ABSTRACT
Passage of the basement membrane (BM), which forms a barrier between the epithelium and the underlying lamina propria, represents an important step in the early pathogenesis of different alphaherpesviruses. Rho GTPase signaling plays an important role in transmigration of cells across the BM during physiological and pathological processes. We reported earlier that the US3 protein kinase of the alphaherpesvirus pseudorabies virus (PRV) interferes with Rho GTPase signaling and causes a reorganization of the host cell cytoskeleton, which as a consequence, enhances viral cell-to-cell spread in epithelial cell cultures. Here, using an ex vivo system of porcine nasal respiratory mucosa explants that allows to study PRV invasion through the BM, we found that a PRV strain that lacks US3 expression (ΔUS3 PRV) showed a reduced spread in mucosal epithelium and was virtually unable to breach the BM, in contrast to isogenic wild-type (WT) or US3 rescue PRV strains. Interestingly, addition of IPA3, an inhibitor of p21-activated kinases that blocks the effects of US3 on the cytoskeleton, suppressed the ability of WT PRV to spread across the BM. In addition, artificial suppression of RhoA signaling using CPC3 (cell-permeable C3 transferase) to mimic the effects of US3 on Rho GTPase signaling, significantly increased passage of ΔUS3 PRV through the BM, whereas it did not significantly affect BM passage of WT or US3 rescue PRV. In conclusion, these data indicate that US3 plays an important role in PRV mucosal invasion across the BM, which involves its interference with Rho GTPase signaling. This is the first report describing an alphaherpesvirus protein that drives viral BM passage.
IMPORTANCE Many viruses, including alphaherpesviruses, primarily replicate in epithelial cells of surface mucosae, such as the respiratory mucosa. Some of these viruses breach the basement membrane underlying these epithelial cells to reach underlying connective tissue and blood vessels and invade the host. Hence, epithelial spread and basement membrane passage represent crucial but still poorly understood early steps in (alphaherpes)virus pathogenesis. Here, using ex vivo porcine respiratory mucosa explants, we show that the conserved US3 protein of the porcine alphaherpesvirus pseudorabies virus (PRV) is critical for passage of PRV across the basement membrane and contributes to efficient viral epithelial spread. In addition, we show that US3-mediated viral epithelial spread and passage across the basement membrane depend at least in part on the ability of this viral protein to modulate cellular Rho GTPase signaling. This is the first report that identifies an alphaherpesvirus protein that drives viral basement membrane passage.
INTRODUCTION
Pseudorabies virus (PRV) is a porcine alphaherpesvirus that is related to other animal alphaherpesviruses, like bovine herpesvirus 1 (BHV1) and equine herpesvirus 1 (EHV1), and the human alphaherpesviruses herpes simplex virus (HSV) and varicella-zoster virus (VZV). Primary replication of many alphaherpesviruses, including PRV, occurs in epithelial cells of the upper respiratory tract. The basement membrane (BM) forms a physical barrier between the epithelial cells and the underlying lamina propria. Several alphaherpesviruses—including PRV, HSV, BHV1, and EHV1—have been shown to breach the BM, thereby reaching the connective tissue (1–4). Passage across the BM may lead to further dissemination of the virus throughout the body via blood vessels and nerves (5–7). Despite representing a crucial step in early pathogenesis, very little is known about the underlying mechanism of herpesvirus passage across the BM. For PRV, trypsin-like serine protease activity has been reported to be involved (8), but no specific viral component has been identified to contribute to BM passage.
BM breakdown and cellular passage of the BM are a well-studied subject in the fields of oncology and developmental biology. Rho GTPase signaling plays an important role in several of these processes. Rho GTPases can be roughly divided into two counteracting branches, with Cdc42/Rac1 signaling on the one hand and RhoA signaling on the other (9). Proteases involved in BM breakdown have been reported to be upregulated by Cdc42 and Rac1 (and downregulated by RhoA) (10, 11). Furthermore, Rho GTPase signaling is involved in invadopodium formation, which can perforate the BM by efficiently coordinating the delivery of proteases (12). In addition, RhoA has a significant role in maintaining BM integrity, and suppression of RhoA activity has been reported to trigger BM breakdown (13). In general, Cdc42/Rac1 signaling appears to be associated with BM breakdown/passage, while RhoA signaling is involved in BM stability.
