ABSTRACT
Receptor-dependent herpes simplex virus (HSV)-induced cell-cell fusion requires glycoproteins gD, gH/gL, and gB. Our current model posits that during fusion, receptor-activated conformational changes in gD activate gH/gL, which subsequently triggers the transformation of the prefusion form of gB into a fusogenic state. To examine the role of each glycoprotein in receptor-dependent cell-cell fusion, we took advantage of our discovery that fusion by wild-type herpes simplex virus 2 (HSV-2) glycoproteins occurs twice as fast as that achieved by HSV-1 glycoproteins. By sequentially swapping each glycoprotein between the two serotypes, we established that fusion speed was governed by gH/gL, with gH being the main contributor. While the mutant forms of gB fuse at distinct rates that are dictated by their molecular structure, these restrictions can be overcome by gH/gL of HSV-2 (gH2/gL2), thereby enhancing their activity. We also found that deregulated forms of gD of HSV-1 (gD1) and gH2/gL2 can alter the fusogenic potential of gB, promoting cell fusion in the absence of a cellular receptor, and that deregulated forms of gB can drive the fusion machinery to even higher levels. Low pH enhanced fusion by affecting the structure of both gB and gH/gL mutants. Together, our data highlight the complexity of the fusion machinery, the impact of the activation state of each glycoprotein on the fusion process, and the critical role of gH/gL in regulating HSV-induced fusion.
IMPORTANCE Cell-cell fusion mediated by HSV glycoproteins requires gD, gH/gL, gB, and a gD receptor. Here, we show that fusion by wild-type HSV-2 glycoproteins occurs twice as fast as that achieved by HSV-1 glycoproteins. By sequentially swapping each glycoprotein between the two serotypes, we found that the fusion process was controlled by gH/gL. Restrictions imposed on the gB structure by mutations could be overcome by gH2/gL2, enhancing the activity of the mutants. Under low-pH conditions or when using deregulated forms of gD1 and gH2/gL2, the fusogenic potential of gB could only be increased in the absence of receptor, underlining the exquisite regulation that occurs in the presence of receptor. Our data highlight the complexity of the fusion machinery, the impact of the activation state of each glycoprotein on the fusion process, and the critical role of gH/gL in regulating HSV-induced fusion.
INTRODUCTION
Herpes simplex viruses 1 (HSV-1) and 2 (HSV-2) and varicella-zoster virus are alphaherpesviruses that infect humans. Like all herpesviruses, they have similar double-stranded DNA genomes (reviewed in reference 1). Alphaherpesviruses establish and maintain a latent infection in sensory ganglia and periodically reactivate at the original site of infection (reviewed in reference 2). During their lytic phase, they productively infect a variety of cells (3).
Although HSV-1 and HSV-2 have a high degree of genomic identity (>80%) and are similar in genome size (4, 5), they display subtle differences in infection and disease. For example, HSV-1 is more likely to cause oral lesions and sporadic encephalitis and establishes latency in the trigeminal ganglia (6), whereas HSV-2 replicates preferentially in the genital mucosa, establishes latency in the sacral ganglia, and is a principal cause of neonatal infections (7, 8). The pattern of reactivation from latency is also different for each virus (9).
Despite multiple antigenic and structural differences (10–18), both HSV-1 and HSV-2 use four essential glycoproteins, gD, gH, gL, and gB. The receptor binding protein gD binds to nectin-1 or -2, herpesvirus entry mediator (HVEM), or 3-0-sulfated heparin sulfate (19–21), to promote fusion of the viral envelope with a cellular membrane during entry. We postulate that HSV glycoproteins fuse cell membranes through a cascade of events initiated by the interaction of gD with one of its cellular receptors (22–25). In this process, the conformation of gD is altered to expose receptor binding residues that are normally hidden (22–24). This change and/or the exposed residues trigger structural changes within the gH/gL complex (26–29), thereby converting it into a functionally active state. Activated gH/gL then interacts with the class III fusion glycoprotein gB (27, 29), converting it from its metastable prefusion form (30) to its stable postfusion form (31). Through an unknown series of conformational changes, gB then completes the process of membrane fusion.
While it is generally accepted that gD is the receptor binding protein (22) and gB the fusogen (31), gH/gL is postulated to be a regulator of gB (26, 28, 29). While it has also been postulated that gH/gL itself plays a more direct role in fusion (32–34), gB and gH/gL can promote fusion when they are in separate cells (in trans), supporting a regulatory role that involves the ectodomain (26, 35, 36). However, the efficiency in this configuration is lower, suggesting a role for the tails of gB and gH/gL in the process. Recent evidence suggests a mechanism for control whereby the cytoplasmic tail of gH clamps to the cytoplasmic tail of gB to stabilize that protein (37).
Because certain mutations convert gB of HSV-1 (gB1) into a hypo- or a hyperfusogenic form (38–40), we assumed that the major regulation of the fusion process would be inherent to gB. In support of this, the overall levels of cell-cell fusion were proportional to the amount of gB expressed on the cell surface (38). However, to further evaluate the specific role of each protein in the process, we undertook a comparative analysis of the gD, gH/gL, and gB glycoproteins from HSV-1 (referred to as gD1, gH1/gL1, and gB1 or type 1 glycoproteins below) and HSV-2 (referred to as gD2, gH2/gL2, and gB2 or type 2 glycoproteins below). Key to this study is the fact that the HSV-2 forms of each of these glycoproteins can replace the HSV-1 forms with no apparent qualitative effect on cell fusion, as estimated by the formation of syncytia (11, 41, 42). To understand the dynamics of fusion, we extended our previous studies using a dual split luciferase-based cell fusion assay (43, 44) to monitor content mixing in real time (38, 45). By mixing and matching different forms of each member of the fusion complex in the presence and absence of receptor, we defined the contribution of each to the overall process.
MATERIALS AND METHODS
Cells.
B78H1 mouse melanoma cells (receptor deficient) were grown in selective Dulbecco modified Eagle medium (DMEM) containing 5% fetal calf serum (FCS) and 100 μg/ml penicillin-streptomycin. For B78-C10 cells stably expressing human nectin-1 (46), the medium was supplemented with 500 μg/ml G418.
Plasmids.
All plasmids have been characterized elsewhere; pEP98 (gB1), pEP99 (gD1), pEP100 (gH1), and pEP101 (gL1) were gifts from P. Spear (47). The construction of the Rluc81–7 and Rluc88–11 reporter constructs is described in reference 48. gB2, gD2, gH2, gL2, gH2Δ48 (the first 28 residues of gH2 are missing), gDV231A, and various gB mutants (bearing mutations W174K [encoding a change of W to K at position 174], F175K, G176K, H177A, Y179K, E260A, A261D, F262L, H263A, R264A, Y265R, Q584A, F641Y, Y649A, H657R, and LL871AA) were described elsewhere (38–40, 49, 50).
Split luciferase assay.
The split luciferase assay has been described in detail elsewhere (38, 45). Briefly, amounts of 5 × 104 B78 cells (effector cells) were seeded on white 96-well luciferase plates treated for cell culture (Corning). Amounts of 5 × 105 C10 cells (target cells) were seeded on 6-well plates. The effector cells were transfected with a mixture containing Lipofectamine 2000 (Invitrogen), plasmids encoding gD, gH, and gL proteins, and a split renilla luciferase plasmid, RLuc81–7. This master mix was divided among three wells. The target cells were transfected with the second luciferase plasmid, RLuc88–11. Twenty-four hours posttransfection, target cells were detached with Versene and resuspended in fusion medium (DMEM without phenol red, supplemented with 50 mM HEPES and 5% fetal calf serum). The membrane-permeable substrate EnduRen (Promega) was added at a final dilution of 1:1,000. Target cells were cocultured with effector cells. The accumulation of the luminescent product (colenteramide) in live cells at the time intervals specified in each figure was monitored with a BioTek plate reader. The luminescence (increase in product accumulation) was normalized by considering the signal at the last point of the time course to be 100%. To determine rates (substrate conversion per hour or minute), the slope of the linear part of the curve (R2 value equal to or higher than 0.96) was calculated. At least three independent experiments were performed (38). To aid in the interpretation of the times of initiation, the results of single representative experiments (performed in triplicate) are shown in Fig. 1 to 5 without standard deviations.
