ABSTRACT
The dynamics of HIV reservoir accumulation off antiretroviral therapy (ART) is underexplored. Levels of integrated HIV DNA in peripheral blood mononuclear cells (PBMCs) were longitudinally monitored before and after antiviral therapy. HIV integration increased over time in both elite controllers (ECs; n = 8) and noncontrollers (NCs; n = 6) before ART, whereas integration remained stable in patients on ART (n = 4). The median annual fold change was higher in NCs than in ECs and negatively correlated with CD4/CD8 T-cell ratio. Cytotoxic T lymphocyte (CTL) function as assessed by infected CD4 T-cell elimination (ICE) and granzyme B activity did not significantly change over time in ECs, suggesting that the gradual increase in integrated HIV DNA observed in ECs was not a result of progressive loss of immune-mediated control. Also, acutely infected (n = 7) but not chronically infected (n = 6) patients exhibited a significant drop in integrated HIV DNA 12 months after ART initiation. In conclusion, in the absence of ART, integrated HIV accumulates over time both in NCs and in ECs, at variable individual rates. Starting ART early in infection leads to a greater drop in integrated HIV DNA than does initiating treatment after years of infection. The increase in integrated HIV DNA over time suggests that early treatment may be of benefit in limiting HIV reservoirs.
IMPORTANCE The establishment of a latent reservoir represents a barrier to cure among HIV-infected individuals. The dynamics of HIV reservoir accumulation over time in patients before antiviral therapy is underexplored, in large part because it is difficult to accurately and reproducibly measure the size of HIV reservoir in this setting. In our study, we compared the dynamics of integrated HIV DNA over time in ECs and NCs before and after ART was initiated. We found that integrated HIV DNA levels progressively increase over time in the absence of ART, but with a higher, albeit variable, rate in NCs compared to ECs. In addition, integrated HIV DNA declines more dramatically when ART is initiated in acute rather than chronic HIV infection, suggesting important differences between acute and chronic infection. Our study highlights the role of HIV replication and CTL control in reservoir accumulation in sanctuary sites and why ART appears to be more effective in acute infection.
INTRODUCTION
The establishment of a latent reservoir represents a barrier to cure among individuals infected with human immunodeficiency virus (HIV). It has been difficult to design assays that can accurately and reproducibly measure the size of HIV reservoir. The quantitative viral outgrowth assay (QVOA) provides a measure of the frequency of replication-competent virus, but this assay is laborious, costly, and subject to high error and may underestimate the reservoir size (1). It is technically difficult to measure replication-competent virus robustly by viral sequencing or viral outgrowth in patients off antiretroviral therapy (ART) due to the contribution of preintegration complexes. This technical limitation has left a gap in HIV reservoir research regarding the accumulation rate of HIV reservoirs over time. Among the nucleic acid intermediates of HIV, the integrated form appears to correlate better with replication competence (2–4) and thus may provide a superior surrogate for reservoir size when the QVOA cannot be employed.
Only a few studies have measured replication-competent virus in patients not receiving ART (5–7), and while these groundbreaking studies established that latent reservoirs were formed early in infection, they did not monitor the dynamics of reservoir accumulation. Additional studies have suggested that reservoirs accumulate very quickly after infection (8). Prior studies provide indirect evidence that reservoirs increase over time without monitoring patients longitudinally (9–11). One of these studies suggested that integration levels may reach a plateau level after a few years in the absence of ART (11). However, the kinetics of reservoir accumulation within an individual off ART have not been monitored over several years. Moreover, it is unclear whether reservoirs increase at differing rates in different individuals. We also sought to compare the impacts of early and late initiation of ART on HIV reservoir size, as patients treated during acute infection have been reported to have lower levels of HIV DNA than patients treated during chronic infection (4, 11–15). It is important to understand even subtle differences between early and late treatment since early treatment has been reported to restrain reservoir size to a level that allows treatment interruptions for a prolonged period without viral rebound (12).
We longitudinally measured integrated HIV DNA as a surrogate for reservoir size in elite controllers (ECs) and noncontrollers (NCs), as well as in acutely and chronically infected individuals before and after ART initiation. We also prospectively assessed the cytotoxic profile of ECs. We found that integrated HIV DNA increased over time in the absence of ART at variable rates depending on the individual, and the rates of accumulation were strikingly different between ECs and NCs. We also found that integrated HIV DNA was more significantly impacted by ART during acute infection, consistent with functional differences between these cohorts.
MATERIALS AND METHODS
Study subjects.
Frozen peripheral blood mononuclear cell (PBMC) samples from ECs, patients on ART, and acutely and chronically infected patients were obtained from the Clinical Research Center, National Institutes of Health (NIH; Bethesda, MD), the Center for AIDS Research at the University of Pennsylvania, and the Options cohort at the University of California, San Francisco (UCSF). All participants signed informed-consent forms approved by their Investigational Review Boards (IRB). The University of Pennsylvania IRB approved the transfer of materials from NIH and UCSF for this study.
This study consisted of two different parts. In the first one, we measured integrated HIV DNA over time in 8 ECs, 6 untreated NCs, and 4 ART-treated patients. In the second part, we measured integrated HIV DNA in 6 individuals in whom ART was initiated in chronic infection (more than 12 months after acquiring infection) and 7 in whom ART was initiated within an estimated 6 months after HIV infection (using HIV testing history and antibody testing) (16). We tested these individuals before and 12 months after starting ART. ECs are part of a cohort studied at NIH under protocol number 02-I-0086 and provide apheresis products every 6 to 12 months for research use (17). These patients have maintained HIV loads persistently below 50 copies/ml for more than 10 years in the absence of ART and a CD4 T-cell count of >400 cells/μl, and thus, we do not have data related to acute infection in this cohort. At enrollment, the median CD4 T-cell count was 811 (interquartile range [IQR], 544 to 980) cells/μl. At the last time point (after 5.4 ± 3.7 years), the median CD4 T-cell count was not significantly different (618 [IQR, 485 to 709] cells/μl; P = 0.1). Analogously, CD4/CD8 cell ratio did not change over time in ECs (from 1.02 [IQR, 0.8 to 1.8] to 1.22 [IQR, 1.08 to 1.79]; P = 0.31). ART-treated patients started treatment during chronic infection and had undetectable viremia (i.e., <40 to 75 HIV RNA copies/ml) for at least 2 years before enrollment. NCs were individuals who enrolled in the Options cohort at UCSF within 6 months of HIV infection but elected not to start ART and who were followed longitudinally during chronic infection, including after starting ART. For individuals who initiated ART during chronic HIV, the mean duration of infection before starting ART was 6.1 ± 3.5 years, while for acute patients, the intervals between estimated date of infection and treatment initiation were 1 month for 4 patients, 2 months for 1 patient, 4 months for 1 patient, and 5 months for 1 patient. At treatment initiation, median CD4 T-cell counts were 271 (IQR, 146 to 461) cells/μl in chronic patients and 598 (IQR, 493 to 896) cells/μl in acute individuals.
