ABSTRACT
The variable regions (VHHs) of two heavy chain-only antibodies, JM2 and JM4, from llamas that have been immunized with a trimeric gp140 bound to a CD4 mimic have been recently isolated (here referred to as VHH JM2 and VHH JM4, respectively). JM2 binds the CD4-binding site of gp120 and neutralizes HIV-1 strains from subtypes B, C, and G. JM4 binds gp120 and neutralizes HIV-1 strains from subtypes A, B, C, A/E, and G in a CD4-dependent manner. In the present study, we constructed glycosylphosphatidylinositol (GPI)-anchored VHH JM2 and JM4 along with an E4 control and transduced them into human CD4+ cell lines and primary CD4 T cells. We report that by genetically linking the VHHs with a GPI attachment signal, VHHs are targeted to the lipid rafts of the plasma membranes. Expression of GPI-VHH JM4, but not GPI-VHH E4 and JM2, on the surface of transduced TZM.bl cells potently neutralizes multiple subtypes of HIV-1 isolates, including tier 2 or 3 strains, transmitted founders, quasispecies, and soluble single domain antibody (sdAb) JM4-resistant viruses. Moreover, transduction of CEMss-CCR5 cells with GPI-VHH JM4, but not with GPI-VHH E4, confers resistance to both cell-free and T cell-T cell transmission of HIV-1 and HIV-1 envelope-mediated fusion. Finally, GPI-VHH JM4-transduced human primary CD4 T cells efficiently resist both cell-free and T cell-T cell transmission of HIV-1. Thus, we conclude that VHH JM4, when targeted to the lipid rafts of the plasma membrane, efficiently neutralizes HIV-1 infection via both cell-free and T cell-T cell transmission. Our findings should have important implications for GPI-anchored antibody-based therapy against HIV-1.
IMPORTANCE Lipid rafts are specialized dynamic microdomains of the plasma membrane and have been shown to be gateways for HIV-1 budding as well as entry into T cells and macrophages. In nature, many glycosylphosphatidylinositol (GPI)-anchored proteins localize in the lipid rafts. In the present study, we developed GPI-anchored variable regions (VHHs) of two heavy chain-only antibodies, JM2 and JM4, from immunized llamas. We show that by genetically linking the VHHs with a GPI attachment signal, VHHs are targeted to the lipid rafts of the plasma membranes. GPI-VHH JM4, but not GPI-VHH JM2, in transduced CD4+ cell lines and human primary CD4 T cells not only efficiently blocks diverse HIV-1 strains, including tier 2 or 3 strains, transmitted founders, quasispecies, and soluble sdAb JM4-resistant strains, but also efficiently interferes T cell-T cell transmissions of HIV-1 and HIV-1 envelope-mediated fusion. Our findings should have important implications in GPI-anchored antibody-based therapy against HIV-1.
INTRODUCTION
Llamas naturally produce heavy chain-only antibodies. The variable regions (VHHs) of these heavy chain-only antibodies exhibit antigen-specific binding affinity comparable to that of conventional immunoglobulins (1). Previously, using trimeric gp140 bound to a CD4 mimic as immunogens in llamas, we isolated a panel of broadly neutralizing VHHs of heavy chain-only antibodies. Among these antibodies, JM2 binds the CD4-binding site (CD4BS) of gp120 and neutralizes human immunodeficiency virus type 1 (HIV-1) strains from subtypes B, C, and G, and JM4 binds gp120 and neutralizes HIV-1 strains from subtypes A, B, C, A/E, and G in a CD4-dependent manner (2). A recent crystal structure of JM4 in the complex of HIV-1 Yu2 gp120 core and a CD4 mimic shows that JM4 binds to an epitope spanning the gp120 bridge sheet, V3 loop, β19 strand, the CD4-binding loop, and the glycan at residue Asn386 (3). The JM4 epitope overlaps the b12 epitope in the CD4BS and the 17b, 48d, X5, and 412d epitopes in the coreceptor-binding site (CRBS) of gp120 (3). Thus, consistent with what was found with binding and mutagenesis analyses (2), JM4 targets a hybrid epitope on gp120 that combines elements from both the CD4-binding and coreceptor-binding sites.
HIV-1 infects cells by both cell-free and cell-cell mechanisms. Viral transmission from infected to uninfected cells occurs via formation of virological and infectious synapses, nanotubes, and filopodia (4, 5). The formation of such structures allows the coordination of viral assembly with viral entry at sites of cell-cell contacts (6). As a result, HIV-1 infection of T cells in vitro by cell-cell transmission has been found to be 100- to 1,000-fold more efficient for spreading virus than cell-free transmission (7, 8). While the relative impact of cell-free and cell-cell transmission in vivo remains to be defined, in a bone marrow-liver-thymus (BLT) humanized mouse model, HIV-1-infected T cells in lymph nodes were found to be mobile and to form virological synapses and syncytia. Of note, a sphingosine 1-phosphate receptor 1 (S1PR1) antagonist, FTY720, blocks the egress of migratory T cells from the lymph nodes into efferent lymph vessels, thereby interrupting T cell recirculation. When used at the onset of HIV-1 infection, it limited HIV-1 dissemination and reduced plasma viremia (9), indicating that the cell-cell transmission of HIV-1 could be important in the establishment of systemic HIV-1 infection.
Neutralizing antibodies and entry inhibitors effectively block cell-free HIV-1. But with few exceptions, they are much less capable of blocking cell-cell viral transmission (7, 8, 10–14). In T cell-T cell coculture, neutralization was demonstrated only when virus-infected donor T cells were pretreated with antibodies before being added to target T cells (7, 8, 10–14). In dendritic cell (DC)-CD4 T cell cocultures, due to variations in assay systems used by different laboratories, the results were quite variable, sometimes even controversial (15–19). For example, Su et al. showed that both anti-gp120 and anti-gp41 antibodies block the trans-infection (15), whereas Sagar et al. showed that only anti-gp41, but not anti-gp120, antibodies could block the trans-infection (19). Moreover, van Montfort et al. showed that HIV-1 bound to antibodies 2F5, 4E10, and 10E8, but not those bound to b12, NIH45-46, and VRC01, could still be captured by DCs and subsequently infect CD4 T cells (17, 18). Finally, Reh et al. recently tested a panel of 16 broadly neutralizing antibodies against 11 HIV-1 strains during both cell-free and cell-cell transmissions and concluded that the capacity of broadly neutralizing antibodies to inhibit cell-cell transmission of HIV-1 is not only strain and epitope dependent but also dependent on the window of action during the entry process (11). Thus, entry inhibitors that can efficiently block both cell-free and cell-cell transmissions of HIV-1 are urgently needed.
Lipid rafts are specialized dynamic microdomains of the plasma membrane that have been shown to be gateways for HIV-1 budding (20, 21), as well as for HIV-1 entry into T cells and macrophages (21). CD4, the receptor for HIV-1 entry, is located in lipid rafts of the plasma membrane (22–24). Previously, we showed that by genetically linking single-chain Fv (scFv) or third-heavy-chain complementarity-determining region (HCDR3) of human anti-HIV-1 envelope antibodies with a glycosylphosphatidylinositol (GPI) attachment signal from decay-accelerating factor (DAF) (25), scFvs and HCDR3s are targeted into lipid rafts of the plasma membrane. GPI-scFv X5 and 48d and GPI-HCDR3s PG9 and PG16 on the surface of transduced human CD4+ cell lines exhibit potent neutralization against diverse cell-free HIV-1 strains (26, 27). Recently, we showed that trimerization of GPI-HCDR3s PG9 and PG16 further improves anti-HIV-1 neutralizing activity (28). However, so far no studies on the effect of GPI-anchored antibody derivatives on human primary CD4 T cells and on HIV-1 transmission from infected T cells to uninfected T cells have been reported.
In the present study, we constructed fusion genes in which sequences encoding the VHH domains of JM2, JM4, or E4 (here referred to as VHH JM2, VHH JM4, and VHH E4, respectively) and a IgG3 hinge region and histidine (his) tag were genetically linked with or without the sequence encoding the GPI attachment signal of DAF (25). VHH E4, originally isolated from a phage display library, recognizes the Trf2 telomeric protein (J. Matz and S. Benichou, unpublished results) and was used as a negative control. These constructs were used to transduce human CD4+ cell lines and human primary CD4 T cells to investigate whether GPI-VHH JM2 or JM4 would confer broad and potent protection against HIV-1. We report here that transduction of human CD4+ cell lines and primary CD4 T cells with GPI-VHH JM4 confers broad and potent neutralization of HIV-1 via both cell-free and T cell-T cell transmission.
MATERIALS AND METHODS
Gene constructs.
Fusion genes encoding VHH E4, JM2, or JM4, the IgG3 hinge, and the his tag, with or without a GPI attachment signal (C-terminal 34 amino acid residues of DAF), were generated by overlapping PCR, ligated into the TA vector system (Invitrogen Life Technologies, San Diego, CA), and sequenced as described previously (29). The fusion genes with the correct sequences were ligated between the BamHI and SalI sites of a third-generation lentiviral transfer vector, pRRLsin-18.PPT.hPGK.Wpre (30). The resulting lentiviral transfer constructs were designated pRRL-VHH E4, JM2, or JM4/hinge/his-tag/DAF and pRRL-VHH E4, JM2, or JM4/hinge/his-tag, respectively.
Fusion genes encoding GPI-VHH E4 or JM4 were generated by PCR and ligated between the BamHI and SalI sites of a third-generation lentiviral transfer vector, pRRLsin-18.PPT.EF1α.2A.GFP.Wpre (31). The resulting lentiviral transfer constructs were designated pRRL-GPI-VHH E4 or JM4-2A-GFP.
The gene encoding rhesus TRIM5α (rhTRIM5α) was also amplified by PCR using pLPCX-rhTRIM5αvector (32) as a template and a pair of primers (5′-GGACTAGTTCCACCATGGCTTCTGGAATCCTGCTTAATGT-3′ and 5′-CCGCTCGAGTCAAGCGTAGTCTGGGACGTCGTATGGGTA-3′, where the underlined sequences are those recognized by restriction enzymes SpeI and XhoI, respectively), ligated into the SpeI and XhoI sites of the lentiviral transfer vector pTRIP-MND-NeffinB6-IRES-GFP (33), and sequenced. The resulting lentiviral transfer construct was designated pTRIP-rhTRIM5α-IRES-GFP. The lentiviral transfer vector containing the correct rhTRIM5α sequence was used to generate recombinant lentiviruses.
The genes encoding soluble VHH E4 and JM4 were amplified using pRRL-VHH E4 or JM4/hinge/his-tag/DAF as templates and a pair of primers (5′-CGGGATCCGAGGTGCAGCTGGTGGAGTC-3′ and 5′-GGCTCGAGCTAGCTCCCATGGTGATGGTGGT-3′, where the underlined sequences are those recognized by two restriction enzymes, BamHI and XhoI, respectively) and ligated into BamHI and XhoI sites of bacterial expression vector pET28b in frame with a his tag coding sequence and sequenced. The pET28E vectors containing the correct VHH E4 and JM4 sequences were used to transform bacterial strain BL21(DE3) (Invitrogen).
Cell lines and human primary CD4 T cells.
The packaging cell line 293FT was purchased from Invitrogen Life Technologies and was maintained in complete Dulbecco's modified Eagle's medium (DMEM) (i.e., high-glucose DMEM supplemented with 10% fetal bovine serum [FBS], 2 mM l-glutamine, 1 mM sodium pyruvate, penicillin [100 U/ml], and streptomycin [100 μg/ml]) plus G418 (500 μg/ml) (Invitrogen Life Technologies). The human CD4 T cell lines CEMss-CCR5 and Jurkat-CCR5 were generated as described previously (27). TZM.bl cells were obtained from the NIH AIDS Research and Reference Reagent Program (ARRRP; Germantown, MD), contributed by J. Kappes and X. Wu (23, 34–37). CEMss-CCR5, Jurkat-CCR5, and TZM.bl cells were maintained in complete DMEM.