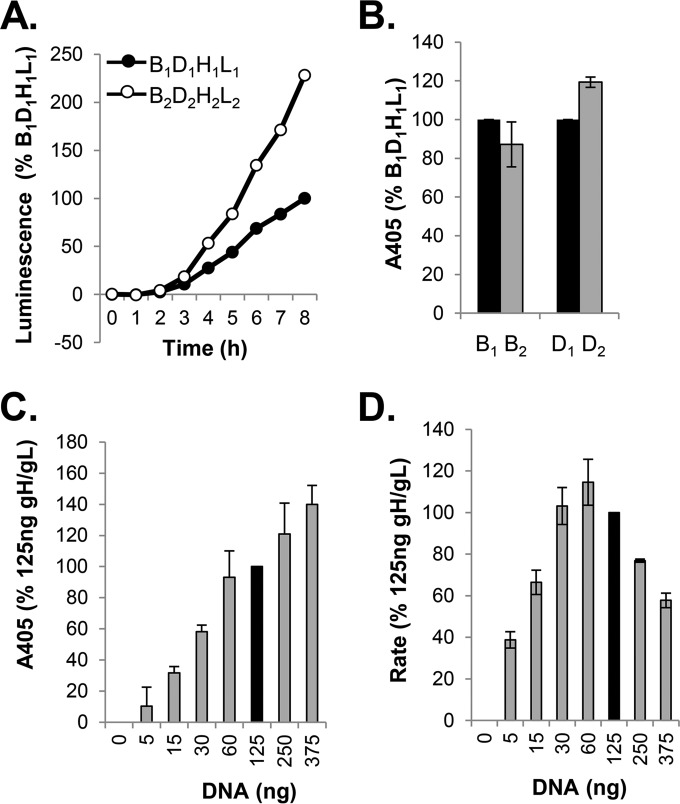
FIG 1.
Split luciferase assay (SLA) and surface expression of all-HSV-1 (all type 1) versus all-HSV-2 (all type 2) glycoproteins. (A) Kinetics of fusion. Cells were transfected with B1D1H1L1 or B2D2 H2 L2, and the rate of fusion was determined by SLA. Data are normalized to the results for all type 1 glycoproteins. (B) Results of CELISA. Cells transfected with all type 1 or all type 2 constructs were used to determine surface expression of gB1 and gB2 (R68 PAb); replica wells were used to determine the expression of gD1 and gD2 (R7 PAb). (C) Surface expression of gH2/gL2 (CELISA). Cells were transfected with various amounts of gH2/gL2 DNA, while DNA of the other two glycoproteins was maintained at 125 ng. The overall DNA concentration in all samples was maintained constant by using pCAGGS empty vector. Data were normalized to the expression of gH2/gL2 when cells were transfected with 125 ng of gH2/gL2 DNA (black bar). (D) Rate of fusion as a function of gH2/gL2 DNA concentration. Cells were transfected as described for panel C. Fusion was monitored for 8 h. Data were normalized to fusion levels measured when cells were transfected with 125 ng of gH2/gL2 DNA (black bar).
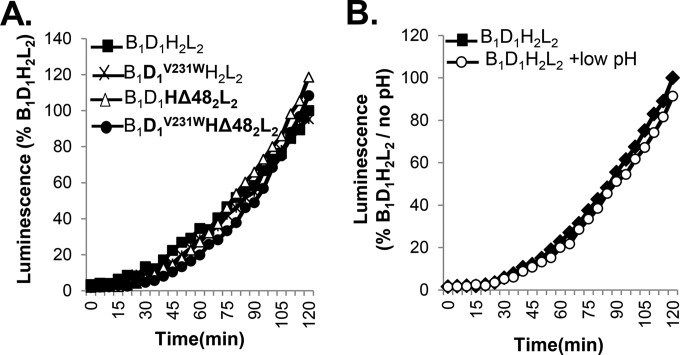
FIG 5.
Testing the regulation of fusion in the presence of receptor. (A) In the presence of receptor, fusion by gD1V231W, gH2Δ48/gL2, or the combination is the same as for wt gD and gH/gL. (B) Treating cells transfected with wt glycoproteins with a low-pH solution does not alter the kinetics of fusion compared to that of an untreated sample.
To evaluate the effect of low pH on cell-cell fusion in the presence of receptor, glycoprotein-expressing cells were treated with 50 mM citrate buffer, pH 5.6, for 3 min. After removal of the low-pH solution, the effector (nectin-1-expressing) cells were overlaid and EnduRen substrate was added. Fusion was monitored as described above.
CELISA.
To detect cell surface expression of the glycoproteins, we used a modified cell-based enzyme-linked immunosorbent assay (CELISA) (51, 52). B78H1 cells growing in 96-well plates were transfected overnight with plasmids encoding gD, gH, gL, and gB proteins at the same concentrations described above for the split luciferase assay. Cells were fixed with 3% paraformaldehyde and rinsed with phosphate-buffered saline (PBS) containing Ca2+ and Mg2+. Cells were incubated with primary antibody diluted in 3% bovine serum albumin (BSA)-PBS and then incubated for 30 min with goat anti-mouse secondary antibody coupled to horseradish peroxidase. Cells were rinsed with 20 mM citrate buffer (pH 4.5), and ABTS [2,2′-azino-di(3-ethylbenzthiazoline) sulfonic acid diammonium salt] peroxidase substrate (Roche) was added. The absorbance at 405 nm was recorded using a BioTek plate reader. The antibodies used were R68 (gB polyclonal antibody [PAb]), R7 (gD PAb), or a cocktail of gH2/gL2 monoclonal antibodies (CHL26, CHL39, and CΔ48L3) (42, 53).
Immunofluorescence and cell-cell fusion assay in the absence of receptor.
Amounts of 2 × 105 B78 cells plated on glass coverslips were transfected for 24 h with the glycoprotein constructs indicated below. For testing the effect of low-pH treatment on cell-cell fusion in the absence of receptor, cells were treated at 6 h posttransfection with 50 mM citrate buffer, pH 5.6, for 5 min. The buffer was removed by aspiration, and cells were rinsed and refed with DMEM supplemented with 10% FCS. Samples were fixed 24 h posttreatment with 3% paraformaldehyde at room temperature (RT), quenched with 50 mM NH4Cl, washed with PBS, blocked in goat serum, and incubated with anti-gH2/gL2 PAb R176 followed by Alexa Fluor 594-conjugated goat anti-IgG secondary antibody (Invitrogen). Coverslips were rinsed with PBS and H2O and mounted in ProLong gold antifade reagent (Invitrogen). As detailed previously (27, 54), samples were examined by confocal microscopy using a Nikon TE-300 inverted microscope coupled to a PerkinElmer imaging system. A two-line argon/krypton laser (488/514 and 568/647 nm) was used to excite the fluorescence of Alexa Fluor 594 (590/617 nm) and enhanced yellow fluorescent protein (EYFP) (515/528 nm).
Quantitation of cell-cell fusion.
After the cells were stained, syncytia were counted on the entire surface of each coverslip at ×60 magnification. A syncytium was defined as such when one fluorescent membrane enclosed three or more nuclei (26). The results of at least three independent experiments were averaged.
RESULTS
Fusion rates mediated by HSV-1 and HSV-2 glycoproteins in nectin-bearing B78-C10 cells differ.
Using the split luciferase assay (43), we monitored cell-cell fusion mediated by the four essential glycoproteins from HSV-1 and HSV-2. Figure 1A shows typical fusion curves from a representative experiment over an 8-h time course (38, 45). Although the rates in each case were constant over time, cells transfected with the type 2 glycoproteins consistently exhibited higher rates of fusion than those transfected with the type 1 constructs, with a two- to threefold difference in kinetics (Fig. 1A). Thus, the serotype of each of the glycoproteins has a characteristic and intrinsic rate of cell-cell fusion.
The CELISA results showed that the difference in kinetics could not be ascribed to differences in the total levels of gB or gD expression on the cell surface, as they were very similar for both serotypes (Fig. 1B). A direct comparison of cell surface expression between gH1/gL1 and gH2/gL2 was not possible due the lack of a type-common polyclonal antibody. Therefore, we looked at the correlations between the concentrations of gH2/gL2 DNA transfected into cells, surface expression levels, and fusion levels and compared them to those observed for gH1/gL1 (38). The level of cell surface expression for gH2/gL2 was DNA concentration dependent (Fig. 1C), as previously observed for gH1/gL1 (38). Furthermore, as shown for gH1/gL1, substantial fusion occurred when as little as 5 ng of DNA was used, and the maximal level occurred using quantities ranging between 30 and 125 ng (Fig. 1D). As the glycoproteins in all type 1 (B1D1H1L1) and all type 2 (B2D2H2L2) backgrounds are expressed at essentially similar levels, this suggested that the increased level of fusion seen with type 2 constructs was governed by independent factors.
Swapping of glycoproteins between type 1 and type 2 serotypes.
In previous studies, we speculated that when cells were transfected with type 1 glycoproteins, the structure of gB determined the rate of fusion (38). However, we and others have hypothesized that the overall regulation of fusion resides with gH/gL (26, 28, 29, 37). Here, we questioned whether the regulation of cell-cell fusion is due to gB, gH/gL, or a combination of the two core fusion proteins. To test this, we exploited differences in the fusion kinetics of HSV-1 and HSV-2, sequentially replacing each type 1 glycoprotein with its type 2 counterpart, and measured the effects on fusion rates. In each case, cells transfected with four homologous glycoproteins served as controls for setting the range of expected fusion levels (Fig. 2, black lines).
