ABSTRACT
Superinfection exclusion (SIE) is an antagonistic virus-virus interaction whereby initial infection by one virus prevents subsequent infection by closely related viruses. Although SIE has been described in diverse viruses infecting plants, humans, and animals, its mechanisms, including involvement of specific viral determinants, are just beginning to be elucidated. In this study, SIE determinants encoded by two economically important wheat viruses, Wheat streak mosaic virus (WSMV; genus Tritimovirus, family Potyviridae) and Triticum mosaic virus (TriMV; genus Poacevirus, family Potyviridae), were identified in gain-of-function experiments that used heterologous viruses to express individual virus-encoded proteins in wheat. Wheat plants infected with TriMV expressing WSMV P1, HC-Pro, P3, 6K1, CI, 6K2, NIa-VPg, or NIb cistrons permitted efficient superinfection by WSMV expressing green fluorescent protein (WSMV-GFP). In contrast, wheat infected with TriMV expressing WSMV NIa-Pro or coat protein (CP) substantially excluded superinfection by WSMV-GFP, suggesting that both of these cistrons are SIE effectors encoded by WSMV. Importantly, SIE is due to functional WSMV NIa-Pro or CP rather than their encoding RNAs, as altering the coded protein products by minimally changing RNA sequences led to abolishment of SIE. Deletion mutagenesis further revealed that elicitation of SIE by NIa-Pro requires the entire protein while CP requires only a 200-amino-acid (aa) middle fragment (aa 101 to 300) of the 349 aa. Strikingly, reciprocal experiments with WSMV-mediated expression of TriMV proteins showed that TriMV CP, and TriMV NIa-Pro to a lesser extent, likewise excluded superinfection by TriMV-GFP. Collectively, these data demonstrate that WSMV- and TriMV-encoded CP and NIa-Pro proteins are effectors of SIE and that these two proteins trigger SIE independently of each other.
IMPORTANCE Superinfection exclusion (SIE) is an antagonistic virus-virus interaction that prevents secondary invasions by identical or closely related viruses in the same host cells. Although known to occur in diverse viruses, SIE remains an enigma in terms of key molecular determinants and action mechanisms. In this study, we found that Wheat streak mosaic virus (WSMV) and Triticum mosaic virus (TriMV) encode two independently functioning cistrons that serve as effectors of SIE at the protein but not the RNA level. The coat protein and NIa-Pro encoded by these two viruses, when expressed from a heterologous virus, exerted SIE to the cognate viruses. The identification of virus-encoded effectors of SIE and their transgenic expression could potentially facilitate the development of virus-resistant crop plants. Additionally, functional conservation of SIE in diverse virus groups suggests that a better understanding of the underlying mechanisms of SIE could facilitate the development of novel antiviral therapies against viral diseases.
INTRODUCTION
Development of reverse genetics systems for viruses has revolutionized the understanding of viral replication, infection, and disease development through identification of viral determinants involved in these processes as well as the elucidation of interactions between viral and host factors (1–3). However, virus-virus interactions in hosts that facilitate superinfection exclusion (SIE) between related viruses or synergistic interactions between unrelated viruses have received less attention (4, 5). Synergistic interactions are facilitative virus-virus interactions between two or more unrelated viruses, and these interactions often cause increased virus accumulation of one or both viruses that could lead to severe disease compared to infection by individual viruses (4). In contrast, SIE is the result of antagonistic virus-virus interactions between closely related viruses (5–7).
SIE, often referred to as “cross-protection” or “homologous interference,” is defined as the phenomenon whereby initial infection by one virus prevents subsequent infection of preinfected cells by closely related viruses. SIE was originally observed between two strains of Tobacco mosaic virus (TMV) (6, 7), followed by observations with bacteriophages (8, 9). In TMV, cross-protection was used to examine the relatedness of newly collected virus isolates as being strains of, or distinct from, existing virus isolates (7, 10). Subsequently, cross-protection has been used for the management of plant viruses by purposefully infecting plants with mild isolates of a virus to prevent infection by severe isolates (11, 12).
The SIE phenomenon has been observed in diverse groups of plant-, human-, and animal-infecting viruses belonging to the reverse transcribing viruses such as Human immunodeficiency virus (13) and Rous sarcoma virus (14); positive-sense RNA viruses such as Alfalfa mosaic virus (15), Barley yellow dwarf virus (16), Citrus tristeza virus (CTV) (17, 18), Hepatitis C virus (HCV) (19, 20), Plum pox virus (21), Potato virus A (PVA) (22), Semliki Forest virus (SFV) (23), Sindbis virus (SINV) (24), TMV (6, 7), Tobacco streak virus (25), West Nile virus (WNV) (26), and Zucchini yellow mosaic virus (ZYMV) (27); negative-sense RNA viruses like Newcastle disease virus (28) and Vesicular stomatitis virus (29); and large double-stranded DNA viruses like herpesviruses (30) and poxviruses (31). SIE is an interesting phenomenon because the primary virus specifically excludes superinfection by closely related viruses but tolerates coinfection by unrelated viruses. Functional conservation of SIE in diverse virus groups suggests that a better understanding of the underlying mechanisms of SIE could facilitate the development of novel antiviral therapies against viral diseases.
Wheat streak mosaic virus (WSMV) is the most economically important wheat (Triticum aestivum L.) virus in the Great Plains region of the United States. WSMV is the type species of the genus Tritimovirus in the family Potyviridae (32). The 9.4-kb RNA genome of WSMV encodes a single large open reading frame (ORF) of ∼350 kDa that is processed into at least 10 mature proteins by the three virus-encoded proteinases P1, HC-Pro, and NIa-Pro (32). HC-Pro is dispensable for systemic infection of wheat but required for wheat curl mite (Aceria tosichella Keifer) transmission (33–35). Recent development of green fluorescent protein (GFP)- or red fluorescent protein (RFP)-tagged WSMV has facilitated the examination of viral determinants required for virion assembly and both cell-to-cell and long-distance movement (36–39). Previously, interference tests were conducted to determine relationships among cereal viruses (40). In particular, a mild strain of WSMV was shown to completely block superinfection by a virulent or yellowing strain of WSMV, thus revealing the relatedness of these two strains (40). Additionally, SIE has been demonstrated between closely related Sidney and Type strains of WSMV (41). However, the mechanistic basis and viral determinants involved in SIE of WSMV are unknown.
Triticum mosaic virus (TriMV) is a recently discovered wheat curl mite-transmitted wheat virus (42, 43). TriMV is the type species of the genus Poacevirus in the family Potyviridae (44, 45). The genomic organization of the 10.3-kb single-stranded RNA genome of TriMV is similar to that of WSMV (45). Through development of an infectious cDNA clone of TriMV, another efficient and stable viral transient expression vector for wheat is now available (46). Both WSMV and TriMV are transmitted mechanically to ∼100% of inoculated wheat seedlings and coinfect wheat systemically (47).
As noted above, SIE is a conserved functional property of many diverse viruses. Although SIE was reported many decades ago, viral factors and mechanisms conferring this phenomenon are just beginning to be understood. Mapping viral determinants involved in SIE is a crucial first step in defining its underlying mechanisms. Candidate viral determinants involved in SIE have been identified by loss-of-function studies employing either introduction of deletions in viral ORFs which are otherwise capable of systemic infection of plants (48, 49) or genomic exchanges between viral strains (22). However, most viruses encode multifunctional proteins, and many deletions adversely affect the ability of viruses to infect plants systemically. Mapping viral determinants by exchanging viral genome segments between strains is limited by the availability of viral strains with sequence differences that exhibit differential SIE activity and requires that chimeric viruses retain the ability to systemically infect plants. A further complication is that SIE between strains of viruses often is not reciprocal (22).
Members of the family Potyviridae utilize a polyprotein genomic expression strategy, and most of the potyviral mature proteins play multiple roles in virus biology (50). Hence, potyviruses may not tolerate extensive deletions in order to map viral determinants involved in SIE. Development of reverse genetics systems for WSMV (51) and TriMV (46), wheat-infecting distinct members of the family Potyviridae, has allowed the use of efficient gene expression vectors (39, 46, 52, 53). In this study, experiments were conducted to identify WSMV and TriMV determinants involved in SIE by inoculating wheat with TriMV harboring WSMV individual cistrons or vice versa as the “primary virus” and WSMV-GFP or TriMV-GFP as the “challenge virus.”
Recently, the gene products of p33 and L1L2 of CTV were demonstrated to be required for SIE, but substitution with a heterologous p33 or L1L2 gene was not sufficient for SIE of either homologous or heterologous virus (49, 54), indicating that other gene products must be involved. Coat protein (CP) and HC-Pro of PVA have been implicated in cross-protection, as PVA with heterologous CP or HC-Pro cistrons failed to cross-protect the homologous strains (22). Thus, the determinants of SIE of CTV and PVA were mapped through loss-of-function experiments. Here, we show through gain-of-function experiments that WSMV CP and NIa-Pro proteins but not their RNA sequences are effectors of SIE. A series of deletions covering the entire NIa-Pro and CP cistrons revealed that complete NIa-Pro and CP amino acids (aa) 101 to 300 but not aa 3 to 100 and 301 to 349 were required for SIE. In reciprocal experiments, wheat infected with WSMV expressing TriMV CP prevented superinfection by TriMV-GFP, while NIa-Pro delayed the onset of local foci but did not prevent systemic infection. Collectively, these data demonstrate that WSMV- and TriMV-encoded CP and NIa-Pro proteins are effectors of SIE and that these two proteins independently trigger SIE activity.
MATERIALS AND METHODS
Viruses.
Wheat cultivar Tomahawk was used in all experiments described in this study. Wild-type WSMV isolate Sidney 81 and TriMV isolate Nebraska were obtained by inoculating wheat seedlings at the single-leaf stage with in vitro transcripts of pSP6-WSMV (51) and pTriMV-R (46), respectively. Cycle 3 GFP-tagged WSMV [pSP6-WSMV-GFP-6K1/CI(7aa)] (53) and TriMV [pTriMV-GFP-NIb/CP(9aa)] (46) were described previously. Wheat seedlings at the single-leaf stage were inoculated with in vitro transcripts of pSP6-WSMV-GFP-6K1/CI(7aa) and pTriMV-GFP-NIb/CP(9aa) to obtain WSMV-GFP and TriMV-GFP, respectively.
Generation of constructs.
Individual WSMV cistrons P1, HC-Pro, P3, 6K1, CI, 6K2, NIa-VPg, NIa-Pro, NIb, and CP were inserted in TriMV between the P1 and HC-Pro cistrons essentially as described before (46) for the development of pTriMV-GFP-NIb/CP(9aa). Individual WSMV cistrons, tagged with the 9-amino-acid (aa) cleavage peptide located between the NIb and CP cistrons of TriMV (46) at the C terminus, were precisely engineered between the P1 and HC-Pro cistrons by overlap extension PCR. Three individual PCR fragments with 18- to 21-bp overlapping sequences were used for overlap extension PCR as described previously (46). Individual PCR and overlap extension PCRs were performed using Herculase II fusion DNA polymerase (Agilent Technologies, Santa Clara, CA). The overlap extension PCR fragments were ligated into pTriMV-R between XbaI (created upstream of an SP6 RNA polymerase promoter sequence) and BssHII (nucleotide [nt] 2831) restriction endonuclease sites.
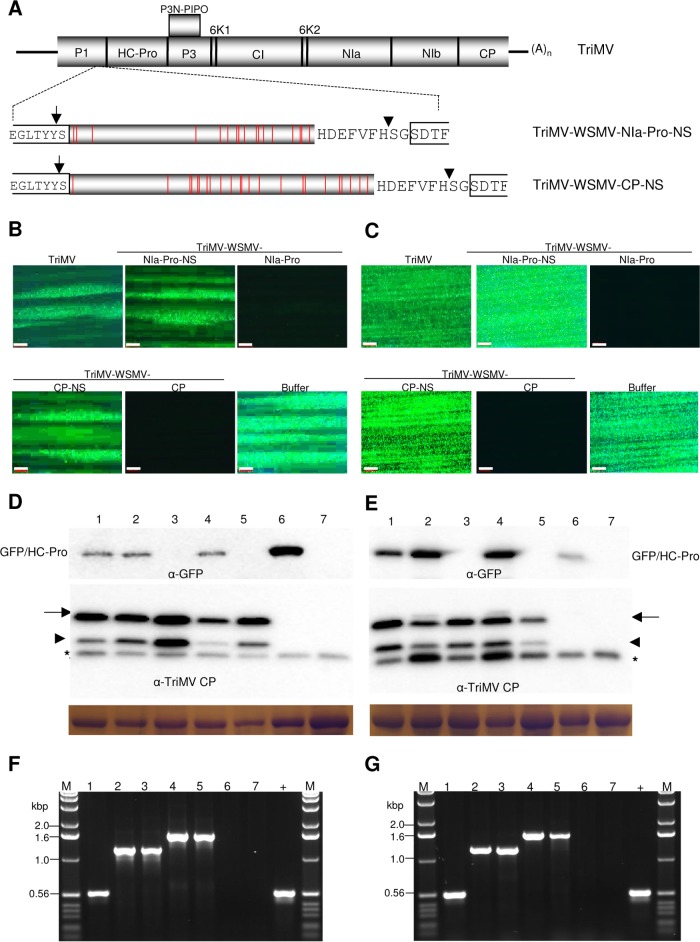
WSMV NIa-Pro and CP cistrons with frameshift and nucleotide substitution mutations were synthesized at GenScript (Piscataway, NJ). Four +2, five +1, and four −1 frameshift mutations were introduced into NIa-Pro sequence to obtain NIa-Pro-NS with a continuous reading frame with 98% nucleotide homology but no homology at the protein level with wild-type NIa-Pro. In the CP cistron, three +2, six +1, and four −1 frameshift mutations; two 2-nt substitutions; and three 1-nt substitutions were introduced to obtain CP-NS with a continuous reading frame with 98% homology at the nucleotide level but no homology at the protein level compared to wild-type CP. WSMV NIa-Pro-NS and CP-NS cistrons with a 9-aa cleavage peptide were inserted into the TriMV genome between the P1 and HC-Pro cistrons using overlap extension PCR as described earlier. Similarly, deletions were introduced into WSMV NIa-Pro and CP cistrons using pTriMV-WSMV-NIa-Pro and pTriMV-WSMV-CP, respectively, as the templates for overlap extension PCR, followed by ligation into pTriMV-R as described above.
HC-Pro, NIa-VPg, NIa-Pro, and CP cistrons of TriMV were engineered between the P1 and HC-Pro cistrons in pSP6-WSMV (51) using overlap extension PCR as previously described (53). The 9-amino-acid cleavage peptide comprising a heptapeptide cleavage site plus a spacer amino acid on either side located between the NIb and CP cistrons of WSMV was fused to the 3′ end of TriMV cistrons by overlap extension PCR. The TriMV HC-Pro, NIa-VPg, NIa-Pro, and CP cistrons, each with a 9-amino-acid cleavage peptide sequence, were inserted between the P1 and HC-Pro cistrons by overlap extension PCR using three individual PCR fragments as described for engineering WSMV cistrons into the TriMV genome. The overlap extension PCR fragments were ligated into pSP6-WSMV (51) between NgoMIV (created upstream of an SP6 RNA polymerase promoter sequence) and AflII (nt 3905) restriction endonuclease sites.