Human peripheral blood mononuclear cells (PBMCs) were obtained from healthy donors through the blood bank of the Changhai Hospital, Shanghai, China. Human primary CD4 T cells were enriched from PBMCs by negatively selecting magnetic beads according to the manufacturer's instructions (Thermo Fisher Scientific) and resuspended in the complete RPMI 1640 medium (i.e., RPMI 1640 medium supplemented with 15% FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, penicillin [100 U/ml], and streptomycin [100 μg/ml]) supplemented with human recombinant interleukin 2 (rIL-2; 100 IU/ml; R&D Systems) before being activated and transduced with recombinant lentiviral vectors.
Generation of recombinant lentiviruses.
Recombinant lentiviruses were generated as described previously (38). Briefly, 4 × 106 293FT cells were seeded onto a P-100 dish in 10 ml of complete DMEM. After culturing overnight, cells were cotransfected with 20 μg of transfer construct (pRRL-VHH E4, JM2, or JM4/hinge/his-tag/DAF, pRRL-VHH E4, JM2, or JM4/hinge/his-tag, pRRL-GPI-VHH E4 or JM4-2A-GFP, or pTRIP-rhTRIM5α-IRES-GFP), 10 μg of packaging construct encoding HIV-1 Gag/Pol (pLP1), 7.5 μg of plasmids encoding the vesicular stomatitis virus G protein (VSV-G) envelope (pLP/VSV-G), and 7.5 μg of HIV-1 Rev protein (pLP2; Invitrogen), using a calcium phosphate precipitation method. Sixteen hours later, culture supernatants were removed and replaced with fresh complete DMEM plus 1 mM sodium butyrate (Sigma). Eight hours later, supernatants were again removed and replaced with fresh DMEM plus 4% FBS. After another 20 h, the culture supernatants were harvested and concentrated by ultracentrifugation as described previously (38). The vector pellets were resuspended in a small volume of DMEM and stored in aliquots in a −80°C freezer. Vector titers were determined as previously described (38). The amount of HIV-1 Gag p24 in concentrated vector stocks was determined by an enzyme-linked immunosorbent assay (ELISA).
Generation of stably transduced CD4+ cell lines and human primary CD4 T cells.
To generate stably transduced TZM.bl cell lines, 5 × 104 TZM.bl cells per well were seeded onto a 24-well plate. After overnight culture, 2 × 106 transducing units (TU) of one of the recombinant lentiviral viruses (expressing VHH E4, JM2, or JM4/hinge/his-tag/DAF or pRRL-VHH E4, JM2, or JM4/hinge/his-tag fusion genes) was added onto a 24-well tissue culture plate in the presence of 8 μg/ml of Polybrene. Twenty-four hours later, cells were extensively washed and cultured in complete DMEM. The expressions of pRRL-VHH E4, JM2, or JM4/hinge/his-tag/DAF and pRRL-VHH E4, JM2, or JM4/hinge/his-tag constructs were measured by fluorescence-activated cell sorter (FACS) analysis and/or Western blot analysis. We usually found that after a single round of transduction, over 98% of cells express transgenes (data not shown). After transduced cells were generated, cells were continuously cultured in complete DMEM and split every 3 or 4 days. Periodically, the expression of transgenes was measured. We found that the level of transgene expression was very stable in the stably transduced cell lines (data not shown).
To generate a stably transduced CEMss-CCR5-rhTRIM5α/GFP cell line, 1 × 105 CEMss-CCR5 cells and 2 × 106 TU of the recombinant lentiviruses containing pTRIP-rhTRIM5α-IRES-GFP were added to a 24-well tissue culture plate in the presence of 8 μg/ml of Polybrene (Sigma). Twenty-four hours later, cells were extensively washed and cultured in complete DMEM. The expression of fusion gene rhTRIM5α-IRES-GFP was analyzed by Western blotting.
To transduce CEMss-CCR5 or CEMss-CCR5-rhTRIM5α/GFP cells, 1 × 105 CEMss-CCR5 or CEMss-CCR5-rhTRIM5α/GFP cells and 2 × 106 TU of one of the recombinant lentiviruses containing pRRL-GPI-VHH E4 or JM4 were added to a 24-well tissue culture plate in the presence of 8 μg/ml of Polybrene (Sigma). Twenty-four hours later, cells were extensively washed and cultured in complete DMEM. The expression of fusion genes rhTRIM5α-IRES-GFP and pRRL-GPI-VHH E4 or JM4 was detected by FACS analysis.
To transduce human primary CD4 T cells, CD4 T cells were enriched from human PBMCs by negatively selecting magnetic beads (Thermo Fisher Scientific). A total of 2.5 × 105 enriched human CD4 T cells per well were activated by mixing with anti-CD3/CD28 antibody-coated microbeads (Thermo Fisher Scientific) at a 1:1 ratio in 500 μl of complete RPMI 1640 medium supplemented with human rIL-2 (100 IU/ml) in 48-well plates. After 24 h, 5 × 106 TU of pseudotyped virions containing pRRL-GPI-VHH E4 or JM4-2A-GFP in complete RPMI 1640 supplemented with human rIL-2 (100 IU/ml) and 8 μg/ml of Polybrene were added into cell suspension at a final volume of 750 μl and a multiplicity of infection (MOI) of 20. The plates were centrifuged at 1,500 × g and 37°C for 2 h to facilitate transduction. After overnight incubation at 37°C, 500 μl of supernatant was removed and 750 μl of fresh complete RPMI 1640 medium supplemented with human rIL-2 (100 IU/ml) were added into cells. The anti-CD3/CD28 antibody-coated microbeads in human CD4 T cells were removed by DynaMag magnet after 4 days of activation. Transduced human CD4 T cells were resuspended in 2 ml of complete RPMI 1640 supplemented with human rIL-2 (100 IU/ml) and cultured in 24-well plates for additional 2 days. The transduction efficiency and CD4, CCR5, and CXCR4 gene expression were estimated by antibody staining followed by FACS analysis before transduced human CD4 T cells were challenged with cell-free HIV-1 and used as recipient cells in T cell-T cell transmission of HIV-1.
The generation of soluble VHH JM4 and E4 and CD4.
To produce soluble VHH JM4 and E4, bacterial strain BL21(DE3) containing the pET28-VHH E4 or JM4 plasmid was cultured in LB medium until the optical density at 600 nm (OD600) reached 0.6 to 0.7. Then 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added and bacteria were incubated for another 8 h at 18°C. Bacteria were lysed and VHH JM4 and E4 proteins were purified from the soluble fraction of the bacterial lysates using the nickel-nitrilotriacetic acid (Ni-NTA) purification system according to the manufacturer's instructions (Invitrogen). Soluble CD4 (sCD4) used in this study was generated as before (27). We purified soluble CD4 and VHH E4 and JM4.
Western blot analysis.
To detect transgene expression, 1 × 106 TZM.bl cells that were mock transduced or transduced with pRRL-VHH E4, JM2, or JM4/hinge/his-tag or pRRL-VHH E4, JM2, or JM4/hinge/his-tag/DAF were grown in DMEM plus 1% FBS on a 6-well plate for 48 h. Cells and supernatant were harvested. Cells were lysed with lysis buffer (100 mM Tris-HCl [pH 8.0], 1% NP-40) in the presence of a protease inhibitor cocktail (Calbiochem). Proteins in supernatant were precipitated by trichloroacetic acid (TCA) and dissolved in a volume of lysis buffer equal to that of the lysed cell pellet. Samples were separated by 12% SDS–PAGE, transferred onto a polyvinylidene difluoride (PVDF) membrane, and detected by mouse anti-his tag antibody (Sigma) and mouse anti-human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody as a control.
To detect the expression of rhTRIM5α/GFP fusion gene, 1 × 106 mock- and rhTRIM5α/GFP fusion gene-transduced CEMss-CCR5 cells were grown in DMEM plus 1% FBS on a 12-well plate for 24 h. Cells were harvested, lysed using lysis buffer in the presence of protease inhibitor cocktail, separated by 12% SDS-PAGE, transferred onto a PVDF membrane (Millipore), and detected by mouse anti-hemagglutinin (anti-HA) tag antibody (Sigma) and mouse anti-GAPDH antibody (Sigma) as a control.
FACS analysis.
To measure green fluorescent protein (GFP) expression, 1 × 105 mock-, rhTRIM5α/GFP-, or GPI-VHH E4- or JM4-2A-GFP-transduced CEMss-CCR5 cells were collected 4 days after transduction (see above), washed twice with FACS buffer (phosphate-buffered saline [PBS] containing 1% bovine serum albumin [BSA] and 0.02% NaN3), and fixed with 1% formaldehyde in 0.5 ml of FACS buffer. FACS analysis was performed with a BD LSRII flow cytometer (BD Biosciences).
To measure the cell surface expression of GPI-VHH E4, JM2, or JM4, 2 × 105 mock- and VHH E4, JM2, or JM4/hinge/his-tag/DAF-transduced TZM.bl cells, mock- and VHH E4- or JM4/hinge/his-tag/DAF-transduced CEMss-CCR5 cells, or mock- and GPI-VHH E4- or JM4-2A-GFP-transduced human primary CD4 T cells were incubated with a mouse anti-his tag antibody for 45 min on ice. Cells were then washed twice with FACS buffer and stained with phycoerythrin (PE)-conjugated goat anti-mouse IgG antibody (Sigma) for another 45 min on ice. Cells then were washed twice with FACS buffer and fixed with 1% formaldehyde in 0.5 ml of FACS buffer. FACS analysis was performed with a BD LSRII flow cytometer.
To determine whether the expression of GPI-VHH E4, JM2, or JM4 is truly targeted through a GPI anchor, 8 × 105 mock- and VHH E4-, JM2-, or JM4/hinge/his-tag/DAF-transduced TZM.bl cells were first incubated with or without 6 U/ml of phosphatidylinositol-specific phospholipase C (PI-PLC; Invitrogen) in 0.5 ml of 1× PBS and rocked at 4°C for 20 min. After incubation, cells were washed twice to remove the remaining PI-PLC and then stained with a mouse anti-his tag antibody as described above.
To determine whether the expression of Sec-VHH or GPI-VHH E4, JM2, or JM4 could alter the cell surface expression of CD4, CCR5, and CXCR4, 2 × 105 mock- and GPI-VHH E4-, JM2-, or JM4- or Sec-VHH E4-, JM2-, or JM4-transduced TZM.bl cells, mock- and GPI-VHH E4- or JM4-transduced CEMss-CCR5 cells, or mock- and pRRL-VHH E4- or JM4-2A-GFP-transduced human primary CD4 T cells were incubated with allophycocyanin (APC)-conjugated anti-human CD4 (Miltenyi), PE-conjugated anti-human CCR5 (BD Science), or PE-conjugated anti-human CXCR4 (BD Science) antibodies for 45 min on ice. Cells were then washed twice with FACS buffer and fixed with 1% formaldehyde in 0.5 ml of FACS buffer. FACS analysis was performed with a BD LSRII flow cytometer.
Intracellular HIV-1 Gag p24 was stained as described before (15). Briefly, at the desired intervals after HIV-1 infection, small portions of cells were harvested, washed with 1 ml of FACS buffer, and fixed and permeabilized with a Cytofix/Cytoperm kit (Becton Dickinson). Cells were resuspended in 50 μl of Perm/Wash buffer with 0.8 μl of PE-conjugated anti-Gag p24 antibody (KC57; Beckman Coulter) and incubated for 30 min on ice. After being washed twice with 1 ml of Perm/Wash buffer, the cells were resuspended in 400 μl of FACS buffer and analyzed by FACS Fortessa (BD Biosciences) using FlowJo and GraphPad Prism software.
Immunofluorescence staining and confocal analysis.
Mock- and GPI-VHH E4-, JM2-, or JM4-transduced TZM.bl cells were seeded (5,000 cells per well) onto a tissue culture-treated glass slide (BD Biosciences) and incubated at 37°C in 5% CO2 for 2 days. Cells were then washed twice with 500 μl of PBS and fixed with fixation buffer (4% formaldehyde in PBS plus 1% BSA) for 15 min. Cells were washed twice with 500 μl of PBS and blocked with blocking buffer (5% goat serum in PBS plus 1% BSA) for 1 h. Cells were stained with Alexa 555-conjugated cholera toxin subunit B (CtxB; Invitrogen Life Technologies) at 4°C for 45 min. After being washed 3 times with PBS, cells were stained with mouse anti-his tag antibody at 4°C for 45 min and then stained with Alexa 488-conjugated goat anti-mouse IgG antibody (Invitrogen) at 4°C. After cells were washed 3 times with PBS, cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) in permeabilization buffer (blocking buffer plus 0.5% saponin) for 5 min. The slides were mounted before being analyzed under a confocal fluorescence microscope (Zeiss model LSM 510).