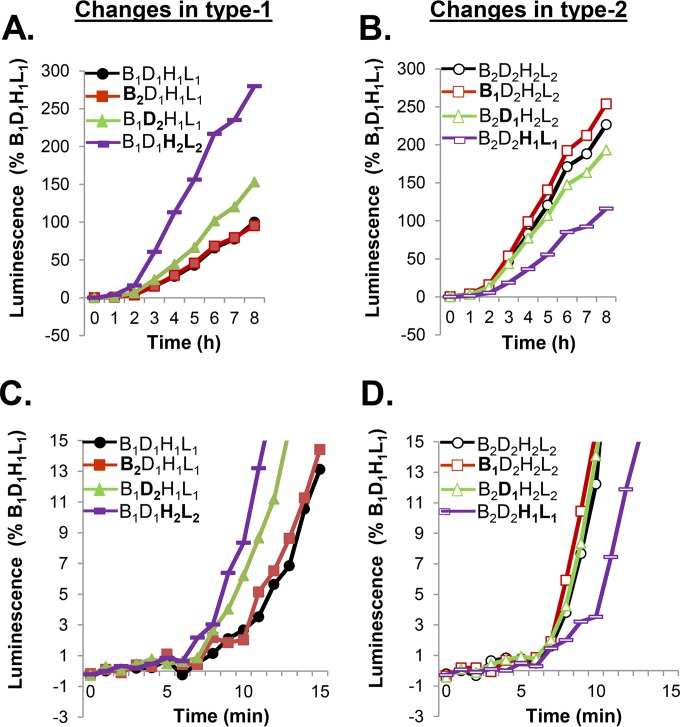
FIG 2.
gH/gL governs the rates of fusion of type 1 and type 2 glycoproteins. (A) Cells were transfected with type 1 glycoproteins (control, black). Each type 1 form was replaced with the type 2 form (gB2, red; gD2, green; gH2/gL2, purple). Fusion was measured over an 8-h period. (B) Cells were transfected with type 2 glycoproteins (control, black). Each type 2 form was replaced with its type 1 counterpart (gB1, red; gD1, green; gH1/gL1, purple). (C, D) The rates of fusion of glycoproteins with substitutions as described for panels A and B were determined over a 15-min period to examine initiation. Each experiment was done multiple times, in duplicate. The results of representative experiments are shown.
Somewhat unexpectedly, swapping gB2 for gB1 in a type 1 background (B1D1H1L1→B2D1H1L1) (Fig. 2A, red lines) (or vice versa, gB1 for gB2 in a type 2 background; Fig. 2B, red lines) did not alter the rate of fusion over 8 h compared with the results using all type 1 or all type 2 glycoproteins (Fig. 2A and B, black lines). However, swapping gD2 for gD1 (Fig. 2A, green) (or vice versa) enhanced fusion marginally. Most notably, however, the replacement of gH1/gL1 with gH2/gL2 greatly enhanced the kinetics of fusion over 8 h (Fig. 2A, purple), equaling the higher fusion rates seen with B2D2H2L2 proteins (Fig. 2B, black). Conversely, when gH2/gL2 was replaced with gH1/gL1 in a type 2 background (Fig. 2B, purple), the low fusion rates were similar to those seen with B1D1H1L1 (Fig. 2A, black). Taken together, the results show that the difference in fusion kinetics for the two serotypes of HSV glycoproteins, i.e., increased or decreased activity, is primarily controlled by the gH/gL serotype.
To better understand the regulatory process, we measured fusion during the first 15 min after triggering (Fig. 2C and D). Initiation was defined as the time when the luminescent signal was at least twofold over the background level (6 to 8 min) (38, 45). Switching gB2 for gB1 (or vice versa) had no effect on the initial rate of fusion (Fig. 2A and B). In contrast, switching gD2 (Fig. 2C, green) for gD1 enhanced the initial rate of fusion, but its effects were more pronounced at earlier than at later times. Exchanging gH2/gL2 for gH1/gL1 (Fig. 2C, purple) induced a sustained threefold increase within 15 min that extended over 8 h. Consistent with this, exchanging gH1/gL1 for gH2/gL2 reduced the rate of type 2 fusion (Fig. 2D, purple). We conclude that the gH/gL complex constitutes the main regulator of cell-cell fusion and that the serotype determines both the initial and extended rates of fusion.
Contribution of gH and gL to regulation of cell-cell fusion.
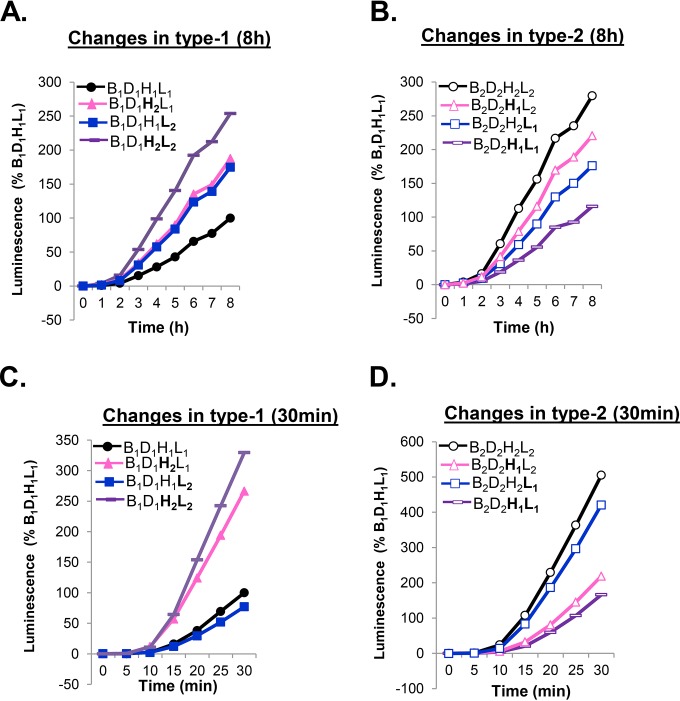
HSV gH and gL function as a heterodimer (29, 55, 56), and the crystal structures of all herpesvirus gH/gL complexes studied demonstrate an intimate physical interaction between gH and gL (28, 57–59). We showed previously that hybrids of gH1/gL2 (or vice versa) were functional in cell-cell fusion (42, 60), but the detailed kinetics of fusion by these heterocomplexes was not determined. Therefore, we asked whether the modulatory activity relies on gH, gL, or the complex. For this, we examined the rates of fusion that occur over a 30-min or 8-h period (Fig. 3), testing each combination of gH and gL with either gB1 or gB2, as well as with gD1 or gD2 (e.g., B1D1H1L2 versus B1D1H2L1). The rates of fusion for all type 1 or all type 2 glycoproteins were as expected for these time frames (Fig. 3, black curves).
FIG 3.
The early stages of fusion are controlled by gH. Cells were transfected with all type 1 glycoproteins (control, black) or a combination of gB1, gD1, and either gH2/gL2 (purple), gH1/gL2 (blue), or gH2/gL1 (pink) and fusion measured over 8 h (A) or 30 min (C). Cells were transfected with all type 2 glycoproteins (control, black) or a combination of gB2, gD2, and either gH1/gL1 (purple), gH1/gL2 (pink), or gH2/gL1 (blue) and fusion measured over 8 h (B) or 30 min (D).
The presence of either a gH2 or a gL2 was sufficient to increase the level of fusion over the result for all type 1 glycoproteins in an 8-h time course (Fig. 3A, blue and magenta lines). However, the gain in activity did not match the total fusion levels observed for gH2/gL2 (Fig. 3A, purple). A similar enhancing effect was found in the presence of B2D2 (Fig. 3B): the heterotypic gH/gL molecule was more active than the type 1 unit but less active than the type 2 (magenta and blue curves). Thus, the heterologous gH/gL unit retained activity.
To extend these studies, we examined early events (30 min). We found that the fusion rate obtained using the gH1/gL2 complex in the background of type 1 gB and gD (Fig. 3C, blue) correlated with that obtained using all type 1 forms (Fig. 3C, black), while the fusion rate for the gH2/gL1 complex approximated the fusion rate for the all type 2 form (Fig. 3C, compare the pink and purple lines). Thus, the serotype of gH plays a major regulatory role in the fusion rate. As expected, the fusion capacity was reduced fivefold by introducing gH1/gL1 into an all type 2 background (Fig. 3D, purple). While replacing gL2 with gL1 alone had little effect (Fig. 3D, blue), replacing gH2 with gH1 (Fig. 3B, pink) led to a fusion rate that matched the lower rate of fusion seen for B2D2H1L1 (Fig. 3D, purple). Together, these results suggest that early fusion rates are governed primarily by gH and that gH2 is primarily responsible for the upregulation of fusion in either a type 1 or a type 2 background. However, both gH and gL are important for the initiation and ongoing fusion.
Determining the relative potential of gB to fuse cells.
