ABSTRACT
Bank vole is a rodent species that shows differential susceptibility to the experimental transmission of different prion strains. In this work, the transmission features of a panel of diverse prions with distinct origins were assayed both in bank vole expressing methionine at codon 109 (Bv109M) and in transgenic mice expressing physiological levels of bank vole PrPC (the BvPrP-Tg407 mouse line). This work is the first systematic comparison of the transmission features of a collection of prion isolates, representing a panel of diverse prion strains, in a transgenic-mouse model and in its natural counterpart. The results showed very similar transmission properties in both the natural species and the transgenic-mouse model, demonstrating the key role of the PrP amino acid sequence in prion transmission susceptibility. However, differences in the PrPSc types propagated by Bv109M and BvPrP-Tg407 suggest that host factors other than PrPC modulate prion strain features.
IMPORTANCE The differential susceptibility of bank voles to prion strains can be modeled in transgenic mice, suggesting that this selective susceptibility is controlled by the vole PrP sequence alone rather than by other species-specific factors. Differences in the phenotypes observed after prion transmissions in bank voles and in the transgenic mice suggest that host factors other than the PrPC sequence may affect the selection of the substrain replicating in the animal model.
INTRODUCTION
Transmissible spongiform encephalopathies (TSEs), or prion diseases, are a group of fatal neurodegenerative diseases that include Creutzfeldt-Jakob disease (CJD) in humans, bovine spongiform encephalopathy (BSE) in cattle, and scrapie in small ruminants. The prion agent consists mainly, if not entirely, of PrPSc, which is an abnormal isoform of the host-encoded prion cellular protein, PrPC (1–4). Prion propagation is considered to occur in a two-step process in which aggregates of PrPSc bind new PrPC monomers and then induce their conformational change into PrPSc. The conversion into PrPSc results in changes in physical properties, such as decreased solubility in nondenaturing detergents, an increase in β-pleated-sheet content, and increased resistance to proteolytic degradation.
The occurrence of different TSE strains has been described in several species. Upon experimental transmission, TSE strains show distinctive incubation periods and neuropathological features that are maintained after subpassage in the same species. These strains correspond to different PrPSc conformations, according to the prion hypothesis (5). Prion strain transmission among different species seems to be driven by the differences in the PrP amino acid sequences and by the prion strain being transmitted (6). Transgenic-mouse models null for the murine Prnp gene and expressing PrPC from another species (such as bovine, ovine, porcine, or human), have been used in the prion research field as useful tools to characterize prion strains and to learn about the susceptibility of the species to different prion strains.
Bank vole (Myodes glareoulus) is a rodent species used for prion transmission studies due to its remarkable susceptibility to prion infection by a wide range of prion strains isolated from both animals and humans (7–12). The bank vole PrP gene is polymorphic at codon 109, coding for methionine or isoleucine (13). It has been shown that homozygous 109Met/Met bank voles (here referred to as Bv109M) show a variable transmission barrier when infected with different prion strains from the same donor species (8), suggesting specific strain preferences for the animal model, which are at least partially independent of the amino acid sequence of the incoming PrPSc. Interestingly, strain preferences were found to be different in Bv109M and wild type (wt) mice, where Bv109M voles were susceptible to some scrapie strains and sporadic CJD (sCJD) but less to BSE while wt mice showed the opposite features (8, 9, 14). By in vitro studies (14) and by comparing the susceptibilities of different rodent species to scrapie and BSE (9), it was possible to identify PrP amino acid variations potentially responsible for the observed differential susceptibility. However, these studies did not allow us to exclude the possibility that factors other than PrP could contribute to the differential susceptibility observed, for instance, binding of PrPC (15) or PrPSc (16, 17) to other proteins or cellular factors. In this work, we investigate whether the transmission barrier encountered in Bv109M depends on the PrPC sequence or on other host factors. The influence of Bv109M PrPC protein on the differential susceptibility to TSE prion strains was analyzed using a transgenic-mouse line expressing Bv109M-PrPC at levels similar to those in Bv109M. In this way, a collection of TSE isolates from several species, representing a panel of diverse prion strains, was compared in a transgenic-mouse model expressing physiological levels of vole PrPC and in its natural counterpart.
MATERIALS AND METHODS
Generation of transgenic mice expressing Bv109M PrPC.
Complete sequencing of the Bv109M PRNP open reading frame (ORF) was achieved using 3′ rapid amplification of cDNA ends. Bv109M mRNA was purified from brain using TRI reagent RNA isolation reagent (Sigma-Aldrich). mRNA was the template in cDNA synthesis using Superscript from Invitrogen and oligo(dT). 3′ cDNA was PCR amplified using a PRNP-specific primer (AACCAGAACAACTTCGTGCACG) and oligo(dA). The PCR product was purified and sequenced. The Bv109M PRNP ORF was isolated by PCR amplification from genomic DNA extracted from Bv109M brain tissue. The primers used created an XhoI restriction enzyme site (italics) adjacent to the translation start and stop sites of the Bv109M PRNP ORF (5′-CTCGAGACTATGGCGAACCTCAGCTACTG-3′ and 5′-CTCGAGTCATCCCACGATCAGGAAGATGAGGAAGGAGATG-3′, respectively).
The PCR fragments obtained were subcloned using the pGEM-T Easy vector system from Promega following the manufacturer's instructions, and the inserts were sequenced, confirming no differences in the inferred amino acid sequences with respect to the Bv109M PrP gene encoding methionine at codon 109 (GenBank accession no. AF367624.1 and EF455012.1). The vole PRNP ORF was excised from the cloning vector using the XhoI restriction enzyme and inserted into the XhoI site from the expression vector MoPrP.Xho (18). The vole transgene was excised from the pMo-bvPrP.XhoI plasmid after NotI digestion, purified, and used for microinjection into pronuclear-stage ova collected from superovulated B6CBAfl females mated with PrP knockout 129/Ola (mu−/−) (19) males. The vole transgene was detected by PCR amplification using specific primers for the vole ORF and mouse PrP exon 2: 5′-GAAGTTCCCGCGCCGTGC-3′ and 5′-GCGAAGAACAAGCAGGAAGG-3′. The murine PrP ORF was identified by PCR amplification using the primers 5′-TAGATGTCAAGGACCTTCAGCC-3′ and 5′-GTTCCACTGATTATGGGTACC-3′. Six different lines (founders) of vole PrPC (bvPrP) and murine PrPC (muPrP) heterozygous transgenic mice were obtained (PrP mu+/− and bv+/−). After crossing with mu−/− mice, hemizygous bvPrP mice were selected (PrP bv+/− and mu−/−), and interbreeding within the same transgenic line was conducted to obtain PrP bv+/+ and mu−/− animals.
Analysis of PrPC expression levels.
Whole brains from animals were homogenized in extraction buffer (0.5% NP-40, 1% sodium deoxycholate, 10 mM EDTA in phosphate-buffered saline [PBS], pH 7.4, and the Complete cocktail of protease inhibitors from Roche). Samples were precleared by centrifugation at 2,000 × g for 5 min, after which an equal volume of 2× SDS reducing sample loading buffer was added to all the samples, and each one was boiled for 5 min before loading on an SDS-12% polyacrylamide gel. The expression levels of vole PrPC in brains from the mouse lines were analyzed by Western blotting (WB) and compared with the PrPC content in Bv109M brain (data not shown) using monoclonal antibody (MAb) 2A11 (which recognizes the 160QVYYRPVDQ168 epitope, numbered according to the vole PrP sequence) as previously described (20). The BvPrP-Tg407 mouse line expressing Bv109M-PrPC (referred to here as BvPrP-Tg407) was selected for further experiments on the basis of PrPC expression.