Measurement of integrated HIV DNA.
Aliquots of frozen PBMCs for each patient were thawed and isolated using the Qiagen Blood and Cell Culture Maxi kit (Qiagen, Valencia, CA). Integrated HIV DNA levels were measured using our previously published method (17). The entire sample was utilized to measure integrated HIV DNA, since many studies have longitudinally measured total HIV DNA (9, 11, 13, 18). As a result, it was possible to increase the sensitivity and robustness of our measurements by performing PCR with more wells at limiting dilution (19). HIV integration was detected in every sample so that no data censoring was required.
GrB cytotoxicity assay.
Cytotoxicity of autologous CD4 T-cell targets by CD8 T cells was assessed by granzyme B (GrB) target cell activity and infected CD4+ T-cell elimination (ICE), as described previously (20, 21). Briefly, CD4 T cells were positively selected from cryopreserved PBMCs by magnetic automated cell sorting (AutoMACS; Miltenyi Biotec, Germany) and polyclonally stimulated for 3 days prior to infection. CD4 T cells were then resuspended in warmed medium containing interleukin 2 (IL-2; Roche Diagnostics, Mannheim, Germany) at 40 IU/ml in 96-well tissue culture plates. Concentrated HIVSF162 was bound to ViroMag beads (OZ Biosciences, Marseille, France). Bead-labeled virus or medium was added to “infected” or “uninfected” control wells, respectively. The plates were centrifuged at 1,600 rpm for 2 min prior to 15 min of incubation on a magnet (OZ Biosciences). Cells were incubated at 37°C for 36 h prior to use as targets in cytotoxicity assays. Effector PBMCs were incubated with HIVSF162-infected targets for 6 days. Day 6 cells were then labeled with immunomagnetic beads prior to negative selection of CD8 T cells by magnetic automated cell sorting (CD8 T-cell isolation kit II; Miltenyi Biotec). HIVSF162-infected and uninfected targets were labeled with a LIVE/DEAD fixable violet stain kit (Invitrogen Molecular Probes) per the manufacturer's instructions and mixed with day 6 enriched CD8 T cells at an effector/target cell (E:T) ratio of 25:1 at 37°C for 1 h. The GranToxiLux killing assay was conducted per the manufacturer's protocols (OncoImmunin), except where otherwise noted. The GrB substrate was used at a 4× dilution. After analysis of GrB activity in live targets by flow cytometry, cells were treated with Cytofix/Cytoperm (BD Biosciences, San Jose, CA) prior to staining for measurement of elimination of p24-expressing cells (KC57 RDI; Beckman Coulter, Inc., Fullerton, CA). ICE was calculated as follows: ([percent p24 expression of infected targets − percent p24 expression of infected targets mixed with day 0 or day 6 effector cells]/percent p24 expression of infected targets) × 100.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA) and Stata 14 (StataCorp, College Station, TX). Correlation between integrated HIV DNA, HIV load, and CD4/CD8 T-cell ratio was calculated using either Pearson (for log-transformed variables) or Spearman correlation coefficients. The Mann-Whitney U test was used to compare continuous variables between groups, and the Wilcoxon signed-rank test was used to compare paired values, such as before- and after-treatment reservoir measures. A linear mixed-effects model was used to estimate the rate of change in log transformed integrated HIV DNA per year in ECs, NCs, and ART-treated patients. Data are presented as mean (±standard deviation [SD]) or median (IQR), as appropriate.
RESULTS
Untreated noncontrollers and elite controllers show an increase in integrated HIV DNA over time (Fig. 1 and 2).
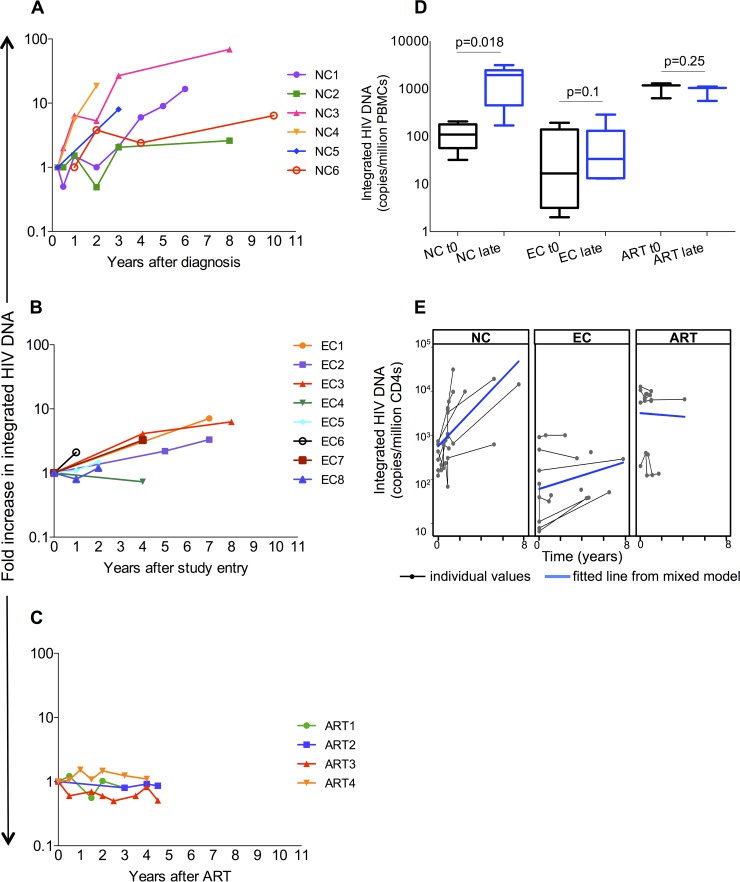
FIG 1.