We previously showed that the overall fusion levels achieved by mutant forms of gB in an all type 1 background ranged from null to impaired to enhanced and that the fusion levels achieved were directly related to the fusion rate (38). As such, we had previously speculated that the restraint/allowance imposed on the structure of gB by individual mutations dictated the extent and rate of fusion. However, our current data paradoxically suggest that gH/gL is the predominant regulator of the fusion rate. To try to reconcile these findings, we again exploited the ability of gH2/gL2 to induce a twofold increase in the fusion rate of gB1 (Fig. 2). We reasoned that if the level of fusogenic activity of gB1 is dictated solely by its structure, the addition of gH2/gL2 should not enhance the fusogenic activity of gB mutants. However, if fusion levels increased, it would suggest that the actual fusogenic activity (its total potential to fuse) resides in gB but that gH/gL controls this process. Thus, we asked whether gH2/gL2 could overrule the restrictions placed on gB1 function by each mutation and thereby upregulate fusion. We tested three sets of gB1 mutants.
(i) Effect of gH2/gL2 on fusion tested by mutations in the FLs of gB1.
We previously constructed gB1 mutants with mutations in the fusion loops (FLs) (39, 61), several of which were unable to promote cell fusion or infection, while others were impaired (38–40, 54, 62). The results in Fig. 4A show the capacity of gB1 with mutations in FL1 (H177A and Y179K) or FL2 (E260A and H263A) to fuse cells when stimulated by gH1/gL1. As observed previously (38–40), Y179K and H263A mutants failed to function in cell-cell fusion (Fig. 4A, diamond and circle) and H177A and E260A mutants were markedly impaired (Fig. 4A, square and triangle). Next, we tested whether gH2/gL2 could override the restrictions placed on gB by its altered structure. The results in Fig. 4B show that mutants with the null mutations, e.g., W174K and Y179K, remained unable to promote fusion but H177A and E260A mutants, which were markedly impaired in the context of gH1/gL1, were significantly enhanced (twofold) by gH2/gL2.
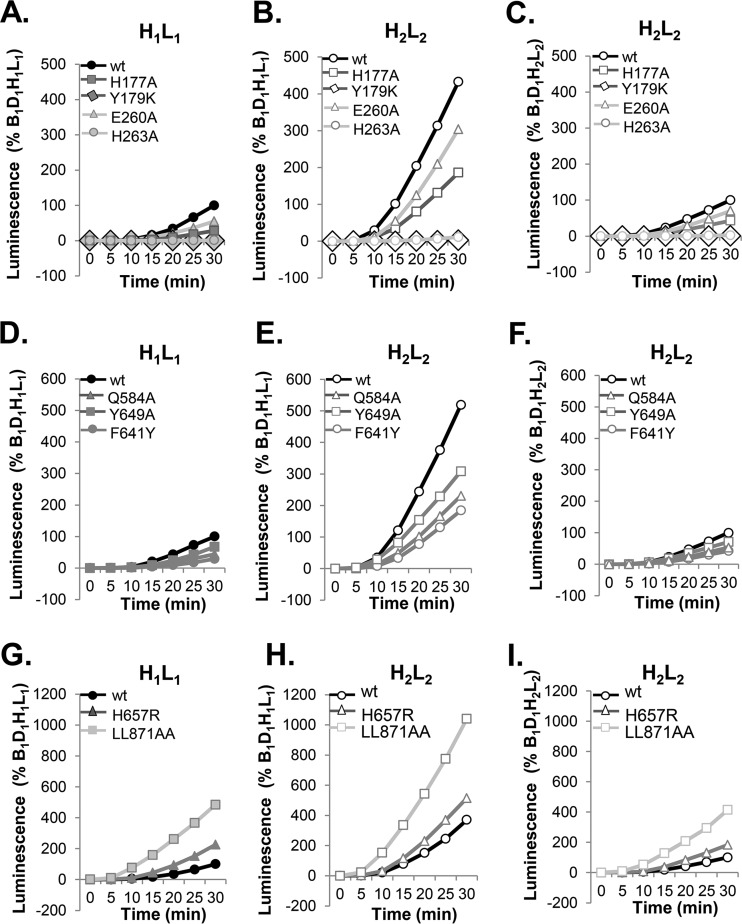
FIG 4.
Determining the relative fusogenic potential of gB. (A, B) The rates of fusion of mutants with select mutations in FR1 controlled by gH1/gL1 (A) or gH2/gL2 (B). (D, E) Rates of fusion of hypofusogenic gB1 FR3 mutants controlled by gH1/gL1 (D) or gH2/gL2 (E). (G, H) Rates of fusion of hyperfusogenic gB1 mutants with mutations in the crown (H657R) and the cytoplasmic tail (LL871AA) controlled by gH1/gL1 (G) or gH2/gL2 (H). Data are shown as percentages of B1D1H1L1 fusion. (C, F, I) Data presented in panels B, E, and H were replotted as percentages of the results for B1D1H2L2 to show the relative fusion activities of gB mutants when combined with gH2/gL2.
(ii) Regulation of fusion by hypofusogenic (slow) mutants tested by gH2/gL2.
Mutants with mutations in the crown of functional region 3 (FR3) (F641Y, Y649A, and Q584A) are functional but exhibit a slow initiation time and diminished overall rate and extent of fusion in a type 1 background (Fig. 4D) (38). When gH2/gL2 was substituted for gH1/gL1, all three mutants exhibited a marked increase in both the initiation time (data not shown) and overall rate of fusion (Fig. 4E). Thus, the mutation in gB dictated the level of function of the system and gH2/gL2 could increase the fusogenic capacity of that mutant.
(iii) Regulation of fusion by hyperfusogenic (fast) gB mutants tested by gH2/gL2.
Hyperfusogenic gB1 with mutations in the crown (H657R) and in the cytoplasmic tail (LL871AA) showed enhanced levels of fusion but were different in their time of initiation of fusion (38, 63). Fast mutants were postulated to represent unlocked forms of gB (further along in the transition path of pre- to postfusion conformation) that were inherently in a higher energy state than wild-type (wt) gB. Particularly striking was the result for gBLL871AA, which we had postulated exhibited the maximal capacity for fusion that could be achieved by gB (38). As before, in the context of the homologous form of gH1/gL1, gBH657R (Fig. 4G, triangle) (38) showed a twofold increase in the rate of fusion compared to that of wt gB (circle), and gBLL871AA showed a fivefold increase (Fig. 4H, squares). Here, we found that gH2/gL2 did in fact induce a twofold increase in the fusion level of hyperfusogenic gB (Fig. 4H, square and triangle) over that seen with gH1/gL1 and that the fusion rates for hyperfusogenic gB1 mutants were significantly enhanced by gH2/gL2 compared to the rates with gH1/gL1. This was true for all gB mutants and was more apparent when the results shown in Fig. 4B, E, and H were expressed as the percentages of the rates for B1D1H2L2 (Fig. 4C, F, and I). We conclude that the relative fusion activities of all three classes of mutant forms of gB were dictated by changes in its structure and that the actual fusogenic capacity of any gB molecule was regulated by gH/gL.
Regulation of fusion in the presence of partially activated gD1 and gH2/gL2 forms.
According to our activation cascade model of receptor-driven fusion, the activity of each protein is regulated in a sequential fashion. Specifically, gD is activated by receptor, gH/gL by gD, and gB by gH/gL, as now documented here. Therefore, we next assessed the requirements needed to fully activate gB as a fusogen (drive it to its maximum capacity) and identified the role of each glycoprotein in fusion activation.
(i) Fusion in the presence of receptor.
Previously, we created mutant forms of gD1 and gH2/gL2 that function, albeit poorly, in the absence of their individual activator: gD1V231W (50) induces low levels of fusion in the absence of receptor, and gH2Δ48/gL2 (49) induces fusion in the absence of gD1 (42). Here,we first asked whether these deregulated forms, either alone or in combination, could alter the fusogenic potential of gB1 in the presence of nectin-1 (considered 100%). The results shown in Fig. 5A show that every combination of gB1 with gD1V231W and/or gH2Δ48/gL2 was indistinguishable from the wt proteins in terms of fusion (49, 50) and that fusion was regulated in a type 2 fashion by gH2Δ48/gL2. Thus, the partially activated gD1 and gH2/gL2 forms can be driven to the same level as the wt forms, indicating that regulation in the presence of a receptor is finely controlled.
(ii) Fusion in the absence of receptor.
We next attempted to quantify fusion in the absence of receptor using the split luciferase assay. However, this proved to have inconsistent results, presumably because glycoprotein-expressing cells (containing one half of the luciferase reporter gene) could fuse with either a cell that expressed the complementary reporter gene (positive signal) or a cell that did not (no signal). As a result, only a fraction of the reporter genes would be reconstituted and not always at the same rate, generating inconsistent readings. As we have shown that fusion and fusion kinetics measured by immunofluorescence or luciferase assay were comparable in the presence of receptor (26, 27, 42, 50, 54), we quantified fusion in the absence of receptor by direct counting of syncytia. Data for each combination of glycoproteins in the absence of receptor were expressed as the percentage of the number of syncytia observed using wt constructs (B1D1H2L2) in the presence of receptor, i.e., 100% (Fig. 6, dashed line). In the absence of receptor, wt gD1 and gH2/gL2 failed to activate fusion by wt gB1 (Fig. 6A, black bar). Individually, gD1V231W and gH2Δ48/gL2 (Fig. 6A, light gray and dark gray bar, respectively) increased fusion to about 10% of the level for the wt/receptor control, thus confirming our previous observations (42, 50). In combination, the two mutants enhanced fusion to 40% of the level for the wt/receptor control (Fig. 6A, white bar), highlighting the effect of gD on the upregulation of gH/gL activity. Even though both gD1V231W and gH2Δ48/gL2 were fully competent in the presence of receptor (Fig. 5), neither could activate gB1 to its full capacity in the absence of receptor. Thus, the full activation of each glycoprotein is dependent on the level of activation of each preceding step in the pathway. gD1V231W (which in part mimics receptor-activated gD) could upregulate the activity of partially activated gH2Δ48/gL2 but could not completely compensate for the lack of receptor.