Transmission studies.
BvPrP-Tg407 mice were inoculated with a panel of experimental or “natural” isolates with distinct origins representative of different TSE agents (Table 1 has descriptions of the isolates used). For that, individually identified 6- to 9-week-old mice (BvPrP-Tg407) were inoculated by the intracerebral route, as previously described (21). The inocula were prepared from brain tissues as 10% (wt/vol) homogenates in 5% glucose. In some cases, brain homogenates from protease-resistant PrP (PrPres)-positive animals were used for further passage. When progression of the disease was evident or at the end of the life span, animals were euthanized for ethical reasons. Once the animals were euthanized, necropsy was performed and brains were frozen for the determination of PrPres by Western blotting, as previously described (22). Survival times were calculated as means ± standard deviations (SD) of the number of days postinoculation (dpi) of all the mice scored positive for PrPres. Survival curves of the Bv109M groups inoculated with BSE-derived prions were generated by the Kaplan-Meier method and verified by the log-rank (Mantel-Cox) test.
TABLE 1.
Isolates used in this study
| Isolate | Description | Suppliera | Reference |
|---|---|---|---|
| Ca-BSE-1 | Naturally BSE-infected cow; Italy (1994) | ISS | 9 |
| Ca-BSE-2 | Naturally BSE-infected cow; UK (PG1199/00) | VLA | 41 |
| Ca-BSE-H-1 | BSE (H type); natural case from Poland | NVRI | |
| Ca-BSE-H-2 | BSE (H type); natural case from France | ANSES | |
| Ca-BSE-L-1 | BSE (L type); natural case from Poland | NVRI | |
| Ca-BSE-L-2 | BSE (L type); natural case from Italy | IZSPLV | |
| Sh-BSE-3 | ARQ/ARQ sheep inoculated with classical BSE | ISS | 42 |
| Sh-BSE-4 | ARQ/ARQ sheep inoculated with classical BSE | ISS | 42 |
| Sh-BSE-5 | ARQ/AHQ sheep inoculated with classical BSE | ISS | |
| Sh-BSE-6 | ARQ/ARH sheep inoculated with classical BSE | ISS | |
| vCJD-Sp | vCJD-infected case Met129Met; Spain | BHUFA | |
| vCJD-UK | vCJD-infected case Met129Met; UK (RU/98/148) | CJDRC | |
| vCJD-Sp/TgHu-340 | Pool of terminally ill human TgHu-340 transgenic mice inoculated with vCJD-Sp | CISA | 21 |
| sCJD MM1 | Type 1 sCJD Met129Met-infected case | ISS | 8 |
| sCJD MM1/TgHu-340 | Pool of terminally ill human TgHu-340 transgenic mice inoculated with sCJD MM1 (after 2 passages in TgHu-340) | CISA | |
| SS-N799-97 | Naturally scrapie-infected sheep, ARQ/ARQ; Spain (799–97) | NRL | |
| SS-10 | Naturally scrapie-infected sheep, ARQ/ARQ; Italy (SS-10) | ISS | |
| 139A/vole (4th passage) | Terminally ill vole inoculated with 139A (3rd passage) | ISS | 9 |
| SS-3/vole (4th passage) | Terminally ill vole inoculated with SS3 ARQ/ARQ from Italian scrapie case (3rd passage) | ISS | 14 |
| SS-7/vole (3rd passage) | Terminally ill vole inoculated with SS-7 (2nd passage) | ISS | 10 |
| Ca-BSE-1/vole (2nd passage) | Terminally ill vole inoculated with Ca-BSE-1 (2nd passage) | ISS | |
| Ca-BSE-1/vole (4th passage) | Terminally ill vole inoculated with Ca-BSE-1 (4th passage) | ISS |
ISS, Istituto Superiore di Sanità, Rome, Italy; VLA, Veterinary Laboratory Agency, New Haw, Addlestone, Surrey, UK; NVRI, National Veterinary Research Institute, Pulawy, Poland; ANSES, Agence Française de Sécurité Sanitaire de l'Alimentation, de l'Environnement et du Travail, Lyon, France; IZSPLV, Istituto Zooprofilattico Sperimentale del Piemonte, Liguria e Valle d'Aosta, Turin, Italy; BHUFA, Biobanco Hospital Universitario Fundación Alcorcón, Alcorcón, Spain; CJDRC, CJD Resource Centre-National Institute for Biological Standards and Control, South Mimms, Potters Bar, UK; CISA, Centro de Investigación en Sanidad Animal, Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria, Valdeolmos, Madrid, Spain; NRL, National Reference Laboratory, Zaragoza, Spain.
Animal experiments were carried out in strict accordance with the recommendations in the guidelines of the Code for Methods and Welfare Considerations in Behavioral Research with Animals (Directive 2010/63/EU), and all efforts were made to minimize suffering. The experiments were approved by the Committee on the Ethics of Animal Experiments of the authors' institution (Instituto Nacional de Tecnologia Agraria y Alimentaria [INIA]), permit numbers CEEA 2011/050 and CEEA 2012/002.
BV109M animals were also inoculated by the intracerebral route following previously described procedures (8). The research protocol, approved by the Service for Biotechnology and Animal Welfare of the Istituto Superiore di Sanità (ISS) and authorized by the Italian Ministry of Health, adhered to the guidelines contained in Italian Legislative Decree 116/92, which adapted or transferred to Italian legislation European Directive 86/609/EEC on Laboratory Animal Protection, and then in Legislative Decree 26/2014, which adapted or transferred to Italian legislation European Directive 2010/63/UE on Laboratory Animal Protection.
Western blotting.
Frozen brain tissue (175 ± 20 mg) was homogenized in 5% glucose in distilled water in grinding tubes (Bio-Rad) and adjusted to 10% (wt/vol) using a TeSeE Precess 48 homogenizer (Bio-Rad) following the manufacturer's instructions. The presence of PrPres in transgenic-mouse brains was determined by Western blotting, following the procedure described below and using the reagents of the commercial enzyme-linked immunosorbent assay (ELISA) (TeSeE; Bio-Rad). Ten to 100 μl of a 10% (wt/vol) brain homogenate was analyzed by Western blotting, as previously described (21) using a 12% Bis-Tris gel (Criterion XT; Bio-Rad). For immunoblotting, monoclonal antibodies Sha31 (23), 12B2 (24), and Saf84 (25) were used at a concentration of 1 μg/ml. Sha31 recognizes the 145-WEDRYYRE-152 epitope, 12B2 recognizes the 89-WGQGG-93 epitope, and Saf84 recognizes the 163-YRPVDQY-169 epitope of the bank vole PrPC sequence. Immunocomplexes were detected by incubating the membranes for 1 h with horseradish peroxidase-conjugated anti-mouse IgG (GE Healthcare Amersham Biosciences). Immunoblots were developed with enhanced chemiluminescence ECL Select (GE Healthcare Amersham Biosciences). Images were captured using the ChemiDoc XRS+ System, and images were processed using Image Lab 5.2.1 software. SigmaPlot 2001 software was used for data analysis.
CSSA.