Chemotaxis assay.
The chemotaxis response of GPI-VHH E4 or JM4-transduced CEMss-CCR5 cells was assessed using Transwell filters (5-μm pore size; Corning) with 100 ng/ml of SDF1α (Peprotech) used as a chemoattractant. While 3 × 105 cells were resuspended in 300 μl of RPMI 1640 in the upper chamber, 600 μl of RPMI 1640 medium with or without 100 ng/ml of SDF1α was added to the lower chamber. Chemotaxis assays were performed at 37°C in 5% CO2 for 3 h. The filter was then removed, and the number of cells in the lower chamber was determined by flow cytometry (Accuri C6).
Capping assay.
To analyze the capping of the CXCR4 coreceptor, mock- and GPI-VHH-JM4- or E4-transduced CEMss-CCR5 cells were stimulated with 100 ng/ml of SDF1α for 10 min and then fixed with 4% paraformaldehyde (PFA) in PBS. Cells were then plated onto coverslips coated with poly-l-lysine (0.002%, wt/vol; Sigma) in H2O2 for 2 min and stained with anti-CXCR4 antibody (R&D Systems; MAB172 at 10 μg/ml) for 30 min and Alexa 488-conjugated anti-mouse IgG Fc antibody (Invitrogen) for 30 min. Cells were then permeabilized in PBS containing 1% BSA and 0.15% Triton X-100 and stained with Alexa 647-conjugated phalloidin (Invitrogen). Coverslips were washed with PBS and mounted using 10 μl of Fluoromount media containing DAPI (Sigma). Images were acquired on a spinning-disk (CSU-X1M1; Yokogawa) inverted microscope (DMI6000, Leica) equipped with a CoolSnap HQ2 camera (Photometrics) with a 63× oil objective using Metamorph software (v7.7.5 Molecular Devices) and processed using Fiji (ImageJ; NIH).
Generation of HIV-1 pseudotypes and viruses.
To generate HIV-1 pseudotypes, 4 × 106 293FT packaging cells were cotransfected with 10 μg of an HIV-1–luciferase transfer vector and 1 μg of a DNA plasmid encoding one of several HIV-1 envelopes (Q168, Q461ENVe2, Yu2, AD8, JRFL, SF162, HxBc2, consensus B and C, PVO.4, QH0692.42, CNE3, CNE5, CNE8, CNE11, CNE15, CNE50, CNE55, 92BR025.9, 93TH966.8, and 92UG975.10) or control VSV-G using a calcium phosphate precipitation method. The pseudotype-containing supernatants were harvested and stored in aliquots in a freezer at −80°C. Titers of pseudotyped virions were determined as previously described (38).
To generate infectious HIV-1, 4 × 106 293FT packaging cells were transfected with 10 μg of one of infectious HIV-1 molecular clones Bru-3, Yu2, AD8, JRCSF, and PBRGX, one of molecular clones of HIV-1 transmitted founder viruses WITO, CH040, THRO, REJO, CH077, and CH106 (39), or a molecular clone of simian immunodeficiency virus (SIV) strain SIVMne027 (40) using a calcium phosphate precipitation method. The virus-containing supernatants were harvested and stored in aliquots in a freezer at −80°C. Virus titers were determined as previously described (27). The amount of HIV-1 p24 in collected supernatants was measured by the RETROtek HIV-1 p24 antigen ELISA (Zeptometrix Corporation).
Single-cycle infectivity assay.
In a single-cycle assay, 1 × 104 TZM.bl cells mock transduced or transduced with pRRL-VHH E4, JM2, or JM4/hinge/his-tag/DAF-, and pRRL-VHH E4, JM2, or JM4/hinge/his-tag were transduced with HIV-1 or 10A1 pseudotype-containing supernatants equivalent to a relative luciferase activity (RLA) of 100,000 to 500,000 overnight or infected with HIV-1 strains Bru-3, Bru-Yu2, AD8, JRCSF, and PBRGX, HIV-1 transmitted founder viruses WITO, CH040, THRO, REJO, CH077, and CH106, and SIV strain SIVMne027 as a control at an MOI of 2 in a final volume of 0.2 ml overnight. Cells were then washed twice with PBS and cultured in complete DMEM for 2 days. Cells were then washed once with PBS and lysed in 100 μl of Glo-lysis buffer. The luciferase activity in 50-μl cell suspensions was measured by a BrightGlo luciferase assay according to the manufacturer's instructions (Promega).
Cell-free HIV-1 infection and p24 assay.
To test the effect of GPI-VHH JM4 on cell-free HIV-1 infection, 1 × 106 GPI-VHH JM4- or E4-transduced CEMss-CCR5 cells were infected with HIV-1 strains Bru-3, Bru-Yu2, JRCSF, AD8, THRO.c, and Mj4 at an MOI of 0.01 in a final volume of 0.5 ml overnight. Cells were then extensively washed with Hanks' balanced salt solution (HBSS), resuspended in 6 ml of complete DMEM, and cultured for 30 days. Every 3 days, 4.5 ml of cell suspensions was harvested and replaced with fresh medium. The supernatants were then collected. HIV-1 Gag p24 in the supernatants was measured by ELISA (Zeptometrix Corporation) according to the manufacturer's instructions.
To test the effect of GPI-VHH JM4 on cell-free HIV-1 infection in transduced human primary CD4 T cells, 1 × 106 human primary CD4 T cells transduced with GPI-VHH E4 or JM4-2A-GFP (see above) were infected with HIV-1 Bru-3 and AD8 at an MOI of 0.01 in a final volume of 0.5 ml overnight. Cells were then extensively washed with HBSS, resuspended in 2 ml of complete RPMI 1640 supplemented with 100 IU/ml of human rIL-2, and cultured on 24-well plates for 9 days. Every 3 days, small portions of cells were harvested and replaced with fresh RPMI 1640 supplemented with 100 IU/ml of human rIL-2. HIV-1 infection was measured by intracellular Gag p24 staining at 3, 6, and 9 days postinfection (see above).
Measurement of T cell-T cell transmission of HIV-1.
To establish HIV-1-infected donor cells, CEMss-CCR5 cells were incubated with HIV-1 JRCSF and Jurkat-CCR5 cells were incubated with HIV-1 Bru-3 or AD8 overnight at an MOI of 5 and washed twice with RPMI 1640. Infected CEMss-CCR5 and Jurkat-CCR5 cells were then cultured for 4 days at 37°C and the percentage of infected cells was assessed by intracellular Gag p24 staining (see above).
To assess the effect of GPI-VHH JM4 on cell-cell transmission of HIV-1 in transduced human CD4+ T cell lines, 1 × 105 HIV-1 JRCSF-infected or uninfected CEMss-CCR5 cells (see above) were cocultured with 5 × 105 CEMss-CCR5-rhTRIM5α/GFP cells transduced with lentiviral vectors expressing VHH E4 or JM4/hinge/his-tag/DAF. Infection was monitored for 12 days after coculture by measuring intracellular Gag p24 protein expression in the target cell population as described in the section on FACS analysis (see above). For the comparison, 1 × 105 HIV-1 JRCSF-infected or uninfected CEMss-CCR5 cells cocultured with 5 × 105 CEMss-CCR5-rhTRIM5α/GFP cells in the presence of 10 or 40 μg/ml of soluble VHH E4 or JM4 were also monitored for 12 days post coculture. The cell-cell transmission of HIV-1, as measured by intracellular Gag p24 staining in target cells and Gag p24 in coculture supernatants, was assessed every other day postcoculture. Percent inhibition was determined by the equation 100 − 100 × [percent infected cells in coculture between HIV-1-infected CEMss-CCR5 cells and CEMss-CCR5-rhTRIM5α/GFP cells transduced with lentiviral vector expressing VHH E4/hinge/his-tag/DAF]/[percent infected cells in coculture between HIV-1-infected CEMss-CCR5 cells and CEMss-CCR5-rhTRIM5α/GFP cells transduced with lentiviral vector expressing VHH JM4/hinge/his-tag/DAF].
For the comparison, JRCSF-infected donor cells and uninfected CEMss-CCR5-rhTRIM5α/GFP cells or CEMss-CCR5-rhTRIM5α/GFP cells transduced with pRRL-VHH E4 or JM4/hinge/his-tag/DAF were also cocultured in Transwell chambers (12-well 0.4-mm polyester-membrane dishes; Corning Life Sciences, Corning, NY), in which JRCSF-infected donor cells added to the Transwell insert and uninfected CEMss-CCR5-rhTRIM5α/GFP cells or CEMss-CCR5-rhTRIM5α/GFP cells transduced with pRRL-VHH E4 or JM4/hinge/his-tag/DAF were seeded as target cells in the bottom chamber. Intracellular Gag p24 staining was performed as described above.
To assess the effect of GPI-VHH JM4 on cell-cell transmission of HIV-1 in transduced human primary CD4 T cells, 1 × 105 HIV-1 AD8- or Bru-3-infected or uninfected Jurkat-CCR5 cells (see above) were cocultured with 4 × 105 GPI-VHH E4- or JM4.2A.eGFP-transduced human primary CD4 T cells in 2 ml of complete RPMI 1640 supplemented with human rIL-2 (100 IU/ml). After 3, 6, and 9 days of coculture at 37°C, the infectivity in transduced human primary CD4 T cells was assessed by intracellular Gag p24 staining (see above).
Cell-cell fusion experiment.
A total of 1 × 105 HIV-1 Bru-3 and JRCSF-infected or uninfected CEMss-CCR5 cells (see above) were cocultured with 5 × 105 CEMss-CCR5-rhTRIM5α/GFP cells transduced with lentiviral vectors expressing VHH E4 and JM4/hinge/his-tag/DAF on a 12-well plate at 37°C and 5% CO2. As controls, 1 × 105 HIV-1 Bru-3- or JRCSF-infected or uninfected CEMss-CCR5 cells were cocultured with 5 × 105 CEMss-CCR5-rhTRIM5α/GFP cells in the presence of 10 or 40 μg/ml of soluble VHH E4 or JM4. Cell-cell fusion was monitored by light microscopy at various intervals and recorded by a charge-coupled-device (CCD) digital camera (Leica DM IRB). The syncytia (3 pictures per coculture sample) were counted manually.
RESULTS
Targeting VHH JM2, JM4, and E4 to the lipid rafts of the plasma membrane through a GPI anchor.
To generate GPI-anchored and secreted VHH, the sequences encoding VHH from the heavy chain-only antibodies E4, JM2, and JM4 were genetically linked with the sequence encoding a his-tagged IgG3 hinge region and with or without the sequence encoding a GPI attachment signal of DAF. The VHH/hinge/his-tag/DAF and the VHH/hinge/his-tag fusion genes were inserted into a third-generation lentiviral vector, pRRLsin-18.PPT.hPGK.Wpre (Fig. 1A). The recombinant viruses were then generated and used to transduce TZM.bl, CEMss-CCR5, and CEMss-CCR5-rhTRIM5α/GFP cells (see below).
FIG 1.