Interestingly, we have found that the PRV protein kinase US3, which is conserved across alphaherpesviruses, interferes with Rho GTPase signaling. PRV US3 activates the Cdc42/Rac1 branch of Rho GTPase signaling by activating the Cdc42/Rac1 effector group I p21-activated kinases (PAK) (14) and at the same time suppresses RhoA signaling by triggering RhoA phosphorylation (15). This modulation of Rho GTPase signaling leads to profound rearrangements of the actin cytoskeleton in cell culture and is associated with enhanced viral cell-to-cell spread in epithelial cell cultures (14–16).
Although ΔUS3 PRV strains show only slightly reduced growth in cell cultures compared to wild-type (WT) PRV (17, 18), US3 is an important virulence factor in vivo. Reduced virulence of a US3 null PRV is particularly evident in the natural host, the pig (19–21). Indeed, intranasal infection of pigs with ΔUS3 PRV resulted in very mild symptoms, such as a slight fever, barely any respiratory signs, and very mild neurological signs compared to severe signs of Aujeszky's disease—including such severe neurological signs as itching, ataxia, and paralysis and a significant mortality rate—in pigs infected with WT PRV (19, 20).
In the present study, we aimed to investigate whether US3 is involved in epithelial spread and BM passage of PRV ex vivo in porcine respiratory mucosa explants and, if so, whether this correlates with the ability of US3 to modulate Rho GTPase signaling.
MATERIALS AND METHODS
Animals.
Four-week-old piglets (Belgian Landrace) were transferred from a high-health-status PRV-free farm in Belgium to the Faculty of Veterinary Medicine, Ghent University, where they were kept in an experimental unit. Upon arrival, they were treated daily for 3 days intramuscularly with antibiotics (6.25 mg/kg body weight enrofloxacin [Baytril; Bayer]) and 6.25 mg/kg body weight lincomycin with 12.5 mg/kg body weight spectinomycin (Linco-Spectin; Zoetis). Piglets were given ad libitum access to food and water. The animals were euthanized at the Faculty of Veterinary Medicine, Ghent University, according to Federation of European Laboratory Animal Science Associations (FELASA) guidelines, as approved by the Ethical Committee of the Faculty of Veterinary Medicine of Ghent University.
Isolation and cultivation of porcine nasal mucosa explants.
Porcine nasal mucosa explants were isolated and cultured as described earlier (22). Briefly, 8-week-old piglets were euthanized by intravenous injection of 12.5 mg/kg body weight pentobarbital (Nembutal; Sanofi) and subsequent exsanguination. The nose was removed, and nasal explants were carefully stripped from the ventral conchea and septum. Pieces of respiratory mucosa were cultured at an air-liquid interface containing serum-free medium (50% RPMI (Life Technologies)–50% Dulbecco's modified Eagle's medium (DMEM; Life Technologies) supplemented with glutamine (0.3 mg/ml) and antibiotics (100 U/ml penicillin, 0.1 mg/ml streptomycin, and 1 μg/ml gentamicin; Life Technologies). Ciliary beating was checked on a daily basis.
Infection and inhibitor treatment.
Mucosa explants were cultivated for 16 h prior to inoculation with 600 μl medium containing 107 tissue culture infective doses (TCID50) of PRV. To investigate the effect of US3 on viral spread in mucosa explants, explants were inoculated with earlier-described wild-type PRV (NiA3 WT), an isogenic US3-negative mutant (NiA3 ΔUS3; M118; carrying a premature stop codon at the 5′ end of the open reading frame), or an isogenic US3 rescue strain (NiA3 US3R; M120 [the M118 virus in which the original US3 sequence is restored]) (19, 23, 24). Explants were washed three times with serum-free medium 1 h postinoculation and subsequently submerged for 1 h in serum-free medium with or without inhibitor. After 1 h, explants were placed back at the air-liquid interface—again with or without inhibitor— and incubated for another 22 or 46 h, depending on the experiment, as specified in the text. IPA3, a selective inhibitor for group I p21-activated kinases (PAK) (25), was used to counteract US3-mediated PAK activation, while the selective RhoA inhibitor CPC3 (cell-permeable C3 transferase, cytoskeleton) was used to mimic the RhoA activity-suppressing effect of US3.
Immunofluorescence.
