viruses that emerged in Indiana in 2016 have been eradicated from those poultry farms, it is impossible to predict when avian influenza viruses will remerge and cause outbreaks in poultry populations in the future. Therefore, it is necessary to continuously monitor the genetic features of emerging avian influenza viruses and assess their abilities to infect and cause disease in mammals.
The assessment of avian influenza transmission in mammals is essential for evaluating the potential risk to public health and for pandemic preparedness. Our current understanding of the molecular mechanisms associated with avian influenza virus transmission in mammals is largely derived from studies using the ferret model. These studies have revealed roles for both strong binding to humanlike α2,6-linked sialic acid receptors and potent replication in the respiratory tract as necessary for efficient transmission in mammals (31). In our study, we identified that the H7N8 LPAI virus has the capacity for limited transmission by direct contact, as one of three contact ferrets displayed virus shedding on day 3 p.c. and also seroconverted by day 21 p.c. Unlike avian influenza H5 wild-type viruses, which generally fail to transmit in either direct contact or respiratory droplet model systems, low to high levels of transmission in the presence of direct contact have been identified among selected H7 viruses from the North American lineage, with partial transmissibility by aerosol also detected among several H7N9 LPAI viruses isolated from China since 2013 (10). Increased receptor binding for α2,6-linked sialic acid, conferred either by mutations in the receptor-binding domain, as seen in H7N9 LPAI virus, or by a deletion in the HA loop, as observed in A/New York/107 (H7N2) virus, has been associated with enhanced transmissibility for some H7 viruses, as has heightened viral replication conferred by the PB2-E627K mutation (14, 16, 34, 35). However, other viruses, such as A/Mallard/Alberta/24/01 (H7N3) virus, which possess typical α2,3-linked sialic acid binding and have no known mammalian adaption mutations, still exhibit partial to full transmission among ferrets in direct contact (21, 36), suggesting that other molecular determinants associated with H7 virus transmission remain unidentified.
Like most H7 viruses that have been characterized so far, both HPAI and LPAI H7N8 viruses can replicate in the respiratory tract of multiple mammalian species without prior adaption. H7N8 HPAI virus exhibited a higher magnitude and greater persistence of viral replication in murine lungs, which may partially contribute to its enhanced virulence compared to that of H7N8 LPAI virus. However, there was no significant difference in viral replication in upper (nasal turbinates) or lower (lung) respiratory tract tissues of ferrets infected with the H7N8 HPAI and LPAI viruses, despite greater morbidity following infection with the H7N8 HPAI virus. These results suggest that efficient viral replication does not always coincide with the disease severity of avian influenza virus infection, at least in ferrets. The enhanced histopathology observed in the lungs of ferrets infected by HPAI virus indicates that extensive bronchopneumonia with characteristic inflammatory cell infiltration may contribute to disease severity. Future studies on how HPAI virus causes severe viral pneumonia and induces strong inflammatory cell infiltration in mammals are necessary.
Wild birds in North America maintain a large pool of genetically diverse avian influenza viruses. It is difficult to predict which avian influenza virus will emerge in poultry and when it will do so. Meanwhile, influenza viruses will continue to evolve, reassort when they cross the species barrier, and establish infection in a new host. The recent H7N8 virus outbreaks in Indiana further emphasize the importance of continued surveillance in wild birds and poultry populations and the important role of risk assessment in animal models.
ACKNOWLEDGMENTS
We thank the National Veterinary Services Laboratory (NVSL), USDA, Ames, IA, for providing access to viruses. We are grateful to the Comparative Medicine Branch at CDC for exceptional animal care.
J.A.P.-P. was supported by the Oak Ridge Institute for Science and Education.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry.
REFERENCES
- 1.Indiana State Department of Health. 2016. Avian influenza H7N8. http://www.in.gov/isdh/26902.htm Accessed May 2016.
- 2.Indiana State Board of Animal Health. 2016. Final Indiana avian influenza quarantine lifted; state achieves AI-free. https://www.in.gov/boah/files/HPAI_Final_Quarantine_Released_b_PR_5-2-16.pdf.