Expression of GPI-VHH and Sec-VHH in transduced TZM.bl cells. (A) Schematic diagram of the lentiviral vectors pRRL-VHH/hinge/his-tag and pRRL-VHH/hinge/his-tag/DAF. VHHs were derived from llama heavy chain-only antibodies E4, JM2, and JM4. Hinge, a human IgG3 hinge region; his-tag: a 6-histidine residue tag; DAF, the C-terminal 34 amino acid residues of decay-accelerating factor. (B) Western blot analysis of VHH E4, JM2, and JM4 in TZM.bl cells transduced with VHH/hinge/his-tag and VHH/hinge/his-tag/DAF. GPI-VHH, GPI-anchored VHH; Sec-VHH, secreted VHH; anti-his, anti-his tag antibody; SN, supernatants; CL, cell lysates. (C) FACS analysis of cell surface expression of GPI-VHH E4, JM2, and JM4 in mock- or VHH/hinge/his-tag/DAF-transduced TZM.bl cells with or without PI-PLC treatment. Red, mock-transduced cells stained with anti-his tag antibody; blue, VHH E4, JM2, or JM4/hinge/his-tag/DAF-transduced cells without PI-PLC treatment stained with anti-his tag antibody; yellow, VHH E4, JM2, or JM4/hinge/his-tag/DAF-transduced cells with PI-PLC treatment stained with anti-his tag antibody. (D) Confocal analysis of mock or pRRL-VHH/hinge/his-tag/DAF-transduced TZM.bl cells. CtxB, cells were stained with Alexa 555-conjugated cholera toxin B subunit; anti-his, cells were stained with mouse anti-his tag antibody followed by Alexa 488-conjugated goat anti-mouse IgG antibody. Scale bar: 10 μm.
Figure 1B shows the expression of VHH/hinge/his-tag/DAF and VHH/hinge/his-tag fusion genes in cell lysates and culture supernatants of transduced TZM.bl cells by Western blotting using anti-his tag and anti-GAPDH antibodies. As expected, without a GPI attachment signal, VHHs were detected in both culture supernatants and cell lysates, with a majority in supernatants (right side). In contrast, with a GPI attachment signal VHH were detected only in cell lysates and not in culture supernatants (left side), indicating that inclusion of a GPI attachment signal allows for cell association and prevents secretion of the VHH.
To determine if VHH/hinge/his-tag/DAF was expressed on the cell surface through a GPI anchor, VHH/hinge/his-tag/DAF E4, JM2, and JM4-transduced TZM.bl cells were treated or not with phosphatidylinositol phospholipase C (PI-PLC) and stained with anti-his tag antibody, followed by FACS analysis. Figure 1C shows that VHH/hinge/his-tag/DAFs were highly expressed at the cell surface and their expression was significantly reduced with PI-PLC treatment, indicating that VHH/hinge/his-tag/DAF is attached to the cell surface through a GPI anchor. Thus, we refer to the VHH/hinge/his-tag/DAF and VHH/hinge/his-tag as GPI-VHH and Sec-VHH, respectively.
To localize GPI-VHH, mock- and GPI-VHH E4-, JM2-, or JM4-transduced TZM.bl cells were seeded onto a glass slide and costained with (i) anti-his tag antibody followed by Alexa 488-conjugated anti-mouse IgG antibody, (ii) Alexa 555-conjugated cholera toxin subunit B (CtxB), and (iii) DAPI. CtxB interacts with GM1 (a lipid raft marker). Figure 1D shows that GPI-VHH E4, JM2, or JM4 is colocalized with GM1 on cell surface, indicating that it is located in the lipid rafts of the plasma membrane.
GPI-VHH JM4 in transduced TZM.bl cells exhibits a remarkable degree of breadth and potency against HIV-1.
Next, we compared CD4, CCR5, and CXCR4 expression in Sec-VHH- and GPI-VHH E4-, JM2-, or JM4-transduced TZM.bl cells by flow cytometry and found that there was no significant difference in their expression compared to that in mock-transduced TZM.bl cells, suggesting that the expression of transgenes does not alter the expression of the receptor and the coreceptors for HIV-1 in the transduced cells (Fig. 2A). Nor did we find that the expression of the transgenes alters the cell growth (data not shown).
FIG 2.
Neutralization by GPI-VHH and Sec-VHH JM2 and JM4 in transduced TZM.bl cells against HIV-1 or VSV-G pseudotypes using a single-cycle infectivity assay. (A) Summary of mean and median fluorescence intensities of CD4, CCR5, and CXCR4 expression in mock-transduced and GPI-VHH and Sec-VHH E4-, JM2-, and JM4-transduced TZM.bl cells. (B) Neutralization by GPI-VHH and Sec-VHH E4, JM2, and JM4 against HIV-1 and VSV-G pseudotypes. The numbers represent the percent inhibition, which was calculated as follows: (RLA in virus alone to a given transduced cell − RLA in no virus to the same transduced cell)/(RLA in virus alone to parental cells − RLA in no virus to parental cell). (C and D) Purified soluble CD4 (sCD4) and soluble VHH (sVHH) JM4 (lane 2) and E4 (lane 3) revealed by SDS-PAGE followed by Coomassie blue staining (C) or Western blotting (D). (E) Determination of the highest concentrations of sCD4 that did not have an inhibitory effect by itself on a given HIV-1 strain (highlighted in red). These sCD4 concentrations were used in the coculture assay with sVHH JM4 and E4. (F) The neutralization activity of various indicated concentrations of sVHH JM2 and JM4 in the presence or absence of sCD4 on infectivity of HIV-1 and VSV-G pseudotypes. The numbers represent the percent inhibition, based on the calculation described above.
To test neutralization activity of Sec-VHH and GPI-VHH E4, JM2, and JM4 against cell-free HIV-1, a panel of 21 HIV-1 pseudotypes and a VSV-G pseudotype control were used to infect transduced TZM.bl cells in a single-round infectivity assay (38). The 21 HIV-1 pseudotypes consist of HIV-1 envelopes derived from subtype A (Q168 and Q461ENVe2), subtype B (Yu2, AD8, JRFL, SF162, HxBc2, consensus B, PVO.4, and QH0692.42), subtype B′ (CNE11), subtype C (consensus C and 92BR025.9), CRF07_B′C recombinant (CNE15 and CNE50), CRF01_AE recombinants (CNE3, CNE5, CNE8, CNE55, and 93TH966.8), and subtype G (92UG975.10). Of note, Q168, Q461ENV2e, Yu2, AD8, JRFL, QH0692.42, CNE5, CNE8, CNE11, CNE15, CNE55, and 92BR025.9 are tier 2 strains and PVO.4 is a tier 3 strain. 93TH966.8 and 92UG975.10 are two soluble sdAb JM4-resistant strains (2). Figure 2B shows that while mock-transduced TZM.bl cells and TZM.bl cells transduced with Sec-VHH E4, JM2, or JM4 and with GPI-VHH E4 or JM2 did not neutralize any of 21 HIV-1 pseudotypes tested, TZM.bl cells transduced with GPI-VHH JM4 had a high degree of potency and breadth against all 21 HIV-1 pseudotypes. Except for 89.4 and 93.5% neutralization activity against HIV-1 92UG975.10 and Q461ENV2e pseudotypes, respectively, over 99% neutralization activity was detected against all other 19 HIV-1 pseudotypes. For the comparison, none of the transduced TZM.bl cells neutralized the VSV-G pseudotype control.
To confirm that 93TH966.8 and 92UG975.10 are indeed sdAb JM4-resistant strains (2), the Yu2, HxBc2, 93TH966.8, and 92UG975.10 pseudotypes, along with 10A1 as a pseudotype control, were incubated with various concentrations (ranging from 0.2 to 40 μg/ml) of sVHH JM4 or E4 in the presence of indicated concentrations of soluble CD4 (sCD4) (Fig. 2C to E). Figure 2F shows that while the Yu2 and HxBc2 pseudotypes were efficiently neutralized by high doses of sVHH JM4, the 93TH966.8 and 92UG975.10 pseudotypes indeed were totally resistant to neutralization by sVHH JM4 in the presence or absence of sCD4.
To test if GPI-VHH JM4-transduced TZM.bl cells are resistant to replication competent HIV-1 infection, GPI-VHH- and Sec-VHH E4-, JM2-, or JM4-transduced TZM.bl cells were infected with HIV-1 strains Yu2, AD8, ADA, JRCSF, and PBRGX, transmitted founder viruses WITO, CH040, THRO, REJO, CH077, and CH106, and quasispecies HKU1721, HKU004, and HKU1447 along with an SIV control, SIVMne027 (40), in a single-round infectivity assay. Table 1 shows that although all transduced TZM.bl cells were equally infected with SIVmne027, TZM.bl cells transduced with GPI-VHH JM4 neutralized all HIV-1 variants tested at a level of >99%. In contrast, TZM.bl cells transduced with GPI-VHH E4 or Sec-VHH E4 had no significant neutralization activity. Interestingly, TZM.bl cells transduced with GPI-VHH JM2 or Sec-VHH JM2 and JM4 exhibited moderate neutralization (ranging from 51 to 81% inhibition) against a few HIV-1 strains (Bru-3, Mj4, THRO, CH040, and CH077). Thus, taken together, these results clearly show that TZM.bl cells transduced with GPI-VHH JM4 exhibit remarkable degrees of breadth and potency against both HIV-1 pseudotypes and replication-competent HIV-1, including tier 2 or 3 strains and strains that are resistant to soluble sdAb JM4.
TABLE 1.
Neutralization activities of GPI-VHH and Sec-VHH E4, JM2, and JM4 against replication-competent HIV-1a
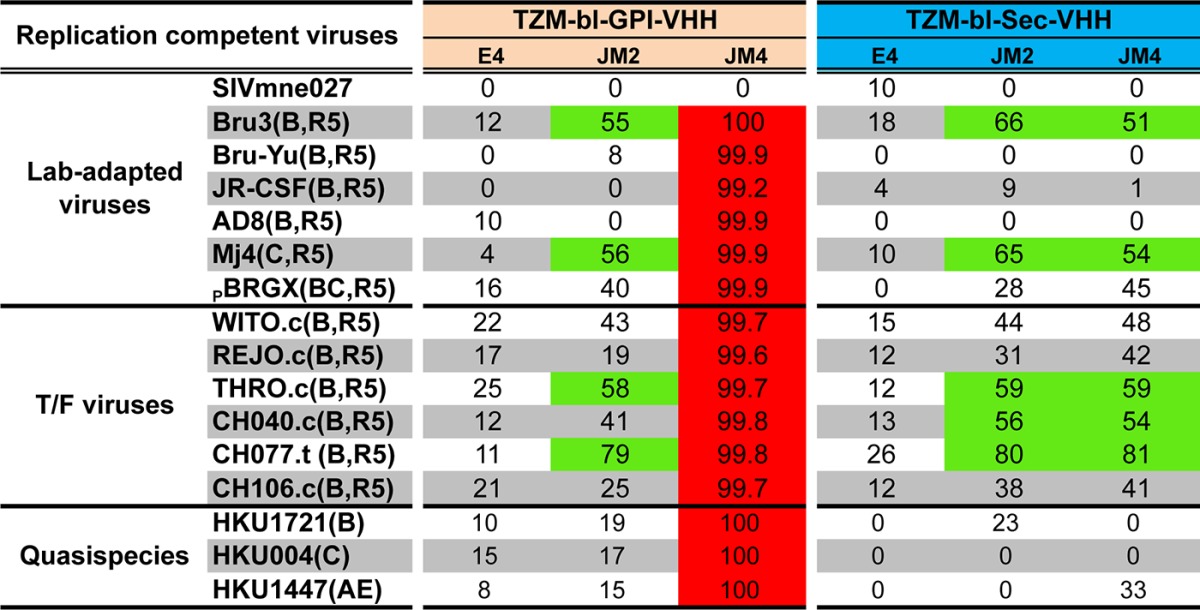
Red indicates over 99% neutralization activity; green indicates from 50% to 89% neutralization activity.
GPI-VHH JM4-transduced CEMss-CCR5 cells completely resist cell-free HIV-1 infection.
While TZM.bl cells, which were engineered with an HIV-1 long terminal repeat (LTR)-driven reporter gene, have been widely used in antibody neutralization assays (23, 34–37), they are of epithelial origin (23). Therefore, we transduced cells of the human CD4+ T cell line CEMss-CCR5 with lentiviral vectors expressing GPI-VHH E4 or JM4. Importantly, both GPI-VHH E4 and JM4 are expressed at high levels on transduced CEMss-CCR5 cells (Fig. 3A and B), and no significant difference in CD4, CCR5, and CXCR4 expression was observed in GPI-VHH E4- and JM4-transduced CEMss-CCR5 cells compared to that in mock-transduced CEMss-CCR5 cells (Fig. 3C). Importantly, when stimulated with 100 ng/ml of SDF1α, mock- and GPI-VHH E4- and JM4-transduced CEMss-CCR5 cells exhibited comparable levels of migration (chemotaxis) (Fig. 3D) as well as capping activities (Fig. 3E), indicating that the functions of CXCR4 coreceptor are not impaired due to the lipid raft expression of GPI-VHHs.