Explants were embedded in Methocel (Fluka) and frozen at −70°C. Twenty-micrometer cryosections were made, fixed in methanol (−20°C), and stained as previously described (22). Basement membrane and lamina propria were visualized by primary goat anti-collagen IV antibodies (Southern Biotech), secondary biotinylated rabbit anti-goat antibodies (Sigma-Aldrich), and tertiary streptavidin-Texas Red antibodies (Molecular Probes). PRV antigens were visualized using fluorescein isothiocyanate (FITC)-labeled porcine polyclonal anti-PRV antibodies (26). Serial images (of 10 individual plaques per condition per pig) were acquired using an Olympus IX81 fluorescence microscope. Plaque width and BM passage were subsequently determined using the software imaging system ImageJ (27). Representative illustrative confocal micrographs were acquired using a Leica SPE confocal microscope.
Statistical analysis.
Data were analyzed by analysis of variance (ANOVA) at the 5% significance level. Post hoc comparisons between different conditions were performed by Tukey's range test. Statistical analysis was performed on 10 plaques from 3 different pigs (thus, 3 × 10 plaques) per condition, using S-PLUS (TIBCO Software, Inc.).
RESULTS
Role of US3 in epithelial spread.
Although US3 has been shown to contribute to cell-to-cell spread in epithelial cell cultures in vitro, this had not yet been investigated in epithelial tissue. Therefore, ex vivo porcine nasal mucosa explants were inoculated with either WT PRV, ΔUS3 PRV, or US3R PRV. Samples were collected at 24 or 48 h postinfection (hpi) and analyzed by fluorescence and confocal laser scanning microscopy. Plaque width at 24 and 48 hpi was quantified, and mean values and standard deviations (SD) from three independent experiments are shown in Fig. 1G and 2G (24 and 48 hpi, respectively). At 24 hpi (Fig. 1G, blue bars), lack of US3 expression resulted in a mild but statistically nonsignificant (P = 0.092) reduction in plaque width, compared to WT or US3R PRV. However, at 48 hpi (Fig. 2G, blue bars), the plaque width of ΔUS3 PRV was significantly (P < 0.001) reduced compared to that of the WT (41.9% ± 3.3% reduction) and US3R (42.7% ± 12.3% reduction) PRV. This confirms earlier in vitro experiments that US3 contributes to, but is not essential for, viral spread in epithelial cell layers.
FIG 1.
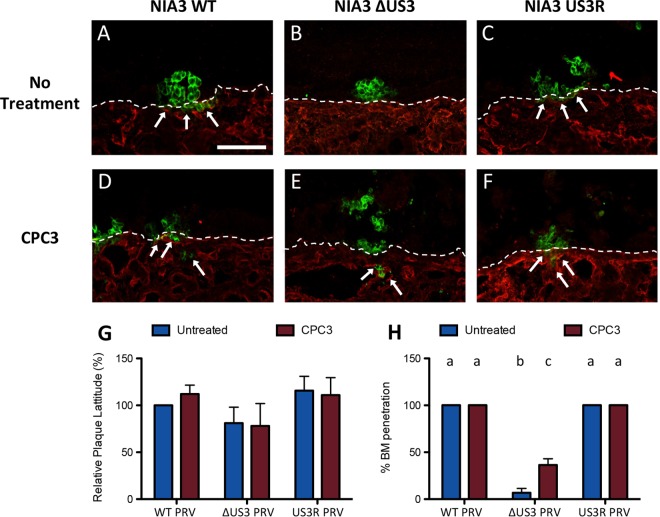
ΔUS3 PRV is impaired in viral passage across the basement membrane (BM) at 24 hpi, and inhibition of RhoA signaling increases BM passage of ΔUS3 PRV. Porcine respiratory mucosa explants were inoculated with WT, ΔUS3, or US3R PRV in the absence or presence of the RhoA inhibitor CPC3. At 24 hpi, plaque width and viral passage across the BM were analyzed by fluorescence microscopy. (A to F) Representative confocal micrographs showing viral antigens (green) and collagen IV (red) for the three different virus strains in the absence (A to C) or presence (D to F) of CPC3. The dashed line indicates localization of the BM, and arrows indicate viral passage across the BM. Scale bar, 100 μm. (G and H) Quantification of plaque width (G) and the percentage of plaques with viral passage across the BM (H) for the three virus strains in the absence (blue bars) or presence (red bars) of CPC3. Data represent means + SD from triplicate independent biological replicates. Different letters indicate statistically significant differences between conditions.