Standard molecular biology techniques such as PCR, ligation, and transformation were performed as described in the work of Green and Sambrook (55). Escherichia coli strain JM109 was used for transformation of ligation reactions. Plasmid DNA from 40-ml overnight-grown cultures was isolated with the Bio-Rad plasmid midiprep kit (Bio-Rad, Hercules, CA). The presence of engineered sequences in pTriMV and pSP6-WSMV was confirmed by nucleotide sequencing using an Applied Biosystems 3730 model sequencer at the ICBR Core DNA Sequencing Facility, University of Florida, Gainesville, FL.
Infection of wheat seedlings.
Wheat seedlings at the single-leaf stage were inoculated with freshly prepared in vitro transcripts of WSMV, TriMV, TriMV containing WSMV cistrons, and WSMV containing TriMV cistrons as described by Tatineni et al. (53). Briefly, the in vitro transcription reaction of each construct was performed in a 40-μl reaction mixture comprising 1.0 μg of linearized plasmid DNA; 1× transcription buffer (New England BioLabs, Ipswich, MA; 40 mM Tris-HCl, pH 7.9, 20 mM dithiothreitol [DTT], 8.5 mM MgCl2, and 2 mM spermidine); 1.2 mM (each) ATP, CTP, UTP, and Cap analog (m7G[5′]ppp[5′]G; Cellscript, Madison, WI); 0.048 mM GTP; 20 U of rRNasin RNase inhibitor (Promega); and 50 U of SP6 RNA polymerase (Cellscript). The GTP concentration was augmented to 0.5 mM after an initial 15-min incubation at 37°C, followed by an additional 2 h of incubation at 37°C. One microliter of in vitro transcription reaction mixtures was tested on a 1.0% native agarose gel in 1× Tris-acetate-EDTA (TAE) buffer. Freshly prepared in vitro transcripts were inoculated onto wheat seedlings at the single-leaf stage as described previously (53). Infected wheat leaves collected at 14 days postinoculation (dpi) were stored at −20°C for inoculation of wheat seedlings for SIE studies.
Superinfection exclusion assay.
Crude sap extracted from in vitro transcript-infected wheat leaves at a 1:20 dilution in 20 mM sodium phosphate buffer, pH 7.0, was mechanically inoculated into wheat seedlings at the single-leaf stage. The symptomatic third leaf of primary virus-infected wheat at 10 dpi was inoculated with the challenge virus by swiping 2 to 3 times from the bottom of the leaf to the top between inoculum-dipped index finger and thumb. Crude sap prepared from wheat leaves freshly infected with WSMV-GFP or TriMV-GFP at 1:40 and 1:30 dilutions, respectively, in 20 mM sodium phosphate buffer, pH 7.0, was used as the challenge virus. Challenge virus-inoculated wheat seedlings were incubated in a growth chamber at 22°C maximum and 20°C minimum temperature with a 14-h photoperiod. At least three independent superinfection exclusion experiments were conducted with two independent clones per construct.
Examination of local and systemic infection.
Challenge-inoculated wheat seedlings were observed for the development of local foci on inoculated leaves at 5 days post-challenge inoculation (dpci), and upper noninoculated leaves were observed for systemic infection at 9 dpci (for WSMV-GFP) or 12 dpci (for TriMV-GFP) under a Zeiss Stereo Discovery V12 fluorescence microscope using GFP filter set 38 (400- to 450-nm excitation and 450- to 490-nm emission) (Carl Zeiss MicroImaging, Inc., New York, NY) as described in the work of Tatineni et al. (46, 53). The fluorescent images of leaves were obtained with an AxioCam MRc5 camera attached to the Discovery V12 fluorescence microscope. Wheat plants inoculated with GFP- and RFP-tagged viruses for coinfection experiments were observed under a Zeiss Stereo Discovery V12 fluorescence microscope using a GFP filter (for GFP-tagged viruses) and RFP filter set 43 (533- to 558-nm excitation and 571- to 641-nm emission, for RFP-tagged viruses). Coinfected wheat leaves were also observed under a Nikon A1 confocal system on a Nikon 90i upright fluorescence microscope (Nikon, Tokyo, Japan) using GFP (at 488-nm excitation and 500- to 550-nm emission) and RFP (at 561.4-nm excitation and 570- to 620-nm emission) filters.
Western blot analyses.
Three 7.5-cm-long inoculated leaf pieces at 5 dpci and 300 mg from upper noninoculated leaves at 9 or 12 dpci were collected into mesh bags (Agdia, Elkhart, IN) and stored at −80°C for total protein extraction as described in the work of Tatineni et al. (53). Total proteins were isolated by macerating tissue with a tissue homogenizer (Agdia) in 2 ml of TPE buffer (53). Thoroughly ground macerate (500 μl) was mixed with 500 μl of 2× sample buffer (100 mM Tris-Cl, pH 6.8, 20% glycerol, 4% SDS, 5% β-mercaptoethanol, and 0.02% bromophenol blue), followed by incubation at 100°C for 3 min. The protein extract was clarified at 16,000 × g for 5 min at room temperature, and 500 μl of supernatant was stored at −20°C for Western blot analyses.
Total proteins were electrophoretically separated on 4 to 20% Tris-glycine-SDS polyacrylamide gels (Invitrogen, Carlsbad, CA). Either the PAGE gels were stained by Coomassie brilliant blue R-250 for RubisCO protein or proteins were transferred onto polyvinylidene difluoride (PVDF) membranes using the iBlot dry blotting system (Invitrogen). The PVDF membranes were incubated in a blocking solution of 5% (wt/vol) nonfat dry milk powder in Tris-buffered saline containing 0.05% Tween 20 (TBS-T), followed by incubation in either GFP-specific monoclonal antibody (Clontech, Mountain View, CA) at a 1:10,000 dilution or TriMV polyclonal antiserum (56) at a 1:20,000 dilution or with WSMV polyclonal antiserum (Agdia) at a 1:15,000 dilution. Either anti-rabbit (for TriMV and WSMV antisera) or anti-mouse (for GFP antibody) antibody-horseradish peroxidase (HRP) conjugate was used at a 1:50,000 dilution as a secondary antibody. Immobilon Western blot substrate (Millipore) was used for the development of PVDF membranes per the manufacturer's instructions (Millipore, Billerica, MA). Immunoreactive protein band images on membranes were obtained using the Molecular Imager ChemiDoc XRS+ with Image Lab software system (Bio-Rad). Coomassie blue-stained SDS-PAGE gels showing the large subunit of wheat RubisCO protein were used as a Western blot loading control.
RT-PCR assay.
Stability of inserted sequences in TriMV and WSMV genomes in challenge virus-inoculated leaves at 5 dpci and in upper noninoculated leaves at 9 or 12 dpci was examined by reverse transcription-PCR (RT-PCR). Total RNA was extracted from three 7.5-cm-long challenge virus-inoculated leaf pieces and 200 mg of upper noninoculated leaves as described previously (57). Reverse transcription was performed in a 10-μl volume with 1 μl of total RNA as a template in the presence of random primers with avian myeloblastosis virus (AMV) reverse transcriptase (Roche, Indianapolis, IN; 0.5 U per μl reaction volume). PCR was performed in a 25-μl reaction volume with 1 μl of cDNA as a template with primers W-211 (corresponding to nt 1021 to 1048) and W-212 (complementary to nt 1249 to 1223) for WSMV or Tr-289 (corresponding to nt 1621 to 1648) and Tr-290 (complementary to nt 2137 to 2110) for TriMV with Herculase II fusion DNA polymerase (Agilent Technologies Inc.). Five microliters of RT-PCR products was analyzed electrophoretically through 1.0% agarose gels in 1× TAE buffer, followed by ethidium bromide staining.
RESULTS
No SIE between WSMV and TriMV in coinfected wheat.
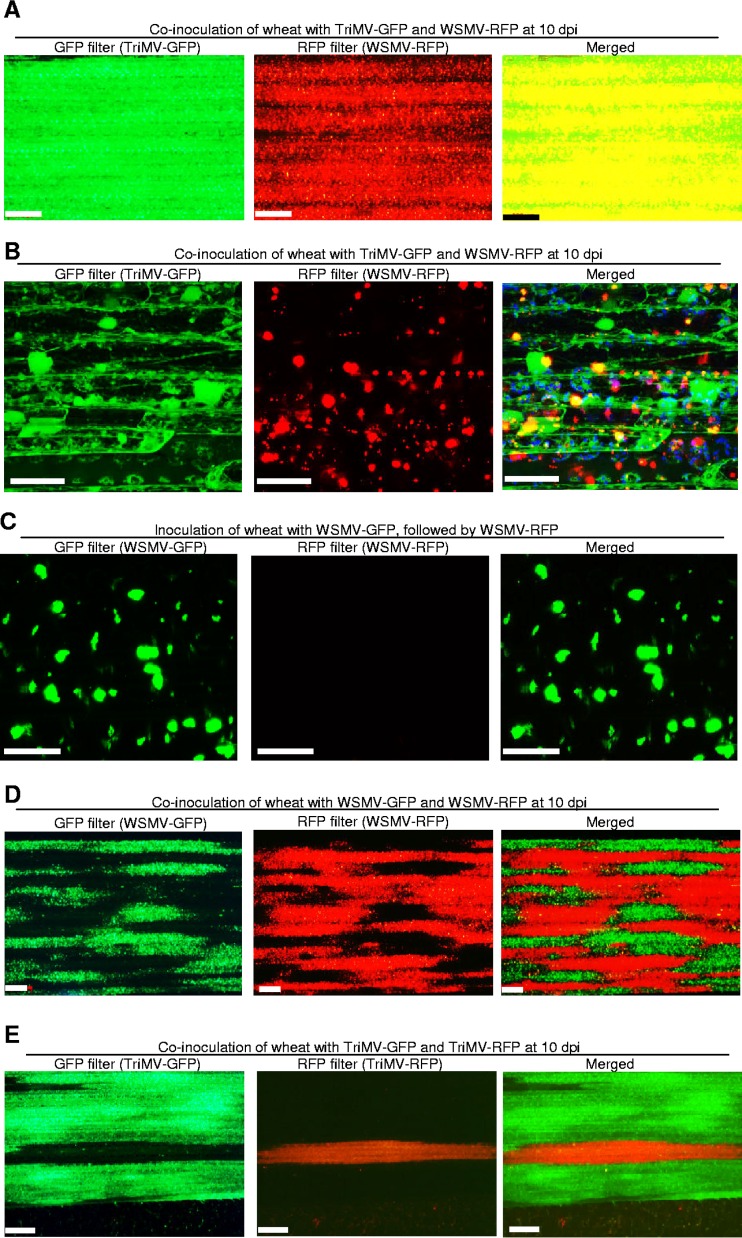
WSMV and TriMV are divergent members of the family Potyviridae with 27% and 18 to 41% amino acid identity at the polyprotein and individual mature protein levels, respectively (32, 45). Previously, we have shown that WSMV and TriMV interact synergistically in coinoculated wheat, but it was not known whether these two viruses could coinfect the same wheat cells. In this study, GFP-tagged TriMV (TriMV-GFP) (46) and RFP-tagged WSMV (WSMV-RFP) (39) were used to examine the nature of coinfection by WSMV and TriMV in a susceptible wheat cultivar, Tomahawk. Wheat seedlings at the single-leaf stage were coinoculated with crude sap of WSMV-RFP and TriMV-GFP at a 1:20 dilution. The upper noninoculated leaves of wheat were examined for systemic infection by WSMV-RFP and TriMV-GFP at 10 dpi under a Zeiss Stereo Discovery V12 fluorescence microscope (Fig. 1A) and a Nikon 90i upright fluorescent confocal microscope (Fig. 1B) using GFP (for TriMV-GFP infection) or RFP (for WSMV-RFP infection) filters.
FIG 1.
Demonstration of coinfection and superinfection exclusion (SIE) phenomena of Wheat streak mosaic virus (WSMV) and Triticum mosaic virus (TriMV) in wheat. (A and B) Coinfection of wheat by WSMV-RFP and TriMV-GFP at 10 days postinoculation (dpi). GFP and RFP in upper noninoculated leaves were observed under a Zeiss Stereo Discovery V12 fluorescence microscope (A) and a Nikon 90i upright fluorescence confocal microscope (B). Superimposed images of GFP and RFP are presented at the right (merged). Bars in panels A and B, 500 and 50 μm, respectively. (C) SIE between GFP- and RFP-tagged variants of WSMV. Wheat seedlings infected with WSMV-GFP were superinoculated with WSMV-RFP. Fluorescent images were taken at 14 days after challenge inoculation by confocal microscopy. Bars, 50 μm. (D and E) SIE phenomenon between the GFP- and RFP-tagged variants of WSMV or TriMV in wheat. Presented images of leaves show the expression of GFP (WSMV-GFP or TriMV-GFP) or RFP (WSMV-RFP or TriMV-RFP) in wheat coinoculated with GFP- and RFP-tagged WSMV or TriMV. Superimposed images in panels D and E show that expression of GFP and RFP is restricted to mutually exclusive regions. Bars, 500 μm.
Wheat leaves observed under a confocal microscope indicated the presence of both GFP and RFP in the same wheat cells. Furthermore, some RFP and GFP were colocalized in the same location within cells, resulting in yellow coloration (Fig. 1B, merged). Additionally, wheat leaves observed under a dissecting fluorescence microscope revealed that a large number of wheat cells were coinfected with both TriMV-GFP and WSMV-RFP, as the superimposed images of GFP and RFP appeared yellow (Fig. 1A, merged). These results revealed that no SIE was found between divergent WSMV and TriMV and that these two viruses efficiently coinfected the same wheat cell.
RFP- or GFP-tagged variants of WSMV and TriMV display reciprocal SIE.
The phenomenon of SIE in WSMV was examined by superinoculating WSMV-GFP-infected wheat at 10 dpi with WSMV-RFP. Examination of upper noninoculated leaves at 12 days post-challenge inoculation (dpci) revealed that WSMV-RFP failed to superinfect WSMV-GFP-infected wheat (Fig. 1C), demonstrating that WSMV-GFP prevented superinfection by WSMV-RFP. In another experiment, wheat seedlings at the single-leaf stage were simultaneously coinoculated with WSMV-RFP and WSMV-GFP. The upper noninoculated leaves were observed for systemic infection by WSMV-GFP and WSMV-RFP at 10 dpi. RFP- and GFP-tagged WSMV were both found in upper noninoculated leaves but in mutually exclusive regions, appearing as tissue islands infected by either RFP- or GFP-tagged virus but not both (Fig. 1D).