The conformational stability and solubility assay (CSSA) was done as previously described (26). Briefly, aliquots of brain homogenates (6% [wt/vol] in 100 mM Tris-HCl, pH 7.4) were added with an equal volume of 100 mM Tris-HCl (pH 7.4), 4% Sarkosyl and incubated for 1 h at 37°C with gentle shaking. Then, aliquots of each sample were treated (1 h at 37°C) with different concentrations of guanidine hydrochloride (GdnHCl) (Pierce) to obtain final concentrations ranging from 0 to 4 M. After the GdnHCl treatment, samples were centrifuged at 20,000 × g for 1 h at 22°C. The pellets were resuspended in 16 μl Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA) and 10% β-mercaptoethanol (Bio-Rad Laboratories, Hercules, CA) and analyzed by Western blotting using Saf84 MAb. The dose-response curves allowed us to estimate the concentration of GdnHCl that was able to solubilize 50% of PrPSc ([GdnHCl]1/2). Individual denaturation curves were analyzed and best fitted by plotting the fraction of PrPSc remaining in the pellet as a function of the GdnHCl concentration and using a four-parameter logistic equation (GraphPad Prism).
RESULTS
Bv109M PrPC expression in transgenic mice.
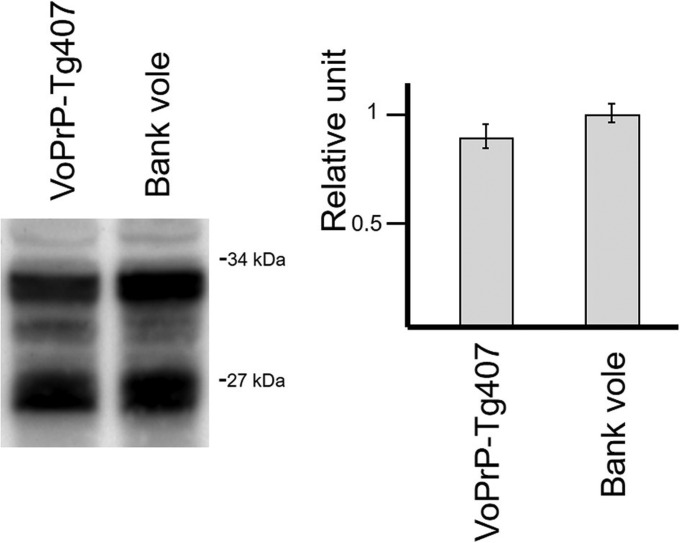
Transgenic PrPC expression in homozygous BvPrP-Tg407 mice was checked by Western blotting using a specific anti-PrP monoclonal antibody (2A11 [20]). Brain PrPC expression levels for the homozygous mice were found to be similar to the PrPC levels found in bank voles, and therefore, BvPrP-Tg407 was chosen as the only transgenic-mouse line used in the study. Interestingly, PrPC from BvPrP-Tg407 mice showed an electrophoretic profile similar to that of the PrPC obtained from the brain of Bv109M (Fig. 1). Neither behavioral defects, such as alterations in reproduction rates, neurological signs, or social deficits, nor reduction in the life span (over 700 days) was observed in animals from this mouse line.
FIG 1.
Brain PrPC expression in a homozygous BvPrP-Tg407 mouse line in comparison to Bv109M brain detected with the 2A11 MAb. The immunoblots illustrate a representative set of three independent experiments, and the diagrams represent the mean quantification using the Image Lab program after capture with ChemiDoc XRS+ under nonsaturating conditions. The data from the Bv109M brain were considered 1 relative unit. Molecular masses (in kDa) are shown on the right of the blot.
Transmission of Bv109M-adapted prions in Bv109M and BvPrP-Tg407.
In a first step, BvPrP-Tg407 mice and Bv109M voles were inoculated with a collection of previously obtained Bv109M-passaged prion strains (Table 2) derived from mouse scrapie, sheep scrapie, and classical BSE (8, 10). In this way, the susceptibilities of the two animal models to prion infection in the absence of amino acid mismatches between donor and host PrPs were compared. Similarly short survival times and 100% attack rates were observed in both animal models for each inoculum (Table 2), supporting the absence of a transmission barrier in both animal models for the inoculated prions. It is important to note that although survival times in the two animal models are not directly comparable because they are distinct species with different physiologies, anatomy, normal life spans, etc., very similar survival times were recorded in both animal models.
TABLE 2.
Transmission of prion inocula generated in Bv109M to BvPrP-Tg407 mice and Bv109M
| Inoculum | Mean survival time (n/n0)a |
|
|---|---|---|
| BvPrP-Tg407 mice | Bv109M | |
| 139A/vole (4th passage) | 80 ± 20 (5/5) | 72 ± 7 (8/8) |
| SS-3/vole (4th passage) | 85 ± 9 (5/5) | 77 ± 3 (6/6) |
| SS-7/vole (3rd passage) | 98 ± 14 (7/7) | 94 ± 3 (8/8) |
| Ca-BSE-1/vole (2nd passage) | 124 ± 3 (5/5) | 92 ± 8 (19/19) |
| Ca-BSE-1/vole (4th passage) | 108 ± 4 (5/5) | 74 ± 3 (11/11) |
| Vole (uninfected brain) | 722 ± 100 (0/5) | >700 (0/10) |
Survival time is indicated as mean number of days postinoculation ± SD of all the mice scored positive for PrPres. n, number of diseased PrPres-positive animals; n0, number of inoculated animals.
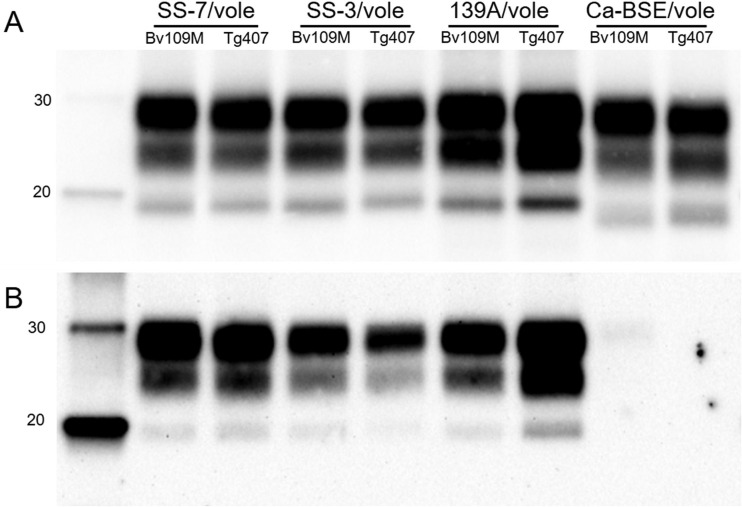
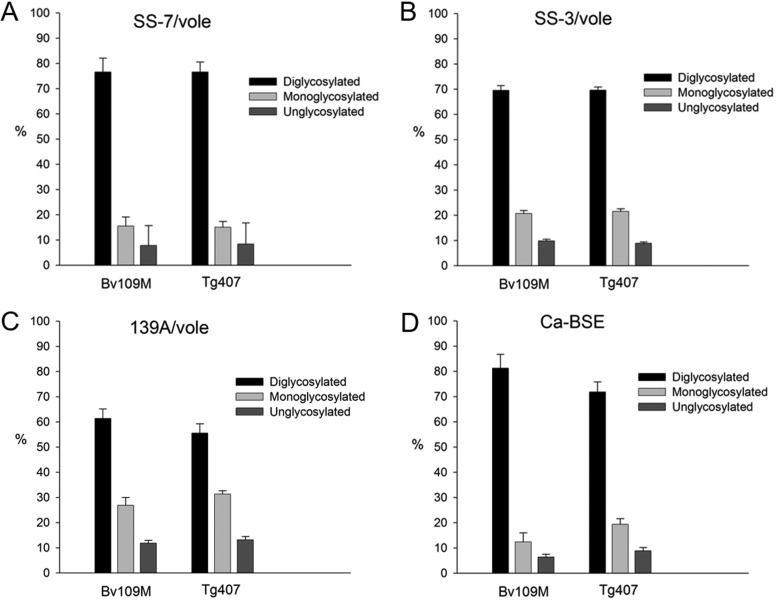
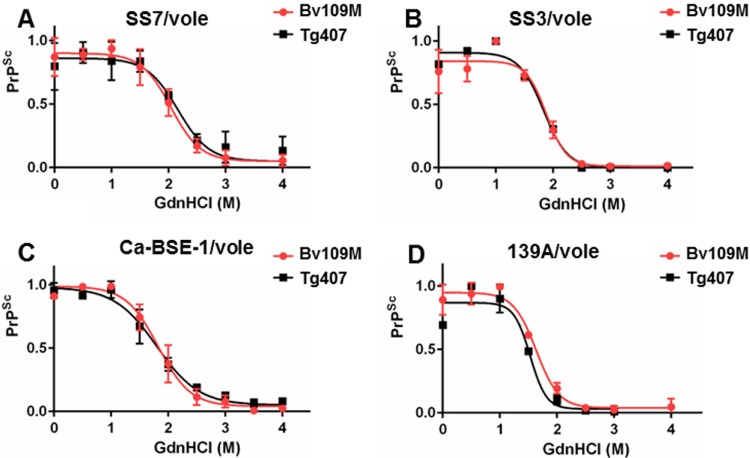
In addition, the brain PrPres generated in BvPrP-Tg407 mice or Bv109M inoculated with the same inoculum showed indistinguishable signatures in Western blots with Sha31 and 12B2 MAbs (Fig. 2), exhibiting similar glycoprofiles (Fig. 3). Further analysis to compare the biophysical properties of PrPSc proteins from both BvPrP-Tg407 mice and Bv109M were done by CSSA, which measures the increase in solubility of PrPSc after exposure to increasing concentrations of GndHCl. The CSSA results revealed identical denaturation profiles (Fig. 4) for PrPSc produced in both animal models (BvPrP-Tg407 mice and Bv109M) when infected with the same inoculum.