FIG 2.
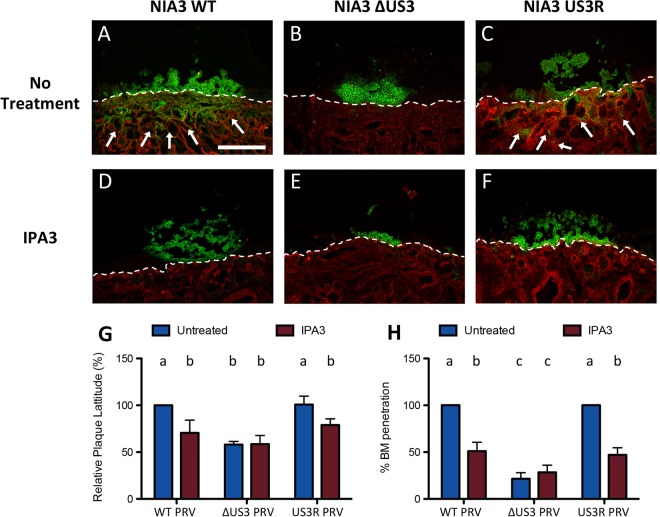
ΔUS3 PRV shows reduced epithelial spread and viral passage across the basement membrane (BM) at 48 hpi, and inhibition of PAK activity reduces epithelial spread and BM passage of WT PRV. Porcine respiratory mucosa explants were inoculated with WT, ΔUS3, or US3R PRV in the absence or presence of the PAK inhibitor IPA3. At 48 hpi, plaque width and viral passage across the BM were analyzed by fluorescence microscopy. (A to F) Representative confocal micrographs showing viral antigens (green) and collagen IV (red) for the three different virus strains in the absence (A to C) or presence (D to F) of IPA3. The dashed line indicates localization of the BM, and arrows indicate viral passage across the BM. Scale bar, 250 μm. (G and H) Quantification of plaque width (G) and the percentage of plaques with viral passage across the BM (H) for the three virus strains in the absence (blue bars) or presence (red bars) of IPA3. Data represent means + SD from triplicate independent biological replicates. Different letters indicate statistically significant differences between conditions.
The critical role of US3 protein kinase in BM breakdown.
In addition to the analysis of epithelial plaque width, the ability of the different virus strains to breach the BM was analyzed at 24 and 48 hpi (Fig. 1H and 2H, respectively, and arrows in Fig. 1A to C and 2A to C). As described earlier in ex vivo explants (1) and in line with in vivo findings in pigs (28), WT PRV and US3R PRV very efficiently breached the BM already at 24 hpi, with 100% (30 out of 30) of plaques showing BM passage at either time point. Viral passage across the BM mainly presented as clusters of infected cells at 24 hpi and as fully infected zones underneath the BM at 48 hpi. Remarkably, ΔUS3 PRV was very strongly and significantly (P < 0.001) impaired in breaching the BM compared to WT or US3R PRV, both at 24 hpi, when only 2 out of 30 analyzed plaques (93.3% ± 4.7% reduction) were able to breach the BM, and at 48 hpi, when only 9 out of 30 analyzed plaques transgressed the BM (78.6% ± 6.5% reduction) (Fig. 1H and 2H, blue bars). These results show that US3 plays a critical role in efficient invasion of PRV across the BM.
US3-mediated viral passage across the BM depends on Rho GTPase signaling.
US3 has been reported to activate group I PAK signaling (14) and to inhibit RhoA signaling (15). To investigate whether these signaling pathways contribute to the observed effects of US3 in viral spread and invasion in mucosa explants, we used drugs that specifically target group I PAK and RhoA signaling. IPA3 is a specific inhibitor of group I PAK (25) and thereby counteracts US3-mediated activation of group I PAK (14). CPC3 is a cell-permeable derivative of Clostridium botulinum coenzyme 3 that inhibits RhoA (29), thereby mimicking the ability of US3 to suppress RhoA signaling (15). CPC3 could be used in 24-h infection assays (not in 48-h infection assays since it caused mild toxicity at that time point), whereas IPA3 did not cause any toxicity and could be used in 48-h assays. Data obtained with CPC3 are shown in Fig. 1 (Fig. 1G and H, red bars, and Fig. 1D to F). Data obtained with IPA3 are shown in Fig. 2 (Fig. 2G and H, red bars, and Fig. 2D to F). Mimicking US3-mediated suppression of RhoA activity using CPC3 led to a marked and significant increase (P = 0.003) in passage of ΔUS3 virus through the BM at 24 hpi (11 out of 30 plaques that breached the BM compared to 2 out of 30 plaques without treatment) (Fig. 1H, compare blue with red bars and arrows in Fig. 1D to F), although it did not fully restore BM passage to WT PRV levels. Representative images are shown in Fig. 1D to F.