Longitudinal measures of integrated HIV DNA in noncontrollers (NCs; n = 6 [A, D, and E]), elite controllers (ECs; n = 8 [B, D, and E]), and antiretroviral therapy (ART)-treated patients (n = 4 [C, D, and E]). Integrated HIV DNA levels increased over time in NCs (from 109 [IQR, 57 to 178] to 1,941 [IQR, 448 to 2,442] copies/million PBMCs after 6 years [P = 0.018] and ECs (from 16 [IQR, 3.2 to 139] to 34 [IQR, 13.2 to 131] copies/million peripheral blood mononuclear cells [PBMCs] at the last time point [P 0.1]) but not in ART-treated patients (from 1,179 [IQR 632 to 1,287] to 1,038 [IQR, 555 to 1,074] copies/million PBMCs [P = 0.25]) (D). The median annual fold change was significantly higher in NCs than in ECs (3 [IQR, 0.53 to 7.9] versus 0.63 [IQR, 0.35 to 0.96] per year [P = 0.022]) (A to C). When normalized to CD4, using a linear mixed-effects model, the increase in integrated HIV DNA levels per year was higher in NCs than in ECs {difference in slope: −0.18 (95% confidence interval [CI]: −0.29, −0.07); P = 0.002} and in ART-treated ones (difference in slope: −0.28 [95% CI: −0.41, −0.15]; P < 0.001) (E). Moreover, integrated HIV DNA levels decreased in ART patients and were statistically different than those in NCs (difference in slope: −0.28 [95% CI: −0.41, −0.15]; P < 0.001) and ECs (difference in slope: −0.1 [95% CI: −0.19, −0.005]; P = 0.04) (E). “t0” refers to the first available time point, while “late” refers to the last available one (i.e., 6.1 ± 3.5 years for NCs, 5.4 ± 3.7 years for ECs, and 4 ± 1.9 years for patients on ART). Integrated HIV DNA levels are reported as fold change compared to baseline in panels A, B, and C, as copies/million CD4 in panel E, and as copies/million PBMCs in panel D. HIV integration levels at t0 and late time points were compared using the Wilcoxon signed-rank test, while Mann-Whitney U test was used to compare fold change across groups.
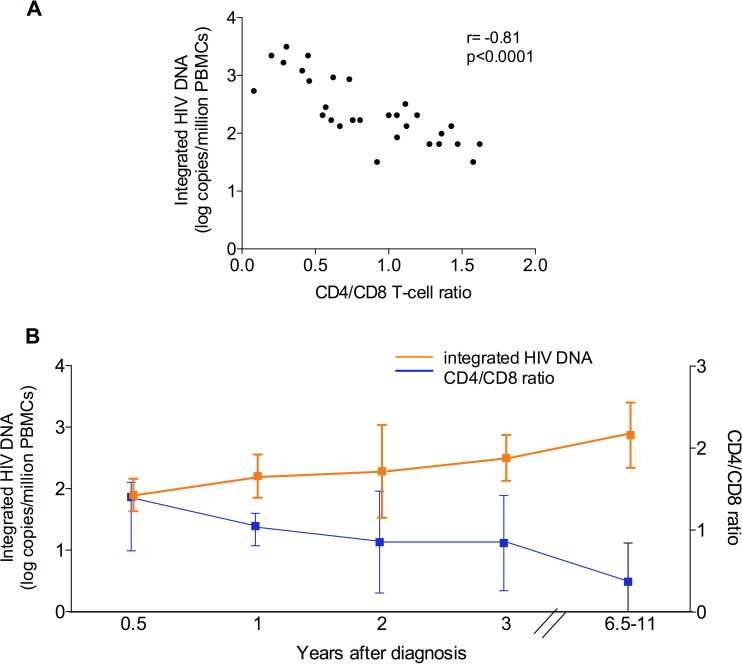
FIG 2.
(A) Correlation between CD4/CD8 cell ratio and integrated HIV DNA in PBMCs (r = −0.81; P < 0.0001) of untreated noncontrollers. (B) Longitudinal measures of CD4/CD8 T-cell ratio and integrated HIV DNA levels over time in PBMCs of untreated noncontrollers. In PBMCs, integrated HIV DNA increased from 109 (IQR, 57 to 178) to 1,941 (IQR, 448 to 2,442) copies/million cells after 6.1 years (P = 0.018). On the other hand, CD4/CD8 cell ratio declined from 0.99 (IQR, 0.68 to 1.2) to 0.29 (IQR, 0.14 to 0.6). Data are medians (interquartile range) for CD4/CD8 cell ratio and mean logs ± standard deviations for integrated HIV DNA.
We sought to determine how reservoir size changed over time in ECs compared to NCs. Integrated HIV DNA was measured in 6 NCs (Fig. 1A and D) and 8 ECs (Fig. 1B and D) over a variety of time points spanning from 1 year to 12 years. The mean observation times were 6.1 ± 3.5 years for NCs and 5.4 ± 3.7 years for ECs.
Integrated HIV DNA increased over time in both groups when normalized per PBMCs. The median annual fold change was significantly higher in NCs than in ECs (P = 0.022). In NCs, HIV DNA level increased from 109 to 1941 copies/million PBMCs after 6 years (P = 0.018) (Fig. 1D) and from 0.23 to 4.11 copies/μl of blood (P = 0.03). The increase in integrated HIV DNA was even higher when normalizing for CD4 T-cell count (from 357 to 12,088 copies/million CD4 T cells; P = 0.03). As expected, CD4 T-cell count declined from 667 to 271 cells/μl and CD4/CD8 cell ratio declined from 0.99 to 0.29 over ∼6 years in NCs. Notably, the rates of accumulation varied between patients, especially among NCs. In fact, two NCs (NC1 and NC2) experienced a drop in integration level early after infection (Fig. 1A), followed by a steady increase in integrated HIV DNA, suggesting initial immune control that was subsequently lost over time.
ECs had low integrated HIV DNA at baseline (median value, 16.6 copies/million PBMCs). However, integrated HIV was higher at subsequent time points, with a median level of 34 copies/million PBMCs at the last time point (P = 0.1) (Fig. 1D). All ECs experienced an increase in integrated HIV DNA over time except for one patient (EC4 [Fig. 1B]). CD4 T-cell counts were relatively constant in the EC cohort such that very similar results were obtained when normalizing for CD4 count (median integration level increased from 99.6 to 181 copies/million CD4 [data not shown]).
As a control for these two cohorts, we also measured integrated HIV DNA in 4 patients on ART with stable undetectable HIV loads. Among these patients, integrated HIV DNA did not significantly change over a 4-year period. The median levels were 1,179 at the start of the study period and 1,038 copies/million PBMCs at the end of the study period (P = 0.25) (Fig. 1C and D).
When normalized to CD4 count, using a linear mixed-effects model, the increase in integrated HIV DNA per each year was found to be higher in NCs than in ECs (P = 0.002) and in ART-treated patients (P < 0.001) (Fig. 1E). Moreover, after normalizing for CD4 count, the integration levels decreased in ART-treated patients and were statistically different than in NCs (P < 0.001) and ECs (P = 0.04) (Fig. 1E).
While it is possible that the increase in integrated HIV DNA represents accumulation of defective proviruses without an increase in reservoir size, the results of a modified viral outgrowth assay developed for ECs correlated with integration levels, suggesting that both replication-competent and defective proviruses likely accumulate over time (2). Moreover, in NCs we found a negative correlation between CD4/CD8 T-cell ratio and integrated HIV DNA levels in PBMCs (r = −0.81; P < 0.0001 [Fig. 2A]). Similar findings were obtained when assessing the correlation between CD4/CD8 T-cell ratio and integrated HIV DNA over time (Fig. 2B), consistent with the idea that integration levels in this cohort provide a surrogate for reservoir size. Taken together, our results show that integrated HIV DNA continues to increase in the absence of ART, and this may reflect growth of the HIV reservoir.