FIG 3.
Neutralization activity of GPI-VHH JM4 in transduced CEMss-CCR5 cells to replication-competent HIV-1. (A) Western blot analysis of mock- and GPI-VHH E4 or JM4-transduced CEMss-CCR5 cells. Anti-his tag, anti-his tag antibody; anti-GAPDH, anti-GAPDH antibody; SN, supernatants; CL, cell lysates. (B) Expression of GPI-VHH E4 and JM4 on the surface of transduced CEMss-CCR5 cells. (Left) Mock- and GPI-VHH E4-transduced CEMss-CCR5 cells; (right) mock- and GPI-VHH JM4-transduced CEMss-CCR5 cells. (C) Summary of mean and median fluorescence intensities of CD4, CCR5, and CXCR4 expression in mock- and GPI-VHH E4- and JM4-transduced CEMss-CCR5 cells. (D) Chemotaxis of mock- and GPI-VHH E4- or JM4-transduced CEMss-CCR5 cells to SDF1α. Cells were loaded onto the upper chamber of a 5-μm Transwell filter, whereas medium containing SDF1α was added in the lower chamber. After 3 h of incubation, the filter was removed and the cell number in the lower chamber was determined by flow cytometry. (E) CXCR4 coreceptor capping of mock- or GPI-VHH E4- or JM4-transduced CEMss-CCR5 cells after stimulation with SDF1α for 10 min followed by stained using anti-CXCR4 antibody, phalloidin, and DAPI and analysis by confocal microscopy. (F) GPI-VHH JM4 confers long-term resistance to HIV-1 Bru-3, Bru-YU2, JRCSF, AD8, THRO.c, and Mj4 in transduced CEMss-CCR5 cells. Mock, mock-transduced CEMss-CCR5 cells; GPI-VHH E4, GPI-VHH E4-transduced CEMss-CCR5 cells; GPI-VHH JM4, GPI-VHH JM4-transduced CEMss-CCR5 cells.
Mock- and GPI-VHH E4- and JM4-transduced CEMss-CCR5 cells were then infected with HIV-1 strains Bru-3, Yu2, JRCSF, AD8, THRO.c, and Mj4 using an MOI of 0.01 as described before (26) and cultured in complete DMEM for 30 days or longer. As shown in Fig. 3F, the replication of all 6 HIV-1 strains was completely inhibited in CEMss-CCR5 cells transduced with GPI-VHH JM4 throughout the experiments. In contrast, robust replication of all 6 HIV-1 strains was observed in mock- and GPI-VHH E4-transduced CEMss-CCR5 cells. Thus, we concluded that GPI-VHH JM4 expression in CEMss-CCR5 cells completely blocks viral replication.
GPI-VHH JM4-transduced CEMss-CCR5 cells resist T cell-T cell HIV-1 transmission.
HIV-1 infection is potently restricted by rhesus TRIM5α (rhTRIM5α) at postentry steps preceding integration (41). Notably, this restriction inhibits infection only by cell-free HIV-1 and not virus by cell-cell transmission (42). Thus, before we examined if GPI-VHH JM4 could block T cell-T cell transmission of HIV-1, we generated CEMss-CCR5 cells stably expressing rhTRIM5α and GFP from a bicistronic expression vector (Fig. 4A). Figure 4B shows that in rhTRIM5α/GFP-transduced CEMss-CCR5 cells, both GFP- and HA-tagged rhTRIM5α was expressed well (lane 2) compared to expression in mock-transduced CEMss-CCR5 cells (lane 1). We then tested the effect of rhTRIM5α on cell-free and cell-cell transmission of HIV-1. Clearly, although the expression of rhTRIM5α does not alter CD4, CCR5, and CXCR4 expression (Fig. 4C), it significantly blocks cell-free HIV-1 (Fig. 4D) but not cell-cell transmission of HIV-1 (Fig. 4E and F). These data are consistent with previous studies (42).
FIG 4.
Effect of rhesus TRIM5α on cell-free and cell-cell transmission of HIV-1. (A) Schematic diagram of the lentiviral vector MND-rhTRIM5α-IRES-GFP. In this vector the rhTRIM5α-IRES-GFP was driven by an internal MND promoter. (B) Western blot analysis of mock- and rhTRIM5α-IRES-GFP-transduced CEMss-CCR5 cells (lanes 1 and 2) as well as GPI-VHH E4 or JM4-transduced CEMss-CCR5-rhTRIM5α-IRES-GFP cells (lanes 3 and 4). Anti-his, anti-his tag antibody; anti-rhTRIM5α-HA, anti-HA tag antibody to detect HA-tagged rhTRIM5α; anti-GFP, anti-GFP antibody; anti-GAPDH, anti-GAPDH antibody. (C) Summary of mean and median fluorescence intensities of CD4, CCR5, and CXCR4 expression in CEMss-CCR5-rhTRIM5α/GFP cells and CEMss-CCR5-rhTRIM5α/GFP cells transduced with GPI-VHH E4 or JM4. (D) rhTRIM5α/GFP-transduced CEMss-CCR5 cells are resistant to cell-free HIV-1 JRCSF infection measured by HIV-1 Gag p24 in culture supernatants. (E and F) rhTRIM5α/GFP-transduced CEMss-CCR5 cells are susceptible to the cell-cell transmission of HIV-1 JRCSF measured by GFP+ Gag+ cells. (E) GFP+ Gag+ cells in coculture between uninfected CEMss-CCR5 cells and rhTRIM5α/GFP-transduced CEMss-CCR5 cells. (F) GFP+ Gag+ cells in coculture between HIV-1 JRCSF-infected CEMss-CCR5 cells and rhTRIM5α/GFP-transduced CEMss-CCR5 cells.
To test if GPI-VHH JM4 blocks T cell-T cell transmission of HIV-1, CEMss-CCR5-rhTRIM5α/GFP cells were transduced with lentiviral vectors expressing GPI-VHH E4 or JM4. Figure 4B shows that both GPI-VHH E4 (lane 3) and JM4 (lane 4) are well expressed. No significant difference in CD4, CCR5, and CXCR4 expression was observed in CEMss-CCR5-rhTRIM5/GFP-GPI-VHH E4 and JM4 cells compared to CEMss-CCR5-rhTRIM5α/GFP cells (Fig. 4C). GPI-VHH E4- or JM4-transduced CEMss-CCR5-rhTRIM5α/GFP cells were then cocultured with HIV-1 JRCSF-infected or uninfected CEMss-CCR5 cells in a regular 24-well plate. For comparison, mock- and GPI-VHH E4- or JM4-transduced CEMss-CCR5-rhTRIM5α/GFP cells were also cocultured with HIV-1 JRCSF-infected CEMss-CCR5 cells in a 24-well Transwell plate. Every 2 days postcoculture, small aliquots of culture supernatants were collected for measuring HIV-1 Gag p24 for a total of 12 days. At 2 and 12 days postcoculture, small aliquots of cells were also harvested, permeabilized, and stained with PE-conjugated anti-HIV-1 Gag p24 antibody. Figure 5A shows the cell-cell transmission of HIV-1 as measured by GFP+ Gag+ cells at 2 days postcoculture. Compared to the coculture between mock-transduced CEMss-CCR5-rhTRIM5α/GFP cells and uninfected donor cells (0.4%) (leftmost image), significant HIV-1 transmission (41.6% or 35.4%, respectively) was observed in the coculture between mock- or GFP-GPI-VHH E4-transduced CEMss-CCR5-rhTRIM5α/GFP cells and HIV-1 JRCSF-infected cells (middle left and middle right images). In contrast, a significant reduction in HIV-1 transmission (5.6%) was found in GFP-GPI-VHH JM4-transduced CEMss-CCR5-rhTRIM5α/GFP cells and HIV-1 JRCSF-infected donor cells (rightmost image). As expected, no cell-cell HIV-1 transmission was observed in coculture between mock-, GFP-GPI-VHH E4-, or JM4-transduced CEMss-CCR5-rhTRIM5α/GFP and HIV-1 JRCSF-infected cells in a Transwell plate (Fig. 5B). A similar pattern of cell-cell HIV-1 transmission was observed at 12 days postcoculture (Fig. 5C).
FIG 5.
GPI-VHH-JM4-transduced CEMss-CCR5-rhTRIMα/GFP cells are resistant to T cell-T cell transmission of HIV-1. (A) GFP+ Gag+ cells in day 2 coculture using regular culture plates between uninfected CEMss-CCR5 and rhTRIM5α/GFP-transduced CEMss-CCR5 cells (far left), GFP+ Gag+ cells in coculture between HIV-1 JRCSF-infected CEMss-CCR5 and rhTRIM5α/GFP-transduced CEMss-CCR5 cells (left middle), GFP+ Gag+ cells in coculture between HIV-1 JRCSF-infected CEMss-CCR5 and GPI-VHH E4-transduced CEMss-CCR5-rhTRIM5α/GFP cells (middle right), and GFP+ Gag+ cells in coculture between HIV-1 JRCSF-infected CEMss-CCR5 and GPI-VHH JM4-transduced CEMss-CCR5-rhTRIM5α/GFP cells (far right). (B) Same coculture as in panel A but using Transwell plates. (C) Same coculture as in panel A but with day 12 coculture samples. (D) GFP+ Gag+ cells in coculture between HIV-1 JRCSF-infected CEMss-CCR5 and rhTRIM5α/GFP-transduced CEMss-CCR5 cells in the presence of 40 and 10 μg/ml of sVHH E4 (far left and middle left) and GFP+ Gag+ cells in coculture between HIV-1 JRCSF-infected CEMss-CCR5 and rhTRIM5α/GFP-transduced CEMss-CCR5 cells in the presence of 40 and 10 μg/ml of soluble JM4 (middle right and far right). (E) HIV-1 replication measured by Gag p24 in coculture supernatants between HIV-1 JRCSF-infected CEMss-CCR5 and rhTRIM5α/GFP-transduced CEMss-CCR5 cells and between HIV-1 JRCSF-infected CEMss-CCR5 and GPI-VHH E4- or JM4-transduced CEMss-CCR5-rhTRIM5α/GFP cells, along with HIV-1 JRCSF-infected CEMss-CCR5 alone.
For comparison, we also did intracellular Gag staining in coculture between CEMss-CCR5-rhTRIM5α/GFP cells and JRCSF-infected CEMss-CCR5 donor cells in the presence of 10 or 40 μg/ml of soluble VHH E4 and JM4. Figure 5D shows that in the presence of 10 or 40 μg/ml of soluble VHH E4, significant HIV-1 transmission (36.2% or 42.6%, respectively) occurred in the coculture between CEMss-CCR5-rhTRIM5α/GFP cells and HIV-1 JRCSF-infected cells (middle left and leftmost images), whereas in the presence of 10 or 40 μg/ml of soluble VHH JM4, HIV-1 transmission was moderately reduced, to 28.7% and 14.2%, respectively, in coculture between JRCSF-infected CEMss-CCR5 donor cells and CEMss-CCR5-rhTRIM5α/GFP cells (rightmost and middle right images). The experiment was repeated three times, with similar results. Thus, taken together, these results indicate that GPI-VHH JM4 effectively blocks T cell-T cell transmission of HIV-1, whereas soluble VHH JM4 has only a moderate effect.
The results of intracellular Gag staining shown above correlate well with Gag in culture supernatants. Robust HIV-1 replication as measured by Gag in culture supernatants was detected in cocultures between mock- or GPI-VHH E4-transduced CEMss-CCR5-rhTRIM5α/GFP cells and HIV-1 JRCSF-infected donor cells. In contrast, HIV-1 replication in cocultures between GPI-VHH JM4-transduced CEMss-CCR5-rhTRIM5α/GFP cells and HIV-1 JRCSF-infected donor cells was significantly lower, similar to the case with HIV-1 JRCSF-infected donor cells alone (Fig. 5E).