In line with this, inhibition of US3-mediated modulation of Rho GTPase signaling using the group I PAK inhibitor IPA3 strongly reduced the passage of the WT and US3R across the BM (14 out of 30 plaques that breached the BM, compared to 30 out of 30 plaques without treatment; P < 0.001), whereas it did not affect the (limited) BM passage of ΔUS3 PRV (Fig. 2H). Also, IPA3 substantially reduced the epithelial plaque width of WT and US3R PRV without affecting the plaque width of ΔUS3 PRV (Fig. 2G).
In conclusion, US3-mediated viral passage of the BM and its contribution to epithelial cell-to-cell spread ex vivo at least in part depend on the ability of US3 to modulate Rho GTPase signaling.
DISCUSSION
In the present study, we found that the US3 protein kinase of PRV plays a crucial role in viral passage across the BM during infection of porcine respiratory mucosa explants and also contributes to epithelial spread in these explants. This is the first description of an alphaherpesvirus protein that drives viral passage across the BM. In addition, we found that US3-mediated spread and invasion in mucosa explants at least in part depend on its ability to modulate Rho GTPase signaling.
These ex vivo experiments support a role for US3 in epithelial spread, in line with the established role of US3 in viral spread in epithelial cell culture (16). In cell cultures, US3-mediated spread was associated with profound cytoskeletal rearrangements, consisting of actin stress fiber disassembly and the formation of long cellular projections. Resolution of confocal analysis of the explants did not allow us to determine whether cell projections are formed under these circumstances. However, in line with the in vitro results (14), US3-mediated spread in respiratory mucosa epithelial cells could be suppressed using the group I PAK inhibitor IPA3, indicating a similar underlying mechanism.
Although epithelial spread of ΔUS3 PRV and IPA3-treated WT PRV was reduced, it was not inhibited. This indicates that epithelial cell-to-cell spread of PRV likely involves additional mechanisms, which may include polarized viral transport via tight junctions as has been described for HSV (30). In addition, cell-free spread of PRV via exocytosis of the virus followed by entry in noninfected neighboring cells may also contribute to viral spread, particularly since ex vivo explants may lack components of the immune system that may limit cell-free virus spread.
More striking than the contribution of US3 to epithelial spread was the observed critical role for US3 in viral crossing of the BM. Indeed, at 24 hpi, ΔUS3 PRV showed a virtually abolished BM passage. Even at 48 hpi, ΔUS3 PRV was still unable to efficiently initiate BM passage. Like the US3-mediated contribution to epithelial spread, the ability of US3 to drive viral passage across the BM depended at least in part on its ability to modulate Rho GTPase signaling. We reported earlier that US3 triggers the Cdc42/Rac1 branch of Rho GTPase signaling by activation of group I PAK and at the same time suppresses RhoA signaling in epithelial cells (14, 15). Suppressing US3-mediated activation of PAK reduced the invasive capacity of WT PRV, whereas mimicking US3-mediated inhibition of RhoA increased BM passage of ΔUS3 PRV. Nevertheless, inhibition of PAK did not completely reduce WT PRV passage across the BM to the level of ΔUS3 PRV, and inhibition of RhoA did not restore BM passage of ΔUS3 PRV to the level of WT PRV. These may have different causes. One explanation may be that the inhibitors used do not completely inhibit/mimic the effects of US3. Indeed, US3 activates PAK and at the same time suppresses RhoA activity (15). IPA3 only blocks the ability of US3 to activate PAK, and CPC3 only mimics the ability of US3 to suppress RhoA activity. Another explanation may be the involvement of other US3-affected cellular signaling pathways. For example, US3 of HSV-1 has been reported to display Akt-like activity (31). Since Akt has been reported to contribute to BM passage—e.g., of mesenchymal stem cells (32)— it will be interesting in future research to investigate whether PRV US3 also displays Akt-like activity and, if so, whether this contributes to US3-mediated viral BM passage. Furthermore, since it has been shown before that different wild-type PRV strains may display somewhat different efficiencies in passage across the BM (1), it might be interesting to analyze potential differences in the sequence, expression, and functionality of US3 of these PRV strains.