Integrated HIV DNA significantly drops after ART initiation in acutely but not chronically infected patients (Fig. 3).
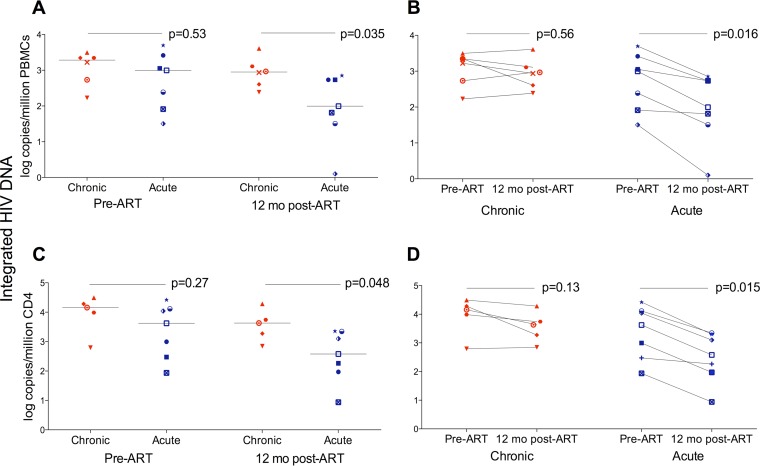
FIG 3.
Integrated HIV DNA in PBMCs (A and B) and CD4 T cells (C and D) from patients with acute and chronic infection at baseline and 12 months after starting ART. (A) Pre-ART integrated HIV DNA levels in PBMCs did not differ between the acute and chronic groups (989 [IQR, 82 to 2,595] versus 1,941 [IQR, 448 to 2,442] copies/million PBMCs, respectively; P = 0.53). Integrated HIV DNA was significantly lower after 12 months of ART in acutely infected individuals (99 [IQR, 32 to 541] versus 893 [IQR, 365 to 1,980] copies/million PBMCs; P = 0.035). Lines represent median log values, with the two groups compared using the Mann-Whitney U test. (B) A significant drop in integrated HIV DNA was observed after 12 months of ART in the acute group (P = 0.016) but not in the chronic one (P = 0.56). Integrated HIV DNA is presented as log copies/million PBMCs. Pre- and post-ART samples were compared using the Wilcoxon signed-rank test. (C) Pre-ART integrated HIV DNA levels in CD4 T cells were not significantly different between the acute and chronic groups (4,213 [IQR, 299 to 13,012] versus 14,342 [IQR, 5,164 to 25,095] copies/million CD4 T cells, respectively; P = 0.27). Integrated HIV DNA was significantly lower after 12 months of ART in acutely infected individuals (380 [IQR, 94 to 2,211] versus 4,257 [IQR, 1,292 to 12,410] copies/million CD4 T cells; P = 0.048). Lines represent median log values, with the two groups compared using the Mann-Whitney U test. (D) The levels of integrated HIV DNA in CD4 T cells significantly declined after 12 months of ART in the acute group (P = 0.015), while a smaller reduction was observed in the chronic one (P = 0.13). Integrated HIV DNA is presented as log copies/million CD4 T cells. Pre- and post-ART samples were compared using the Wilcoxon signed-rank test.
We next tested the impact of the timing of ART initiation on integrated HIV DNA in chronically and acutely infected subjects. Integrated HIV DNA levels were not markedly different before starting ART (989 copies/million PBMCs in acutely infected subjects versus 1,941 in chronically infected subjects [P = 0.53]) (Fig. 3A). Acutely infected patients showed a significant drop in integrated HIV DNA 12 months after starting ART, while integration levels barely decreased among chronically infected individuals over the first 12 months of treatment. Specifically, the median level declined ∼10-fold, from 989 to 99 copies/million PBMCs, in the acute cohort (P = 0.016) (Fig. 3B), while only decreasing from 1,941 to 893 copies/million PBMCs in the chronic patients (P = 0.56) (Fig. 3B). Analogous results were obtained when correcting for CD4 T-cell count. In fact, integrated HIV DNA declined from 14,342 to 4,257 copies/million CD4 in chronically infected patients (P = 0.13) and from 4,213 to 380 copies/million CD4 in acutely infected ones (P = 0.015) (Fig. 3C and D). Similarly, integration levels decreased from 1.24 to 0.3 copies/μl blood in acute patients (P = 0.015) and from 3.8 to 1.36 copies/μl blood in chronic ones (P = 0.67). While our cohort is small, our results are consistent with findings of Koelsch and colleagues (22) and suggest that cells carrying integrated HIV DNA are cleared more effectively when ART is initiated during acute infection.
Pre-ART integrated HIV DNA relates to viral burden in acutely and chronically infected individuals.
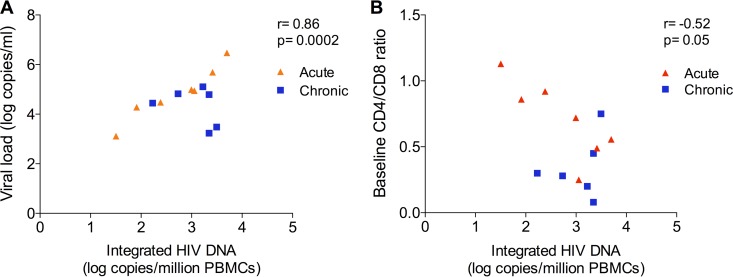
We chose to measure the frequency of integrated HIV DNA in PBMCs as a surrogate of reservoir size because we could do this technically with the small samples provided. Moreover, it is technically challenging to measure replication-competent virus by QVOA in the absence of ART because of the presence of preintegration complexes. To address the functional meaning of integration level, we asked if pre-ART integration levels correlated with viral load or CD4/CD8 T-cell ratio. Integrated HIV DNA correlated with viral load (r = 0.86; P = 0.0002 [Fig. 4A]) and was negatively associated with CD4/CD8 T-cell ratio (r = −0.52; P = 0.05 [Fig. 4B]), again consistent with data showing that integration levels provide a surrogate of reservoir size (2–4).
FIG 4.
Correlation between pre-ART integrated HIV DNA and HIV load (r = 0.86; P = 0.0002) (A) and CD4/CD8 T-cell ratio (r = −0.52; P = 0.05) (B) in chronically (n = 6) and acutely infected (n = 7) patients.