The ability of TriMV to exhibit the SIE phenomenon was also examined using GFP- and RFP-tagged variants of TriMV (46). Systemic infection of wheat by GFP- or RFP-tagged TriMV completely prevented superinfection by RFP- or GFP-tagged TriMV, respectively (data not shown). Furthermore, in coinoculated wheat, mutually exclusive regions of TriMV-GFP or TriMV-RFP were observed in upper noninoculated leaves (Fig. 1E), similar to that of WSMV. These data revealed that GFP and RFP variants of TriMV exhibit SIE in wheat, as do GFP and RFP variants of WSMV.
Screening WSMV cistrons involved in SIE.
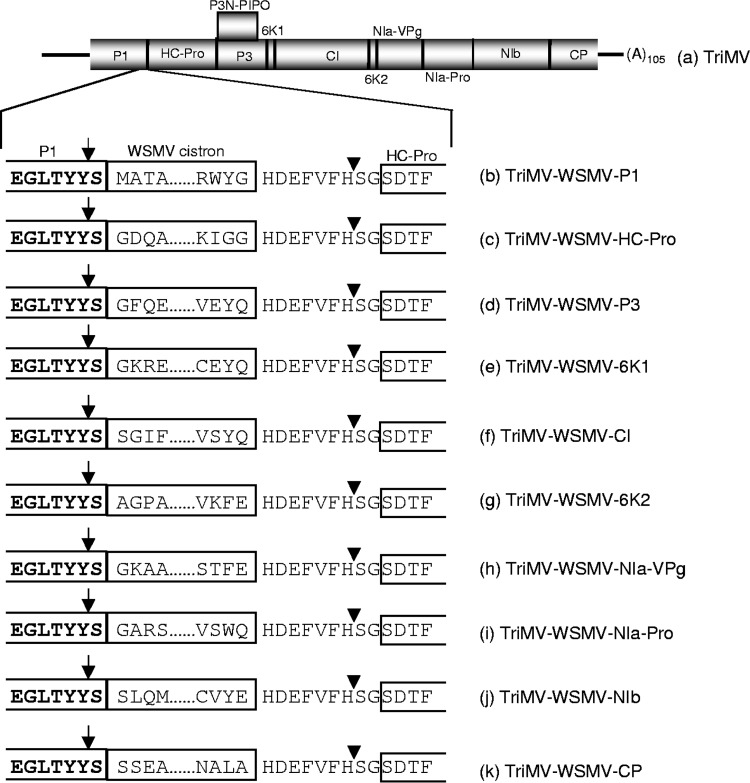
Previously, we demonstrated that TriMV efficiently and stably expressed GFP or RFP as free soluble protein by fusing a 9-amino-acid cleavage peptide compared to that of the heptapeptide cleavage site (46). In this study, each WSMV cistron was engineered into the TriMV genome between the P1 and HC-Pro cistrons with a 9-amino-acid cleavage peptide located between the NIb and CP cistrons of TriMV (Fig. 2). WSMV proteins from the TriMV polyprotein were expected to be released by a cis cleavage by the TriMV P1 at the C terminus of P1 and a trans cleavage by the TriMV NIa-Pro at the engineered cleavage peptide at the C-terminal end of WSMV proteins (Fig. 2).
FIG 2.
Schematic diagrams of the genomic organization of Triticum mosaic virus (TriMV) (a) and TriMV expression vectors with Wheat streak mosaic virus (WSMV) cistrons (b to k). The proteins encoded by TriMV are shown along with the positions of cleavage sites, indicated with vertical lines inside the polyprotein. Expanded schematic diagrams presented below the TriMV genomic organization are the C-terminal region of P1, each WSMV cistron inserted between the P1 and HC-Pro cistrons, and the N-terminal region of HC-Pro. The C terminus of each WSMV citron was fused to a 9-amino-acid cleavage peptide derived from the junction of NIb/CP cistrons of TriMV. The N- and C-terminal 4 amino acids of each WSMV cistron inserted in the TriMV genome are indicated. Predicted cleavage sites on either side of the WSMV cistrons are indicated with arrows and arrowheads.
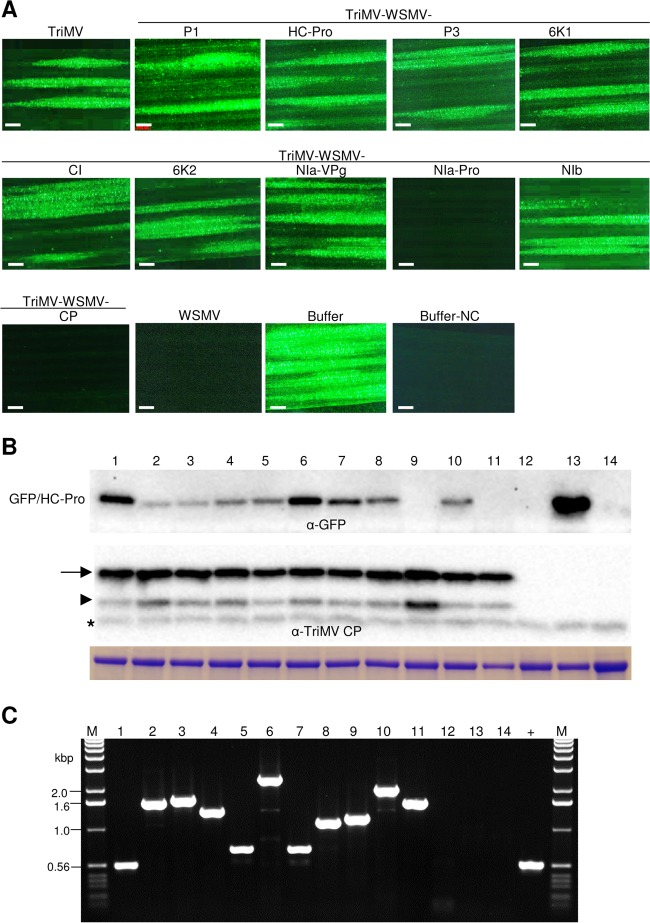
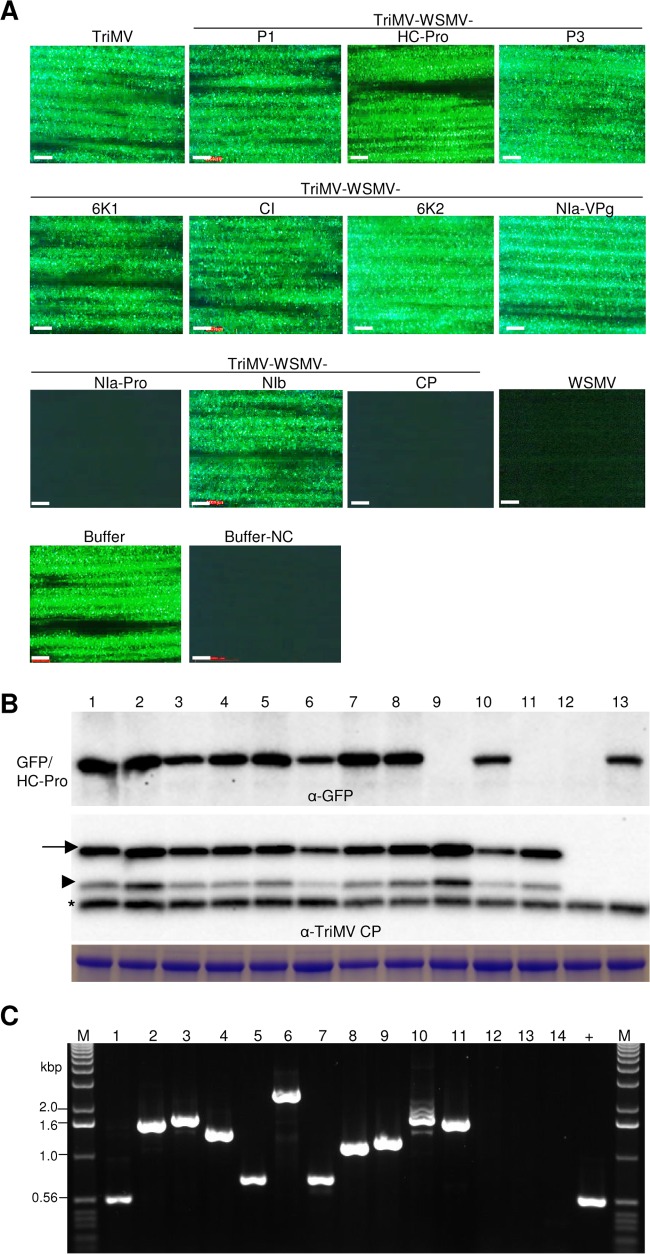
Wheat seedlings at the single-leaf stage were inoculated with crude sap from 14-dpi wheat leaves infected with in vitro transcripts of TriMV containing WSMV cistrons. The symptomatic third leaf of wheat at 10 dpi was challenge inoculated with WSMV-GFP. At 5 dpci, WSMV-GFP elicited 19 to 62 (experiment 1) and 15 to 32 (experiment 2) foci per inoculated leaf of wheat infected with wild-type TriMV or TriMV expressing WSMV P1, HC-Pro, P3, 6K1, CI, 6K2, NIa-VPg, or NIb cistrons (Fig. 3A; Table 1). In contrast, wheat infected with TriMV expressing WSMV NIa-Pro or CP allowed only 0.1 to 0.7 smaller focus per inoculated leaf (Fig. 3A; Table 1). The upper noninoculated leaves of wheat were examined for systemic infection by the challenge virus at 9 dpci (Fig. 4). Wheat infected with wild-type TriMV or TriMV expressing WSMV P1, HC-Pro, P3, 6K1, CI, 6K2, NIa-VPg, or NIb allowed efficient systemic infection by WSMV-GFP in 77 to 100% of plants (Fig. 4A; Table 1). In contrast, systemic infection by WSMV-GFP was severely debilitated in wheat infected with TriMV expressing WSMV NIa-Pro or CP with only 8 to 14% and 14 to 17% of plants systemically infected, respectively, with a few isolated foci per leaf (Fig. 4A; Table 1). Total proteins analyzed from inoculated and upper noninoculated leaves at 5 and 9 dpci, respectively, indicated that accumulation of TriMV CP was essentially the same in wheat leaves preinfected with either TriMV or TriMV with WSMV cistrons (Fig. 3B and 4B, lower panels). These data indicate that the presence of WSMV cistrons did not affect TriMV replication in wheat. GFP accumulated in challenge virus-inoculated and upper noninoculated leaves of wheat infected with TriMV or TriMV expressing WSMV P1, HC-Pro, P3, 6K1, CI, 6K2, NIa-VPg, or NIb (Fig. 3A and B and 4A and B), confirming replication and movement of the challenge virus in inoculated and upper noninoculated leaves. However, GFP did not accumulate at detectable levels in wheat infected with TriMV expressing WSMV NIa-Pro or CP (Fig. 3A and B and 4A and B), demonstrating that replication and/or movement of WSMV-GFP was greatly reduced in the presence of WSMV NIa-Pro or CP. RT-PCR analyses with primers flanking insert sequences obtained expected-size products, indicating that TriMV retained WSMV cistrons stably at 5 and 9 dpci (Fig. 3C and 4C).
FIG 3.
Wheat streak mosaic virus (WSMV)-encoded NIa-Pro and coat protein (CP) function in superinfection exclusion at the local infection level. (A) Local foci elicited by the challenge virus (WSMV-GFP) on wheat infected by TriMV expressing individual WSMV cistrons and wild-type TriMV and WSMV at 5 days post-challenge inoculation (dpci). Buffer and buffer-NC are buffer-inoculated wheat, subsequently challenge inoculated or not challenge inoculated with WSMV-GFP, respectively. Bars, 500 μm. (B) Western blot assays of total proteins from the challenge virus (WSMV-GFP)-inoculated leaves at 5 dpci. Blots were probed with GFP monoclonal antibody (upper blot) and TriMV polyclonal antibodies (lower blot). Positions of full-length and truncated TriMV CP are indicated by an arrow and arrowhead, respectively. An asterisk indicates a wheat protein reacting nonspecifically with TriMV antiserum. Below is an SDS-PAGE gel stained with Coomassie blue showing the amount of the large subunit of wheat RubisCO protein as the amount of protein loaded per well. (C) RT-PCR analysis showing the stability of WSMV cistrons in TriMV at 5 dpci. Lanes in panels B and C: lane 1, challenge-inoculated wheat preinfected with TriMV; lanes 2 to 11, TriMV expressing WSMV P1 (lane 2), HC-Pro (lane 3), P3 (lane 4), 6K1 (lane 5), CI (lane 6), 6K2 (lane 7), NIa-VPg (lane 8), NIa-Pro (lane 9), NIb (lane 10), or coat protein (lane 11); lane 12, wheat infected with WSMV; lane 13, buffer-inoculated wheat; lane 14, buffer-inoculated wheat with no challenge inoculation; lane +, pTriMV as a PCR control; lanes M, 1.0-kbp DNA ladder.
TABLE 1.
Examination of Wheat streak mosaic virus (WSMV) cistrons for superinfection exclusion activity in wheata
| Primary virus | Challenge virus | Expt 1 |
Expt 2 |
||||
|---|---|---|---|---|---|---|---|
| Avg no. of local foci/leaf ± SE | No. of plants systemically infected/no. of plants inoculatedb | % of plants systemically infected with WSMV-GFP | Avg no. of local foci/leaf ± SE | No. of plants systemically infected/no. of plants inoculatedb | % of plants systemically infected with WSMV-GFP | ||
| TriMV | WSMV-GFP | 33.3 ± 4.6 | 11/11 | 100 | 31.8 ± 3.8 | 16/16 | 100 |
| TriMV-WSMV-P1 | WSMV-GFP | 59.6 ± 6.6 | 11/11 | 100 | 18.5 ± 1.7 | 20/20 | 100 |
| TriMV-WSMV-HC-Pro | WSMV-GFP | 19.9 ± 4.2 | 10/13 | 77 | 15.7 ± 0.8 | 15/15 | 100 |
| TriMV-WSMV-P3 | WSMV-GFP | 37.9 ± 6.2 | 10/10 | 100 | 15.4 ± 1.2 | 17/17 | 100 |
| TriMV-WSMV-6K1 | WSMV-GFP | 18.9 ± 2.2 | 15/15 | 100 | 19.1 ± 1.3 | 16/16 | 100 |
| TriMV-WSMV-CI | WSMV-GFP | 61.5 ± 10.1 | 8/8 | 100 | 32.0 ± 4.5 | 18/18 | 100 |
| TriMV-WSMV-6K2 | WSMV-GFP | 29.6 ± 3.3 | 13/13 | 100 | 29.6 ± 2.5 | 16/16 | 100 |
| TriMV-WSMV-NIa-VPg | WSMV-GFP | 54.1 ± 6.4 | 12/12 | 100 | 21.5 ± 2.3 | 17/17 | 100 |
| TriMV-WSMV-NIa-Pro | WSMV-GFP | 0.1 ± 0.1 | 2/14* | 14 | 0.2 ± 0.1 | 2/24* | 8 |
| TriMV-WSMV-NIb | WSMV-GFP | 60.4 ± 8.6 | 15/15 | 100 | 30.3 ± 2.6 | 22/22 | 100 |
| TriMV-WSMV-CP | WSMV-GFP | 0.7 ± 0.4 | 2/14* | 14 | 0.7 ± 0.3 | 3/18* | 17 |
| WSMV | WSMV-GFP | 0.0 ± 0.0 | 0/12 | 0 | 0.31 ± 0.2 | 0/16 | 0 |
| Buffer | WSMV-GFP | 108.0 ± 6.1 | 15/15 | 100 | 62.4 ± 8.2 | 14/14 | 100 |
Wheat plants systemically infected with Triticum mosaic virus (TriMV) harboring WSMV cistrons were challenge inoculated with WSMV-GFP at 10 dpi. Local foci and systemic infection by the challenge virus (WSMV-GFP) on primary virus-infected wheat were examined at 5 and 9 days post-challenge inoculation for local fluorescent foci and the number of systemically infected plants, respectively. Presented local foci per leaf are average numbers of foci from 15 to 20 inoculated leaves.