FIG 2.
Western blot analysis of brain PrPres from Bv109M or from BvPrP-Tg407 (Tg407) mice detected with Sha31 (A) or 12B2 (B) MAb. Animals were infected with the different Bv109M-adapted prions as indicated at the top. Similar quantities of PrPres (according to the Sha31 MAb) were loaded in each lane for better comparison. The same quantities of PrPres were loaded in both panels. Molecular masses (in kDa) are shown at the left of the blots.
FIG 3.
Glycoform analysis of PrPres from Bv109M and BvPrP-Tg407 (Tg407) inoculated with SS7/vole (A), SS3/vole (B), 139A/vole (C), or Ca-BSE/vole (D). PrPres was detected by Western blotting using the Sha31 MAb as for Fig. 2. The data shown are the means of at least four measurements in two or more different Western blots using the Image Lab program after capture with ChemiDoc XRS+ under nonsaturating conditions. The error bars indicate SD.
FIG 4.
Conformational stability and solubility assay of different prion strains in BvPrP-Tg407 and Bv109M. Shown are dose-response curves of conformational stability of brain PrPSc from BvPrP-Tg407 (Tg407) and Bv109M inoculated with SS-7/vole (A), SS-3/vole (B), Ca-BSE-1/vole (C), and 139 A/vole (D). The curves were obtained by plotting the fraction of PrPSc remaining in the pellet as a function of the GdnHCl concentration and best fitted using a four-parameter logistic equation. Individual curves were combined within each strain group. The [GdnHCl]1/2 values of SS-7/vole, SS-3/vole, Ca-BSE-1/vole, and 139/vole were 2.04 M, 1.88 M, 1.82 M, and 1.64 M, respectively, in Bv109M and 2.16 M, 1.83 M, 1.80 M, and 1.53 M in BvPrP-Tg407. The error bars indicate SD.
Overall, these findings indicate that the newly generated transgenic-mouse line efficiently replicates vole prions without an apparent transmission barrier and preserves the biochemical features of vole PrPSc.
Bv109M and BvPrP-Tg407 are susceptible to atypical BSE-H bovine prions.
We next aimed to investigate the role of Bv109M PrP in driving the susceptibility to TSE isolates deriving from different species, i.e., in the presence of amino acid mismatches between donor and host PrPs. To this end, a panel of isolates with distinct origins (bovine, ovine, and human) representative of different TSE agents were selected and inoculated into Bv109M and BvPrP-Tg407 animals. The results are summarized in Table 3.
TABLE 3.
Transmission of bovine, sheep, and human prion isolates to Bv109M and BvPrP-Tg407 mice
| PrPSc sequence | Inoculum | Mean survival time (n/n0)a |
|
|---|---|---|---|
| BvPrP-Tg407 | Bv109M | ||
| Bovine | Ca-BSE-1 | ND | >1,044 (0/6)b |
| Ca-BSE-2 | 519 (1/5) | >934 (0/6) | |
| Ca-BSE-L-1 | >650 (0/5) | ND | |
| Ca-BSE-L-2 | ND | 760 ± 166 (13/17) | |
| Ca-BSE-H-1 | 471 ± 34 (6/6) | ND | |
| Ca-BSE-H-2 | ND | 404 ± 46 (10/10) | |
| Sheep | Sh-BSE-3 | 508 ± 151 (6/7) | 571 ± 171 (7/14) |
| SS-10 | 174 ± 42 (13/13) | 172 ± 16 (6/6) | |
| SS-N799-97 | 729 ± 14 (4/6) | 560 ± 202 (5/12) | |
| Human | sCJD MM1 | 272 ± 51 (8/8) | 188 ± 22 (16/16)c |
| sCJD MM1/TgHu-340 | 234 ± 2 (6/6) | ND | |
| vCJD-Sp | 612 ± 47 (5/5) | ND | |
| vCJD-UK | ND | 760 ± 107 (7/12) | |
| vCJD-Sp/TgHu-340 | 644 ± 31 (5/5) | ND | |
Cattle-derived TSEs comprise classical BSE and two atypical BSE strains, H-type BSE and L-type BSE. Wt mice are susceptible to classical BSE but much less to atypical BSE strains (27, 28). In contrast, Bv109M voles were reported to be resistant to primary transmission with classical BSE, and transmission was apparent only after a blind second passage (9). In keeping with these data, the primary transmission of epidemic classical BSE evidenced the existence of a strong transmission barrier in both Bv109M and BvPrP-Tg407. Only one out of five classical-BSE-inoculated BvPrP-Tg407 mice was scored positive for brain PrPres at a late stage of 519 dpi and none in Bv109M. L-type BSE also encountered a strong transmission barrier in both animal models, as it was transmitted inefficiently and with very long incubation times in Bv109M and not at all in BvPrP-Tg407 mice. In contrast, both Bv109M and BvPrP-Tg407 mice were susceptible to H-type BSE isolates, with a 100% attack rate and similar survival times (Table 3). Bv109M and BvPrP-Tg407 mice are the first animal models described as showing a preferential susceptibility to infection with H-type BSE while presenting a strong barrier to infection with L-type BSE or epidemic classical BSE.
Bv109M and BvPrP-Tg407 are susceptible to Italian scrapie prions.