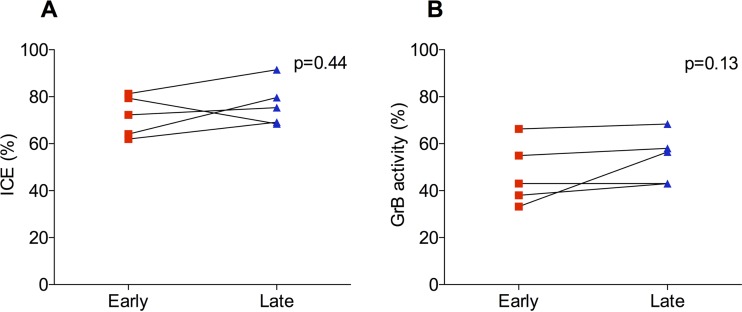
ECs have intact CTL function over time (Fig. 5).
FIG 5.
Infected CD4 T-cell elimination (ICE) (A) and granzyme B (GrB) activity (B) in paired samples of ECs. Cytotoxic functions were measured over time in paired samples of 5 ECs, corresponding to the first (early) and last (late) available time points (mean observation time, 6 ± 1.6 years). No significant changes were detected over time in either median ICE (from 72.3% [IQR, 63 to 80.4%] to 75.4% [IQR, 68.8 to 85.6%]; P = 0.44) (A) or GrB (from 43% [IQR, 35.6 to 60.6%] to 56.5% [IQR, 43 to 63.2%]; P = 0.13) (B) activity. The Wilcoxon signed-rank test was used for comparisons.
To test whether the increase in HIV DNA levels we observed in ECs was due to a decline in cytotoxic T lymphocyte (CTL) function, we measured granzyme B activity and ICE in paired samples of 5 ECs, which were collected at the first and last available time points (mean observation time, 6 ± 1.6 years).
We found that both ICE (from 72.3 to 75.4%; P = 0.44 [Fig. 5A]) and granzyme B activity (from 43 to 56.5%; P = 0.13 [Fig. 5B]) did not change significantly over time. While ECs have on occasion been reported to become viremic, requiring therapy (23–25), our patients maintained CTL control. Thus, loss of CTL does not explain the increase in integrated HIV DNA in our cohort.
DISCUSSION
We found that integrated HIV DNA levels progressively increase over time in the absence of ART, but with a higher rate in NCs than in ECs, consistent with the idea that the immune response can slow reservoir accumulation. Whether this is due to immune control of reservoirs, immune control of viral replication, or both was not addressed by our study. In addition, integrated HIV DNA declines more dramatically in acutely infected individuals than chronic ones following initiation of ART. Taken together, our results suggest important differences in the provirus or immune response between the acute and chronic stages of HIV infection.
Previous studies have shown that HIV reservoirs are established very early after HIV infection. Immediate treatment (as early as 10 days after the onset of symptoms of primary HIV infection [PHI]) does not prevent the establishment of latently infected CD4 T cells harboring integrated HIV DNA (8) but limits the size of the reservoir as measured by HIV DNA (4, 11–15) and may minimize immune activation. Importantly, early treatment likely reduces the amount of replication-competent proviruses, though making these measurements robustly is challenging. In the Visconti cohort, some adults with primary HIV infection who received ART within the first 2 months after infection were able to control viremia for several years after treatment interruption, suggesting that replication-competent proviruses were significantly reduced and/or the replication-competent proviruses that remained with early treatment were less likely to be expressed, perhaps due to less immune activation (12). Similarly, the “Mississippi child,” who started ART at 30 h of age, remained virally suppressed for 27 months in the absence of ART (26). Jain et al. (27) showed that early ART (within 6 months of infection) was associated with lower levels of cell-associated HIV DNA and RNA as well as lower levels of CD4 and CD8 T-cell activation. Again, the authors suggested timing of ART to be a critical determinant of reservoir size, consistent with prior studies (11, 14, 15, 28–30).
One possible explanation for differential rates of integrated HIV DNA accumulation is the individual level of CTL function. We noted that two NCs (NC1 and NC2) had slow reservoir growth early on, suggesting strong CTL activity that was lost over time. It is clear that ECs with effective CTL have much smaller reservoirs than patients with less effective CTL (17, 31, 32), and in this study, we found that they have a lower rate of expansion than NCs (Fig. 1). The increase in integration levels in ECs over time, while initially surprising, is consistent with evidence of ongoing replication (33, 34) and detectable viral loads in ECs (20, 35). We first thought that the increase in integration might reflect loss of CTL function, but CTL functions remained intact (Fig. 5). Given the steady but slow increase in integration levels in ECs, it seems likely that this may be due to continuous viral replication in sanctuary sites. Several studies suggest that B-cell follicles would provide such a sanctuary since they are relatively deficient in functional CD8 T cells (36, 37). This hypothesis has recently been confirmed in rhesus monkey ECs (38): in fact, Fukazawa and colleagues have shown not only that in ECs potent simian immunodeficiency virus (SIV)-specific CD8 T cells are able to restrict HIV production to follicular helper CD4 T cells but also that CD8 depletion is associated with a dramatic redistribution of SIV production to extrafollicular sites. These findings further reinforce the concept that although they are functionally intact, CD8 T cells from ECs may not be able to fully control HIV replication, leading to a slow but continuous increase in reservoir size.
Our results also suggest that ART can induce a greater reduction in the number of cells carrying integrated HIV DNA when administered early during infection. While our sample size is small, these results are consistent with those obtained in another similar study (22, 39). Several mechanisms may be responsible for the greater reduction in primary infection. One possible explanation is the lack of escape CTL in the very early stage of infection (40, 41). As recently reported by Deng and colleagues (41), CTL escape variants dominate the HIV reservoir of individuals starting ART during chronic but not acute infection; moreover, these CTL escape variants can be reactivated and released after latency reversal. From this perspective, treating HIV early means not only limiting the reservoir size but also limiting proviral diversity, as the overwhelming presence of archived CTL escape mutations clearly represents a hurdle for viral eradication strategies based on therapeutic vaccination.
In addition, CTL may be more functional early in infection (40, 42). Several studies have investigated the functional profile of CD8 T cells during primary infection. Streeck et al. (43) reported that HIV-specific CD8 T-cell responses during acute but not chronic infection were associated with the subsequent viral set point and CD4 T-cell dynamics. The same group (42) also showed that HIV-specific CTLs have increased cytotoxicity early in HIV infection compared to that in chronic infection, though they are more prone to apoptosis as a result of sustained activation and metabolic stress (42).
Another possible explanation for enhanced reservoir containment during acute infection is that early treatment may protect central memory CD4 T cells (TCM) from infection, as fewer TCM have been reported to be infected in both posttreatment controllers (12) and ECs (44), although one study found that TCM were not protected by early treatment (10).