GPI-VHH JM4 completely blocks HIV-1 envelope-mediated cell-cell fusion.
Figure 6A and D show the HIV-1 envelope-mediated cell-cell fusion as measured by syncytium formation at 2 days postcoculture. Compared to the cocultures between CEMss-CCR5-rhTRIM5α/GFP cells and uninfected donor cells (Fig. 6A, leftmost image), significant HIV-1 envelope-mediated cell-cell fusion (on average, there were 40 and 41 syncytia per picture, respectively) was observed in cocultures between CEMss-CCR5-rhTRIM5α/GFP or CEMss-CCR5-rhTRIM5α/GFP-GPI-VHH E4 cells and HIV-1 JRCSF-infected cells (Fig. 6A and D, middle left and middle right images). In contrast, no HIV-1 envelope-mediated cell-cell fusion was observed in cocultures between GPI-VHH JM4-transduced CEMss-CCR5-rhTRIM5α/GFP cells and HIV-1 JRCSF-infected donor cells (Fig. 6A and D, rightmost image). As expected, no cell-cell fusion was observed in coculture between CEMss-CCR5-rhTRIM5α/GFP cells and HIV-1 JRCSF-infected cells in a Transwell plate (Fig. 6B).
FIG 6.
GPI-VHH JM4-transduced CEMss-CCR5-rhTRIMα/GFP are resistant to HIV-1 envelope-mediated cell-cell fusion. (A) Absence of cell-cell fusion in coculture between uninfected CEMss-CCR5 and rhTRIM5α/GFP-transduced CEMss-CCR5 cells (far left), dramatic cell-cell fusion in coculture between HIV-1 JRCSF-infected CEMss-CCR5 and rhTRIM5α/GFP-transduced CEMss-CCR5 cells (middle left), dramatic cell-cell fusion in coculture between HIV-1 JRCSF-infected CEMss-CCR5 and GPI-VHH E4-transduced CEMss-CCR5-rhTRIM5α/GFP cells (middle right), and absence of cell-cell fusion in coculture between HIV-1 JRCSF-infected CEMss-CCR5 and GPI-VHH JM4-transduced CEMss-CCR5-rhTRIM5α/GFP cells (far right). (B) Same fusion assay as in panel A but using Transwell plates. (C) Dramatic cell-cell fusion in coculture between HIV-1 JRCSF-infected CEMss-CCR5 and rhTRIM5α/GFP-transduced CEMss-CCR5 cells in the presence of 40 and 10 μg/ml of sVHH E4 (far left and middle left) and moderate reduction in cell-cell fusion in coculture between HIV-1 JRCSF-infected CEMss-CCR5 and rhTRIM5α/GFP-transduced CEMss-CCR5 cells in the presence of 40 and 10 μg/ml of soluble JM4 (middle right and far right). (D) Mean and standard deviation (SD) of the number of syncytia in coculture between HIV-1 JRCSF-infected CEMss-CCR5 and mock-transduced CEMss-CCR5-rhTRIM5α/GFP cells (red bar), between HIV-1 JRCSF-infected CEMss-CCR5 and GPI-VHH E4-transduced CEMss-CCR5-rhTRIM5α/GFP cells (brown bar); between HIV-1 JRCSF-infected CEMss-CCR5 and GPI-VHH JM4-transduced CEMss-CCR5-rhTRIM5α/GFP cells (gray bar), between HIV-1 JRCSF-infected CEMss-CCR5 and rhTRIM5α/GFP-transduced CEMss-CCR5 cells in the presence of 40 μg/ml of sVHH E4 (yellow bar), between HIV-1 JRCSF-infected CEMss-CCR5 and rhTRIM5α/GFP-transduced CEMss-CCR5 cells in the presence of 10 μg/ml of sVHH E4 (orange bar), between HIV-1 JRCSF-infected CEMss-CCR5 and rhTRIM5α/GFP-transduced CEMss-CCR5 cells in the presence of 40 μg/ml of sVHH JM4 (blue bar), and between HIV-1 JRCSF-infected CEMss-CCR5 and rhTRIM5α/GFP-transduced CEMss-CCR5 cells in the presence of 10 μg/ml of sVHH JM4 (green bar).
For comparison, we also recorded cell-cell fusion in coculture between CEMss-CCR5-rhTRIM5α/GFP cells and JRCSF-infected CEMss-CCR5 donor cells in the presence of 10 or 40 μg/ml of sVHH E4 and JM4 (Fig. 6C and D). Coculture between JRCSF-infected CEMss-CCR5 donor cells and CEMss-CCR5-rhTRIM5α/GFP cells in the presence of 10 and 40 μg/ml of sVHH E4 did not have any inhibitory effect on HIV-1 envelope-mediated cell-cell fusion of HIV-1 (with average numbers of syncytia of 43 and 44, respectively) (Fig. 6C and D, leftmost and middle left images). In contrast, coculture between JRCSF-infected CEMss-CCR5 donor cells and CEMss-CCR5-rhTRIM5α/GFP cells in the presence of 10 or 40 μg/ml of soluble VHH JM4 resulted in moderate reduction in cell-cell transmission of HIV-1 (with average numbers of syncytia of 35 and 28, respectively) (Fig. 6C and D, middle right and rightmost images). The experiment was repeated three times, with similar results. Thus, taken together, these results indicate that GPI-VHH JM4 completely blocks HIV-1 envelope-mediated cell-cell fusion, whereas high concentrations of soluble VHH JM4 have only a moderate effect.
GPI-VHH JM4 in transduced human primary CD4 T cells blocks both cell-free and T cell-T cell transmission of HIV-1.
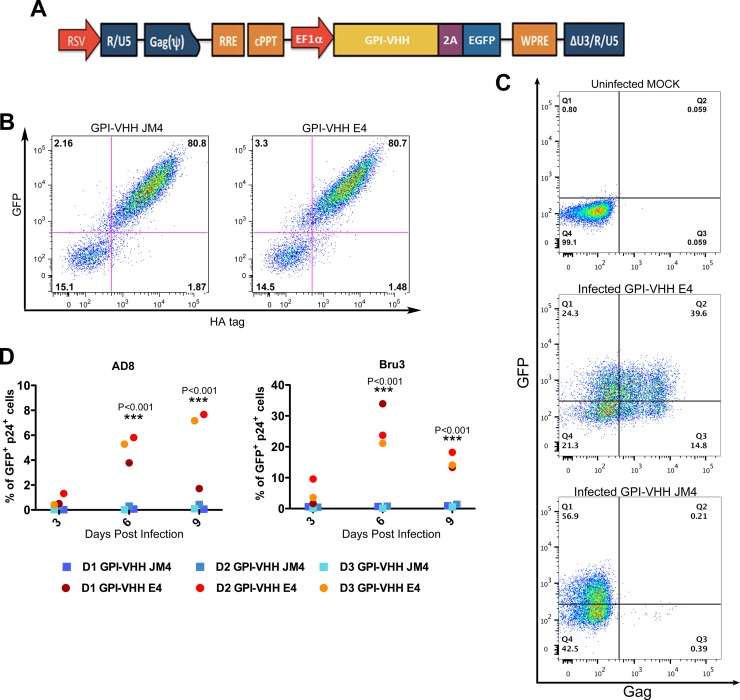
Having demonstrated that GPI-VHH JM4 in transduced CEMss-CCR5 cells potently blocks both cell-free and T cell-T cell transmission of HIV-1, we went on to test if human primary CD4 T cells could be efficiently transduced with GPI-VHH JM4 or E4 and, if so, whether GPI-VHH JM4-transduced human primary CD4 T cells could efficiently resist cell-free and T cell-T cell transmission of HIV-1. To facilitate monitoring of transduced human primary CD4 T cells, we inserted genes encoding GPI-VHH JM4 or E4 into the pRRLsin-18.PPT.EF1α0.2A.GFP.Wpre (31). The resulting pRRL-GPI-VHH JM4 or E4-2A-GFP transfer vectors (Fig. 7A) were used to produce recombinant lentiviruses. The latter were used to transduce human primary CD4 T cells. Figure 7B shows that after a single round of transduction, over 80% of human primary CD4 T cells became transgene positive as measured by GFP and GPI-VHH JM4 (left image) or E4 (right image) expression.
FIG 7.
GPI-VHH JM4-transduced human primary CD4 T cells are resistant to cell-free HIV-1 infection. (A) Schematic diagram of lentiviral transfer vector pRRL-GPI-VHH E4 or JM4-2A-GFP. RRE, Rev response element; cPPT, central polypurine tract; EF1α, promoter derived from elongating factor 1α; GFP, green fluorescent protein. (B) Expression of GFP and GPI-VHH JM4 (left) or E4 (right) in human primary CD4 T cells transduced with recombinant lentiviral vectors containing pRRL-GPI-VHH JM4 or E4-2A-GFP, respectively, after a single round of transduction. (C) Representative gating of Gag+ GFP+ cells in human primary CD4 T cells transduced with recombinant lentiviral vectors containing GPI-VHH JM4 (bottom) or E4 (middle) infected with HIV-1 Bru-3 at 6 days postcoculture, along with an uninfected mock-transduced control (top). (D) Summary of percentages of Gag+ GFP+ cells at 3, 6, and 9 days postinfection of culture of GPI-VHH E4- or JM4-transduced human CD4 T cells from all three donors infected with HIV-1 AD8 (left) and Bru-3 (right).
To measure the resistance or susceptibility of GPI-VHH JM4 or E4-transduced human primary CD4 T cells to cell-free HIV-1 infection, at 6 days posttransduction, cells were infected with HIV-1 AD8 or Bru-3 at an MOI of 0.1. After infection, HIV-1 replication in transduced human primary CD4 T cells as measured by intracellular Gag staining was monitored for 9 days. Figure 7C shows representative gating of GFP+ Gag+ cells in GPI-VHH JM4- or GPI-VHH E4-transduced primary CD4 T cells 6 days after HIV-1 Bru-3 infection along with the uninfected CEMss-CCR5 cell control (top image). Of human primary CD4 T cells transduced with GPI-VHH E4 control 39.6% were GFP+ Gag+ (middle image), whereas of cells transduced with GPI-VHH JM4, less than 0.1% were GFP+ Gag+ (bottom image). Figure 7D summarizes the percentage of GFP+ Gag+ cells in GPI-VHH E4- or JM4-transduced human CD4 T cells from all three donors at 3, 6, and 9 days after HIV-1 AD8 (left image) or Bru-3 (right image) infection. Clearly, while robust replication of HIV-1 AD8 and Bru-3 was seen in GPI-VHH E4-transduced human primary CD4 T cells from all three donors, GPI-VHH JM4-transduced human primary CD4 T cells were completely resistant to cell-free HIV-1 AD8 or Bru-3 infection.
To measure the resistance or susceptibility of GPI-VHH JM4- or E4-transduced human primary CD4 T cells to T cell-T cell transmission of HIV-1, transduced cells 6 days postransduction were cocultured with HIV-1 AD8- or Bru-3-infected Jurkat-CCR5 cells (Fig. 8A). Figure 8B shows representative gating on GFP+ Gag+ cells in GPI-VHH JM4- or E4-transduced primary CD4 T cells 6 days after GPI-VHH JM4- or E4-transduced cells were cocultured with HIV-1 Bru-3-infected Jurkat-CCR5 cells along with an uninfected CEMss-CCR5 cell control (left image). Of human primary CD4 T cells transduced with the GPI-VHH E4 control, 36% were GFP+ Gag+ (middle image), whereas of cells transduced with GPI-VHH JM4, less than 0.3% were GFP+ Gag+ (right image). Figure 8C summarizes the percentage of GFP+ Gag+ cells in GPI-VHH E4- or JM4-transduced human CD4 T cells from all three donors at 3, 6, and 9 days after transduced cells were cocultured with HIV-1 AD8-infected (left image) or Bru-3-infected (right image) Jurkat-CCR5 cells. Clearly, GPI-VHH JM4-transduced human primary CD4 T cells from the first two donors were almost completely resistant to T cell-T cell transmission of both AD8 and Bru-3 HIV-1. GPI-VHH JM4-transduced human primary CD4 T cells from the third donor were almost completely resistant to T cell-T cell transmission of HIV-1 AD8 but significantly reduced T cell-T cell transmission of HIV-1 Bru-3. Thus, we concluded that GPI-VHH JM4 in transduced human primary CD4 T cells substantially blocks both cell-free and T cell-T cell transmission of HIV-1.