Past studies showed that the efficiency of transmission of sheep TSEs in Bv109M depends on the prion strain, with Italian scrapie being more easily transmitted than sheep BSE (8) and some Spanish scrapie isolates (R. Nonno and U. Agrimi, personal communication). Thus, three ovine isolates sharing the same PrP amino acid sequence (ARQ/ARQ genotype), including both sheep BSE and sheep scrapie isolates from Spain and Italy, were used to study their transmissibility in BvPrP-Tg407 mice in comparison to Bv109M animals. Although variable transmission efficiency was observed among isolates, very similar results were observed in the two animal models for a given isolate. Bv109M and BvPrP-Tg407 mice were highly susceptible to the Italian SS-10 scrapie isolate (an attack rate of 100% and survival to ∼173 dpi), while sheep BSE and the Spanish SS-N799-97 scrapie isolate were partially transmitted to both animal models with long incubation times (Table 3). A higher infectious titer for the SS-10 isolate seems unlikely, as the SS-N799-97 scrapie isolate and sheep BSE were chosen due to the isolate's high PrPres content (data not shown). Furthermore, the same scrapie SS10 and sheep BSE inocula were inoculated into wt RIII mice, which, as expected from previous studies, were susceptible to sheep BSE (386 ± 51 dpi and a 100% attack rate) but much less to SS10 (only 1 out of 9 mice positive at 667 dpi). Thus, replacing mouse PrP with BvPrP in BvPrP-Tg407 mice was sufficient to confer a vole-like pattern of susceptibility to scrapie and sheep BSE. It is interesting that sheep BSE showed a lower transmission barrier in both animal models than classical BSE; a higher attack rate and reduced survival times were observed after transmission of the Sh-BSE-1 inoculum in both BvPrP-Tg407 mice (450 ± 141 dpi) and Bv109M (571 ± 171 dpi).
Bv109M and BvPrP-Tg407 are susceptible to sCJD.
Among human TSEs, sCJD MM1 was shown to be easily transmissible into Bv109M, but not into mice (8). In contrast, mice are known to be susceptible to other human strains, such as variant CJD (vCJD) (29). Hence, we also compared the transmissibilities of sCJD (MM1 subtype) and vCJD in both BvPrP-Tg407 mice and Bv109M. Once again, different transmission properties were observed for each prion strain, but the two animal models showed similar transmission features for a given strain (Table 3). Sporadic CJD was efficiently transmitted, with short survival times in both BvPrP-Tg407 mice (272 ± 51 dpi) and Bv109M (188 ± 22 dpi), showing that BvPrP expression in mice was able to confer full susceptibility to sCJD MM1 infection. Similarly to classical BSE and sheep BSE, vCJD was transmitted in BvPrP-Tg407 mice and Bv109M after long survival times (612 ± 47 and 760 ± 107 dpi, respectively) but only partially in Bv109M. Interestingly, when BvPrP-Tg407 mice were inoculated with sCJD (MM1 subtype) or vCJD after passage of each prion strain in transgenic mice expressing human prion protein (21), the same strain-dependent behavior was observed (234 ± 2 dpi for sCJD and 644 ± 31 dpi for vCJD).
Different PrPres in Bv109M brains than in BvPrP-Tg407 after inoculation with prions.
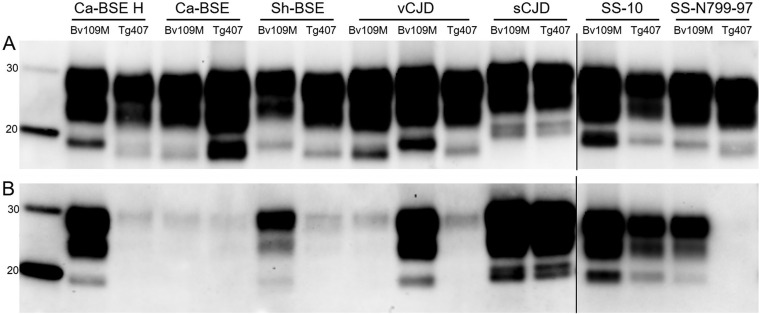
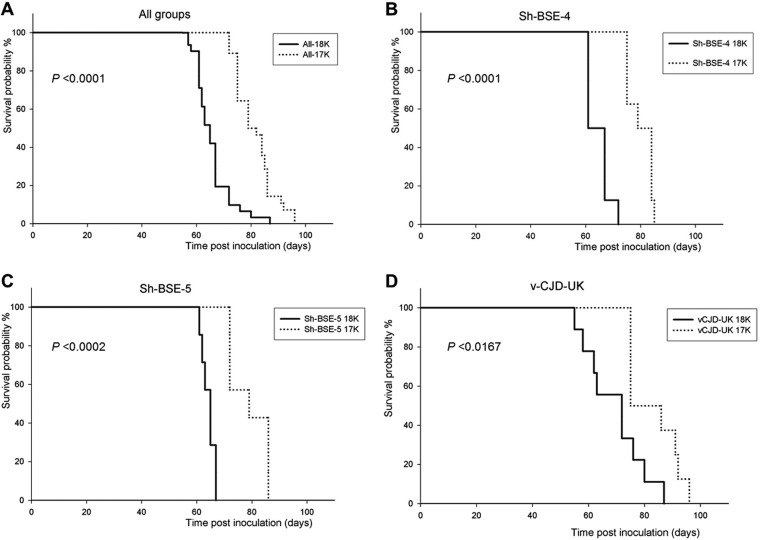
Comparison of the PrPres from brains collected from Bv109M versus PrPres from those collected from BvPrP-Tg407 mice infected with the same inoculum revealed the same molecular pattern in Western blots for Ca-BSE, sCJD, and SS-10 prions (Fig. 5). In contrast, discrepant PrPres profiles were observed in animals inoculated with Ca-BSE H, Sh-BSE, and SS-N799-97 prions (Fig. 5). Indeed, for all these TSEs, the relative mobility of the unglycosylated band was higher in Bv109M (∼18 kDa) than in BvPrP-Tg407 (∼17 kDa), as confirmed by the 12B2 MAb, which failed to detect the ∼17-kDa PrPres (Fig. 5). Finally, for vCJD, partially discrepant results were observed. Interestingly, the expected ∼17-kDa PrPres was reproduced in BvPrP-Tg407, while two different brain PrPres profiles were observed by Western blotting in individual Bv109M voles infected with vCJD. The relative mobility of the unglycosylated band was either ∼18 or ∼17 kDa in vCJD-infected Bv109M brains (Fig. 5). The possibility of different endogenous proteolytic cleavages being responsible for the two different brain PrPres profiles was excluded, because neither ∼18- nor ∼17-kDa fragments were detectable in non-proteinase K-treated brain samples (data not shown). In parallel experiments aimed at characterizing BSE-related strains derived from different species, we observed similar behavior in Bv109M inoculated with several BSE sources besides vCJD (unpublished data). Some sheep BSE isolates gave both ∼18- and ∼17-kDa PrPres, while others resulted in only ∼18- or only ∼17-kDa patterns (Table 4; full details of these experiments will be reported elsewhere). Furthermore, second passages initiated from the brains of sheep BSE- or vCJD-infected Bv109M with ∼18- or ∼17-kDa PrPres (Table 4) showed that the PrPres signature was preserved on subpassage. In all subpassages, a Bv109M-adapted prion with a short survival time (between 63 and 69 dpi) was associated with ∼18-kDa PrPres, while groups of Bv109M with ∼17-kDa PrPres had longer survival times (between 80 and 92 dpi). Moreover, in all cases, these differences in survival times between ∼18- and ∼17-kDa PrPres Bv109M-adapted prions were statistically significant (Fig. 6). These findings strongly argue that the same 2 distinct vole-adapted strains were isolated in Bv109M from different BSE sources, including vCJD and sheep BSE. This is at variance with the finding that the ∼18-kDa PrPres signature was not observed in BvPrP-Tg407 mice, as 17 out of 17 positive mice inoculated with BSE-related TSEs had ∼17-kDa PrPres (Table 3).