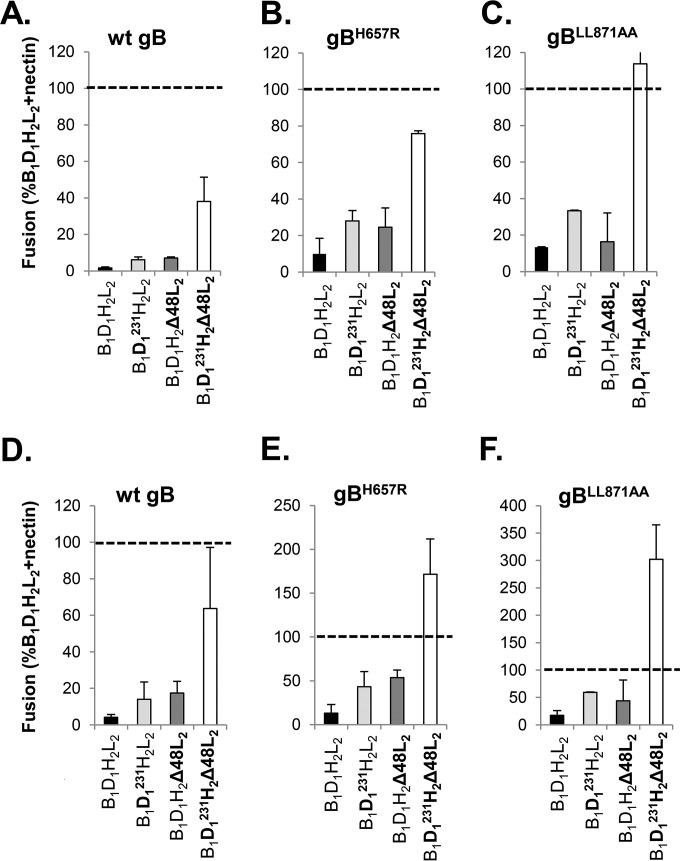
FIG 6.
Effects of mutant forms of gD and gH/gL on fusion in the absence of receptor. (A) Fusion in the absence of receptor by gB1 is enhanced by gD1V231W (D1231; light gray) or gH2Δ48/gL2 (dark gray) compared to the results for wt gD and gH/gL (black bar). The combination of gD1V231W and gH2Δ48/gL2 (white bar) represents the maximum fusogenic potential of gB1 under these conditions. (B, C) Compared to the results for the wt, hyperfusogenic gB1 mutants gBH657R (B) or gBLL871AA (C) show increased fusion levels when combined with gD1V231W (light gray), gH2Δ48/gL2 (dark gray), or both (white bar). (D, E, F) Low-pH treatment increases the fusion levels in the absence of receptor by wt gB1 (D), gBH657R (E), or gBLL871AA (F). Data were normalized to fusion levels by wt gB1, gD1, and gH2/gL2 in nectin-1-expressing C10 cells (dashed line).
We next hypothesized that hyperfusogenic gB1 mutants (gB1H657R and gB1LL871AA) (38, 63, 64) in combination with gD1V231W and gH2Δ48/gL2 might completely uncouple the process and enhance fusion to its theoretical maximum. In the presence of wt D1H2L2, the addition of gB1H657R (Fig. 6B, black bar) or gB1LL871AA (Fig. 6C, black bar) had a small positive effect. Together with gD1V231W (Fig. 6B and C, light gray bars) or gH2Δ48/gL2 (Fig. 6B and C, dark gray bars), fusion was moderately enhanced (now ∼30% of the level for wt/receptor). Combining all three deregulated glycoproteins, gD1V231W, gH2Δ48/gL2, and gB1H657R (Fig. 6B, white bar), markedly increased fusion to 80% of the level for the receptor-driven situation and to 100% for gB1LL871AA (Fig. 6C, white bar). Thus, in combining hyperfusogenic gB1 with a receptor-independent gD1 and a gD-independent gH/gL, we have mimicked the main steps in the fusion pathway required for the activation of wt glycoproteins in the presence of receptor.
Effect of low pH on fusion in a completely deregulated system.
Low pH can induce conformational changes in gB and gH/gL (61, 65–67; unpublished data) that affect virus infection of certain cell types (68–70). To determine whether low pH could drive gB to a higher energy level as evidenced by enhanced fusion, transfected cells were treated to lower the pH (see Materials and Methods) and the extent of fusion determined by counting syncytia. The protocol for the experiments was set up as shown in Fig. 6A to C. In the presence of receptor, low-pH (pH 5.6) treatment had no effect on cell-cell fusion mediated by the wt glycoproteins or any combination of the deregulated proteins (Fig. 5B). However, in the absence of receptor, the pH treatment had a consistent and marked effect on the extent of fusion by either wt or deregulated glycoproteins (compare Fig. 6D to F with A to C). For wt gB (Fig. 6A and D, white bars), low pH enhanced the fusion levels from 40% to 65%. For gB1H657R (Fig. 6B and E, white bars), low pH enhanced the level of fusion from 75% to 170%. For the highly hyperfusogenic gB1LL871AA (previously thought to be gB at its maximum), low pH increased the level of fusion further, from 110% to 300%.
Thus, as previously shown, receptor-driven fusion is highly regulated and cannot be perturbed by low pH (Fig. 5B), presumably because each glycoprotein has already reached a fully activated state. In contrast, acid treatment had a positive effect on the fusion levels in a deregulated system where the glycoproteins were partially activated and the level of activation was dependent on the state of deregulation and the structure of gB. As reported before, low-pH-mediated enhancement appears to be driven by both gB and gH/gL but not gD (data not shown). However, at this point, it is unclear whether pH-modified gH/gL was driven to a higher energy level to work on gB (also altered by low pH) or whether low pH affected the gB-gH/gL interaction.
In conclusion, we have shown that the extent and the ongoing rates of fusion by HSV glycoproteins were primarily governed by gH/gL, with gH being the main contributor. Fusion in the presence or absence of a cellular receptor involved similar regulatory steps of the fusion cascade. Finally, efficient activation of each step was required in order for each glycoprotein to reach its maximum activity.
DISCUSSION
Fusion mediated by glycoproteins from different serotypes occurs at specific rates.
In this study, we analyzed the fusion kinetics of human nectin-1-expressing B78 mouse melanoma cells following the expression of either HSV-1 or HSV-2 entry glycoproteins gD, gH/gL, and gB using the highly sensitive split luciferase fusion assay (38, 43, 45, 48). We found that the rate of cell-cell fusion for type 2 forms of the glycoproteins was enhanced at least twofold over that caused by the type 1 forms (Fig. 1). To determine which glycoprotein(s) was responsible for the difference in the fusion rates between the two serotypes, we took advantage of previous studies which demonstrated that the four essential glycoproteins from either HSV-1 or HSV-2 are functionally interchangeable in a cell-cell fusion assay (11, 41). While these studies provided information about the requirements for homotypic interactions and the general regions important for these interactions, the extent of fusion was only evaluated at late time points (18 to 24 h) and underlying changes in the kinetics of fusion were not addressed. By using the split luciferase assay, we continuously monitored the initial and ongoing rates of fusion driven by HSV glycoproteins. While we had originally assumed that the fusion rate would be heavily influenced by gB (38), our subsequent finding that gH/gL regulated this process (26, 28) raised the question of which played the more dominant role in governing the fusion process. We found that switching gB1 for gB2 (or vice versa) made no difference in the initiation, the overall rate, or the total amount of fusion (Fig. 2), indicating that these two forms of HSV gB are functionally equivalent in their ability to fuse membranes.
Consistent with the functional equivalence of gB1 and gB2 in our split luciferase assay, gB is evolutionarily more highly conserved between HSV-1 and HSV-2 than are the other members of the HSV fusion complex (71). The conservation is reflected not only in the high sequence identity between gB1 and gB2 but also in the crystal structures of gB molecules across human herpesviruses (31, 72–76) and likely reflects the essential nature of gB as the viral fusogen. This also explains why it was possible to exchange HSV gB with those of herpesviruses that infect other species without compromising fusion, including human-squirrel (HSV-1/saimiriine herpesvirus 1 [SaHV-1]) (77, 78), human-pig (HSV-1/pseudorabies virus [PrV]) (79), pig-cow (PrV/bovine herpesvirus [BHV]) (80), and human-monkey (HSV-1/cercopithecine herpesvirus 2 [CeHV-2]) (81) exchanges.