FIG 8.
GPI-VHH JM4-transduced human primary CD4 T cells are resistant to cell-cell transmission of HIV-1. (A) Schematic diagram of transducing human CD4 T cells with lentiviral vectors expressing GPI-VHH E4 or JM4, establishing HIV-1 AD8- or Bru-3-infected Jurkat cells; coculture between infected or uninfected Jurkat cells, and GPI-VHH JM4- or E4-transduced human primary CD4 T cells. (B) Representative gating of Gag+ GFP+ cells in human primary CD4 T cells transduced with GPI-VHH JM4 (right) or E4 (middle) cocultured with HIV-1 Bru-3-infected Jurkat cells at 6 days postcoculture along with uninfected mock-transduced control cells (left). (C) Summary of percentages of Gag+ GFP+ cells at 3, 6, and 9 days postinfection of coculture between GPI-VHH E4- or JM4-transduced human CD4 T cells from all three donors and HIV-1 AD8-infected (left) and Bru-3-infected (right) human T cells.
DISCUSSION
To date, llamas are an only animal species in which immunization has elicited broadly neutralizing antibodies against HIV-1 (1, 2). The broadly neutralizing antibodies elicited in llamas are heavy chain-only antibodies, and their VHHs exhibit antigen-specific binding affinity comparable to that of conventional immunoglobulins (1). Due to their high-level expression in yeasts and bacteria as well as extreme thermal and pH stability, VHHs have been developed as microbicides against HIV-1 both in gels and in commensal bacteria (43). In the present study, we developed GPI-anchored VHH JM2 and JM4 from two heavy chain-only antibodies isolated from immunized llamas (2). We demonstrated that by genetically linking the VHHs with a GPI attachment signal, VHHs are targeted to the lipid rafts of the plasma membranes through a GPI anchor (Fig. 1). GPI-VHH JM4, but not GPI-VHH JM2, efficiently blocks cell-free HIV-1 infection in transduced CD4+ cell lines and human primary CD4 T cells (Fig. 2, 3, and 7 and Table 1). HIV-1 viruses neutralized by GPI-VHH JM4 include diverse strains of various subtypes, including tier 2 and 3 strains, transmitted founders, and quasispecies as well as strains resistant to neutralization by sVHH JM4 plus sCD4 (Fig. 2 and Table 1). Thus, GPI-VHH JM4 is truly a much more potent and broader entry inhibitor than sVHH JM4.
Increasing evidence indicates that the cell-cell transmission of retroviruses plays an important role in the establishment of systemic infection as well as in virus spread within lymphoid tissues in vivo (9). For example, in a BLT humanized mouse model, Murooka et al. showed that HIV-1-infected T cells in lymph nodes form virological synapses and block the egress of T cells from the lymph nodes into efferent lymph vessels at the onset of limited systemic HIV-1 infection (9). Sewald et al. showed that in secondary lymph tissues, HIV-1 and murine leukemia virus (MLV) are captured by sinus-lining macrophages via CD169. MLV-captured by macrophages can then trans-infect B-1 cells. Infected B-1 cells then migrate into the lymph node to spread the infection through virological synapses (44). HIV-1 transmission from infected T cells to uninfected T cells occurs via virological synapses (4). The formation of such structures allows the coordination of viral assembly with viral entry at sites of cell-cell contacts, resulting in a high local concentration and high rate of transmission of HIV-1 (6). As a result, T cell-T cell transmission of HIV-1 is much more efficient for spreading virus and is much less susceptible to neutralizing antibodies and entry inhibitors than cell-free HIV-1 (7, 8, 10–14). In the present study, we demonstrated that GPI-VHH JM4 in transduced CEMss-CCR5 cells exerts a potent block on T cell-T cell transmission of HIV-1 (Fig. 5) and HIV-1 envelope protein-mediated cell-cell fusion (Fig. 6). GPI-VHH JM4-transduced human primary CD4 T cells are resistant to T cell-T cell transmission of HIV-1 (Fig. 8).
The potent neutralization of cell-cell transmission of HIV-1 by GPI-VHH JM4 is likely due to the high local concentration of GPI-VHH JM4 in the lipid rafts of the cell plasma membrane. When viral transmission from infected T cells to uninfected T cells and HIV-1 envelope-mediated cell-cell fusion occur at sites of cell-cell contacts, GPI-VHH JM4 is able to capture its CD4-induced epitope. This may block further conformational change of the HIV-1 envelope protein, thereby preventing HIV-1 envelope-mediated membrane fusion and virus entry.
Table 2 summarizes neutralization breadth and potency of GPI-anchored antibody derivatives (scFv, HCDR3, and VHH) that we have developed in this study or previous studies (26–28) compared to those of soluble whole antibodies, scFvs and VHHs, that serve as sources for GPI-anchored antibody derivatives (2, 45–50). GPI-anchored antibody derivatives that we have tested include CD4BS, coreceptor-binding site (CRBS), V1/V2 loop, C-helix, and MPER antibodies. Interestingly, GPI-VHH JM2, like other anti-CD4BSs (GPI-scFv VRC01, and GPI-HCDR3 b12) that we tested previously, has no or very limited neutralization activity, whereas the soluble whole antibodies VRC01, b12, and JM2 exhibit various potencies and breadths of neutralization activity (2, 45, 46). In contrast, GPI-VHH JM4, like the other anti-CRBSs GPI-scFv X5 and E51, has extremely potent and broad neutralization activity, whereas the soluble whole antibodies, scFv and VHH, of these anti-CRBS antibodies have lower or little neutralization activity or breadth (26, 27, 47, 49). Moreover, anti-V1/V2 loop GPI-HCDR3 PG9 and PG16 have potent and broad neutralization activities similar to those of the soluble whole antibodies PG9 and PG16 (26, 28, 46). Furthermore, anti-C-helix GPI-scFv TG15 exhibited moderate neutralization against 8 out of 12 HIV-1 strains tested, whereas soluble whole antibody TG15, which was originally defined as a nonneutralizing antibody, does not have any neutralization activity (27, 48). Finally, anti-MPER GPI-scFv 4E10 significantly increases neutralization potency compared to that of soluble whole antibody 4E10 (27, 46, 50). Thus, it appears that the broad and the potent neutralization by GPI-anchored antibody derivatives is epitope specific and only partially overlaps those of soluble broadly neutralizing antibodies.
TABLE 2.
Breadth and the potency of GPI-anchored antibody derivatives (scFv, HCDR3, and VHH) and soluble IgG or scFv and sdAb of various human monoclonal antibodiesa

Neutralization breadth is presented as the number of HIV-1 strains neutralized per number of HIV-1 strains tested. Colors represent the neutralization potency. For GPI-anchored antibody derivatives, red stands for an average inhibition of ≥98%, green an average inhibition between 50 and 80%, and gray an average inhibition between 0 and 50%. For soluble IgG, scFv, or VHH, red stands for a median 50% inhibitory concentration (IC50) of <1 μg/ml, yellow an IC50 between 1 and 10 μg/ml, green an IC50 between >10 and 50 μg/ml, and gray an IC50 of >50 μg/ml or no neutralization. ND, not done.
Finally, GPI-VHH JM4, with such remarkable neutralization breadth and potency against both cell-free and T cell-T cell transmission of HIV-1, should have the potential to be developed into an anti-HIV-1 agent either alone or in combination with other anti-HIV-1 gene constructs. For example, GPI-VHH JM4 could be delivered or codelivered with trimeric GPI-HCDR3 PG16 (28) into autologous hematopoietic progenitor cells or CD4+ T cells of HIV-1 patients ex vivo using lentiviral vectors. The modified cells could then be transfused into the patients as recently described by DiGiusto et al. (51) or by Tebas et al. (52). However, before being tested in HIV-1-infected individuals, the safety, immunogenicity, potential “toxicity” of GPI-anchored antibody expression on the surface of CD4 cells or its impact on CD4 T cell functions, immune reconstitution, and therapeutic potential of the GPI-VHH JM4-transduced primary CD4+ T cells and/or hematopoietic progenitor cells should first be tested in relevant animal models, such as the simian-human immunodeficiency virus (SHIV) rhesus macaque infection model or HIV-1 humanized mouse infection model.
ACKNOWLEDGMENTS
We thank L. Naldini at the University of Torino Medical School, Turin, Italy, for lentiviral transfer vector, B. H. Hahn at the University of Pennsylvania for DNA plasmids encoding consensus B and C HIV-1 envelope proteins, and Linqi Zhang at Tsinghua University for DNA plasmid encoding CNE3, CNE5, CNE8, CNE11, CNE15, CNE50, and CNE55. Cell line TZM.bl, molecular clones of HIV-1 Bru-3, AD8, Mj4, and Yu2, transmitted founder viruses WITO, CH040, THRO, REJO, and CH077, pNL4-3.luc.R-E- transfer vector, and DNA plasmids encoding Q168, Q461ENVe2, Yu2, AD8, JRFL, SF162, HxBc2, PVO.4, QH0692.42, 92BR025.9, 93TH966.8, and 92UG975.10 envelope proteins were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Germantown, MD. The relevant reagents were originally developed and contributed by J. Kappes, X. Wu, N. Landau, R. Risser, J. Overbaugh, E. Freed, A. Adachi, M. A. Martin, I. R. Chen, G. W. Shaw, and B. H. Hahn.