FIG 5.
Western blot analysis of brain PrPres from either Bv109M or BvPrP-Tg407 (Tg407) mice infected with prion isolates from different species detected with Sha31 (A) or 12B2 (B) MAb: cattle (Ca-BSE H and Ca-BSE), sheep (Sh-BSE, SS-10, and SS-N799-97), or human (sCJD and vCJD). PrPres of brain from Bv109M infected with Ca-BSE was from the second passage in Bv109M. Similar quantities of PrPres (according to the Sha31 MAb) were loaded in each lane for better comparison. The same quantities of PrPres were loaded in both panels. Molecular masses (in kDa) are shown at the left of the blots.
TABLE 4.
Transmission of classical-BSE and BSE-derived inocula in Bv109M mice
| Inoculum | 1st passage |
2nd passage |
||
|---|---|---|---|---|
| Mean survival timea | PrPres type | Mean survival timea | PrPres type | |
| Ca-BSE 1 | >1,044 (0/6) | ND | 92 ± 8 (15/15) | 17 |
| Sh-BSE-3 | 571 ± 171 (7/14) | 18 | 63 ± 3 (7/7) | 18 |
| Sh-BSE-4 | 695 ± 110 (9/11) | 17 | 80 ± 5 (8/8) | 17 |
| 18 | 65 ± 4 (8/8) | 18 | ||
| Sh-BSE-5 | 449 ± 121 (7/13) | 17 | 79 ± 7 (7/7) | 17 |
| 18 | 64 ± 2 (7/7) | 18 | ||
| Sh-BSE-6 | 631 ± 221 (3/11) | 17 | 81 ± 3 (4/4) | 17 |
| vCJD-UK | 760 ± 107 (7/17) | 17 | 83 ± 9 (8/8) | 17 |
| 18 | 69 ± 11 (9/9) | 18 | ||
Survival time is indicated as mean number of days postinfection ± SD of all the mice scored positive for PrPres. n, number of diseased PrPres-positive animals; n0, number of inoculated animals. ND, no data.
FIG 6.
Kaplan-Meier survival curves of Bv109M inoculated on second passages with brains of sheep-BSE- or vCJD-infected Bv109M with 18 kDa (solid lines) or 17 kDa (dashed lines) PrPres. The original inoculum used for each group is indicated at the top of each panel, except for panel A, which compiles all groups of Bv109M-inoculated animals included in panels B, C, and D. P was calculated by the log-rank (Mantel-Cox) test.
DISCUSSION
This work comprises the first systematic comparison of the transmission features of a collection of TSE isolates representing a panel of diverse prion strains in a transgenic-mouse model and in its natural counterpart. The results showed very similar transmissibilities in both the natural species and the transgenic-mouse model for vole-adapted strains and for TSE isolates derived from naturally affected species with different PrP amino acid sequences.
As expected from previous studies with transgenic mice expressing heterologous PrPs (30), vole PrP expression in mice abolished the vole-to-mouse species barrier, as evidenced by the ease of transmission in BvPrP-Tg407 mice of vole-adapted prion strains (Table 2). In fact, the vole-adapted strain 139A encountered a substantial transmission barrier upon back passage in mice (9), resulting in a very long incubation time of 463 ± 62 dpi, while we report here that it was transmissible in only 80 dpi in BvPrP-Tg407 mice. The mouse scrapie strain 139A is characterized by a mainly monoglycosylated PrPres, which shifts to a mainly diglycosylated PrPres upon transmission in voles and reverts to the original monoglycosylated form upon back passage in mice (9). Interestingly, here, we observed that 139A-infected BvPrP-Tg407 mice reproduced the vole-specific diglycosylated pattern, strongly suggesting that this phenotype is driven by the PrP amino acid sequence.
More importantly, we investigated the impact of BvPrP on interspecies transmission of prions and found that BvPrP conferred on mice a vole-like susceptibility to natural TSE strains. Remarkable examples of this behavior were provided by the high susceptibility of BvPrP-Tg407 mice to infection with sCJD MM1 and Italian scrapie, two TSE strains known for being transmissible to voles but much less or not at all to wt mice (references 8–10, 14, and 29 and this study). In contrast, in keeping with the low susceptibility of voles to BSE, the susceptibility of BvPrP-Tg407 mice to BSE-derived strains was low compared to that of wt mice. Taken together, these results demonstrate that the differential susceptibility of Bv109M to prion strains observed here and also previously reported (8, 9) can be modeled in transgenic mice expressing vole PrPC, suggesting that this selective susceptibility is mainly modulated by the vole PrP sequence rather than by other species-specific factors. Overall, our results confirm that the interplay between the host PrP sequence and the prion strain involved plays a pivotal role in determining the transmission barrier for prions (6).
Detection of PrPres is due to the conformational changes produced after the PrPC-to-PrPSc transition, resulting in PrPres fragments that can be representative of different prion strains for a given species, as is the case for the different BSE strains described in cattle (31). Different PrPres electrophoretic mobilities and alterations in the binding of antibodies specific for the PrPres N-terminal regions, such as the 12B2 MAb, allow accurate discrimination among prion strains (24, 32, 33). To investigate the effect of PrP amino acid sequence versus other host factors on strain replication, we compared PrPres in Bv109M voles and BvPrP-Tg407 mice. Our results showed that the two animal models invariably replicated the same PrPres patterns when infected with vole-adapted TSE strains but only in some instances with the TSE strains derived from other species. This suggests that during experimental transmissions involving a species barrier, additional host factors other than PrPC might have played a role in driving either the fidelity of prion replication or the selection of substrains being preferentially replicated in the new hosts. In a recent work, the transmission features of two strains of transmissible mink encephalopathy (TME) and two strains of chronic wasting disease (CWD) were compared in hamster species and in transgenic mice overexpressing hamster PrP (34). While the properties of TME strains were maintained in both hosts, the properties of CWD strains were different in hamsters and transgenic mice. These data reinforce the idea that for some strains the fidelity of prion strain replication may be either maintained or modified in the new host, depending on host factors other than PrPC.
In this context, the results we have obtained with BSE-related sources offer some insight that favors the “substrain selection” hypothesis. Although low-molecular-weight, 12B2-negative PrPres is considered the molecular signature of classical BSE in naturally or experimentally infected species, exceptions to this rule have been reported. Indeed, upon inoculation with BSE and/or vCJD, two different BSE-derived strains with distinct PrPres proteins have been isolated in different lines of Prnpa inbred mice (35), in transgenic mice expressing human PrP (36, 37), in transgenic mice expressing chimeric mouse/human PrP (38), and in transgenic mice expressing ovine PrP (39). In agreement with these previous findings, our results showed that a similar bifurcation of the BSE strain characteristics was also observed in Bv109M, but not in BvPrP-Tg407 mice, inoculated with BSE-derived prions, resulting in distinct vole-adapted strains on subpassage. Taken together, these findings led us to hypothesize that more than one substrain can be selected from BSE sources in new hosts and that vole-specific factors other than PrP, absent in BvPrP-Tg407 mice, favored the replication of an ∼18-kDa substrain. Furthermore, these host factors present in the Bv109M voles would explain the differences in the PrPres types found between BvPrP-Tg407 and Bv109M animal models infected with Ca-BSE H or SS-N799-97 scrapie. Other transgenic-mouse lines overexpressing bank vole 109M-PrPC and challenged with type I sCJD, vCJD, or classical BSE showed brain PrPres signatures apparently similar to those found here in BvPrP-Tg407 (40), supporting the idea that host-specific factors other than PrPC are responsible for the differences in the PrPres types found in BvPrP-Tg407 and Bv109M animal models.