Asterisks indicate that 1 to 10 isolated infection foci per leaf were observed in upper noninoculated leaves.
FIG 4.
Wheat streak mosaic virus (WSMV)-encoded NIa-Pro and coat protein (CP) function in superinfection exclusion at the systemic infection level. (A) Presented fluorescent images are systemic infection by the challenge virus (WSMV-GFP) in wheat infected by TriMV expressing WSMV proteins as indicated. TriMV and WSMV, wheat systemically infected with wild-type TriMV and WSMV, respectively. Buffer and buffer-NC are buffer-inoculated wheat subsequently challenge inoculated or not challenge inoculated with WSMV-GFP, respectively. Bars, 500 μm. (B) Western blot assay of total proteins from upper noninoculated leaves of challenge virus-inoculated wheat that were previously infected by TriMV expressing WSMV cistrons. The blots were probed with GFP monoclonal antibody (upper blot) and TriMV polyclonal antibodies (lower blot). Positions of full-length and truncated TriMV CP are indicated by an arrow and arrowhead, respectively. An asterisk indicates a wheat protein reacting nonspecifically with TriMV antiserum. Below is an SDS-PAGE gel stained with Coomassie blue showing the amount of the large subunit of wheat RubisCO protein as a Western blot loading control. (C) RT-PCR analysis showing the stability of WSMV cistrons in TriMV at 9 dpci in upper noninoculated leaves. Lanes in panels B and C are the same as indicated in the legend for Fig. 3.
Taken together, these data revealed that superinfection of WSMV-GFP was severely hampered in wheat systemically infected with TriMV expressing WSMV NIa-Pro or CP cistrons. These data also demonstrate that WSMV NIa-Pro and CP independently affect SIE when expressed from TriMV while other WSMV-encoded proteins do not play a significant role in SIE.
WSMV CP and NIa-Pro proteins but not RNA sequences are effectors of SIE.
Wheat infected with TriMV expressing WSMV NIa-Pro or CP cistron severely debilitated superinfection by WSMV-GFP, but it is not clear whether an RNA sequence or protein encoded by NIa-Pro and CP is involved in SIE activity. WSMV NIa-Pro and CP are translated in the TriMV genome as polyproteins; hence, introduction of a stop codon or frameshift mutation in WSMV NIa-Pro or CP cistron in the TriMV genome would be lethal to TriMV. To overcome this problem, a series of frameshift mutations and nucleotide substitutions were introduced in WSMV NIa-Pro and CP cistrons to maintain a continuous polypeptide reading frame but prevent expression of either protein, followed by insertion into the TriMV genome to obtain TriMV-WSMV-NIa-Pro-NS and TriMV-WSMV-CP-NS, respectively (Fig. 5A). The third leaf of wheat preinfected with TriMV-WSMV-NIa-Pro-NS, -WSMV-CP-NS, -WSMV-NIa-Pro, or -WSMV-CP at 10 dpi was challenge inoculated with WSMV-GFP. WSMV-GFP elicited efficient local foci on wheat leaves systemically infected with TriMV expressing WSMV NIa-Pro-NS or CP-NS (13 to 42 foci per leaf) but not with wild-type NIa-Pro or CP (Fig. 5B; Table 2).
FIG 5.
Wheat streak mosaic virus NIa-Pro and CP but not RNA sequence is required for superinfection exclusion. (A) Schematic diagram of the genomic organization of Triticum mosaic virus (TriMV) with expanded views of WSMV NIa-Pro and CP cistrons with a series of frameshifts or nucleotide substitution mutations (indicated with vertical red lines) for a continuous reading frame but with a different amino acid sequence. NIa-Pro-NS and CP-NS cistrons of WSMV with a 9-amino-acid NIb/CP cleavage peptide of TriMV were inserted between the P1 and HC-Pro cistrons. (B and C) Fluorescent images shown are local foci at 5 days post-challenge inoculation (dpci) (B) and systemic infection at 9 dpci (C) with WSMV-GFP (challenge virus) on wheat leaves systemically infected with TriMV or TriMV expressing WSMV NIa-Pro-NS, NIa-Pro, CP-NS, or CP cistrons. Buffer, buffer-inoculated wheat subsequently challenge inoculated. (D and E) Western blot assays of total proteins from inoculated (D) and upper noninoculated (E) leaves at 5 and 9 dpci, respectively. The blots were probed with GFP monoclonal antibody and TriMV polyclonal antibodies as indicated. Coomassie blue-stained SDS-PAGE gels show the amount of the large subunit of wheat RubisCO protein as loading controls for the amount of protein loaded per well in Western blot assays. Full-length and truncated TriMV CP are indicated with arrows and arrowheads, respectively. Asterisks indicate a wheat protein reacting nonspecifically with TriMV antiserum. (F and G) Presented ethidium bromide-stained agarose gels are RT-PCR products of WSMV cistrons using TriMV-specific primers from inoculated (F) and upper noninoculated (G) wheat leaves at 5 and 9 dpci, respectively. (D to G) Lanes 1 to 5, wheat infected with TriMV (lane 1) or TriMV with WSMV NIa-Pro-NS (lane 2), NIa-Pro (lane 3), CP-NS (lane 4), and CP (lane 5); lane 6, buffer-inoculated wheat subsequently challenge inoculated; lane 7, buffer-inoculated wheat with no challenge inoculation; lane +, pTriMV as a positive control; lanes M, 1.0-kbp DNA ladder.
TABLE 2.
Wheat streak mosaic virus (WSMV) NIa-Pro and CP, but not RNA sequences, induce superinfection exclusiona
| Primary virus | Challenge virus | Expt 1 |
Expt 2 |
||||
|---|---|---|---|---|---|---|---|
| Avg no. of local foci/leaf ± SE | No. of plants systemically infected/no. of plants inoculatedb | % of plants systemically infected with WSMV-GFP | Avg no. of local foci/leaf ± SE | No. of plants systemically infected/no. of plants inoculatedb | % of plants systemically infected with WSMV-GFP | ||
| TriMV | WSMV-GFP | 17.8 ± 1.1 | 12/12 | 100 | 14.9 ± 1.9 | 16/16 | 100 |
| TriMV-WSMV-NIa-Pro-NS | WSMV-GFP | 42.4 ± 5.7 | 13/14 | 93 | 18.0 ± 2.2 | 14/14 | 100 |
| TriMV-WSMV-NIa-Pro | WSMV-GFP | 0.1 ± 0.1 | 3/16* | 19 | 0.1 ± 0.1 | 1/17* | 6 |
| TriMV-WSMV-CP-NS | WSMV-GFP | 16.7 ± 1.5 | 20/21 | 95 | 13.4 ± 1.2 | 17/17 | 100 |
| TriMV-WSMV-CP | WSMV-GFP | 0.2 ± 0.1 | 1/16* | 6 | 0.3 ± 0.1 | 1/17* | 6 |
| WSMV | WSMV-GFP | 0.0 ± 0.0 | 0/12 | 0 | 0.0 ± 0.0 | 0/15 | 0 |
| Buffer | WSMV-GFP | 95.9 ± 3.2 | 17/17 | 100 | 43.6 ± 6.8 | 16/16 | 100 |
Wheat plants systemically infected with Triticum mosaic virus (TriMV) harboring WSMV cistrons were challenge inoculated with WSMV-GFP at 10 dpi. NS, a series of frameshift and nucleotide substitution mutations were introduced to retain a continuous reading frame but expressed a different protein. Local foci and systemic infection by the challenge virus (WSMV-GFP) on primary virus-infected wheat were examined at 5 and 9 days post-challenge inoculation for local fluorescent foci and the number of systemically infected plants, respectively. Presented local foci per leaf are average numbers of foci from 15 to 20 inoculated leaves.
Asterisks indicate that 1 to 10 isolated infection foci per leaf were observed in upper noninoculated leaves.
Examining the upper noninoculated leaves at 9 dpci for systemic infection by the challenge virus revealed that wheat preinfected with TriMV-WSMV-NIa-Pro-NS or TriMV-WSMV-CP-NS efficiently permitted superinfection by WSMV-GFP in 93 to 100% of inoculated plants (Fig. 5C; Table 2). In contrast, superinfection by WSMV-GFP was severely impaired in wheat infected with TriMV expressing WSMV NIa-Pro or CP, with 6 to 19% of plants systemically infected with a few isolated foci per leaf (Fig. 5C; Table 2). Furthermore, in Western blot assays, accumulation of GFP was readily detected in challenge virus-inoculated and upper noninoculated leaves of wheat infected with TriMV expressing WSMV NIa-Pro-NS or CP-NS but not with wild-type NIa-Pro or CP (Fig. 5D and E, top panels). TriMV CP efficiently accumulated in leaves observed for local and systemic infection by the challenge virus (Fig. 5D and E, bottom panels), demonstrating that TriMV harboring WSMV cistrons efficiently replicated in wheat. TriMV maintained WSMV sequences stably in wheat at 5 and 9 dpci, since expected RT-PCR products were obtained with no detectable deletions in inserts (Fig. 5F and G). Taken together, these data indicate that WSMV CP and NIa-Pro proteins but not RNA sequences are effectors of SIE.
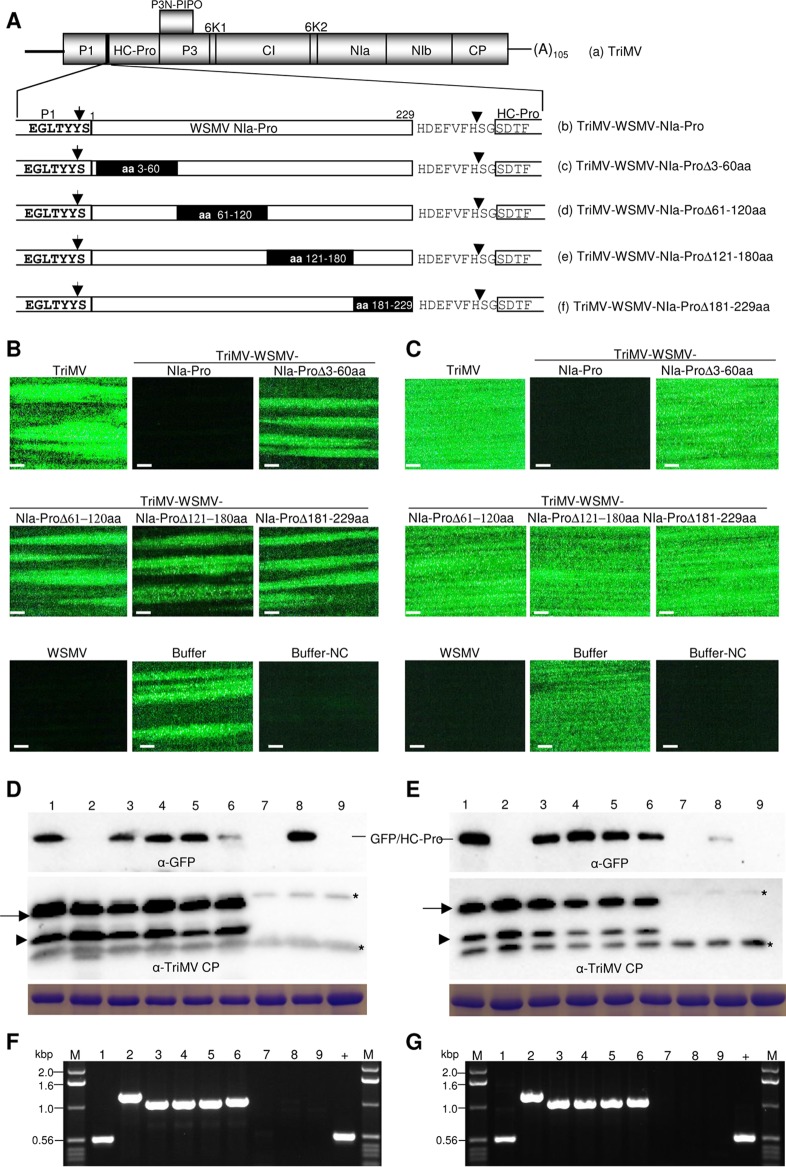
The entire NIa-Pro of WSMV is required for SIE activity.
The role of WSMV NIa-Pro in SIE was further examined by introducing a series of nonoverlapping deletions comprising aa 3 to 60, 61 to 120, 121 to 180, and 181 to 229 and inserting them into TriMV between the P1 and HC-Pro cistrons (Fig. 6A). The third leaf of wheat infected with TriMV expressing WSMV NIa-Pro deletions at 10 dpi was challenge inoculated with WSMV-GFP. Wheat infected with TriMV expressing WSMV NIa-Pro deletion mutants allowed efficient local foci and systemic infection by WSMV-GFP (Fig. 6B and C; Table 3). WSMV-GFP elicited 26 to 43 (experiment 1) and 15 to 22 (experiment 2) fluorescent foci per inoculated leaf of wheat infected with TriMV expressing WSMV NIa-Pro deletion mutants, while it elicited only 0.2 to 1.1 focus per leaf on TriMV with wild-type WSMV NIa-Pro-infected wheat (Fig. 6B; Table 3). Wheat infected with TriMV expressing WSMV NIa-Pro deletions but not wild-type NIa-Pro allowed efficient systemic infection by WSMV-GFP in 94 to 100% of inoculated plants (Fig. 6C; Table 3).
FIG 6.