Evidence that gH/gL governs the rate of fusion.
Here, we showed that the gH/gL serotype determines both the initial and extended rates of fusion, indicating that the gH/gL complex is the key regulator of gB-mediated cell-cell fusion. The early fusion rate was governed primarily by gH, as gH2 was the major factor controlling the differential fusion between the type 1 and type 2 backgrounds (Fig. 3). However, both gH and gL are important for initiation and ongoing fusion. While heterotypic gH/gL molecules achieve similar early rates of fusion, homotypic pairs are superior later on in the process, suggesting that a perfect marriage of structures at interface for the complex might be critical for this stage of the fusion process.
The fusogenic capacity of gB is governed by its structure.
Wild-type gB has an inherent fusogenic capacity that can be influenced by structural restrictions imposed by various mutations (38). Regardless of the structure-imposed phenotype, fusion by each of the functional mutants that we tested was enhanced by their interaction with gH2/gL2 (Fig. 4). A possibility is that gH2/gL2 could have a higher affinity for gB and in this way activate a greater number of gB trimers, thereby enhancing the fusion levels. The fusion activity of mutants with specific mutations in the fusion loops of gB prevented the interaction between gB and gH/gL (54) and was not enhanced by gH2/gL2, indicating that these single-amino-acid changes truly destroyed the ability of gB to function in cell-cell fusion. In contrast, mutants with other mutations which did not totally disrupt the interaction were significantly enhanced by gH2/gL2. We speculated that the gH/gL complex regulated a step in the fusion pathway that occurs somewhere after the insertion of gB fusion loops into lipid membranes but before the activation of gB to a full fusogenic state (54).
What is the effect of mutants that bypass steps in the activation pathway?
While wt gB has an inherent fusogenic capacity, we next wanted to evaluate the full fusogenic capacity of gB and identify the regulatory steps in the fusion pathway that could lead to this activation. Specifically, we wanted to assess whether the activity of gB could be increased to higher levels by gD and gH2/gL2 mutants known to bypass key steps in fusion activation in the context of either receptor-induced fusion or a receptor-free (deregulated) situation. To address these questions, we tested the effects of two partially activated, presumably higher-energy-state glycoproteins, gD1V231W (enables gD1 to facilitate fusion in the absence of receptor) (50) and gH2Δ48/gL2 (promotes cell fusion in the absence of gD) (42). Notably, we observed that fusion by gB1 and either of the two gD1 and gH2/gL2 mutants was tightly regulated by receptor and was driven to the same levels as the wt glycoproteins (49, 50). In the absence of receptor, we found that full activation of each glycoprotein (attaining the same level of fusion activity as that achieved in the presence of receptor) depended on the level of activation of each preceding step in the pathway. For example, by mimicking the conformation of gD bound to receptor (using gD1V231W), recapitulating the movement of the N terminus of gH2/gL2 (gH2Δ48/gL2), and placing gB in a higher fusogenic state (gBLL871AA), we achieved fusion levels that were similar to those found with wt B1D1H2L2 in the presence of receptor (Fig. 6C). Thus, to attain wt levels of fusion, each unit of the machinery needs to operate at its fully activated state to drive the prefusion form of gB to its fully activated state.
Delineation of the role of each component in the fusion core machinery, and particularly gH/gL, at different stages of fusion activation is challenging in the presence of receptor. However, in the absence of receptor, the levels of fusion by wt glycoproteins are essentially null. Clearly, fusion levels can be regulated by partially activated forms of gD and gH/gL (42, 50). Although less efficiently than in the presence of receptor, gD1V231W can upregulate gH2/gL2 in a receptor-independent manner to partially trigger fusion by gB1 (50). gH2Δ48/gL2 alone can also activate gB1 to a higher activation level than wt gH2/gL2 (42). However, the level of fusion induced by the combination of the two mutant proteins represents more than an additive effect (40% for the combination versus 10% for each mutant). Thus, gD1V231W can raise the activation level of gH2Δ48/gL2 (Fig. 6A). Furthermore, while the structural changes induced by gD1V231W and gH2Δ48/gL2 are similar to those induced by receptor, they are clearly not sufficient to fully trigger wt gB1. This suggests that each glycoprotein in turn exhibits a range of activity that is totally dependent on the level of activation of the protein in a previous step. As far as we can tell, full activation of this cascade can only be initiated by conformational changes in gD triggered by binding to receptor.
Effect of acidic conditions on cell-cell fusion.
Low pH is required for HSV entry by endocytosis (68, 82), and pH affects the conformation of gB present in the virion or transfected cells (65–67, 83). Acidic conditions also affect the structure of gH/gL, allowing it to form a complex with truncated gB that associates with lipids in a liposome flotation assay (61). Here, we found that while low pH had no effect on fusion in the presence of receptor, low pH further enhanced the fusogenic capacity of the fully deregulated glycoproteins (gD1V231W, gH2Δ48/gL2, and gBLL871AA) in the absence of receptor (Fig. 6F). Whether the maximum level of fusion by the hyperfusogenic gBLL871AA was achieved remains to be determined. Notably, the final levels of fusion achieved in the deregulated system at low pH were dependent on the structure of the gB mutant, indicating that the final extent of fusion was dictated by the gB molecule. pH affects the antigenic structure of both gB and gH/gL (unpublished data), suggesting that specific acid-induced structural changes to both proteins are necessary for cell-cell fusion, and this observation may be relevant for fusion of the virus during pH-dependent endocytosis.
We conclude that HSV-induced cell-cell fusion is a tightly regulated process and each glycoprotein can be present in multiple activation states to drive fusion. Furthermore, the maximum working capacity of each glycoprotein can be achieved only after efficient activation of the preceding steps of the fusion cascade.
ACKNOWLEDGMENTS
Research reported in this publication was supported by NIH grants R01-AI018289 (G.H.C.) and R01-AI056045 (R.J.E.).
We thank Claude Krummenacher and all the members of our laboratory for helpful advice and Leslie King, School of Veterinary Medicine, for her wonderful editorial input.
The content of this paper is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
REFERENCES
- 1.Baines JD, Pellett PE. 2007. Genetic comparison of human alphaherpesvirus genomes, p 61–69. In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom. [PubMed] [Google Scholar]
- 2.Roizman B, Whitley RJ. 2013. An inquiry into the molecular basis of HSV latency and reactivation. Annu Rev Microbiol 67:355–374. doi: 10.1146/annurev-micro-092412-155654. [DOI] [PubMed] [Google Scholar]
- 3.Roizman B, Taddeo B. 2007. The strategy of herpes simplex virus replication and takeover of the host cell, p 163–173. In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, United Kingdom. [PubMed] [Google Scholar]
- 4.McGeoch DJ, Dalrymple MA, Davison AJ, Dolan A, Frame MC, McNab D, Perry LJ, Scott JE, Taylor P. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol 69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 5.Dolan A, Jamieson FE, Cunningham C, Barnett BC, McGeoch DJ. 1998. The genome sequence of herpes simplex virus type 2. J Virol 72:2010–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitley RJ, Kimberlin DW, Roizman B. 1998. Herpes simplex viruses. Clin Infect Dis 26:541–553. doi: 10.1086/514600. [DOI] [PubMed] [Google Scholar]
- 7.Zheng M, Conrady CD, Ward JM, Bryant-Hudson KM, Carr DJ. 2012. Comparison of the host immune response to herpes simplex virus 1 (HSV-1) and HSV-2 at two different mucosal sites. J Virol 86:7454–7458. doi: 10.1128/JVI.00702-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitley RJ, Roizman B. 2001. Herpes simplex virus infections. Lancet 357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 9.Krause PR, Ostrove JM, Straus SE. 1991. The nucleotide sequence, 5′ end, promoter domain, and kinetics of expression of the gene encoding the herpes simplex virus type 2 latency-associated transcript. J Virol 65:5619–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spear PG, Longnecker R. 2003. Herpesvirus entry: an update. J Virol Methods 77:10179–10185. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zago A, Spear PG. 2003. Differences in the N termini of herpes simplex virus type 1 and 2 gDs that influence functional interactions with the human entry receptor Nectin-2 and an entry receptor expressed in Chinese hamster ovary cells. J Virol 77:9695–9699. doi: 10.1128/JVI.77.17.9695-9699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheshenko N, Herold BC. 2002. Glycoprotein B plays a predominant role in mediating herpes simplex virus type 2 attachment and is required for entry and cell-to-cell spread. J Gen Virol 83:2247–2255. doi: 10.1099/0022-1317-83-9-2247. [DOI] [PubMed] [Google Scholar]