This work was supported by research grants from the Chinese National Natural Science Foundation (number 31170871) and the National Science and Technology Major Project (number 2014ZX10001-001) to P.Z.; by research grants from the National Institutes of Health (R56 AI108467 to J.T.K.) and the Baylor-UTHouston CFAR (P30AI036211); by a Chinese National Natural Science Foundation-National Institutes of Health joint grant (number 81361120406-R01 AI106574) to P.Z. and J.T.K.; by a Chinese National Natural Science Foundation-Hong Kong RGC joint grant (number 81161160569-N_HKU709/11) to P.Z. and Z.C.; and by research grants from the French National Agency for AIDS Research to S.B. S.B. obtained a Visiting Professorship from the Chinese Academy of Sciences (number 2012T1S0024 and number 2016VBA062).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.McCoy LE, Quigley AF, Strokappe NM, Bulmer-Thomas B, Seaman MS, Mortier D, Rutten L, Chander N, Edwards CJ, Ketteler R, Davis D, Verrips T, Weiss RA. 2012. Potent and broad neutralization of HIV-1 by a llama antibody elicited by immunization. J Exp Med 209:1091–1103. doi: 10.1084/jem.20112655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matz J, Kessler P, Bouchet J, Combes O, Ramos OH, Barin F, Baty D, Martin L, Benichou S, Chames P. 2013. Straightforward selection of broadly neutralizing single-domain antibodies targeting the conserved CD4 and coreceptor binding sites of HIV-1 gp120. J Virol 87:1137–1149. doi: 10.1128/JVI.00461-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acharya P, Luongo TS, Georgiev IS, Matz J, Schmidt SD, Louder MK, Kessler P, Yang Y, McKee K, O'Dell S, Chen L, Baty D, Chames P, Martin L, Mascola JR, Kwong PD. 2013. Heavy chain-only IgG2b llama antibody effects near-pan HIV-1 neutralization by recognizing a CD4-induced epitope that includes elements of coreceptor- and CD4-binding sites. J Virol 87:10173–10181. doi: 10.1128/JVI.01332-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiffner T, Sattentau QJ, Duncan CJ. 2013. Cell-to-cell spread of HIV-1 and evasion of neutralizing antibodies. Vaccine 31:5789–5797. doi: 10.1016/j.vaccine.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Rudnicka D, Feldmann J, Porrot F, Wietgrefe S, Guadagnini S, Prevost MC, Estaquier J, Haase AT, Sol-Foulon N, Schwartz O. 2009. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J Virol 83:6234–6246. doi: 10.1128/JVI.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hübner W, McNerney GP, Chen P, Dale BM, Gordon RE, Chuang FYS, Li X-D, Asmuth DM, Huser T, Chen BK. 2009. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science 323:1743–1747. doi: 10.1126/science.1167525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malbec M, Porrot F, Rua R, Horwitz J, Klein F, Halper-Stromberg A, Scheid JF, Eden C, Mouquet H, Nussenzweig MC, Schwartz O. 2013. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J Exp Med 210:2813–2821. doi: 10.1084/jem.20131244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abela IA, Berlinger L, Schanz M, Reynell L, Gunthard HF, Rusert P, Trkola A. 2012. Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies. PLoS Pathog 8:e1002634. doi: 10.1371/journal.ppat.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murooka TT, Deruaz M, Marangoni F, Vrbanac VD, Seung E, von Andrian UH, Tager AM, Luster AD, Mempel TR. 2012. HIV-infected T cells are migratory vehicles for viral dissemination. Nature 490:283–287. doi: 10.1038/nature11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P, Hubner W, Spinelli MA, Chen BK. 2007. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol 81:12582–12595. doi: 10.1128/JVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reh L, Magnus C, Schanz M, Weber J, Uhr T, Rusert P, Trkola A. 2015. Capacity of broadly neutralizing antibodies to inhibit HIV-1 cell-cell transmission is strain- and epitope-dependent. PLoS Pathog 11:e1004966. doi: 10.1371/journal.ppat.1004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durham ND, Yewdall AW, Chen P, Lee R, Zony C, Robinson JE, Chen BK. 2012. Neutralization resistance of virological synapse-mediated HIV-1 infection is regulated by the gp41 cytoplasmic tail. J Virol 86:7484–7495. doi: 10.1128/JVI.00230-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin N, Welsch S, Jolly C, Briggs JA, Vaux D, Sattentau QJ. 2010. Virological synapse-mediated spread of human immunodeficiency virus type 1 between T cells is sensitive to entry inhibition. J Virol 84:3516–3527. doi: 10.1128/JVI.02651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massanella M, Puigdomenech I, Cabrera C, Fernandez-Figueras MT, Aucher A, Gaibelet G, Hudrisier D, Garcia E, Bofill M, Clotet B, Blanco J. 2009. Antigp41 antibodies fail to block early events of virological synapses but inhibit HIV spread between T cells. AIDS 23:183–188. doi: 10.1097/QAD.0b013e32831ef1a3. [DOI] [PubMed] [Google Scholar]
- 15.Su B, Xu K, Lederle A, Peressin M, Biedma ME, Laumond G, Schmidt S, Decoville T, Proust A, Lambotin M, Holl V, Moog C. 2012. Neutralizing antibodies inhibit HIV-1 transfer from primary dendritic cells to autologous CD4 T lymphocytes. Blood 120:3708–3717. doi: 10.1182/blood-2012-03-418913. [DOI] [PubMed] [Google Scholar]
- 16.Frankel SS, Steinman RM, Michael NL, Kim SR, Bhardwaj N, Pope M, Louder MK, Ehrenberg PK, Parren PW, Burton DR, Katinger H, VanCott TC, Robb ML, Birx DL, Mascola JR. 1998. Neutralizing monoclonal antibodies block human immunodeficiency virus type 1 infection of dendritic cells and transmission to T cells. J Virol 72:9788–9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Montfort T, Nabatov AA, Geijtenbeek TBH, Pollakis G, Paxton WA. 2007. Efficient capture of antibody neutralized HIV-1 by cells expressing DC-SIGN and transfer to CD4+ T lymphocytes. J Immunol 178:3177–3185. doi: 10.4049/jimmunol.178.5.3177. [DOI] [PubMed] [Google Scholar]
- 18.van Montfort T, Thomas AAM, Pollakis G, Paxton WA. 2008. Dendritic cells preferentially transfer CXCR4-using human immunodeficiency virus type 1 variants to CD4+ T lymphocytes in trans. J Virol 82:7886–7896. doi: 10.1128/JVI.00245-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sagar M, Akiyama H, Etemad B, Ramirez N, Freitas I, Gummuluru S. 2012. Transmembrane domain membrane proximal external region but not surface unit-directed broadly neutralizing HIV-1 antibodies can restrict dendritic cell-mediated HIV-1 trans-infection. J Infect Dis 205:1248–1257. doi: 10.1093/infdis/jis183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao Z, Cimakasky LM, Hampton R, Nguyen DH, Hildreth JE. 2001. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res Hum Retroviruses 17:1009–1019. doi: 10.1089/088922201300343690. [DOI] [PubMed] [Google Scholar]
- 21.Chazal N, Gerlier D. 2003. Virus entry, assembly, budding, and membrane rafts. Microbiol Mol Biol Rev 67:226–237. doi: 10.1128/MMBR.67.2.226-237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popik W, Alce TM, Au WC. 2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4(+) T cells. J Virol 76:4709–4722. doi: 10.1128/JVI.76.10.4709-4722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol 72:2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter GC, Bernstone L, Sangani D, Bee JW, Harder T, James W. 2009. HIV entry in macrophages is dependent on intact lipid rafts. Virology 386:192–202. doi: 10.1016/j.virol.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medof ME, Kinoshita T, Nussenzweig V. 1984. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med 160:1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Wen M, Wang W, Wang S, Yang L, Liu Y, Qian M, Zhang L, Shao Y, Kimata JT, Zhou P. 2011. Potent and broad anti-HIV-1 activity exhibited by a glycosyl-phosphatidylinositol-anchored peptide derived from the CDR H3 of broadly neutralizing antibody PG16. J Virol 85:8467–8476. doi: 10.1128/JVI.00520-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen M, Arora R, Wang H, Liu L, Kimata JT, Zhou P. 2010. GPI-anchored single chain Fv–an effective way to capture transiently-exposed neutralization epitopes on HIV-1 envelope spike. Retrovirology 7:79. doi: 10.1186/1742-4690-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Wang W, Yang L, Ren H, Kimata JT, Zhou P. 2013. Trimeric glycosylphosphatidylinositol-anchored HCDR3 of broadly neutralizing antibody PG16 is a potent HIV-1 entry inhibitor. J Virol 87:1899–1905. doi: 10.1128/JVI.01038-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou P, Cao H, Smart M, David C. 1993. Molecular basis of genetic polymorphism in major histocompatibility complex-linked proteasome gene (Lmp-2). Proc Natl Acad Sci U S A 90:2681–2684. doi: 10.1073/pnas.90.7.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L. 2000. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet 25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Ye C, Liu J, Zhang D, Kimata JT, Zhou P. 2014. CCR5 gene disruption via lentiviral vectors expressing Cas9 and single guided RNA renders cells resistant to HIV-1 infection. PLoS One 9:e115987. doi: 10.1371/journal.pone.0115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thippeshappa R, Polacino P, Yu Kimata MT, Siwak EB, Anderson D, Wang W, Sherwood L, Arora R, Wen M, Zhou P, Hu SL, Kimata JT. 2011. Vif substitution enables persistent infection of pig-tailed macaques by human immunodeficiency virus type 1. J Virol 85:3767–3779. doi: 10.1128/JVI.02438-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sirven A, Ravet E, Charneau P, Zennou V, Coulombel L, Guetard D, Pflumio F, Dubart-Kupperschmitt A. 2001. Enhanced transgene expression in cord blood CD34(+)-derived hematopoietic cells, including developing T cells and NOD/SCID mouse repopulating cells, following transduction with modified trip lentiviral vectors. Mol Ther 3:438–448. doi: 10.1006/mthe.2001.0282. [DOI] [PubMed] [Google Scholar]
- 34.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeuchi Y, McClure MO, Pizzato M. 2008. Identification of gammaretroviruses constitutively released from cell lines used for human immunodeficiency virus research. J Virol 82:12585–12588. doi: 10.1128/JVI.01726-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol 74:8358–8367. doi: 10.1128/JVI.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Platt EJ, Bilska M, Kozak SL, Kabat D, Montefiori DC. 2009. Evidence that ecotropic murine leukemia virus contamination in TZM-bl cells does not affect the outcome of neutralizing antibody assays with human immunodeficiency virus type 1. J Virol 83:8289–8292. doi: 10.1128/JVI.00709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai C, Caillet C, Hu H, Zhou F, Ding H, Zhang G, Zhou B, Wang S, Lu S, Buchy P, Deubel V, Vogel FR, Zhou P. 2009. Measurement of neutralizing antibody responses against H5N1 clades in immunized mice and ferrets using pseudotypes expressing influenza hemagglutinin and neuraminidase. Vaccine 27:6777–6790. doi: 10.1016/j.vaccine.2009.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, Zajic L, Iyer SS, Decker JM, Kumar A, Hora B, Berg A, Cai F, Hopper J, Denny TN, Ding H, Ochsenbauer C, Kappes JC, Galimidi RP, West AP Jr, Bjorkman PJ, Wilen CB, Doms RW, O'Brien M, Bhardwaj N, Borrow P, Haynes BF, Muldoon M, Theiler JP, Korber B, Shaw GM, Hahn BH. 2013. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci U S A 110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimata JT, Kuller L, Anderson DB, Dailey P, Overbaugh J. 1999. Emerging cytopathic and antigenic simian immunodeficiency virus variants influence AIDS progression. Nat Med 5:535–541. doi: 10.1038/8414. [DOI] [PubMed] [Google Scholar]
- 41.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 42.Richardson MW, Carroll RG, Stremlau M, Korokhov N, Humeau LM, Silvestri G, Sodroski J, Riley JL. 2008. Mode of transmission affects the sensitivity of human immunodeficiency virus type 1 to restriction by rhesus TRIM5alpha. J Virol 82:11117–11128. doi: 10.1128/JVI.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorlani A, Brouwers J, McConville C, van der Bijl P, Malcolm K, Augustijns P, Quigley AF, Weiss R, De Haard H, Verrips T. 2012. Llama antibody fragments have good potential for application as HIV type 1 topical microbicides. AIDS Res Hum Retroviruses 28:198–205. doi: 10.1089/aid.2011.0133. [DOI] [PubMed] [Google Scholar]
- 44.Sewald X, Ladinsky MS, Uchil PD, Beloor J, Pi R, Herrmann C, Motamedi N, Murooka TT, Brehm MA, Greiner DL, Shultz LD, Mempel TR, Bjorkman PJ, Kumar P, Mothes W. 2015. Retroviruses use CD169-mediated trans-infection of permissive lymphocytes to establish infection. Science 350:563–567. doi: 10.1126/science.aab2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol GPI, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dorfman T, Moore MJ, Guth AC, Choe H, Farzan M. 2006. A tyrosine-sulfated peptide derived from the heavy-chain CDR3 region of an HIV-1-neutralizing antibody binds gp120 and inhibits HIV-1 infection. J Biol Chem 281:28529–28535. doi: 10.1074/jbc.M602732200. [DOI] [PubMed] [Google Scholar]
- 48.Takeda S, Dorfman NA, Robert-Guroff M, Notkins AL, Rando RF. 1995. Two-phase approach for the expression of high-affinity human anti-human immunodeficiency virus immunoglobulin Fab domains in Escherichia coli. Hybridoma 14:9–18. doi: 10.1089/hyb.1995.14.9. [DOI] [PubMed] [Google Scholar]
- 49.Zhang MY, Shu Y, Rudolph D, Prabakaran P, Labrijn AF, Zwick MB, Lal RB, Dimitrov DS. 2004. Improved breadth and potency of an HIV-1-neutralizing human single-chain antibody by random mutagenesis and sequential antigen panning. J Mol Biol 335:209–219. doi: 10.1016/j.jmb.2003.09.055. [DOI] [PubMed] [Google Scholar]
- 50.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiGiusto DL, Krishnan A, Li L, Li H, Li S, Rao A, Mi S, Yam P, Stinson S, Kalos M, Alvarnas J, Lacey SF, Yee JK, Li M, Couture L, Hsu D, Forman SJ, Rossi JJ, Zaia JA. 2010. RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma. Sci Transl Med 2:36ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G, Holmes MC, Gregory PD, Ando DG, Kalos M, Collman RG, Binder-Scholl G, Plesa G, Hwang WT, Levine BL, June CH. 2014. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 370:901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]