Complete Wheat streak mosaic virus (WSMV) NIa-Pro is required for superinfection exclusion. (A) Schematic diagram of Triticum mosaic virus (TriMV) genome (a) with expanded views of WSMV NIa-Pro cistron (b) or NIa-Pro with a series of deletions covering the entire NIa-Pro cistron (c to f). Deleted amino acids in the NIa-Pro cistron are indicated with black rectangles. (B and C) Presented fluorescent images are local foci (B) at 5 days post-challenge inoculation (dpci) and systemic infection (C) at 9 dpci by the challenge virus (WSMV-GFP) on wheat infected with TriMV and TriMV with WSMV NIa-Pro or NIa-Pro deletions. WSMV, wheat infected with WSMV; buffer and buffer-NC, buffer-inoculated wheat subsequently challenge inoculated or not challenge inoculated, respectively. Bars, 500 μm. (D and E) Western blotting of total proteins from local foci at 5 dpci (D) and systemic infection at 9 dpci with challenge virus (E). The blots are probed with GFP monoclonal antibody and TriMV polyclonal antibodies as indicated. Below are SDS-PAGE gels stained with Coomassie blue showing the amount of the large subunit of wheat RubisCO protein as a Western blot loading control. Asterisks indicate wheat proteins reacting nonspecifically with TriMV antiserum. (F and G) RT-PCR products of WSMV cistrons using TriMV-specific primers to demonstrate the stability of inserts in the challenge virus-inoculated (F) and upper noninoculated (G) wheat leaves at 5 and 9 dpci, respectively. (D to G) Lanes 1 to 6, wheat infected with TriMV (lane 1) or TriMV with WSMV NIa (lane 2), NIa-ProΔ3-60aa (lane 3), NIa-ProΔ61-120aa (lane 4), NIa-ProΔ121-180aa (lane 5), or NIa-ProΔ181-229aa (lane 6); lane 7, WSMV-infected wheat; lane 8, buffer-inoculated wheat with challenge inoculation; lane 9, buffer-inoculated wheat with no challenge inoculation; lane +, pTriMV as a positive control; lane M, 1.0-kbp DNA ladder.
TABLE 3.
Mapping minimal regions of Wheat streak mosaic virus (WSMV) NIa-Pro and CP required for induction of superinfection exclusiona
| Primary virus | Challenge virus | Expt 1 |
Expt 2 |
||||
|---|---|---|---|---|---|---|---|
| Avg no. of local foci/leaf ± SE | No. of plants systemically infected/no. of plants inoculatedb | % of plants systemically infected with WSMV-GFP | Avg no. of local foci/leaf ± SE | No. of plants systemically infected/no. of plants inoculatedb | % of plants systemically infected with WSMV-GFP | ||
| TriMV | WSMV-GFP | 32.0 ± 3.6 | 16/16 | 100 | 30.4 ± 2.8 | 19/19 | 100 |
| TriMV-WSMV-NIa-Pro | WSMV-GFP | 0.2 ± 0.1 | 2/24* | 8 | 1.1 ± 0.5 | 8/20* | 40 |
| TriMV-WSMV-NIa-ProΔ3-60aa | WSMV-GFP | 35.2 ± 4.3 | 15/15 | 100 | 14.8 ± 1.8 | 15/15 | 100 |
| TriMV-WSMV-NIa-ProΔ61-120aa | WSMV-GFP | 40.4 ± 4.2 | 19/19 | 100 | 19.8 ± 2.6 | 18/18 | 100 |
| TriMV-WSMV-NIa-ProΔ121-180aa | WSMV-GFP | 25.8 ± 2.9 | 18/18 | 100 | 14.9 ± 1.8 | 17/18 | 94 |
| TriMV-WSMV-NIa-ProΔ181-229aa | WSMV-GFP | 42.5 ± 4.6 | 19/20 | 95 | 21.7 ± 2.7 | 19/19 | 100 |
| WSMV | WSMV-GFP | 0.3 ± 0.2 | 0/17 | 0 | 0.0 ± 0.0 | 0/18 | 0 |
| Buffer | WSMV-GFP | 62.4 ± 8.2 | 15/15 | 100 | 48.5 ± 4.6 | 18/18 | 100 |
| TriMV | WSMV-GFP | 12.1 ± 1.0 | 16/16 | 100 | 79.9 ± 4.6 | 19/19 | 100 |
| TriMV-WSMV-CP | WSMV-GFP | 0.3 ± 0.2 | 4/21* | 19 | 1.7 ± 0.5 | 4/18* | 22 |
| TriMV-WSMV-CPΔ3-35aa | WSMV-GFP | 0.3 ± 0.2 | 2/20* | 10 | 1.4 ± 0.5 | 7/19* | 37 |
| TriMV-WSMV-CPΔ36-84aa | WSMV-GFP | 0.1 ± 0.1 | 7/28* | 25 | 5.1 ± 0.9 | 6/17* | 35 |
| TriMV-WSMV-CPΔ85-100aa | WSMV-GFP | 0.5 ± 0.2 | 2/18* | 11 | 1.5 ± 0.5 | 1/16* | 6 |
| TriMV-WSMV-CPΔ101-200aa | WSMV-GFP | 15.6 ± 1.7 | 19/19 | 100 | 34.8 ± 3.9 | 16/16 | 100 |
| TriMV-WSMV-CPΔ201-300aa | WSMV-GFP | 12.1 ± 0.9 | 19/19 | 100 | 40.5 ± 5.0 | 16/16 | 100 |
| TriMV-WSMV-CPΔ294-349aa | WSMV-GFP | 0.5 ± 0.3 | 2/16* | 13 | 1.6 ± 0.2 | 5/17* | 29 |
| TriMV-WSMV-CPaa101-300 | WSMV-GFP | 0.3 ± 0.2 | 4/15* | 27 | 2.2 ± 0.4 | 7/17* | 41 |
| WSMV | WSMV-GFP | 0.0 ± 0.0 | 0/17 | 0 | 0.1 ± 0.1 | 0/18 | 0 |
| Buffer | WSMV-GFP | 62.4 ± 8.2 | 15/15 | 100 | 118.9 ± 5.5 | 18/18 | 100 |
Wheat plants systemically infected with Triticum mosaic virus (TriMV) harboring WSMV cistrons were challenge inoculated with WSMV-GFP at 10 dpi. Local foci and systemic infection by the challenge virus (WSMV-GFP) on primary virus-infected wheat were examined at 5 and 9 days post-challenge inoculation for local fluorescent foci and the number of systemically infected plants, respectively. Presented local foci per leaf are average numbers of foci from 15 to 20 inoculated leaves.
Asterisks indicate that 1 to 10 isolated infection foci per leaf were observed in upper noninoculated leaves.
Western blot analysis indicated that TriMV CP efficiently accumulated in all wheat leaves observed for local and systemic infection by the challenge virus (Fig. 6D and E, lower panels). In contrast, GFP was readily detected in inoculated and systemic leaves of wheat infected with TriMV-WSMV-NIa-Pro deletion mutants but not with wild-type NIa-Pro (Fig. 6D and E, upper panels). RT-PCR analysis of total RNA from leaves observed for local and systemic infection by WSMV-GFP obtained expected products with no detectable deletions (Fig. 6F and G), indicating that TriMV maintained WSMV NIa-Pro sequences stably in inoculated and upper noninoculated leaves. Collectively, these data indicate that the entire NIa-Pro sequence is required for induction of SIE.
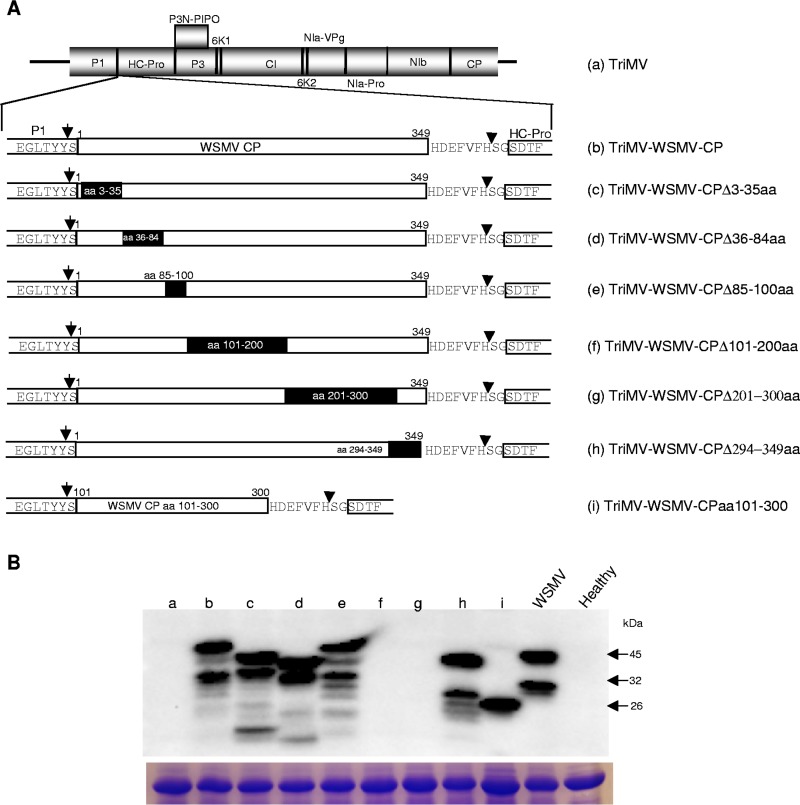
WSMV CP aa 101 to 300 are sufficient for SIE activity.
The minimal CP region required to induce SIE was examined by introducing a series of deletions comprising aa 3 to 35, 36 to 84, 85 to 100, 101 to 200, 201 to 300, and 294 to 349 into the WSMV CP cistron, followed by insertion into the TriMV genome between the P1 and HC-Pro cistrons (Fig. 7A). Total proteins extracted from wheat leaves infected with TriMV harboring deletions in WSMV CP at 14 dpi were examined by Western blotting using WSMV CP polyclonal antisera. TriMV containing WSMV CP with a deletion of aa 3 to 35, 36 to 84, 85 to 100, or 294 to 349 accumulated truncated WSMV CP in wheat with expected sizes (Fig. 7B). However, WSMV CP with a deletion of aa 101 to 200 or 201 to 300 failed to react with WSMV polyclonal antisera (Fig. 7B), most likely due to deletion of aa 101 to 300 abolishing the reactivity of WSMV polyclonal antisera against virions. This notion is supported by the finding that reactivity of WSMV polyclonal antisera was restored with the accumulation of an ∼26-kDa truncated protein from plants systemically infected with TriMV expressing WSMV CP aa 101 to 300 (Fig. 7B, lane i; see below). These data indicate that WSMV CP and all of its truncated versions are efficiently expressed in wheat using TriMV as an expression vector.
FIG 7.
Expression of Wheat streak mosaic virus (WSMV) full-length and truncated coat protein (CP) in wheat infected by Triticum mosaic virus (TriMV) expressing WSMV CP or its deletion mutants. (A) Schematic diagram of the TriMV genome (a) with WSMV CP cistron (b) and CP cistron with a series of deletions (c to h) inserted between the P1 and HC-Pro cistrons. WSMV CP amino acids 101 to 300 were inserted into the TriMV genome (i). (B) Western blot assay of total proteins from wheat infected with TriMV or TriMV with WSMV CP or CP deletions at 14 days postinoculation. Below is an SDS-PAGE gel stained with Coomassie blue showing the amount of protein loaded per well for the Western blot assay. The blot was probed with WSMV polyclonal antibodies. Note that WSMV CP with deletion of aa 101 to 200 or 201 to 300 did not react with WSMV polyclonal antibodies while expression of WSMV CP aa 101 to 300 restored reactivity with WSMV antibodies. Letters a to i are as defined in panel A.
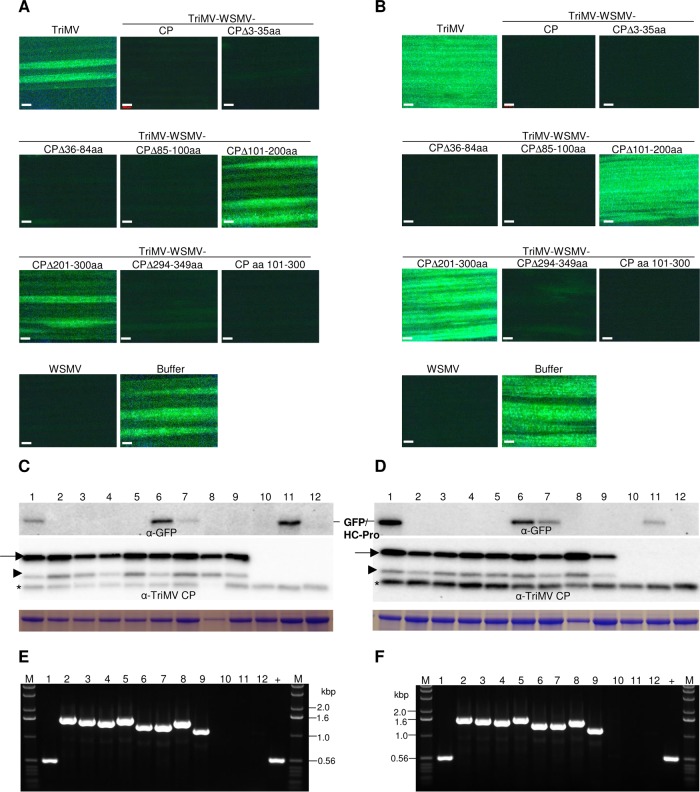
The third leaf of wheat infected with TriMV expressing WSMV CP deletion mutants at 10 dpi was challenge inoculated with WSMV-GFP, followed by observation for the development of local foci at 5 dpci and systemic infection at 9 dpci by the challenge virus. Wheat infected with TriMV expressing WSMV CP with a deletion of aa 3 to 35, 36 to 84, 85 to 100, or 294 to 349 allowed 0.1 to 0.5 (experiment 1) and 1.4 to 5.1 (experiment 2) smaller foci per inoculated leaf by WSMV-GFP (Fig. 8A; Table 3), indicating that WSMV CP aa 3 to 100 and 294 to 349 are not needed to effect SIE activity. In contrast, wheat infected with TriMV expressing WSMV CP with a deletion of aa 101 to 200 or 201 to 300 allowed 12 to 16 (experiment 1) and 35 to 41 (experiment 2) foci per leaf by WSMV-GFP (Fig. 8A; Table 3). Similarly, systemic infection by WSMV-GFP was severely debilitated in wheat preinfected with TriMV with deletion of WSMV CP aa 3 to 35, 36 to 84, 85 to 100, or 294 to 349 with only 11 to 25% (experiment 1) and 6 to 37% (experiment 2) of plants systemically infected with a few foci per leaf (Fig. 8B; Table 3). However, wheat infected with TriMV expressing WSMV CP with a deletion of aa 101 to 200 or 201 to 300 efficiently allowed systemic infection by the challenge virus in 100% of inoculated plants (Fig. 8B; Table 3).
FIG 8.