- 3.Bevins SN, Pedersen K, Lutman MW, Baroch JA, Schmit BS, Kohler D, Gidlewski T, Nolte DL, Swafford SR, DeLiberto TJ. 2014. Large-scale avian influenza surveillance in wild birds throughout the United States. PLoS One 9:e104360. doi: 10.1371/journal.pone.0104360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirst M, Astell CR, Griffith M, Coughlin SM, Moksa M, Zeng T, Smailus DE, Holt RA, Jones S, Marra MA, Petric M, Krajden M, Lawrence D, Mak A, Chow R, Skowronski DM, Tweed SA, Goh S, Brunham RC, Robinson J, Bowes V, Sojonky K, Byrne SK, Li Y, Kobasa D, Booth T, Paetzel M. 2004. Novel avian influenza H7N3 strain outbreak, British Columbia. Emerg Infect Dis 10:2192–2195. doi: 10.3201/eid1012.040743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2012. Notes from the field: highly pathogenic avian influenza A (H7N3) virus infection in two poultry workers—Jalisco, Mexico, July 2012. MMWR Morb Mortal Wkly Rep 61:726–727. [PubMed] [Google Scholar]
- 6.Steinhauer DA. 1999. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology 258:1–20. doi: 10.1006/viro.1999.9716. [DOI] [PubMed] [Google Scholar]
- 7.Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJ, Koch G, Bosman A, Koopmans M, Osterhaus AD. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A 101:1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. 2016. Influenza. Monthly risk assessment summary: influenza at the human-animal interface. WHO, Geneva, Switzerland: http://www.who.int/influenza/human_animal_interface/HAI_Risk_Assessment/en Accessed 16 May 2016. [Google Scholar]
- 9.Belser JA, Bridges CB, Katz JM, Tumpey TM. 2009. Past, present, and possible future human infection with influenza virus A subtype H7. Emerg Infect Dis 15:859–865. doi: 10.3201/eid1506.090072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belser JA, Tumpey TM. 2014. Mammalian models for the study of H7 virus pathogenesis and transmission. Curr Top Microbiol Immunol 385:275–305. doi: 10.1007/82_2014_383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belser JA, Szretter KJ, Katz JM, Tumpey TM. 2009. Use of animal models to understand the pandemic potential of highly pathogenic avian influenza viruses. Adv Virus Res 73:55–97. doi: 10.1016/S0065-3527(09)73002-7. [DOI] [PubMed] [Google Scholar]
- 12.Belser JA, Lu X, Maines TR, Smith C, Li Y, Donis RO, Katz JM, Tumpey TM. 2007. Pathogenesis of avian influenza (H7) virus infection in mice and ferrets: enhanced virulence of Eurasian H7N7 viruses isolated from humans. J Virol 81:11139–11147. doi: 10.1128/JVI.01235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Wit E, Munster VJ, Spronken MI, Bestebroer TM, Baas C, Beyer WE, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2005. Protection of mice against lethal infection with highly pathogenic H7N7 influenza A virus by using a recombinant low-pathogenicity vaccine strain. J Virol 79:12401–12407. doi: 10.1128/JVI.79.19.12401-12407.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belser JA, Gustin KM, Pearce MB, Maines TR, Zeng H, Pappas C, Sun X, Carney PJ, Villanueva JM, Stevens J, Katz JM, Tumpey TM. 2013. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature 501:556–559. doi: 10.1038/nature12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maines TR, Chen LM, Matsuoka Y, Chen H, Rowe T, Ortin J, Falcon A, Nguyen TH, Mai le Q, Sedyaningsih ER, Harun S, Tumpey TM, Donis RO, Cox NJ, Subbarao K, Katz JM. 2006. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc Natl Acad Sci U S A 103:12121–12126. doi: 10.1073/pnas.0605134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belser JA, Blixt O, Chen LM, Pappas C, Maines TR, Van Hoeven N, Donis R, Busch J, McBride R, Paulson JC, Katz JM, Tumpey TM. 2008. Contemporary North American influenza H7 viruses possess human receptor specificity: implications for virus transmissibility. Proc Natl Acad Sci U S A 105:7558–7563. doi: 10.1073/pnas.0801259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu H, Wang D, Kelvin DJ, Li L, Zheng Z, Yoon SW, Wong SS, Farooqui A, Wang J, Banner D, Chen R, Zheng R, Zhou J, Zhang Y, Hong W, Dong W, Cai Q, Roehrl MH, Huang SS, Kelvin AA, Yao T, Zhou B, Chen X, Leung GM, Poon LL, Webster RG, Webby RJ, Peiris JS, Guan Y, Shu Y. 2013. Infectivity, transmission, and pathology of human-isolated H7N9 influenza virus in ferrets and pigs. Science 341:183–186. doi: 10.1126/science.1239844. [DOI] [PubMed] [Google Scholar]
- 18.Zeng H, Goldsmith C, Thawatsupha P, Chittaganpitch M, Waicharoen S, Zaki S, Tumpey TM, Katz JM. 2007. Highly pathogenic avian influenza H5N1 viruses elicit an attenuated type I interferon response in polarized human bronchial epithelial cells. J Virol 81:12439–12449. doi: 10.1128/JVI.01134-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am J Hyg 27:493–497. [Google Scholar]