How does US3 interference with Rho GTPase signaling contribute to BM passage? Several hypotheses can be put forward, based on the role of Rho GTPase signaling in breaching the BM during metastasis, embryogenesis, and angiogenesis. First, during embryogenesis, disturbed cytoskeletal integrity in epithelial cells by suppressed RhoA activity has been reported to negatively affect structural integrity of the BM (9, 13). This may be in line with our previous findings that PRV US3 suppresses RhoA activity and substantially disturbs normal cytoskeletal structure in epithelial cells (15, 16). Second, formation of cellular protrusions, particularly invadopodia, may penetrate the BM and may play a vital role in metastasis of cancer cells (12). Interestingly, the US3-mediated cell projections described in cell culture show substantial homology with invadopodia: both types of cell projections contain both actin and microtubules, both depend on modulation of PAK and RhoA activity for formation, and formation of both also depends on the actin-regulating molecule cofilin (12, 14–16, 33–35). Nevertheless, our confocal analyses and earlier electron microscopy studies (36) did not indicate evidence for invadopodium formation during PRV passage across the BM.
Earlier research showed that PRV makes use of a trypsin-like serine protease to penetrate the BM (8). Trypsin-like serine proteases may be upregulated by activation of Cdc42/Rac signaling (37) and inhibition of RhoA (38). Since US3 similarly affects these signaling pathways, it will be interesting to investigate which proteases are involved in BM passage of PRV and whether US3 expression modulates expression or activity of these proteases.
In conclusion, the present article shows that US3 is critically involved in passage of PRV across the BM and contributes to epithelial spread in porcine respiratory mucosal explants. These US3-mediated effects at least in part depend on the ability of US3 to interfere with Rho GTPase signaling. The present findings shed new light on the role of US3 as an alphaherpesvirus virulence factor and may contribute to the rational design of attenuated alphaherpesvirus vaccines that elicit mucosal immune responses but are impaired in host invasion.
ACKNOWLEDGMENTS
We thank the ID-DLO Institute (The Netherlands) for kindly providing the different NIA3 strains and J. Chernoff (Fox Chase Cancer Center, Philadelphia, PA) for the group I PAK inhibitor (IPA3). Furthermore, we thank Rudy Cooman and Zeger Van Den Abeele for animal caretaking and Cliff Van Waesberghe, Lieve Sys, and Carine Boone for excellent technical assistance.
This research was supported by grants from the Special Research Fund of Ghent University (grant no. 01G01311), F.W.O.-Vlaanderen (grant no. G.0176.15N), and the Belgian Science Policy (BELSPO) via the BELVIR Consortium (IAP, phase VII).
REFERENCES
- 1.Glorieux S, Favoreel HW, Meesen G, de Vos W, Van den Broeck W, Nauwynck HJ. 2009. Different replication characteristics of historical pseudorabies virus strains in porcine respiratory nasal mucosa explants. Vet Microbiol 136:341–346. doi: 10.1016/j.vetmic.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Steukers L, Weyers S, Yang X, Vandekerckhove AP, Glorieux S, Cornelissen M, Van den Broeck W, Temmerman M, Nauwynck HJ. 2014. Mimicking herpes simplex virus 1 and herpes simplex virus 2 mucosal behavior in a well-characterized human genital organ culture. J Infect Dis 210:209–213. doi: 10.1093/infdis/jiu036. [DOI] [PubMed] [Google Scholar]
- 3.Vandekerckhove AP, Glorieux S, Gryspeerdt AC, Steukers L, Duchateau L, Osterrieder N, Van de Walle GR, Nauwynck HJ. 2010. Replication kinetics of neurovirulent versus non-neurovirulent equine herpesvirus type 1 strains in equine nasal mucosal explants. J Gen Virol 91:2019–2028. doi: 10.1099/vir.0.019257-0. [DOI] [PubMed] [Google Scholar]