Wheat streak mosaic virus (WSMV) coat protein (CP) amino acids 101 to 300 induce superinfection exclusion. (A and B) Superinfection of WSMV-GFP on wheat systemically infected with TriMV expressing WSMV CP or CP deletions. TriMV and WSMV, wheat systemically infected with wild-type TriMV and WSMV, respectively. Buffer and buffer-NC, buffer-inoculated wheat subsequently challenge inoculated or not challenge inoculated with WSMV-GFP, respectively. Images shown are local foci at 5 days post-challenge inoculation (dpci) (A) and systemic infection at 9 dpci (B) by WSMV-GFP (challenge virus). Bars, 500 μm. (C and D) Western blot assay of total proteins from the challenge virus-inoculated leaves at 5 dpci (C) and upper noninoculated leaves at 9 dpci (D). Full-length and truncated TriMV CP are indicated with arrows and arrowheads, respectively. Asterisks indicate a wheat protein reacting nonspecifically with TriMV polyclonal antiserum. Presented Coomassie blue-stained SDS-PAGE gels show the large subunit of wheat RubisCO protein for the amount of protein loaded per well in Western blot assays. (E and F) Ethidium bromide-stained agarose gels of RT-PCR products with TriMV-specific primers on either side of insert showing the stability of inserted sequences at 5 dpci of inoculated (E) and 9 dpci of upper noninoculated (F) leaves. (C to F) Lanes 1 to 8, wheat infected with TriMV (lane 1) or TriMV expressing WSMV full-length CP (lane 2) or CP with deletion of amino acids 3 to 35 (lane 3), 36 to 84 (lane 4), 85 to 100 (lane 5), 101 to 200 (lane 6), 201 to 300 (lane 7), and 294 to 349 (lane 8); lane 9, TriMV expressing WSMV CP amino acids 101 to 300; lane 10, wheat infected with WSMV; lane 11, buffer-inoculated wheat subsequently challenge inoculated; lane 12, buffer-inoculated wheat with no challenge inoculation; lane +, pTriMV as a positive control; lanes M, 1.0-kbp DNA ladder.
TriMV CP accumulated efficiently in challenge virus-inoculated and upper noninoculated leaves (Fig. 8C and D, bottom panels), indicating that wheat plants are infected efficiently by primary viruses. Western blot analyses with GFP antibody indicated that GFP accumulated in the challenge virus-inoculated (at 5 dpci) and upper noninoculated (9 dpci) leaves of wheat infected with TriMV expressing WSMV CP with deletion of aa 101 to 200 or 201 to 300 but not with deletions comprising aa 3 to 100 or 294 to 349 (Fig. 8C and D, top panels). RT-PCR analysis of total RNA from wheat leaves examined for local and systemic infection by the challenge virus yielded expected products, indicating that TriMV stably maintained WSMV CP sequences at 5 and 9 dpci (Fig. 8E and F).
The above data revealed that WSMV CP lacking aa 101 to 300 allowed efficient superinfection by WSMV-GFP. We next examined whether wheat expressing WSMV CP aa 101 to 300 is enough to induce SIE activity. Nucleotide sequence encoding WSMV CP aa 101 to 300 was inserted into the TriMV genome between the P1 and HC-Pro cistrons to obtain TriMV-WSMV-CPaa101-300 (Fig. 7A, construct i). Wheat infected with TriMV-WSMV-CPaa101-300 expressed truncated WSMV CP with an ∼26-kDa protein (Fig. 7B, lane i). These data indicated that reactivity of WSMV polyclonal antibodies was lost with the deletion of aa 101 to 300 while antibody reactivity was restored with the expression of aa 101 to 300 (Fig. 7B, lanes f, g, and i). Superinfection of TriMV-WSMV-CPaa101-300-infected wheat by WSMV-GFP was severely hindered with 0.3 to 2.2 smaller foci per inoculated leaf at 5 dpci and 27 to 41% of plants infected systemically with a few foci per leaf at 9 dpci (Fig. 8A and B), indicating that expression of WSMV CP aa 101 to 300 in wheat induced SIE activity. Western blot analysis of total proteins from inoculated and upper noninoculated leaves of challenge virus-inoculated wheat revealed efficient accumulation of TriMV CP but no detectable levels of GFP (Fig. 8C and D, lane 9). RT-PCR analysis of total RNA from wheat leaves observed for local foci and systemic infection by the challenge virus revealed that TriMV stably retained WSMV CP aa 101 to 300 (Fig. 8E and F, lane 9). These data revealed that expression of WSMV CP aa 101 to 300 in wheat is necessary and sufficient for induction of SIE activity.
TriMV CP and NIa-Pro are effectors of SIE.
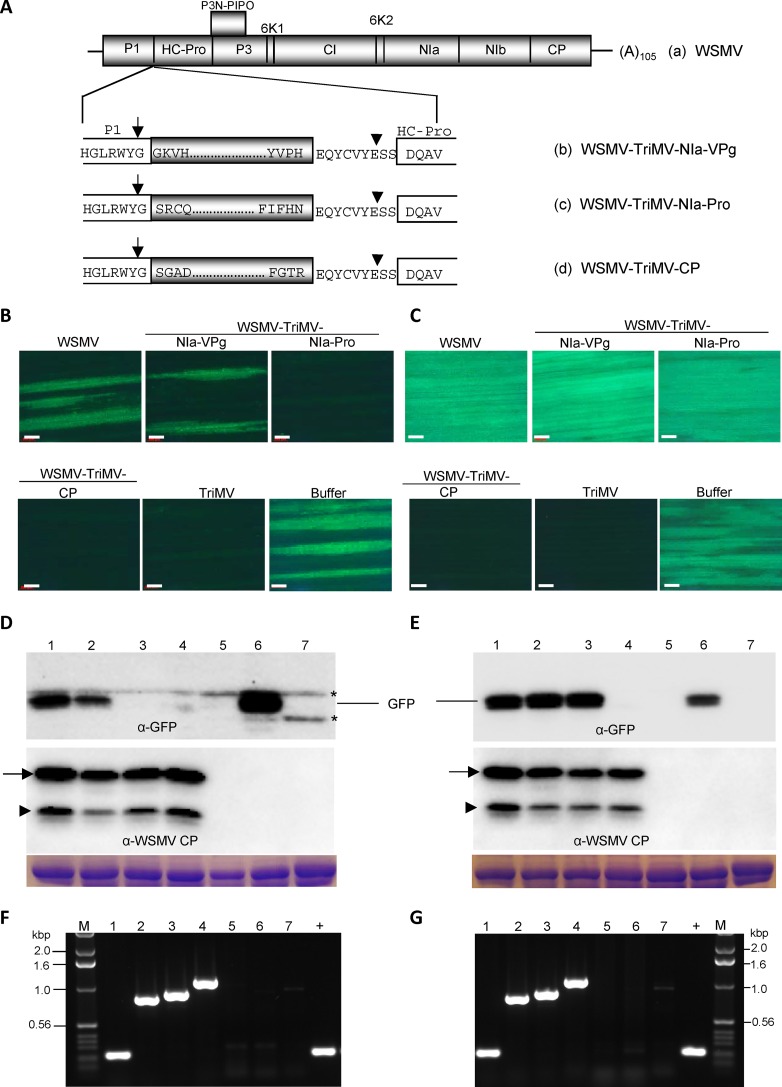
We next examined whether TriMV CP and NIa-Pro are similarly involved in SIE activity by expressing select TriMV cistrons in wheat through WSMV as an expression vector, followed by challenge inoculation with TriMV-GFP. HC-Pro, NIa-VPg, NIa-Pro, or CP cistrons of TriMV were inserted into the WSMV genome between the P1 and HC-Pro cistrons (Fig. 9A). To enable efficient cleavage of TriMV proteins from WSMV polyprotein, a hexapeptide cleavage site with a spacer amino acid on either side was fused to the C terminus of TriMV proteins. TriMV proteins were released by P1 cleavage at the C terminus of WSMV P1 and a trans cleavage by WSMV NIa-Pro at engineered 9-amino-acid cleavage peptides (Fig. 9A). In vitro transcripts of WSMV containing TriMV HC-Pro, NIa-VPg, NIa-Pro, or CP cistrons efficiently infected wheat. In wheat at 14 dpi, WSMV stably maintained TriMV NIa-VPg, NIa-Pro, or CP cistrons but not HC-Pro. RT-PCR product from total RNA isolated from wheat infected with WSMV-TriMV-HC-Pro was smaller than the expected size at 14 dpi (data not shown). SIE studies, hence, were performed only with NIa-VPg, NIa-Pro, and CP cistrons of TriMV.
FIG 9.
Triticum mosaic virus (TriMV) coat protein (CP) and NIa-Pro induced complete and partial superinfection exclusion activity, respectively. (A) Schematic diagram of the genomic organization of Wheat streak mosaic virus (WSMV) (a). Below is an expanded WSMV genomic diagram showing the C terminus of P1, an inserted TriMV cistron with a 9-amino-acid NIb/CP cleavage peptide of WSMV, and the N terminus of HC-Pro. Four amino acids located at the N- and C-terminal regions of TriMV cistrons inserted into the WSMV genome are indicated. Positions of cleavages by P1 and NIa-Pro on either side of inserted TriMV cistrons are indicated with arrows and arrowheads, respectively. (B and C) Superinfection of TriMV-GFP on wheat systemically infected with WSMV or WSMV expressing TriMV NIa-VPg, NIa-Pro, or CP. Local foci at 5 days post-challenge inoculation (dpci) (B) and systemic infection at 12 dpci (C) by TriMV-GFP (challenge virus). Bars, 500 μm. (D and E) Western blot assays of total proteins from superinoculated leaves at 5 dpci (D) and upper noninoculated leaves at 12 dpci (E) of wheat systemically infected with WSMV or WSMV expressing TriMV cistrons. The upper and lower blots were probed with GFP monoclonal antibody and WSMV polyclonal antibodies, respectively. The positions of full-length and truncated CPs are indicated with arrows and arrowheads, respectively. Asterisks indicate wheat proteins reacting nonspecifically with GFP monoclonal antibody. SDS-PAGE gels stained with Coomassie blue show the amount of large subunit of wheat RubisCO protein loaded per well. (F and G) RT-PCR products showing the stability of inserts in wheat systemically infected with WSMV expressing TriMV cistrons at 5 dpci (F) and 12 dpci (G) with primers specific to WSMV. (D to G) Lane 1, wheat infected with WSMV; lanes 2 to 4, wheat infected with WSMV expressing TriMV NIa-VPg (lane 2), NIa-Pro (lane 3), or CP (lane 4); lane 5, wheat infected with TriMV; lane 6, buffer-inoculated wheat subsequently challenge inoculated with TriMV-GFP; lane 7, buffer-inoculated wheat with no challenge inoculation; lane +, pSP6-WSMV as a positive control; lane M, 1.0-kbp DNA ladder.
The third leaf of wheat infected with WSMV-TriMV-NIa-VPg, -NIa-Pro, or -CP at 10 dpi was challenge inoculated with TriMV-GFP, followed by observation for local and systemic infection by TriMV-GFP at 5 and 12 dpci, respectively. Wheat infected with WSMV-TriMV-NIa-VPg efficiently allowed superinfection by TriMV-GFP in inoculated leaves (17 to 19 foci per leaf) (Fig. 9B; Table 4) and upper noninoculated leaves (100% of plants infected systemically) (Fig. 9C; Table 4), indicating that TriMV NIa-VPg is not involved in SIE activity. In contrast, wheat infected with WSMV-TriMV-NIa-Pro reduced formation of foci by TriMV-GFP on inoculated leaves (0.8 focus per leaf) (Fig. 9B; Table 4) but did not reduce systemic infection (Fig. 9C; Table 4), indicating that TriMV NIa-Pro effected SIE only in inoculated leaves. Examination of challenge virus-inoculated leaves preinfected with WSMV-TriMV-NIa-Pro at 8 dpci revealed that there were smaller foci in inoculated leaves, indicating that expression of TriMV NIa-Pro in wheat leaves delayed the onset of local foci by TriMV-GFP. Wheat preinfected with WSMV-TriMV-CP allowed the development of only 0 to 0.1 focus per inoculated leaf and systemic infection in 5% of plants with a few foci per leaf by TriMV-GFP, indicating that expression of TriMV CP in wheat efficiently debilitated superinfection by TriMV-GFP. These data revealed that TriMV CP is a strong effector of SIE (Fig. 9B and C; Table 4).
TABLE 4.
Examining Triticum mosaic virus (TriMV)-encoded NIa-VPg, NIa-Pro, and CP cistrons for superinfection exclusion activity in wheata
| Primary virus | Challenge virus | Expt 1 |
Expt 2 |
||||
|---|---|---|---|---|---|---|---|
| Avg no. of local foci/leaf ± SE | No. of plants systemically infected/no. of plants inoculatedb | % of plants systemically infected with TriMV-GFP | Avg no. of local foci/leaf ± SE | No. of plants systemically infected/no. of plants inoculated | % of plants systemically infected with TriMV-GFP | ||
| WSMV | TriMV-GFP | 18.6 ± 1.8 | 18/18 | 100 | 12.6 ± 0.8 | 17/17 | 100 |
| WSMV-TriMV-NIa-VPg | TriMV-GFP | 16.6 ± 1.3 | 22/22 | 100 | 19.2 ± 2.7 | 20/20 | 100 |
| WSMV-TriMV-NIa-Pro | TriMV-GFP | 0.8 ± 0.2 | 20/20 | 100 | 0.8 ± 0.3 | 19/19 | 100 |
| WSMV-TriMV-CP | TriMV-GFP | 0.1 ± 0.1 | 1/22* | 5 | 0.0 ± 0.0 | 1/19 | 5 |
| TriMV | TriMV-GFP | 0.1 ± 0.1 | 0/18 | 0 | 0.0 ± 0.0 | 0/14 | 0 |
| Buffer | TriMV-GFP | 89.8 ± 7.0 | 16/16 | 100 | 79.3 ± 6.7 | 15/15 | 100 |
Wheat plants systemically infected with Wheat streak mosaic virus (WSMV) harboring TriMV NIa-VPg, NIa-Pro, or CP were challenge inoculated with TriMV-GFP. Formation of local foci and systemic infection by the challenge virus were observed at 5 and 12 days post-challenge inoculation.
The asterisk indicates that 1 to 10 isolated infection foci per leaf were observed in upper noninoculated leaves.
In Western blot assays, WSMV CP accumulated efficiently in all superinoculated and upper noninoculated leaves, indicating that WSMV expression vectors (primary viruses) replicated efficiently in wheat (Fig. 9D and E, bottom panels). However, GFP accumulated in challenge virus-inoculated leaves of wheat infected with WSMV expressing TriMV NIa-VPg but not NIa-Pro or CP (Fig. 9D, top panel). At 12 dpci, GFP accumulated efficiently in upper noninoculated leaves of wheat preinfected with WSMV-TriMV-NIa-VPg or -NIa-Pro but not with WSMV-TriMV-CP (Fig. 9E, top panel). TriMV sequences were stably maintained in WSMV in challenge-inoculated and upper noninoculated leaves since expected RT-PCR products were obtained with no detectable truncated products (Fig. 9F and G). These data revealed that TriMV CP is a strong effector of SIE, while NIa-Pro affected the development of local foci at 5 dpci but did not prevent systemic infection by the challenge virus.