- 13.Gerber SI, Belval BJ, Herold BC. 1995. Differences in the role of glycoprotein C of HSV-1 and HSV-2 in viral binding may contribute to serotype differences in cell tropism. Virology 214:29–39. doi: 10.1006/viro.1995.9957. [DOI] [PubMed] [Google Scholar]
- 14.Herold BC, Gerber SI, Belval BJ, Siston AM, Shulman N. 1996. Differences in the susceptibility of herpes simplex virus types 1 and 2 to modified heparin compounds suggest serotype differences in viral entry. J Virol 70:3461–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herold BC, Visalli RJ, Susmarski N, Brandt CR, Spear PG. 1994. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J Gen Virol 75(Pt 6):1211–1222. doi: 10.1099/0022-1317-75-6-1211. [DOI] [PubMed] [Google Scholar]
- 16.Laquerre S, Argnani R, Anderson DB, Zucchini S, Manservigi R, Glorioso JC. 1998. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J Virol 72:6119–6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rux AH, Lou H, Lambris JD, Friedman HM, Eisenberg RJ, Cohen GH. 2002. Kinetic analysis of glycoprotein C of herpes simplex virus types 1 and 2 binding to heparin, heparan sulfate, and complement component C3b. Virology 294:324–332. doi: 10.1006/viro.2001.1326. [DOI] [PubMed] [Google Scholar]
- 18.Tal-Singer R, Peng C, Ponce De Leon M, Abrams WR, Banfield BW, Tufaro F, Cohen GH, Eisenberg RJ. 1995. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol 69:4471–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. 2011. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat Rev Microbiol 9:369–381. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpes virus fusion and entry: a story with many characters. Viruses 4:800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krummenacher C, Carfi A, Eisenberg RJ, Cohen GH. 2013. Entry of herpesviruses into cells: the enigma variations. Adv Exp Med Biol 790:178–195. doi: 10.1007/978-1-4614-7651-1_10. [DOI] [PubMed] [Google Scholar]
- 22.Carfi A, Willis SH, Whitbeck JC, Krummenacher C, Cohen GH, Eisenberg RJ, Wiley DC. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol Cell 8:169–179. doi: 10.1016/S1097-2765(01)00298-2. [DOI] [PubMed] [Google Scholar]
- 23.Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C, Carfi A. 2011. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog 7:e1002277. doi: 10.1371/journal.ppat.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krummenacher C, Supekar VM, Whitbeck JC, Lazear E, Connolly SA, Eisenberg RJ, Cohen GH, Wiley DC, Carfi A. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J 24:4144–4153. doi: 10.1038/sj.emboj.7600875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazear E, Carfi A, Whitbeck JC, Cairns TM, Krummenacher C, Cohen GH, Eisenberg RJ. 2008. Engineered disulfide bonds in herpes simplex virus type 1 gD separate receptor binding from fusion initiation and viral entry. J Virol 82:700–709. doi: 10.1128/JVI.02192-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atanasiu D, Saw WT, Cohen GH, Eisenberg RJ. 2010. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J Virol 84:12292–12299. doi: 10.1128/JVI.01700-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atanasiu D, Whitbeck JC, Cairns TM, Reilly B, Cohen GH, Eisenberg RJ. 2007. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc Natl Acad Sci U S A 104:18718–18723. doi: 10.1073/pnas.0707452104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chowdary TK, Cairns TM, Atanasiu D, Cohen GH, Eisenberg RJ, Heldwein EE. 2010. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat Struct Mol Biol 17:882–888. doi: 10.1038/nsmb.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stampfer SD, Heldwein EE. 2013. Stuck in the middle: structural insights into the role of the gH/gL heterodimer in herpesvirus entry. Curr Opin Virol 3:13–19. doi: 10.1016/j.coviro.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeev-Ben-Mordehai T, Vasishtan D, Hernandez Duran A, Vollmer B, White P, Prasad Pandurangan A, Siebert CA, Topf M, Grunewald K. 2016. Two distinct trimeric conformations of natively membrane-anchored full-length herpes simplex virus 1 glycoprotein B. Proc Natl Acad Sci U S A 113:4176–4181. doi: 10.1073/pnas.1523234113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 32.Galdiero S, Falanga A, Vitiello M, Browne H, Pedone C, Galdiero M. 2005. Fusogenic domains in herpes simplex virus type 1 glycoprotein H. J Biol Chem 280:28632–28643. doi: 10.1074/jbc.M505196200. [DOI] [PubMed] [Google Scholar]
- 33.Galdiero S, Falanga A, Vitiello M, D'Isanto M, Collins C, Orrei V, Browne H, Pedone C, Galdiero M. 2007. Evidence for a role of the membrane-proximal region of herpes simplex virus type 1 glycoprotein h in membrane fusion and virus inhibition. Chembiochem 8:885–895. doi: 10.1002/cbic.200700044. [DOI] [PubMed] [Google Scholar]
- 34.Galdiero S, Vitiello M, D'Isanto M, Falanga A, Collins C, Raieta K, Pedone C, Browne H, Galdiero M. 2006. Analysis of synthetic peptides from heptad-repeat domains of herpes simplex virus type 1 glycoproteins H and B. J Gen Virol 87:1085–1097. doi: 10.1099/vir.0.81794-0. [DOI] [PubMed] [Google Scholar]
- 35.Vanarsdall AL, Ryckman BJ, Chase MC, Johnson DC. 2008. Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans. J Virol 82:11837–11850. doi: 10.1128/JVI.01623-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chesnokova LS, Ahuja MK, Hutt-Fletcher LM. 2014. Epstein-Barr virus glycoprotein gB and gHgL can mediate fusion and entry in trans, and heat can act as a partial surrogate for gHgL and trigger a conformational change in gB. J Virol 88:12193–12201. doi: 10.1128/JVI.01597-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogalin HB, Heldwein EE. 2015. Interplay between the herpes simplex virus 1 gB cytodomain and the gH cytotail during cell-cell fusion. J Virol 89:12262–12272. doi: 10.1128/JVI.02391-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atanasiu D, Saw WT, Gallagher JR, Hannah BP, Matsuda Z, Whitbeck JC, Cohen GH, Eisenberg RJ. 2013. Dual split protein-based fusion assay reveals that mutations to herpes simplex virus (HSV) glycoprotein gB alter the kinetics of cell-cell fusion induced by HSV entry glycoproteins. J Virol 87:11332–11345. doi: 10.1128/JVI.01700-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hannah BP, Cairns TM, Bender FC, Whitbeck JC, Lou H, Eisenberg RJ, Cohen GH. 2009. Herpes simplex virus glycoprotein B associates with target membranes via its fusion loops. J Virol 83:6825–6836. doi: 10.1128/JVI.00301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hannah BP, Heldwein EE, Bender FC, Cohen GH, Eisenberg RJ. 2007. Mutational evidence of internal fusion loops in herpes simplex virus glycoprotein B. J Virol 81:4858–4865. doi: 10.1128/JVI.02755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muggeridge MI. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J Gen Virol 81:2017–2027. doi: 10.1099/0022-1317-81-8-2017. [DOI] [PubMed] [Google Scholar]
- 42.Atanasiu D, Cairns TM, Whitbeck JC, Saw WT, Rao S, Eisenberg RJ, Cohen GH. 2013. Regulation of herpes simplex virus gB-induced cell-cell fusion by mutant forms of gH/gL in the absence of gD and cellular receptors. mBio 4:e00046-13. doi: 10.1128/mBio.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kondo N, Miyauchi K, Matsuda Z. 2011. Monitoring viral-mediated membrane fusion using fluorescent reporter methods. Curr Protoc Cell Biol Chapter 26:Unit 26.9. doi: 10.1002/0471143030.cb2609s50. [DOI] [PubMed] [Google Scholar]
- 44.Kondo N, Miyauchi K, Meng F, Iwamoto A, Matsuda Z. 2010. Conformational changes of the HIV-1 envelope protein during membrane fusion are inhibited by the replacement of its membrane-spanning domain. J Biol Chem 285:14681–14688. doi: 10.1074/jbc.M109.067090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saw WT, Matsuda Z, Eisenberg RJ, Cohen GH, Atanasiu D. 2015. Using a split luciferase assay (SLA) to measure the kinetics of cell-cell fusion mediated by herpes simplex virus glycoproteins. Methods 90:68–75. doi: 10.1016/j.ymeth.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller CG, Krummenacher C, Eisenberg RJ, Cohen GH, Fraser NW. 2001. Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol Ther 3:160–168. doi: 10.1006/mthe.2000.0240. [DOI] [PubMed] [Google Scholar]
- 47.Pertel PE, Fridberg A, Parish ML, Spear PG. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313–324. doi: 10.1006/viro.2000.0713. [DOI] [PubMed] [Google Scholar]
- 48.Ishikawa H, Meng F, Kondo N, Iwamoto A, Matsuda Z. 2012. Generation of a dual-functional split-reporter protein for monitoring membrane fusion using self-associating split GFP. Protein Eng Des Sel 25:813–820. doi: 10.1093/protein/gzs051. [DOI] [PubMed] [Google Scholar]