Finally, integrated HIV DNA decreases more dramatically after ART therapy during acute infection because there may be a larger fraction of cells containing replication-competent proviruses. It follows that there may be a larger fraction of productively infected cells containing proviruses with a short life span in the early stages of infection (45, 46). Thus, those short-lived cells die and the level of integrated HIV DNA decreases accordingly. This alternate explanation underlies a limitation of our study: the lack of information on the proportion of integrated HIV DNA that is replication competent. One possibility is that defective proviruses continue to accumulate over time (47), while replication-competent HIV reaches a plateau early after infection (8). In this scenario, the large reduction in HIV DNA would reflect the effectiveness of ART against replication-competent proviruses. Meanwhile, in chronic infection, defective proviruses predominate and only a small fraction is reduced by ART. Nonetheless, the facts that HIV proviruses accumulate more slowly in ECs and the lower level of proviruses (17) corresponds to lower levels of replication-competent proviruses in ECs (2, 32) underscore that CTLs likely play a role in clearance of replication-competent proviruses and possibly defective proviruses as well (48, 49). Alternatively, lower viral replication may occur due to poor viral fitness or due to immune restriction of viral replication (50). Studies from Blankson and colleagues suggest that the fitness of virus from ECs is similar to that of virus from chronic progressors and a combination of host immune factors is able to control viral replication (51, 52).
While other studies have indirectly suggested that HIV reservoirs accumulate over time (9–11), especially early after infection, our study is the first to directly assess the dynamics of integrated HIV DNA by monitoring longitudinally the same individuals from the early to the chronic phase of infection. Murray et al. (11) estimated that half of the integrated HIV DNA accumulates within the first 2 years after infection, after which accumulation drastically slows down and finally plateaus, as a consequence of the balance between input (i.e., viral production) and output (i.e., cell activation/loss). Most of the patients in this study were treated within the first 3 years of infection. In contrast, our study suggests that HIV integration levels continue to increase for several years after initial infection. The lack of data on the proportion of defective proviruses during primary and chronic infection limits our ability to make strong conclusions about reservoir accumulation. Nonetheless, there is evidence that suggests that integration levels correlate with the level of replication-competent proviruses (2–4). Proviral sequencing data will eventually resolve these issues; however, techniques able to separate integrated from unintegrated HIV DNA robustly will be required to obtain definitive answers. Notably, different populations may have a different pattern of accumulation of integrated HIV DNA (9, 53). Future studies in this direction may improve our understanding of the contribution of distinct cell subsets as well as of cellular activation status to the growth of HIV reservoirs.
In conclusion, in the absence of ART, integrated HIV DNA accumulates over time both in NCs and, more slowly, in ECs despite robust CTL activity. Low but continued reservoir accumulation in ECs might be due to ongoing, low-level rounds of replication in immunologic sanctuaries where CTLs are excluded. Meanwhile, the rate of accumulation is more brisk in NCs, who have poor CTL function. Moreover, starting ART during acute infection leads to a more dramatic drop in integrated HIV DNA levels than starting ART during chronic infection. Taken together, our findings further reinforce the importance of early diagnosis and treatment to limit reservoir size possibly even in controllers (54), though the risks and benefits have yet to be defined. This work highlights the need to analyze proviral sequences over time to understand the dynamics of replication-competent and defective proviral accumulation. A better understanding of the kinetics of these two forms of HIV may have important implications for cure.
ACKNOWLEDGMENTS
We have no conflict of interest to declare.
This research was supported by amfAR and NIH grants R21AI096993, R21AI106557, R21AI116216, and T32AI007632.
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF. 2013. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendoza D, Johnson SA, Peterson BA, Natarajan V, Salgado M, Dewar RL, Burbelo PD, Doria-Rose NA, Graf EH, Greenwald JH, Hodge JN, Thompson WL, Cogliano NA, Chairez CL, Rehm CA, Jones S, Hallahan CW, Kovacs JA, Sereti I, Sued O, Peel SA, O'Connell RJ, O'Doherty U, Chun TW, Connors M, Migueles SA. 2012. Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood 119:4645–4655. doi: 10.1182/blood-2011-10-381996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graf EH, O'Doherty U. 2013. Quantitation of integrated proviral DNA in viral reservoirs. Current opinion in HIV and AIDS 8:100–105. doi: 10.1097/COH.0b013e32835d8132. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, Bosch RJ, Lai J, Chioma S, Emad F, Abdel-Mohsen M, Hoh R, Hecht F, Hunt P, Somsouk M, Wong J, Johnston R, Siliciano RF, Richman DD, O'Doherty U, Palmer S, Deeks SG, Siliciano JD. 2013. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog 9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 7.Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. 1995. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med 1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 8.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. 1998. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A 95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buzon MJ, Martin-Gayo E, Pereyra F, Ouyang Z, Sun H, Li JZ, Piovoso M, Shaw A, Dalmau J, Zangger N, Martinez-Picado J, Zurakowski R, Yu XG, Telenti A, Walker BD, Rosenberg ES, Lichterfeld M. 2014. Long-term antiretroviral treatment initiated at primary HIV-1 infection affects the size, composition, and decay kinetics of the reservoir of HIV-1-infected CD4 T cells. J Virol 88:10056–10065. doi: 10.1128/JVI.01046-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray JM, McBride K, Boesecke C, Bailey M, Amin J, Suzuki K, Baker D, Zaunders JJ, Emery S, Cooper DA, Koelsch KK, Kelleher AD. 2012. Integrated HIV DNA accumulates prior to treatment while episomal HIV DNA records ongoing transmission afterwards. AIDS 26:543–550. doi: 10.1097/QAD.0b013e328350fb3c. [DOI] [PubMed] [Google Scholar]