Overall, the similar transmission properties observed in both the natural species and the transgenic-mouse model evidence the key role of the PrP amino acid sequence in the efficiency of interspecies prion transmission. However, differences in brain PrPres Western blot profiles observed between the two animal models inoculated with the same inoculum support the hypothesis (34, 35) that the genetic background can impact the strain features of the prion agent.
ACKNOWLEDGMENTS
We declare no competing financial interests.
We thank the following providers of tissues: Animal and Plant Health Agency (New Haw, Addlestone, Surrey, United Kingdom), M. Polak (National Veterinary Research Institute, Pulawy, Poland), C. Casalone (Istituto Zooprofiattico Sperimentale del Piemonte, Liguria e Valle d'Aosta, Turin, Italy), T. Baron (Agence Nationale de Sécurité Sanitaire de l'Alimentation, de l'Environnement et du Travail, Lyon, France), C. Guerrero (Biobanco Hospital Universitario Fundación Alcorcón), J. Cooper (CJD Resource Centre-National Institute for Biological Standards and Control, South Mimms, Potters Bar, United Kingdom).
This work was supported by grants from the Italian Ministry of Health (RF-2009-1474624), the Spanish Ministerio de Economía y Competitividad (AGL2012-37988-C04-04 and RTA2012-00004-00-00 and fellowship BES-2010-040922 to P.A.-C.), and European Union projects FOOD-CT-2006-36353 (GoatBSE) and 219235 ERA-NET EMIDA (GOAT-TSE-FREE).
REFERENCES
- 1.Prusiner SB. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 2.Castilla J, Saa P, Hetz C, Soto C. 2005. In vitro generation of infectious scrapie prions. Cell 121:195–206. doi: 10.1016/j.cell.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Wang F, Wang X, Yuan CG, Ma J. 2010. Generating a prion with bacterially expressed recombinant prion protein. Science 327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makarava N, Kovacs GG, Bocharova O, Savtchenko R, Alexeeva I, Budka H, Rohwer RG, Baskakov IV. 2010. Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol 119:177–187. doi: 10.1007/s00401-009-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peretz D, Williamson RA, Legname G, Matsunaga Y, Vergara J, Burton DR, DeArmond SJ, Prusiner SB, Scott MR. 2002. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron 34:921–932. doi: 10.1016/S0896-6273(02)00726-2. [DOI] [PubMed] [Google Scholar]
- 6.Collinge J, Clarke AR. 2007. A general model of prion strains and their pathogenicity. Science 318:930–936. doi: 10.1126/science.1138718. [DOI] [PubMed] [Google Scholar]
- 7.Chandler RL, Turfrey BA. 1972. Inoculation of voles, Chinese hamsters, gerbils and guinea-pigs with scrapie brain material. Res Vet Sci 13:219–224. [PubMed] [Google Scholar]
- 8.Nonno R, Bari MA, Cardone F, Vaccari G, Fazzi P, Dell'omo G, Cartoni C, Ingrosso L, Boyle A, Galeno R, Sbriccoli M, Lipp HP, Bruce M, Pocchiari M, Agrimi U. 2006. Efficient transmission and characterization of Creutzfeldt-Jakob disease strains in bank voles. PLoS Pathog 2:e12. doi: 10.1371/journal.ppat.0020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agrimi U, Nonno R, Dell'Omo G, Di Bari MA, Conte M, Chiappini B, Esposito E, Di Guardo G, Windl O, Vaccari G, Lipp HP. 2008. Prion protein amino acid determinants of differential susceptibility and molecular feature of prion strains in mice and voles. PLoS Pathog 4:e1000113. doi: 10.1371/journal.ppat.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Bari MA, Chianini F, Vaccari G, Esposito E, Conte M, Eaton SL, Hamilton S, Finlayson J, Steele PJ, Dagleish MP, Reid HW, Bruce M, Jeffrey M, Agrimi U, Nonno R. 2008. The bank vole (Myodes glareolus) as a sensitive bioassay for sheep scrapie. J Gen Virol 89:2975–2985. doi: 10.1099/vir.0.2008/005520-0. [DOI] [PubMed] [Google Scholar]
- 11.Di Bari MA, Nonno R, Castilla J, D'Agostino C, Pirisinu L, Riccardi G, Conte M, Richt J, Kunkle R, Langeveld J, Vaccari G, Agrimi U. 2013. Chronic wasting disease in bank voles: characterisation of the shortest incubation time model for prion diseases. PLoS Pathog 9:e1003219. doi: 10.1371/journal.ppat.1003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirisinu L, Di Bari MA, D'Agostino C, Marcon S, Riccardi G, Poleggi A, Cohen ML, Appleby BS, Gambetti P, Ghetti B. 2016. Gerstmann-Sträussler-Scheinker disease subtypes efficiently transmit in bank voles as genuine prion diseases. Sci Rep 6:20443. doi: 10.1038/srep20443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cartoni C, Schinina ME, Maras B, Nonno R, Vaccari G, Di Baria MA, Conte M, Liu QG, Lu M, Cardone F, Windl O, Pocchiari M, Agrimi U. 2005. Identification of the pathological prion protein allotypes in scrapie-infected heterozygous bank voles (Clethrionomys glareolus) by high-performance liquid chromatography-mass spectrometry. J Chromatogr A 1081:122–126. doi: 10.1016/j.chroma.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 14.Piening N, Nonno R, Di Bari M, Walter S, Windl O, Agrimi U, Kretzschmar HA, Bertsch U. 2006. Conversion efficiency of bank vole prion protein in vitro is determined by residues 155 and 170, but does not correlate with the high susceptibility of bank voles to sheep scrapie in vivo. J Biol Chem 281:9373–9384. doi: 10.1074/jbc.M512239200. [DOI] [PubMed] [Google Scholar]
- 15.Telling GC, Scott M, Mastrianni J, Gabizon R, Torchia M, Cohen FE, DeArmond SJ, Prusiner SB. 1995. Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell 83:79–90. doi: 10.1016/0092-8674(95)90236-8. [DOI] [PubMed] [Google Scholar]
- 16.Hijazi N, Kariv-Inbal Z, Gasset M, Gabizon R. 2005. PrPSc incorporation to cells requires endogenous GAGs expression. J Biol Chem 280:17057–17061. doi: 10.1074/jbc.M411314200. [DOI] [PubMed] [Google Scholar]
- 17.Mishra RS, Basu S, Gu Y, Luo X, Zou WQ, Mishra R, Li R, Chen SG, Gambetti P, Fujioka H, Singh N. 2004. Protease-resistant human prion protein and ferritin are cotransported across Caco-2 epithelial cells: implications for species barrier in prion uptake from the intestine. J Neurosci 24:11280–11290. doi: 10.1523/JNEUROSCI.2864-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borchelt DR, Davis J, Fischer M, Lee MK, Slunt HH, Ratovitsky T, Regard J, Copeland NG, Jenkins NA, Sisodia SS, Price DL. 1996. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal 13:159–163. doi: 10.1016/S1050-3862(96)00167-2. [DOI] [PubMed] [Google Scholar]
- 19.Manson JC, Clarke AR, Hooper ML, Aitchison L, McConnell I, Hope J. 1994. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol Neurobiol 8:121–127. doi: 10.1007/BF02780662. [DOI] [PubMed] [Google Scholar]