DISCUSSION
In this study, interaction of virus-encoded proteins with cognate viruses was examined by expressing virus-encoded proteins in wheat through TriMV or WSMV as an expression vector, followed by challenge inoculation with corresponding GFP-tagged viruses. These experiments allowed a robust system to identify effectors of SIE encoded by WSMV and TriMV through gain-of-function. Wheat systemically infected with TriMV expressing WSMV NIa-Pro or CP substantially prevented superinfection by WSMV-GFP, and deletion analyses revealed that complete NIa-Pro or CP aa 101 to 300 are sufficient for SIE activity. In reciprocal experiments, wheat infected with WSMV expressing TriMV CP efficiently prevented superinfection by TriMV-GFP. However, expression of TriMV NIa-Pro in wheat, while substantially delaying the onset of local infection, had no significant effect on systemic infection.
Efficient coinfection of wheat by WSMV and TriMV, together with superinfection exclusion between GFP- and RFP-tagged variants of WSMV or TriMV, facilitated the mapping of SIE effectors encoded by WSMV and TriMV. Wheat infected with TriMV expressing WSMV P1, HC-Pro, P3, 6K1, CI, 6K2, NIa-VPg, or NIb allowed efficient superinfection by the challenge virus. In contrast, wheat infected with TriMV expressing WSMV NIa-Pro or CP inhibited the development of local and systemic infection by the challenge virus. These data revealed that WSMV NIa-Pro and CP are effectors of SIE, while other WSMV cistrons do not appear to have a significant role in SIE. Screening select TriMV cistrons for SIE activity by expressing TriMV-encoded proteins through WSMV revealed that TriMV CP is a strong effector of SIE, while NIa-Pro delayed the onset of local foci but not systemic infection. WSMV replicates more efficiently in wheat than TriMV (39, 47), but expression of foreign proteins through WSMV vectors forms mostly aggregate-like structures (39, 53), compared to mostly soluble proteins through TriMV vectors (46). The weak SIE activity found for TriMV NIa-Pro could be due to this protein being expressed as insoluble aggregate-like structures in wheat through the WSMV expression vector. However, similarly expressed TriMV CP elicited strong SIE activity. It is conceivable that CP and NIa-Pro might function in SIE in different conformations: CP as aggregate-like structures and NIa-Pro in free soluble form. This possibility was supported by our recent observations that GFP-NIa-Pro and GFP-CP were expressed using a binary vector in Nicotiana benthamiana as free soluble protein and large aggregate-like structures, respectively (S. Tatineni, unpublished data). Experiments reported here tested the effect of individual proteins encoded by WSMV contributing to SIE. Hence, the possibility of synergistic effects provided by other WSMV- or TriMV-encoded proteins in combination in SIE activity cannot be ruled out.
Taken together, we mapped the effectors of superinfection exclusion encoded by WSMV and TriMV through gain-of-function assays. In contrast, investigations with CTV identified p33 and L1L2 proteinases as determinants of SIE through loss-of-function (49, 54). CTV-encoded p33 was found to be a determinant of SIE since CTV lacking the p33 ORF or with heterologous p33 failed to trigger SIE against identical superinfectors (49). Additionally, CTV L1L2 from the heterologous T68 strain failed to elicit SIE, thus confirming the requirement of L1L2 for CTV SIE (54). Yet, neither of the CTV proteins was able to effect SIE when expressed heterologously. Remarkably, we also found that two WSMV-encoded proteins, NIa-Pro and CP, independently elicit SIE. However, it is not clear why WSMV has two effectors of SIE when either one is sufficient to induce SIE.
Superinfection exclusion has been reported from diverse groups of viruses infecting bacteria, plants, and animals with different possible mechanisms (e.g., references 6, 13, 18, 20, 22, 25, 31, 40, and 58 to 60). Superinfection exclusion in animal viruses has been attributed to inhibition of entry of superinfecting viruses into cells (14, 61) as well as interference with translation or replication of the challenge virus (14, 19, 20, 26, 61–63). In the latter examples, superinfecting viruses were able to enter the primary virus-infected cells and produce some of the virus-encoded proteins but failed to replicate, suggesting that SIE targets the replication step of the superinfecting virus. Additionally, replication-related proteins such as NS4 and 2K of WNV (26) and NS5A of HCV (60) were identified as targets of superinfection exclusion activity. SIE at the level of virus entry into cells may not be conserved between plant and animal viruses, as there is no indication that plant viruses have specific cell surface receptors for virus entry into plant cells. It is possible that SIE targeting viral replication is a conserved mechanism of SIE among plant and animal viruses. The NIa-Pro protease of WSMV could function through a mechanism proposed for SINV and SFV in which viral proteases downregulate or completely block replication of the secondary virus by incorrectly processing its replicase into proteins incapable of minus-strand RNA synthesis (63, 64). Furthermore, WSMV NIa-Pro deletion analyses revealed that the sequence encoding complete NIa-Pro is required for SIE activity, thus suggesting that protease activity is essential to induce SIE. Recently, L1L2 proteases of CTV were also implicated in SIE as the primary virus with a heterologous leader protease sequence facilitating efficient superinfection by the challenge virus (54). However, the exact mechanisms of plant viral proteases in SIE need further research.
Another postulated mechanism of SIE in plant viruses is that the CP of the primary virus prevents uncoating of the superinfecting virus as described for TMV (65, 66). The ability of TMV CP to interact with viral RNA and self-association in a helical fashion are essential for CP activity in SIE (67). The CP of PVA was also reported to be involved in SIE, and it has been suggested that virus infection-induced RNA silencing, not “reencapsidation,” is a possible mechanism of SIE in this virus (22). We also found that wheat plants expressing CP of WSMV or TriMV triggered SIE of the respective cognate viruses. Deletion analyses of WSMV CP suggest that involvement of CP in SIE is likely independent of virion formation, since expression of aa 101 to 300 of the 349-aa sequence is enough to induce SIE. Moreover, expression of CP through a heterologous virus is unlikely to cause virion formation as this virus lacks the origin of assembly of CP to initiate virion assembly. Thus, involvement of virions of the primary virus in negatively affecting the uncoating of the superinfecting virus is an unlikely mechanism with WSMV CP and possibly with TriMV CP as well.
RNA silencing has been proposed as a possible mechanism of SIE in plant viruses (22, 68, 69). RNA silencing is a systemically induced host defense mechanism against viruses infecting plants and invertebrate animals (70–73) by targeting nearly identical RNA sequences. Introduction of viral sequences into heterologous viruses or development of transgenic plants with viral RNA sequences would result in generation of small interfering RNAs to guide a nucleotide sequence-specific process that induces mRNA degradation or translation inhibition (74–77). It is a logical hypothesis that RNA silencing is a possible mechanism of SIE, as both of these phenomena are homology dependent. However, WSMV NIa-Pro and CP cistrons containing frameshift and nucleotide substitution mutations for a continuous translation of different proteins but with 98% sequence homology at the RNA level failed to trigger SIE. These data revealed that CP and NIa-Pro protein sequences but not RNA sequences are involved in SIE activity, suggesting that SIE is mechanistically distinct from RNA silencing.
Previously, HC-Pro and CP of PVA were identified as determinants of SIE, and RNA silencing was proposed as a possible mechanism of SIE (22). We also found in several experiments that wheat plants expressing WSMV HC-Pro allowed slightly smaller foci by the challenge virus but had no significant effect on systemic infection of wheat. Nevertheless, HC-Pro is not a primary determinant of WSMV SIE. For PVA, the role of NIa-Pro in SIE was not examined (22); hence, it is unknown whether PVA NIa-Pro is a determinant of SIE. WSMV-GFP elicited a substantially reduced number of local foci on wheat leaves expressing WSMV NIa-Pro or CP, suggesting that these proteins might have inhibited replication of the challenge virus. However, further studies are required to discern the mechanistic roles of NIa-Pro and CP in SIE. Also, a more detailed study of the involvement of the other TriMV cistrons in SIE is warranted.
Although SIE has an important role in the management of plant and animal viral diseases, so far only a few viral determinants involved in SIE have been identified (e.g., references 22, 49, and 78). Identifying viral determinants with a role in SIE would facilitate the development of virus-resistant transgenic plants expressing effectors of SIE as an alternative to traditional cross-protection. This method would eliminate potential spillover of protecting viruses to other susceptible hosts and/or unintended synergistic interactions with unrelated viruses (4). Resistance observed in transgenic tobacco plants with NIa or NIa-NIb-CP sequences of Potato virus Y (79) could be due to the involvement of NIa-Pro and CP as effectors of SIE. Similarly, resistance of CP-based transgenic plants against Papaya ring spot virus, Zucchini yellow mosaic virus, and Watermelon mosaic virus (80, 81) could at least partially be due to CP as an effector of SIE. However, the RNA transcripts of these transgenes themselves also may have provided some degree of resistance.
ACKNOWLEDGMENTS
We thank Feng Qu for sharing his data prior to publication and for critical reading of the manuscript, Christian Elowsky for his help with confocal microscopy, and Stephen N Wegulo for critical reading and editorial comments. We thank Jonathan Horrell and Jeffrey Alexander for technical assistance.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Funding Statement
This work was funded by USDA ARS CRIS (5440-21000-031-00D). This work was also funded, in part, by USDA | National Institute of Food and Agriculture (NIFA) (2013-68004-20358).
REFERENCES
- 1.Shulla A, Randall G. 2016. (+) RNA virus replication compartments: a safe home for (most) viral replication. Curr Opin Microbiol 32:82–88. doi: 10.1016/j.mib.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang AM. 2015. Dissecting the molecular network of virus-plant interactions: the complex roles of host factors. Annu Rev Phytopathol 53:45–66. doi: 10.1146/annurev-phyto-080614-120001. [DOI] [PubMed] [Google Scholar]
- 3.Xu K, Nagy PD. 2014. Expanding use of multi-origin subcellular membranes by positive-strand RNA viruses during replication. Curr Opin Virol 9:119–126. doi: 10.1016/j.coviro.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Syller J. 2012. Facilitative and antagonistic interactions between plant viruses in mixed infections. Mol Plant Pathol 13:204–216. doi: 10.1111/j.1364-3703.2011.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syller J, Grupa A. 2016. Antagonistic within-host interactions between plant viruses: molecular basis and impact on viral and host fitness. Mol Plant Pathol 17:769–782. doi: 10.1111/mpp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKinney HH. 1926. Virus mixtures that may not be detected in young tobacco plants. Phytopathology 16:893. [Google Scholar]
- 7.McKinney HH. 1929. Mosaic diseases in the Canary Islands, West Africa and Gibraltar. J Agric Res 39:557–578. [Google Scholar]
- 8.Dulbecco R. 1952. Mutual exclusion between related phages. J Bacteriol 63:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visconti N. 1953. Resistance to lysis from without in bacteria infected with T2 bacteriophage. J Bacteriol 66:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salaman RN. 1933. Protective inoculation against a plant virus. Nature 131:468. doi: 10.1038/131468a0. [DOI] [Google Scholar]
- 11.Fulton RW. 1986. Practices and precautions in the use of cross protection for plant virus disease control. Annu Rev Phytopathol 24:67–81. doi: 10.1146/annurev.py.24.090186.000435. [DOI] [Google Scholar]
- 12.Gal-On A, Shiboleth YM. 2005. Cross protection, p 261–288. In Loebenstein G, Carr JP (ed), Natural resistance mechanisms of plants to viruses. Springer, Dordrecht, The Netherlands. [Google Scholar]
- 13.Nethe M, Berkhout B, van der Kuyl AC. 2005. Retroviral superinfection resistance. Retrovirology 2:52. doi: 10.1186/1742-4690-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steck FT, Rubin H. 1966. The mechanism of interference between an avian leukosis virus and Rous sarcomavirus. II. Early steps of infection by RSV of cells under conditions of interference. Virology 29:642–653. [DOI] [PubMed] [Google Scholar]
- 15.Hull R, Plaskitt A. 1970. Electron microscopy on the behavior of two strains of Alfalfa mosaic virus in mixed infections. Virology 42:773–776. doi: 10.1016/0042-6822(70)90322-3. [DOI] [PubMed] [Google Scholar]
- 16.Wen F, Lister M, Fattouh FA. 1991. Cross protection among strains of barley yellow dwarf virus. J Gen Virol 72:791–799. doi: 10.1099/0022-1317-72-4-791. [DOI] [PubMed] [Google Scholar]
- 17.Costa AS, Müller GW. 1980. Tristeza control by cross protection. Plant Dis 6:538–541. [Google Scholar]
- 18.Folimonova SY, Robertson CJ, Shilts T, Folimonov AS, Hilf ME, Garnsey SM, Dawson WO. 2010. Infection with strains of Citrus tristeza virus does not exclude superinfection by other strains of the virus. J Virol 84:1314–1325. doi: 10.1128/JVI.02075-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaller T, Appel N, Koutsoudakis G, Kallis S, Lohmann V, Pietschmann T, Bartenschlager R. 2007. Analysis of hepatitis C virus superinfection exclusion by using novel fluorochrome gene tagged viral genomes. J Virol 81:4591–4603. doi: 10.1128/JVI.02144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tscherne DM, Evans MJ, von Hahn T, Jones CT, Stamataki Z, McKeating JA, Lindenbach BD, Rice CM. 2007. Superinfection exclusion in cells infected with hepatitis C virus. J Virol 81:3693–3703. doi: 10.1128/JVI.01748-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capote N, Gorris MT, Martínez MC, Asensio M, Olmos A, Cambra M. 2006. Interference between D and M types of Plum pox virus in Japanese plum assessed by specific monoclonal antibodies and quantitative real-time reverse transcription-polymerase chain reaction. Phytopathology 96:320–325. doi: 10.1094/PHYTO-96-0320. [DOI] [PubMed] [Google Scholar]