- 12.Sáez-Cirión A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C. 2013. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rouzioux C, Richman D. 2013. How to best measure HIV reservoirs? Curr Opin HIV AIDS 8:170–175. doi: 10.1097/COH.0b013e32835fc619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ananworanich J, Schuetz A, Vandergeeten C, Sereti I, de Souza M, Rerknimitr R, Dewar R, Marovich M, van Griensven F, Sekaly R, Pinyakorn S, Phanuphak N, Trichavaroj R, Rutvisuttinunt W, Chomchey N, Paris R, Peel S, Valcour V, Maldarelli F, Chomont N, Michael N, Phanuphak P, Kim JH. 2012. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 7:e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strain MC, Little SJ, Daar ES, Havlir DV, Gunthard HF, Lam RY, Daly OA, Nguyen J, Ignacio CC, Spina CA, Richman DD, Wong JK. 2005. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis 191:1410–1418. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 16.Hecht FM, Busch MP, Rawal B, Webb M, Rosenberg E, Swanson M, Chesney M, Anderson J, Levy J, Kahn JO. 2002. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS 16:1119–1129. doi: 10.1097/00002030-200205240-00005. [DOI] [PubMed] [Google Scholar]
- 17.Graf EH, Mexas AM, Yu JJ, Shaheen F, Liszewski MK, Di Mascio M, Migueles SA, Connors M, O'Doherty U. 2011. Elite suppressors harbor low levels of integrated HIV DNA and high levels of 2-LTR circular HIV DNA compared to HIV+ patients on and off HAART. PLoS Pathog 7:e1001300. doi: 10.1371/journal.ppat.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cillo AR, Sobolewski MD, Bosch RJ, Fyne E, Piatak M Jr, Coffin JM, Mellors JW. 2014. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 111:7078–7083. doi: 10.1073/pnas.1402873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Spiegelaere W, Malatinkova E, Lynch L, Van Nieuwerburgh F, Messiaen P, O'Doherty U, Vandekerckhove L. 2014. Quantification of integrated HIV DNA by repetitive-sampling Alu-HIV PCR on the basis of Poisson statistics. Clin Chem 60:886–895. doi: 10.1373/clinchem.2013.219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, Rood JE, Berkley AM, Sacha JB, Cogliano-Shutta NA, Lloyd M, Roby G, Kwan R, McLaughlin M, Stallings S, Rehm C, O'Shea MA, Mican J, Packard BZ, Komoriya A, Palmer S, Wiegand AP, Maldarelli F, Coffin JM, Mellors JW, Hallahan CW, Follman DA, Connors M. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Migueles SA, Weeks KA, Nou E, Berkley AM, Rood JE, Osborne CM, Hallahan CW, Cogliano-Shutta NA, Metcalf JA, McLaughlin M, Kwan R, Mican JM, Davey RT Jr, Connors M. 2009. Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol 83:11876–11889. doi: 10.1128/JVI.01153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koelsch KK, Boesecke C, McBride K, Gelgor L, Fahey P, Natarajan V, Baker D, Bloch M, Murray JM, Zaunders J, Emery S, Cooper DA, Kelleher AD. 2011. Impact of treatment with raltegravir during primary or chronic HIV infection on RNA decay characteristics and the HIV viral reservoir. AIDS 25:2069–2078. doi: 10.1097/QAD.0b013e32834b9658. [DOI] [PubMed] [Google Scholar]
- 23.Bailey JR, Zhang H, Wegweiser BW, Yang HC, Herrera L, Ahonkhai A, Williams TM, Siliciano RF, Blankson JN. 2007. Evolution of HIV-1 in an HLA-B*57-positive patient during virologic escape. J Infect Dis 196:50–55. doi: 10.1086/518515. [DOI] [PubMed] [Google Scholar]
- 24.Migueles SA, Connors M. 2010. Long-term nonprogressive disease among untreated HIV-infected individuals: clinical implications of understanding immune control of HIV. JAMA 304:194–201. doi: 10.1001/jama.2010.925. [DOI] [PubMed] [Google Scholar]
- 25.Leon A, Perez I, Ruiz-Mateos E, Benito JM, Leal M, Lopez-Galindez C, Rallon N, Alcami J, Lopez-Aldeguer J, Viciana P, Rodriguez C, Grau E, Iribarren J, Gatell JM, Garcia F, EC and Immune Pathogenesis Working Group of the Spanish AIDS Research Network . 2016. Rate and predictors of progression in elite and viremic HIV-1 controllers. AIDS 30:1209–1220. doi: 10.1097/QAD.0000000000001050. [DOI] [PubMed] [Google Scholar]
- 26.Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M Jr, Chun TW, Strain M, Richman D, Luzuriaga K. 2013. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 369:1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, Epling L, Lee TH, Busch MP, McCune JM, Pilcher CD, Hecht FM, Deeks SG. 2013. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis 208:1202–1211. doi: 10.1093/infdis/jit311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hocqueloux L, Avettand-Fenoel V, Jacquot S, Prazuck T, Legac E, Melard A, Niang M, Mille C, Le Moal G, Viard JP, Rouzioux C. 2013. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother 68:1169–1178. doi: 10.1093/jac/dks533. [DOI] [PubMed] [Google Scholar]
- 29.Laanani M, Ghosn J, Essat A, Melard A, Seng R, Gousset M, Panjo H, Mortier E, Girard PM, Goujard C, Meyer L, Rouzioux C. 2015. Impact of the timing of initiation of antiretroviral therapy during primary HIV-1 infection on the decay of cell-associated HIV-DNA. Clin Infect Dis 60:1715–1721. doi: 10.1093/cid/civ171. [DOI] [PubMed] [Google Scholar]
- 30.Koelsch KK, Liu L, Haubrich R, May S, Havlir D, Gunthard HF, Ignacio CC, Campos-Soto P, Little SJ, Shafer R, Robbins GK, D'Aquila RT, Kawano Y, Young K, Dao P, Spina CA, Richman DD, Wong JK. 2008. Dynamics of total, linear nonintegrated, and integrated HIV-1 DNA in vivo and in vitro. J Infect Dis 197:411–419. doi: 10.1086/525283. [DOI] [PubMed] [Google Scholar]
- 31.Julg B, Pereyra F, Buzon MJ, Piechocka-Trocha A, Clark MJ, Baker BM, Lian J, Miura T, Martinez-Picado J, Addo MM, Walker BD. 2010. Infrequent recovery of HIV from but robust exogenous infection of activated CD4(+) T cells in HIV elite controllers. Clin Infect Dis 51:233–238. doi: 10.1086/653677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blankson JN, Bailey JR, Thayil S, Yang HC, Lassen K, Lai J, Gandhi SK, Siliciano JD, Williams TM, Siliciano RF. 2007. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol 81:2508–2518. doi: 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Connell KA, Brennan TP, Bailey JR, Ray SC, Siliciano RF, Blankson JN. 2010. Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus. J Virol 84:7018–7028. doi: 10.1128/JVI.00548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mens H, Kearney M, Wiegand A, Shao W, Schonning K, Gerstoft J, Obel N, Maldarelli F, Mellors JW, Benfield T, Coffin JM. 2010. HIV-1 continues to replicate and evolve in patients with natural control of HIV infection. J Virol 84:12971–12981. doi: 10.1128/JVI.00387-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereyra F, Palmer S, Miura T, Block BL, Wiegand A, Rothchild AC, Baker B, Rosenberg R, Cutrell E, Seaman MS, Coffin JM, Walker BD. 2009. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis 200:984–990. doi: 10.1086/605446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connick E, Mattila T, Folkvord JM, Schlichtemeier R, Meditz AL, Ray MG, McCarter MD, Mawhinney S, Hage A, White C, Skinner PJ. 2007. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol 178:6975–6983. doi: 10.4049/jimmunol.178.11.6975. [DOI] [PubMed] [Google Scholar]