- 20.Brun A, Castilla J, Ramirez MA, Prager K, Parra B, Salguero FJ, Shiveral D, Sanchez C, Sanchez-Vizcaino JM, Douglas A, Torres JM. 2004. Proteinase K enhanced immunoreactivity of the prion protein-specific monoclonal antibody 2A11. Neurosci Res 48:75–83. doi: 10.1016/j.neures.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Padilla D, Béringue V, Espinosa JC, Andreoletti O, Jaumain E, Reine F, Herzog L, Gutierrez-Adan A, Pintado B, Laude H, Torres JM. 2011. Sheep and goat BSE propagate more efficiently than cattle BSE in human PrP transgenic mice. PLoS Pathog 7:e1001319. doi: 10.1371/journal.ppat.1001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres JM, Andreoletti O, Lacroux C, Prieto I, Lorenzo P, Larska M, Baron T, Espinosa JC. 2011. Classical bovine spongiform encephalopathy by transmission of H-type prion in homologous prion protein context. Emerg Infect Dis 17:1636–1644. doi: 10.3201/eid1709.101403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feraudet C, Morel N, Simon S, Volland H, Frobert Y, Creminon C, Vilette D, Lehmann S, Grassi J. 2005. Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J Biol Chem 280:11247–11258. doi: 10.1074/jbc.M407006200. [DOI] [PubMed] [Google Scholar]
- 24.Yull HM, Ritchie DL, Langeveld JP, van Zijderveld FG, Bruce ME, Ironside JW, Head MW. 2006. Detection of type 1 prion protein in variant Creutzfeldt-Jakob disease. Am J Pathol 168:151–157. doi: 10.2353/ajpath.2006.050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demart S, Fournier JG, Creminon C, Frobert Y, Lamoury F, Marce D, Lasmezas C, Dormont D, Grassi J, Deslys JP. 1999. New insight into abnormal prion protein using monoclonal antibodies. Biochem Biophys Res Commun 265:652–657. doi: 10.1006/bbrc.1999.1730. [DOI] [PubMed] [Google Scholar]
- 26.Pirisinu L, Di Bari M, Marcon S, Vaccari G, D'Agostino C, Fazzi P, Esposito E, Galeno R, Langeveld J, Agrimi U, Nonno R. 2010. A new method for the characterization of strain-specific conformational stability of protease-sensitive and protease-resistant PrP. PLoS One 5:e12723. doi: 10.1371/journal.pone.0012723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masujin K, Shu Y, Yamakawa Y, Hagiwara K, Sata T, Matsuura Y, Iwamaru Y, Imamura M, Okada H, Mohri S, Yokoyama T. 2008. Biological and biochemical characterization of L-type-like bovine spongiform encephalopathy (BSE) detected in Japanese black beef cattle. Prion 2:123–128. doi: 10.4161/pri.2.3.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bencsik A, Leboidre M, Debeer S, Aufauvre C, Baron T. 2013. Unique properties of the classical bovine spongiform encephalopathy strain and its emergence from H-type bovine spongiform encephalopathy substantiated by VM transmission studies. J Neuropathol Exp Neurol 72:211–218. doi: 10.1097/NEN.0b013e318285c7f9. [DOI] [PubMed] [Google Scholar]
- 29.Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ. 1997. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 30.Scott M, Foster D, Mirenda C, Serban D, Coufal F, Walchli M, Torchia M, Groth D, Carlson G, DeArmond SJ, Westaway D, Prusiner SB. 1989. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell 59:847–857. doi: 10.1016/0092-8674(89)90608-9. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs JG, Langeveld JP, Biacabe AG, Acutis PL, Polak MP, Gavier-Widen D, Buschmann A, Caramelli M, Casalone C, Mazza M, Groschup M, Erkens JH, Davidse A, van Zijderveld FG, Baron T. 2007. Molecular discrimination of atypical bovine spongiform encephalopathy strains from a geographical region spanning a wide area in Europe. J Clin Microbiol 45:1821–1829. doi: 10.1128/JCM.00160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Espinosa JC, Andreoletti O, Castilla J, Herva ME, Morales M, Alamillo E, San-Segundo FD, Lacroux C, Lugan S, Salguero FJ, Langeveld J, Torres JM. 2007. Sheep-passaged bovine spongiform encephalopathy agent exhibits altered pathobiological properties in bovine-PrP transgenic mice. J Virol 81:835–843. doi: 10.1128/JVI.01356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stack MJ, Chaplin MJ, Clark J. 2002. Differentiation of prion protein glycoforms from naturally occurring sheep scrapie, sheep-passaged scrapie strains (CH1641 and SSBP1), bovine spongiform encephalopathy (BSE) cases and Romney and Cheviot breed sheep experimentally inoculated with BSE using two monoclonal antibodies. Acta Neuropathol 104:279–286. [DOI] [PubMed] [Google Scholar]
- 34.Crowell J, Hughson A, Caughey B, Bessen RA. 2015. Host determinants of prion strain diversity independent of prion protein genotype. J Virol 89:10427–10441. doi: 10.1128/JVI.01586-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lloyd SE, Linehan JM, Desbruslais M, Joiner S, Buckell J, Brandner S, Wadsworth JD, Collinge J. 2004. Characterization of two distinct prion strains derived from bovine spongiform encephalopathy transmissions to inbred mice. J Gen Virol 85:2471–2478. doi: 10.1099/vir.0.79889-0. [DOI] [PubMed] [Google Scholar]
- 36.Asante EA, Linehan JM, Desbruslais M, Joiner S, Gowland I, Wood AL, Welch J, Hill AF, Lloyd SE, Wadsworth JD, Collinge J. 2002. BSE prions propagate as either variant CJD-like or sporadic CJD-like prion strains in transgenic mice expressing human prion protein. EMBO J 21:6358–6366. doi: 10.1093/emboj/cdf653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beringue V, Herzog L, Reine F, Le Dur A, Casalone C, Vilotte JL, Laude H. 2008. Transmission of atypical bovine prions to mice transgenic for human prion protein. Emerg Infect Dis 14:1898–1901. doi: 10.3201/eid1412.080941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giles K, Glidden DV, Patel S, Korth C, Groth D, Lemus A, DeArmond SJ, Prusiner SB. 2010. Human prion strain selection in transgenic mice. Ann Neurol 68:151–161. doi: 10.1002/ana.22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapuis J, Moudjou M, Reine F, Herzog L, Jaumain E, Chapuis C, Quadrio I, Boulliat J, Perret-Liaudet A, Dron M. 2016. Emergence of two prion subtypes in ovine PrP transgenic mice infected with human MM2-cortical Creutzfeldt-Jakob disease prions. Acta Neuropathol Commun 4:10. doi: 10.1186/s40478-016-0284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watts JC, Giles K, Patel S, Oehler A, Dearmond SJ, Prusiner SB. 2014. Evidence that bank vole PrP is a universal acceptor for prions. PLoS Pathog 10:e1003990. doi: 10.1371/journal.ppat.1003990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castilla J, Gutierrez Adan A, Brun A, Pintado B, Ramirez MA, Parra B, Doyle D, Rogers M, Salguero FJ, Sanchez C, Sanchez-Vizcaino JM, Torres JM. 2003. Early detection of PrP(res) in BSE-infected bovine PrP transgenic mice. Arch Virol 148:677–691. doi: 10.1007/s00705-002-0958-4. [DOI] [PubMed] [Google Scholar]
- 42.Vaccari G, D'Agostino C, Nonno R, Rosone F, Conte M, Di Bari MA, Chiappini B, Esposito E, De Grossi L, Giordani F, Marcon S, Morelli L, Borroni R, Agrimi U. 2007. Prion protein alleles showing protective effect on the susceptibility of sheep to scrapie and BSE. J Virol 81:7306–7309. doi: 10.1128/JVI.02880-06. [DOI] [PMC free article] [PubMed] [Google Scholar]