- 4.Glorieux S, Bachert C, Favoreel HW, Vandekerckhove AP, Steukers L, Rekecki A, Van den Broeck W, Goossens J, Croubels S, Clayton RF, Nauwynck HJ. 2011. Herpes simplex virus type 1 penetrates the basement membrane in human nasal respiratory mucosa. PLoS One 6:e22160. doi: 10.1371/journal.pone.0022160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pomeranz LE, Reynolds AE, Hengartner CJ. 2005. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev 69:462–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nauwynck H, Glorieux S, Favoreel H, Pensaert M. 2007. Cell biological and molecular characteristics of pseudorabies virus infections in cell cultures and in pigs with emphasis on the respiratory tract. Vet Res 38:229–241. doi: 10.1051/vetres:200661. [DOI] [PubMed] [Google Scholar]
- 7.Steukers L, Glorieux S, Vandekerckhove AP, Favoreel HW, Nauwynck HJ. 2012. Diverse microbial interactions with the basement membrane barrier. Trends Microbiol 20:147–155. doi: 10.1016/j.tim.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glorieux S, Favoreel HW, Steukers L, Vandekerckhove AP, Nauwynck HJ. 2011. A trypsin-like serine protease is involved in pseudorabies virus invasion through the basement membrane barrier of porcine nasal respiratory mucosa. Vet Res 42:58. doi: 10.1186/1297-9716-42-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaffe AB, Hall A. 2005. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 10.Ispanovic E, Serio D, Haas TL. 2008. Cdc42 and RhoA have opposing roles in regulating membrane type 1-matrix metalloproteinase localization and matrix metalloproteinase-2 activation. Am J Physiol Cell Physiol 295:C600–C610. doi: 10.1152/ajpcell.00460.2007. [DOI] [PubMed] [Google Scholar]
- 11.Parri M, Chiarugi P. 2010. Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal 8:23. doi: 10.1186/1478-811X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. 2010. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol 189:541–556. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakaya Y, Sukowati EW, Wu Y, Sheng G. 2008. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol 10:765–775. doi: 10.1038/ncb1739. [DOI] [PubMed] [Google Scholar]
- 14.Van den Broeke C, Radu M, Deruelle M, Nauwynck H, Hofmann C, Jaffer ZM, Chernoff J, Favoreel HW. 2009. Alphaherpes US3-mediated reorganization is mediated by group A p21-activated kinases. Proc Natl Acad Sci U S A 106:8707–8712. doi: 10.1073/pnas.0900436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob T, Van den Broeke C, Van Waesberghe C, Van Troys L, Favoreel HW. 2015. Pseudorabies virus US3 triggers RhoA phosphorylation to reorganize the actin cytoskeleton. J Gen Virol 96:2328–2335. doi: 10.1099/vir.0.000152. [DOI] [PubMed] [Google Scholar]
- 16.Favoreel HW, Van Minnebruggen G, Adriaensen D, Nauwynck HJ. 2005. Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc Natl Acad Sci U S A 102:8990–8995. doi: 10.1073/pnas.0409099102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Minnebruggen G, Favoreel HW, Jacobs L, Nauwynck HJ. 2003. Pseudorabies virus US3 protein kinase mediates actin stress fiber breakdown. J Virol 77:9074–9080. doi: 10.1128/JVI.77.16.9074-9080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coller KE, Smith GA. 2008. Two viral kinases are required for sustained long-distance axon transport of a neuroinvasive herpesvirus. Traffic 9:1458–1470. doi: 10.1111/j.1600-0854.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimman TG, de Wind N, Oei-Lie N, Pol JM, Berns AJ, Gielkens AL. 1992. Contribution of single genes within the unique short region of Aujeszky's disease virus (suid herpesvirus type 1) to virulence, pathogenesis and immunogenicity. J Gen Virol 73:243–251. doi: 10.1099/0022-1317-73-2-243. [DOI] [PubMed] [Google Scholar]
- 20.Kimman TG, De Wind N, De Bruin T, de Visser Y, Voermans J. 1994. Inactivation of glycoprotein gE and thymidine kinase or the US3-encoded protein kinase synergistically decreases in vivo replication of pseudorabies virus and the induction of protective immunity. Virology 205:511–518. doi: 10.1006/viro.1994.1672. [DOI] [PubMed] [Google Scholar]