- 22.Valkonen JP, Rajamäki ML, Kekarainen T. 2002. Mapping of viral genomic regions important in cross-protection between strains of a potyvirus. Mol Plant Microbe Interact 15:683–692. doi: 10.1094/MPMI.2002.15.7.683. [DOI] [PubMed] [Google Scholar]
- 23.Singh IR, Suomalainen M, Varadarajan S, Garoff H, Helenius A. 1997. Mechanisms for the inhibition of entry and uncoating of superinfecting Semliki Forest virus. Virology 231:59–71. doi: 10.1006/viro.1997.8492. [DOI] [PubMed] [Google Scholar]
- 24.Johnston RE, Wan K, Bose HR. 1974. Homologous interference induced by Sindbis virus. J Virol 14:1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fulton RW. 1978. Superinfection by strains of tobacco streak virus. Virology 85:1–8. doi: 10.1016/0042-6822(78)90406-3. [DOI] [PubMed] [Google Scholar]
- 26.Zou G, Zhang B, Lim P-Y, Yuan Z, Bernard KA, Shi PY. 2009. Exclusion of West Nile virus superinfection through RNA replication. J Virol 83:11765–11776. doi: 10.1128/JVI.01205-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lecoq H, Lemaire JM, Wipf-Scheibel C. 1991. Control of Zucchini yellow mosaic virus in squash by cross protection. Plant Dis 75:208–211. doi: 10.1094/PD-75-0208. [DOI] [Google Scholar]
- 28.Bratt MA, Rubin H. 1968. Specific interference among strains of Newcastle disease virus. 3. Mechanism of interference. Virology 35:395–407. [DOI] [PubMed] [Google Scholar]
- 29.Whitaker-Dowling PA, Youngner JS, Widnell CC, Wilcox DK. 1983. Superinfection exclusion by vesicular stomatitis virus I. Virology 131:137–143. doi: 10.1016/0042-6822(83)90540-8. [DOI] [PubMed] [Google Scholar]
- 30.Kobiler O, Lipman Y, Therkelsen K, Daubechies I, Enquist LW. 2010. Herpesviruses carrying a Brainbow cassette reveal replication and expression of limited numbers of incoming genomes. Nat Commun 1:146. doi: 10.1038/ncomms1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laliberte JP, Moss B. 2014. A novel mode of poxvirus superinfection exclusion that prevents fusion of the lipid bilayers of viral and cellular membranes. J Virol 88:9751–9768. doi: 10.1128/JVI.00816-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stenger DC, Hall JS, Choi IR, French R. 1998. Phylogenetic relationships within the family Potyviridae: Wheat streak mosaic virus and Brome streak mosaic virus are not members of the genus Rymovirus. Phytopathology 88:782–787. doi: 10.1094/PHYTO.1998.88.8.782. [DOI] [PubMed] [Google Scholar]
- 33.Stenger DC, French R, Gildow FE. 2005. Complete deletion of Wheat streak mosaic virus HC-Pro: a null mutant is viable for systemic infection. J Virol 79:12077–12080. doi: 10.1128/JVI.79.18.12077-12080.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stenger DC, Hein GL, Gildow FE, Horken KM, French R. 2005. Plant virus HC-Pro is a determinant of eriophyid mite transmission. J Virol 79:9054–9061. doi: 10.1128/JVI.79.14.9054-9061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenger DC, Hein GL, French R. 2006. Nested deletion analysis of Wheat streak mosaic virus HC-Pro: mapping of domains affecting polyprotein processing and eriophyid mite transmission. Virology 350:465–474. doi: 10.1016/j.virol.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Tatineni S, Van Winkle DH, French R. 2011. The N-terminal region of Wheat streak mosaic virus coat protein is a host- and strain-specific long-distance transport factor. J Virol 85:1718–1731. doi: 10.1128/JVI.02044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatineni S, Kovacs F, French R. 2014. Wheat streak mosaic virus infects systemically despite extensive coat protein deletions: identification of virion assembly and cell-to-cell movement determinants. J Virol 88:1366–1380. doi: 10.1128/JVI.02737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tatineni S, French R. 2014. The C-terminus of Wheat streak mosaic virus coat protein is involved in differential infection of wheat and maize through host-specific long-distance transport. Mol Plant Microbe Interact 27:150–162. doi: 10.1094/MPMI-09-13-0272-R. [DOI] [PubMed] [Google Scholar]
- 39.Tatineni S, Wosula SN, Bartels M, Hein GL, Graybosch RA. 2016. Temperature-dependent Wsm1 and Wsm2 gene-specific blockage of viral long-distance transport provides resistance to Wheat streak mosaic virus and Triticum mosaic virus in wheat. Mol Plant Microbe Interact 29:724–738. doi: 10.1094/MPMI-06-16-0110-R. [DOI] [PubMed] [Google Scholar]
- 40.McKinney HH. 1956. Interference and synergy, their possible use in identifying certain mosaic viruses of cereals and indicating degrees of relationship. Plant Dis Rep 40:898–903. [Google Scholar]
- 41.Hall JS, French R, Hein GL, Morris TJ, Stenger DC. 2001. Three distinct mechanisms facilitates genetic isolation of sympatric Wheat streak mosaic virus lineages. Virology 282:230–236. doi: 10.1006/viro.2001.0841. [DOI] [PubMed] [Google Scholar]
- 42.McMechan AJ, Tatineni S, French R, Hein GL. 2014. Differential transmission of Triticum mosaic virus by wheat curl mite populations collected in the Great Plains. Plant Dis 98:806–810. doi: 10.1094/PDIS-06-13-0582-RE. [DOI] [PubMed] [Google Scholar]
- 43.Seifers DL, Martin TJ, Harvey TL, Fellers JP, Michaud JP. 2009. Identification of the wheat curl mite as the vector of Triticum mosaic virus. Plant Dis 93:25–29. doi: 10.1094/PDIS-93-1-0025. [DOI] [PubMed] [Google Scholar]
- 44.Fellers JP, Seifers D, Ryba-White M, Martin TJ. 2009. The complete genome sequence of Triticum mosaic virus, a new wheat-infecting virus of the High Plains. Arch Virol 154:1511–1515. doi: 10.1007/s00705-009-0462-1. [DOI] [PubMed] [Google Scholar]
- 45.Tatineni S, Ziems AD, Wegulo SN, French R. 2009. Triticum mosaic virus: a distinct member of the family Potyviridae with an unusually long leader sequence. Phytopathology 99:943–950. doi: 10.1094/PHYTO-99-8-0943. [DOI] [PubMed] [Google Scholar]
- 46.Tatineni S, McMechan AJ, Bartels M, Hein GL, Graybosch RA. 2015. In vitro transcripts of wild-type and fluorescent protein-tagged Triticum mosaic virus (family Potyviridae) are biologically active in wheat. Phytopathology 105:1496–1505. doi: 10.1094/PHYTO-06-15-0138-R. [DOI] [PubMed] [Google Scholar]
- 47.Tatineni S, Graybosch RA, Hein GL, Wegulo SN, French R. 2010. Wheat cultivar-specific disease synergism and alteration of virus accumulation during co-infection with Wheat streak mosaic virus and Triticum mosaic virus. Phytopathology 100:230–238. doi: 10.1094/PHYTO-100-3-0230. [DOI] [PubMed] [Google Scholar]
- 48.Tatineni S, Robertson CJ, Garnsey SM, Bar-Joseph M, Gowda S, Dawson WO. 2008. Three genes of Citrus tristeza virus are dispensable for infection and movement throughout some varieties of citrus trees. Virology 376:297–307. doi: 10.1016/j.virol.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 49.Folimonova SY. 2012. Superinfection exclusion is an active virus controlled function that requires a specific protein. J Virol 86:5554–5561. doi: 10.1128/JVI.00310-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Revers F, Garcia JA. 2015. Molecular biology of potyviruses. Adv Virus Res 92:101–199. doi: 10.1016/bs.aivir.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Choi IR, French R, Hein GL, Stenger DC. 1999. Fully biologically active in vitro transcripts of the eriophyid mite-transmitted Wheat streak mosaic tritimovirus. Phytopathology 89:1182–1185. doi: 10.1094/PHYTO.1999.89.12.1182. [DOI] [PubMed] [Google Scholar]
- 52.Choi IR, Stenger DC, Morris TJ, French R. 2000. A plant virus vector for systemic expression of foreign genes in cereals. Plant J 23: 547–555. doi: 10.1046/j.1365-313x.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- 53.Tatineni S, McMechan JA, Hein GL, French R. 2011. Efficient and stable expression of GFP through Wheat streak mosaic virus-based vectors in cereal hosts using a range of cleavage sites: formation of dense fluorescent aggregates for sensitive virus tracking. Virology 410:268–281. doi: 10.1016/j.virol.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 54.Atallah OO, Kang SH, El-Mohtar CA, Shilts T, Bergua M, Folimonova SY. 2016. A 5′-proximal region of the Citrus tristeza virus genome encoding two leader proteases is involved in virus superinfection exclusion. Virology 489:108–115. doi: 10.1016/j.virol.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Green MR, Sambrook J. 2012. Molecular cloning: a laboratory manual, 4th ed, p 20–28 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 56.Tatineni S, Sarath G, Seifers D, French R. 2013. Immunodetection of Triticum mosaic virus by DAS- and DAC-ELISA using antibodies produced against coat protein expressed in Escherichia coli: potential for high-throughput diagnostic methods. J Virol Methods 189:196–203. doi: 10.1016/j.jviromet.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 57.McNeil JE, French R, Hein GL, Baenziger PS, Eskridge KM. 1996. Characterization of genetic variability among natural populations of Wheat streak mosaic virus. Phytopathology 86:1222–1227. doi: 10.1094/Phyto-86-1222. [DOI] [Google Scholar]
- 58.Huang CY, Lu TY, Bair CH, Chang YS, Jwo JK, Chang W. 2008. A novel cellular protein, VPEF, facilitates vaccinia virus penetration in to HeLa cells through fluid phase endocytosis. J Virol 82:7988–7999. doi: 10.1128/JVI.00894-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simon KO, Cardamone JJ Jr, Whitaker-Dowling A, Youngner JS, Widnell CC. 1990. Cellular mechanisms in the superinfection exclusion of vesicular stomatitis virus. Virology 177:375–379. doi: 10.1016/0042-6822(90)90494-C. [DOI] [PubMed] [Google Scholar]
- 60.Webster B, Ott M, Greene WC. 2013. Evasion of superinfection exclusion and elimination of primary viral RNA by an adapted strain of hepatitis C virus. J Virol 87:13354–13369. doi: 10.1128/JVI.02465-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee YM, Tscherne DM, Yun SI, Frolov I, Rice CM. 2005. Dual mechanisms of pestiviral superinfection exclusion at entry and RNA replication. J Virol 79:3231–3242. doi: 10.1128/JVI.79.6.3231-3242.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adams RH, Brown DT. 1985. BHK cells expressing Sindbis virus-induced homologous interference allow the translation of nonstructural genes of superinfecting virus. J Virol 54:351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karpf AR, Lenches E, Strauss EG, Strauss JH, Brown DT. 1997. Superinfection exclusion of alphaviruses in three mosquito cell lines persistently infected with Sindbis virus. J Virol 71:7119–7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ehrengruber MU, Goldin AL. 2007. Semliki Forest virus vectors with mutations in the nonstructural protein 2 gene permit extended superinfection of neuronal and non-neuronal cells. J Neurovirol 13:353–363. doi: 10.1080/13550280701393204. [DOI] [PubMed] [Google Scholar]
- 65.Beachy RN. 1999. Coat-protein-mediated resistance to tobacco mosaic virus: discovery mechanisms and exploitation. Philos Trans R Soc Lond 354:659–664. doi: 10.1098/rstb.1999.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherwood JL, Fulton RW. 1982. The specific involvement of coat protein in tobacco mosaic virus cross-protection. Virology 119:150–158. doi: 10.1016/0042-6822(82)90072-1. [DOI] [PubMed] [Google Scholar]
- 67.Lu B, Stubbs G, Culver JN. 1998. Coat protein interactions involved in tobacco mosaic tobamovirus cross protection. Virology 248:188–198. doi: 10.1006/viro.1998.9280. [DOI] [PubMed] [Google Scholar]
- 68.Ratcliff F, Harrison BD, Baulcombe DC. 1997. A similarity between viral defense and gene silencing in plants. Science 276:1558–1560. doi: 10.1126/science.276.5318.1558. [DOI] [PubMed] [Google Scholar]
- 69.Ratcliff F, MacFarlane S, Baulcombe DC. 1999. Gene silencing without DNA. RNA-mediated cross-protection between viruses. Plant Cell 11:1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mlotshwa S, Voinnet O, Mette MF, Matzke M, Vaucheret H, Ding SW, Pruss G, Vance VB. 2002. RNA silencing and the mobile silencing signal. Plant Cell 14:S289–S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palauqui JC, Elmayan T, Pollien JM, Vaucheret H. 1997. Systemic acquired silencing: transgene-specific posttranscriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J 16:4738–4745. doi: 10.1093/emboj/16.15.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vance V, Vaucheret H. 2001. RNA silencing in plants: defense and counterdefense. Science 292:2277–2280. doi: 10.1126/science.1061334. [DOI] [PubMed] [Google Scholar]
- 73.Voinnet O, Lederer C, Baulcombe DC. 2000. A viral movement protein prevents spread of the gene silencing signal in Nicotiana benthamiana. Cell 103:157–167. doi: 10.1016/S0092-8674(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 74.Brodersen P, Voinnet O. 2006. The diversity of RNA silencing pathways in plants. Trends Genet 22:268–280. doi: 10.1016/j.tig.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Castel SE, Martienssen RA. 2013. RNA interference in the nucleus: roles for small RNAs in transcription, epigenetics and beyond. Nat Rev Genet 14:100–112. doi: 10.1038/nrg3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vaucheret H. 2006. Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes Dev 20:759–771. doi: 10.1101/gad.1410506. [DOI] [PubMed] [Google Scholar]
- 77.Voinnet O. 2001. RNA silencing as a plant immune system against viruses. Trends Genet 17:449–459. doi: 10.1016/S0168-9525(01)02367-8. [DOI] [PubMed] [Google Scholar]
- 78.Cumby N, Edwards AM, Davidson AR, Maxwell KL. 2012. The bacteriophage HK97 gp15 moron element encodes a novel superinfection exclusion protein. J Bacteriol 194:5012–5019. doi: 10.1128/JB.00843-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vardi E, Sela I, Edelbaum O, Livneh O, Kuznetsova L, Stram Y. 1993. Plants transformed with a cistron of a Potato virus Y protease (NIa) are resistant to virus infection. Proc Natl Acad Sci U S A 90:7513–7517. doi: 10.1073/pnas.90.16.7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klas FE, Fuchs M, Gonsalves D. 2006. Comparative spatial spread overtime of Zucchini yellow mosaic virus (ZYMV) and Watermelon mosaic virus (WMV) in fields of transgenic squash expressing the coat protein genes of ZYMV and WMV, and in fields of nontransgenic squash. Transgenic Res 15:527–541. doi: 10.1007/s11248-006-9001-y. [DOI] [PubMed] [Google Scholar]
- 81.Fuchs M, Gonsalves D. 1995. Resistance of transgenic hybrid squash ZW-20 expressing the coat protein genes of zucchini yellow mosaic virus and watermelon mosaic virus 2 to mixed infections of both potyviruses. Nat Biotechnol 13:1466–1473. doi: 10.1038/nbt1295-1466. [DOI] [Google Scholar]