- 49.Cairns TM, Friedman LS, Lou H, Whitbeck JC, Shaner MS, Cohen GH, Eisenberg RJ. 2007. N-terminal mutants of herpes simplex virus type 2 gH are transported without gL but require gL for function. J Virol 81:5102–5111. doi: 10.1128/JVI.00097-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallagher JR, Saw WT, Atanasiu D, Lou H, Eisenberg RJ, Cohen GH. 2013. Displacement of the C terminus of herpes simplex virus gD is sufficient to expose the fusion-activating interfaces on gD. J Virol 87:12656–12666. doi: 10.1128/JVI.01727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milne RS, Connolly SA, Krummenacher C, Eisenberg RJ, Cohen GH. 2001. Porcine HveC, a member of the highly conserved HveC/nectin 1 family, is a functional alphaherpesvirus receptor. Virology 281:315–328. doi: 10.1006/viro.2000.0798. [DOI] [PubMed] [Google Scholar]
- 52.Geraghty RJ, Jogger CR, Spear PG. 2000. Cellular expression of alphaherpesvirus gD interferes with entry of homologous and heterologous alphaherpesviruses by blocking access to a shared gD receptor. Virology 268:147–158. doi: 10.1006/viro.1999.0157. [DOI] [PubMed] [Google Scholar]
- 53.Cairns TM, Shaner MS, Zuo Y, Ponce-de-Leon M, Baribaud I, Eisenberg RJ, Cohen GH, Whitbeck JC. 2006. Epitope mapping of herpes simplex virus type 2 gH/gL defines distinct antigenic sites, including some associated with biological function. J Virol 80:2596–2608. doi: 10.1128/JVI.80.6.2596-2608.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atanasiu D, Whitbeck JC, de Leon MP, Lou H, Hannah BP, Cohen GH, Eisenberg RJ. 2010. Bimolecular complementation defines functional regions of Herpes simplex virus gB that are involved with gH/gL as a necessary step leading to cell fusion. J Virol 84:3825–3834. doi: 10.1128/JVI.02687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hutchinson L, Browne H, Wargent V, Davis-Poynter N, Primorac S, Goldsmith K, Minson AC, Johnson DC. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol 66:2240–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roop C, Hutchinson L, Johnson DC. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol 67:2285–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Backovic M, DuBois RM, Cockburn JJ, Sharff AJ, Vaney MC, Granzow H, Klupp BG, Bricogne G, Mettenleiter TC, Rey FA. 2010. Structure of a core fragment of glycoprotein H from pseudorabies virus in complex with antibody. Proc Natl Acad Sci U S A 107:22635–22640. doi: 10.1073/pnas.1011507107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsuura H, Kirschner AN, Longnecker R, Jardetzky TS. 2010. Crystal structure of the Epstein-Barr virus (EBV) glycoprotein H/glycoprotein L (gH/gL) complex. Proc Natl Acad Sci U S A 107:22641–22646. doi: 10.1073/pnas.1011806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ciferri C, Chandramouli S, Donnarumma D, Nikitin PA, Cianfrocco MA, Gerrein R, Feire AL, Barnett SW, Lilja AE, Rappuoli R, Norais N, Settembre EC, Carfi A. 2015. Structural and biochemical studies of HCMV gH/gL/gO and pentamer reveal mutually exclusive cell entry complexes. Proc Natl Acad Sci U S A 112:1767–1772. doi: 10.1073/pnas.1424818112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cairns TM, Landsburg DJ, Whitbeck JC, Eisenberg RJ, Cohen GH. 2005. Contribution of cysteine residues to the structure and function of herpes simplex virus gH/gL. Virology 332:550–562. doi: 10.1016/j.virol.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 61.Cairns TM, Whitbeck JC, Lou H, Heldwein EE, Chowdary TK, Eisenberg RJ, Cohen GH. 2011. Capturing the herpes simplex virus core fusion complex (gB-gH/gL) in an acidic environment. J Virol 85:6175–6184. doi: 10.1128/JVI.00119-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright CC, Wisner TW, Hannah BP, Eisenberg RJ, Cohen GH, Johnson DC. 2009. Fusion between perinuclear virions and the outer nuclear membrane requires the fusogenic activity of herpes simplex virus gB. J Virol 83:11847–11856. doi: 10.1128/JVI.01397-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beitia Ortiz de Zarate I, Cantero-Aguilar L, Longo M, Berlioz-Torrent C, Rozenberg F. 2007. Contribution of endocytic motifs in the cytoplasmic tail of herpes simplex virus type 1 glycoprotein B to virus replication and cell-cell fusion. J Virol 81:13889–13903. doi: 10.1128/JVI.01231-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silverman JL, Greene NG, King DS, Heldwein EE. 2012. Membrane requirement for folding of the herpes simplex virus 1 gB cytodomain suggests a unique mechanism of fusion regulation. J Virol 86:8171–8184. doi: 10.1128/JVI.00932-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dollery SJ, Delboy MG, Nicola AV. 2010. Low pH-induced conformational change in herpes simplex virus glycoprotein B. J Virol 84:3759–3766. doi: 10.1128/JVI.02573-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dollery SJ, Wright CC, Johnson DC, Nicola AV. 2011. Low-pH-dependent changes in the conformation and oligomeric state of the prefusion form of herpes simplex virus glycoprotein B are separable from fusion activity. J Virol 85:9964–9973. doi: 10.1128/JVI.05291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muggeridge MI. 2012. Glycoprotein B of herpes simplex virus 2 has more than one intracellular conformation and is altered by low pH. J Virol 86:6444–6456. doi: 10.1128/JVI.06668-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milne RS, Nicola AV, Whitbeck JC, Eisenberg RJ, Cohen GH. 2005. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J Virol 79:6655–6663. doi: 10.1128/JVI.79.11.6655-6663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nicola AV, Aguilar HC, Mercer J, Ryckman B, Wiethoff CM. 2013. Virus entry by endocytosis. Adv Virol 2013:469538. doi: 10.1155/2013/469538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nicola AV, McEvoy AM, Straus SE. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol 77:5324–5332. doi: 10.1128/JVI.77.9.5324-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamers SL, Newman RM, Laeyendecker O, Tobian AA, Colgrove RC, Ray SC, Koelle DM, Cohen J, Knipe DM, Quinn TC. 2015. Global diversity within and between human herpesvirus 1 and 2 glycoproteins. J Virol 89:8206–8218. doi: 10.1128/JVI.01302-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Backovic M, Jardetzky TS. 2009. Class III viral membrane fusion proteins. Curr Opin Struct Biol 19:189–196. doi: 10.1016/j.sbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Backovic M, Longnecker R, Jardetzky TS. 2009. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proc Natl Acad Sci U S A 106:2880–2885. doi: 10.1073/pnas.0810530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chandramouli S, Ciferri C, Nikitin PA, Calo S, Gerrein R, Balabanis K, Monroe J, Hebner C, Lilja AE, Settembre EC, Carfi A. 2015. Structure of HCMV glycoprotein B in the postfusion conformation bound to a neutralizing human antibody. Nat Commun 6:8176. doi: 10.1038/ncomms9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma S, Wisner TW, Johnson DC, Heldwein EE. 2013. HCMV gB shares structural and functional properties with gB proteins from other herpesviruses. Virology 435:239–249. doi: 10.1016/j.virol.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oliver SL, Sommer M, Zerboni L, Rajamani J, Grose C, Arvin AM. 2009. Mutagenesis of varicella-zoster virus glycoprotein B: putative fusion loop residues are essential for viral replication, and the furin cleavage motif contributes to pathogenesis in skin tissue in vivo. J Virol 83:7495–7506. doi: 10.1128/JVI.00400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fan Q, Longnecker R, Connolly SA. 2014. Substitution of herpes simplex virus 1 entry glycoproteins with those of saimiriine herpesvirus 1 reveals a gD-gH/gL functional interaction and a region within the gD profusion domain that is critical for fusion. J Virol 88:6470–6482. doi: 10.1128/JVI.00465-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan Q, Longnecker R, Connolly SA. 2015. A functional interaction between herpes simplex virus 1 glycoprotein gH/gL domains I and II and gD is defined by using alphaherpesvirus gH and gL chimeras. J Virol 89:7159–7169. doi: 10.1128/JVI.00740-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mettenleiter TC, Spear PG. 1994. Glycoprotein gB (gII) of pseudorabies virus can functionally substitute for glycoprotein gB in herpes simplex virus type 1. J Virol 68:500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rauh I, Weiland F, Fehler F, Keil GM, Mettenleiter TC. 1991. Pseudorabies virus mutants lacking the essential glycoprotein gII can be complemented by glycoprotein gI of bovine herpesvirus 1. J Virol 65:621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fan Q, Amen M, Harden M, Severini A, Griffiths A, Longnecker R. 2012. Herpes B virus utilizes human nectin-1 but not HVEM or PILRα for cell-cell fusion and virus entry. J Virol 86:4468–4476. doi: 10.1128/JVI.00041-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nicola AV, Hou J, Major EO, Straus SE. 2005. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J Virol 79:7609–7616. doi: 10.1128/JVI.79.12.7609-7616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stampfer SD, Lou H, Cohen GH, Eisenberg RJ, Heldwein EE. 2010. Structural basis of local, pH-dependent conformational changes in glycoprotein B from herpes simplex virus type 1. J Virol 84:12924–12933. doi: 10.1128/JVI.01750-10. [DOI] [PMC free article] [PubMed] [Google Scholar]