- 21.Olsen LM, Ch'ng TH, Card JP, Enquist LW. 2006. Role of pseudorabies virus Us3 protein kinase during neuronal infection. J Virol 80:6387–6398. doi: 10.1128/JVI.00352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glorieux S, Van den Broeck W, van der Meulen KM, Van Reeth K, Favoreel HW, Nauwynck HJ. 2007. In vitro culture of porcine respiratory nasal mucosa explants for studying the interaction of porcine viruses with the respiratory tract. J Virol Methods 142:105–112. [DOI] [PubMed] [Google Scholar]
- 23.Baskerville A, McFerran JB, Dow C. 1973. Aujeszky's disease in pigs. Vet Bull 43:465–480. [Google Scholar]
- 24.van Zijl M, van der Gulden H, de Wind N, Gielkens A, Berns A. 1990. Identification of two genes in the unique short region of pseudorabies virus: comparison with herpes simplex virus and varicella-zoster virus. J Gen Virol 71:1747–1755. doi: 10.1099/0022-1317-71-8-1747. [DOI] [PubMed] [Google Scholar]
- 25.Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. 2008. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol 15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nauwynck HJ, Pensaert MB. 1995. Effect of specific antibodies on the cell-associated spread of pseudorabies virus in monolayers of different cell types. Arch Virol 140:1137–1146. doi: 10.1007/BF01315422. [DOI] [PubMed] [Google Scholar]
- 27.Rasband WS. 2016. ImageJ. National Institutes of Health, Bethesda, MD: http://imagej.nih.gov/ij/. [Google Scholar]
- 28.Pol JM, Gielkens AL, van Oirschot JT. 1989. Comparative pathogenesis of three strains of pseudorabies virus in pigs. Microb Pathog 7:361–371. doi: 10.1016/0882-4010(89)90039-9. [DOI] [PubMed] [Google Scholar]
- 29.Kuribara H, Tago K, Yokozeki T, Sasaki T, Takai Y, Morii N, Narumiya S, Katada T, Kanaho Y. 1995. Synergistic activation of rat brain phospholipase D by ADP-ribosylation factor and rhoA p21, and its inhibition by Clostridium botulinum C3 exoenzyme. J Biol Chem 270:25667–25671. doi: 10.1074/jbc.270.43.25667. [DOI] [PubMed] [Google Scholar]
- 30.Johnson DC, Webb M, Wisner TW, Brunetti C. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J Virol 75:821–833. doi: 10.1128/JVI.75.2.821-833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chuluunbaatar U, Mohr I. 2011. A herpesvirus kinase that masquerades as Akt: you don't have to look like Akt, to act like it. Cell Cycle 10:2064–2068. doi: 10.4161/cc.10.13.16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X, Gao X, Zhou L, Sun L, Lu C. 2013. PDGF-BB-induced MT1-MMP expression regulates proliferation and invasion of mesenchymal stem cells in 3-dimensional collagen via MEK/ERK1/2 and PI3K/AKT signaling. Cell Signal 25:1279–1287. doi: 10.1016/j.cellsig.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 33.Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tetè S, Luini A, Buccione R. 2008. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci 121:369–378. doi: 10.1242/jcs.008037. [DOI] [PubMed] [Google Scholar]
- 34.Jacob T, Van den Broeke C, van Troys M, Waterschoot D, Ampe C, Favoreel HW. 2013. Alphaherpesviral US3 kinase induces cofilin dephosphorylation to reorganize the actin cytoskeleton. J Virol 87:4121–4126. doi: 10.1128/JVI.03107-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spuul P, Ciufici P, Veillat V, Leclercq A, Daubon T, Kramer IJ, Génot E. 2014. Importance of RhoGTPases in formation, characteristics, and functions of invadosomes. Small GTPases 5:e28195. doi: 10.4161/sgtp.28195,10.4161/sgtp.28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glorieux S. 2009. Invasion of pseudorabies virus in porcine nasal respiratory mucosa explants. Ph.D. thesis Ghent University, Ghent, Belgium. [Google Scholar]
- 37.Appledorn DM, Dao KH, O'Reilly S, Maher VM, McCormick JJ. 2010. Rac1 and Cdc42 are regulators of HRasV12-transformation and angiogenic factors in human fibroblasts. BMC Cancer 10:13. doi: 10.1186/1471-2407-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunoyer-Geindre S, Fish RJ, Kruithof EK. 2011. Regulation of the endothelial plasminogen activator system by fluvastatin. Role of Rho family proteins, actin polymerisation and p38 MAP kinase. Thromb Haemost 105:461–472. doi: 10.1160/TH10-07-0444. [DOI] [PubMed] [Google Scholar]