- 20.Wilson DE, Chosewood LC (ed). 2009. Biosafety in microbiological and biomedical laboratories, 5th ed Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 21.Belser JA, Davis CT, Balish A, Edwards LE, Zeng H, Maines TR, Gustin KM, Martinez IL, Fasce R, Cox NJ, Katz JM, Tumpey TM. 2013. Pathogenesis, transmissibility, and ocular tropism of a highly pathogenic avian influenza A (H7N3) virus associated with human conjunctivitis. J Virol 87:5746–5754. doi: 10.1128/JVI.00154-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belser JA, Wadford DA, Xu J, Katz JM, Tumpey TM. 2009. Ocular infection of mice with influenza A (H7) viruses: a site of primary replication and spread to the respiratory tract. J Virol 83:7075–7084. doi: 10.1128/JVI.00535-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reuman PD, Keely S, Schiff GM. 1989. Assessment of signs of influenza illness in the ferret model. J Virol Methods 24:27–34. doi: 10.1016/0166-0934(89)90004-9. [DOI] [PubMed] [Google Scholar]
- 24.CDC. 2013. Modified hemagglutination inhibition (HI) assay using horse RBCs for serologic detection of antibodies to H7 subtype avian influenza virus in human sera. Centers for Disease Control and Prevention, Atlanta, GA: https://consise.tghn.org/site_media/media/articles/160713_Modified_Hemagglutination_Inhibition_Assay_Using_Horse_RBCs.pdf. [Google Scholar]
- 25.Belser JA, Gustin KM, Maines TR, Blau DM, Zaki SR, Katz JM, Tumpey TM. 2011. Pathogenesis and transmission of triple-reassortant swine H1N1 influenza viruses isolated before the 2009 H1N1 pandemic. J Virol 85:1563–1572. doi: 10.1128/JVI.02231-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bottcher-Friebertshauser E, Stein DA, Klenk HD, Garten W. 2011. Inhibition of influenza virus infection in human airway cell cultures by an antisense peptide-conjugated morpholino oligomer targeting the hemagglutinin-activating protease TMPRSS2. J Virol 85:1554–1562. doi: 10.1128/JVI.01294-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joseph T, McAuliffe J, Lu B, Jin H, Kemble G, Subbarao K. 2007. Evaluation of replication and pathogenicity of avian influenza A H7 subtype viruses in a mouse model. J Virol 81:10558–10566. doi: 10.1128/JVI.00970-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. 1992. Evolution and ecology of influenza A viruses. Microbiol Rev 56:152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Killian ML, Kim-Torchetti M, Hines N, Yingst S, DeLiberto T, Lee DH. 2016. Outbreak of H7N8 low pathogenic avian influenza in commercial turkeys with spontaneous mutation to highly pathogenic avian influenza. Genome Announc 4:e00457-16. doi: 10.1128/genomeA.00457-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheiblauer H, Kendal AP, Rott R. 1995. Pathogenicity of influenza A/Seal/Mass/1/80 virus mutants for mammalian species. Arch Virol 140:341–348. doi: 10.1007/BF01309867. [DOI] [PubMed] [Google Scholar]
- 31.Schrauwen EJ, Fouchier RA. 2014. Host adaptation and transmission of influenza A viruses in mammals. Emerg Microbes Infect 3:e9. doi: 10.1038/emi.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Jong RM, Stockhofe-Zurwieden N, Verheij ES, de Boer-Luijtze EA, Ruiter SJ, de Leeuw OS, Cornelissen LA. 2013. Rapid emergence of a virulent PB2 E627K variant during adaptation of highly pathogenic avian influenza H7N7 virus to mice. Virol J 10:276. doi: 10.1186/1743-422X-10-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taubenberger JK, Kash JC. 2010. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 7:440–451. doi: 10.1016/j.chom.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Q, Shi J, Deng G, Guo J, Zeng X, He X, Kong H, Gu C, Li X, Liu J, Wang G, Chen Y, Liu L, Liang L, Li Y, Fan J, Wang J, Li W, Guan L, Li Q, Yang H, Chen P, Jiang L, Guan Y, Xin X, Jiang Y, Tian G, Wang X, Qiao C, Li C, Bu Z, Chen H. 2013. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science 341:410–414. doi: 10.1126/science.1240532. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, Bao L, Deng W, Dong L, Zhu H, Chen T, Lv Q, Li F, Yuan J, Xiang Z, Gao K, Xu Y, Huang L, Li Y, Liu J, Yao Y, Yu P, Li X, Huang W, Zhao X, Lan Y, Guo J, Yong W, Wei Q, Chen H, Zhang L, Qin C. 2014. Novel avian-origin human influenza A(H7N9) can be transmitted between ferrets via respiratory droplets. J Infect Dis 209:551–556. doi: 10.1093/infdis/jit474. [DOI] [PubMed] [Google Scholar]
- 36.Song H, Wan H, Araya Y, Perez DR. 2009. Partial direct contact transmission in ferrets of a mallard H7N3 influenza virus with typical avian-like receptor specificity. Virol J 6:126. doi: 10.1186/1743-422X-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.OIE World Organisation for Animal Health. 2008. Avian influenza, p 465–481. In Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees), 6th ed Office International des Epizooties, Paris, France. [Google Scholar]
- 38.Zitzow LA, Rowe T, Morken T, Shieh WJ, Zaki S, Katz JM. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J Virol 76:4420–4429. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]