- 37.Connick E, Folkvord JM, Lind KT, Rakasz EG, Miles B, Wilson NA, Santiago ML, Schmitt K, Stephens EB, Kim HO, Wagstaff R, Li S, Abdelaal HM, Kemp N, Watkins DI, MaWhinney S, Skinner PJ. 2014. Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J Immunol 193:5613–5625. doi: 10.4049/jimmunol.1401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, Morcock D, Swanson T, Legasse AW, Axthelm MK, Hesselgesser J, Geleziunas R, Hirsch VM, Edlefsen PT, Piatak M Jr, Estes JD, Lifson JD, Picker LJ. 2015. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 21:132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hey-Cunningham WJ, Murray JM, Natarajan V, Amin J, Moore CL, Emery S, Cooper DA, Zaunders J, Kelleher AD, Koelsch KK. 2015. Early antiretroviral therapy with raltegravir generates sustained reductions in HIV reservoirs but not lower T-cell activation levels. AIDS 29:911–919. doi: 10.1097/QAD.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 40.Radebe M, Gounder K, Mokgoro M, Ndhlovu ZM, Mncube Z, Mkhize L, van der Stok M, Jaggernath M, Walker BD, Ndung'u T. 2015. Broad and persistent Gag-specific CD8+ T-cell responses are associated with viral control but rarely drive viral escape during primary HIV-1 infection. AIDS 29:23–33. doi: 10.1097/QAD.0000000000000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, Lai J, McHugh HL, Hao H, Zhang H, Margolick JB, Gurer C, Murphy AJ, Valenzuela DM, Yancopoulos GD, Deeks SG, Strowig T, Kumar P, Siliciano JD, Salzberg SL, Flavell RA, Shan L, Siliciano RF. 2015. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 517:381–385. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trautmann L, Mbitikon-Kobo FM, Goulet JP, Peretz Y, Shi Y, Van Grevenynghe J, Procopio FA, Boulassel MR, Routy JP, Chomont N, Haddad EK, Sekaly RP. 2012. Profound metabolic, functional, and cytolytic differences characterize HIV-specific CD8 T cells in primary and chronic HIV infection. Blood 120:3466–3477. doi: 10.1182/blood-2012-04-422550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Streeck H, Jolin JS, Qi Y, Yassine-Diab B, Johnson RC, Kwon DS, Addo MM, Brumme C, Routy JP, Little S, Jessen HK, Kelleher AD, Hecht FM, Sekaly RP, Rosenberg ES, Walker BD, Carrington M, Altfeld M. 2009. Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells. J Virol 83:7641–7648. doi: 10.1128/JVI.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Descours B, Avettand-Fenoel V, Blanc C, Samri A, Melard A, Supervie V, Theodorou I, Carcelain G, Rouzioux C, Autran B. 2012. Immune responses driven by protective human leukocyte antigen alleles from long-term nonprogressors are associated with low HIV reservoir in central memory CD4 T cells. Clin Infect Dis 54:1495–1503. doi: 10.1093/cid/cis188. [DOI] [PubMed] [Google Scholar]
- 45.Ma ZM, Stone M, Piatak M Jr, Schweighardt B, Haigwood NL, Montefiori D, Lifson JD, Busch MP, Miller CJ. 2009. High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. J Virol 83:3288–3297. doi: 10.1128/JVI.02423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, Zajic L, Iyer SS, Decker JM, Kumar A, Hora B, Berg A, Cai F, Hopper J, Denny TN, Ding H, Ochsenbauer C, Kappes JC, Galimidi RP, West AP Jr, Bjorkman PJ, Wilen CB, Doms RW, O'Brien M, Bhardwaj N, Borrow P, Haynes BF, Muldoon M, Theiler JP, Korber B, Shaw GM, Hahn BH. 2013. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci U S A 110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohn LB, Silva IT, Oliveira TY, Rosales RA, Parrish EH, Learn GH, Hahn BH, Czartoski JL, McElrath MJ, Lehmann C, Klein F, Caskey M, Walker BD, Siliciano JD, Siliciano RF, Jankovic M, Nussenzweig MC. 2015. HIV-1 integration landscape during latent and active infection. Cell 160:420–432. doi: 10.1016/j.cell.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imamichi H, Natarajan V, Adelsberger JW, Rehm CA, Lempicki RA, Das B, Hazen A, Imamichi T, Lane HC. 2014. Lifespan of effector memory CD4+ T cells determined by replication-incompetent integrated HIV-1 provirus. AIDS 28:1091–1099. doi: 10.1097/QAD.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 49.Imamichi H, Dewar R, Adelsberger JW, Rehm C, O'Doherty U, Paxinos EE, Fauci AS, Lane HC. 2016. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc Natl Acad Sci U S A 113:8783–8788. doi: 10.1073/pnas.1609057113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buzon MJ, Seiss K, Weiss R, Brass AL, Rosenberg ES, Pereyra F, Yu XG, Lichterfeld M. 2011. Inhibition of HIV-1 integration in ex vivo-infected CD4 T cells from elite controllers. J Virol 85:9646–9650. doi: 10.1128/JVI.05327-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buckheit RW III, Allen TG, Alme A, Salgado M, O'Connell KA, Huculak S, Falade-Nwulia O, Williams TM, Gallant JE, Siliciano RF, Blankson JN. 2012. Host factors dictate control of viral replication in two HIV-1 controller/chronic progressor transmission pairs. Nat Commun 3:716. doi: 10.1038/ncomms1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salgado M, Swanson MD, Pohlmeyer CW, Buckheit RW III, Wu J, Archin NM, Williams TM, Margolis DM, Siliciano RF, Garcia JV, Blankson JN. 2014. HLA-B*57 elite suppressor and chronic progressor HIV-1 isolates replicate vigorously and cause CD4+ T cell depletion in humanized BLT mice. J Virol 88:3340–3352. doi: 10.1128/JVI.03380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray JM, Zaunders JJ, McBride KL, Xu Y, Bailey M, Suzuki K, Cooper DA, Emery S, Kelleher AD, Koelsch KK, Team PS. 2014. HIV DNA subspecies persist in both activated and resting memory CD4+ T cells during antiretroviral therapy. J Virol 88:3516–3526. doi: 10.1128/JVI.03331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hatano H, Yukl SA, Ferre AL, Graf EH, Somsouk M, Sinclair E, Abdel-Mohsen M, Liegler T, Harvill K, Hoh R, Palmer S, Bacchetti P, Hunt PW, Martin JN, McCune JM, Tracy RP, Busch MP, O'Doherty U, Shacklett BL, Wong JK, Deeks SG. 2013. Prospective antiretroviral treatment of asymptomatic, HIV-1 infected controllers. PLoS Pathog 9:e1003691. doi: 10.1371/journal.ppat.1003691. [DOI] [PMC free article] [PubMed] [Google Scholar]