Abstract
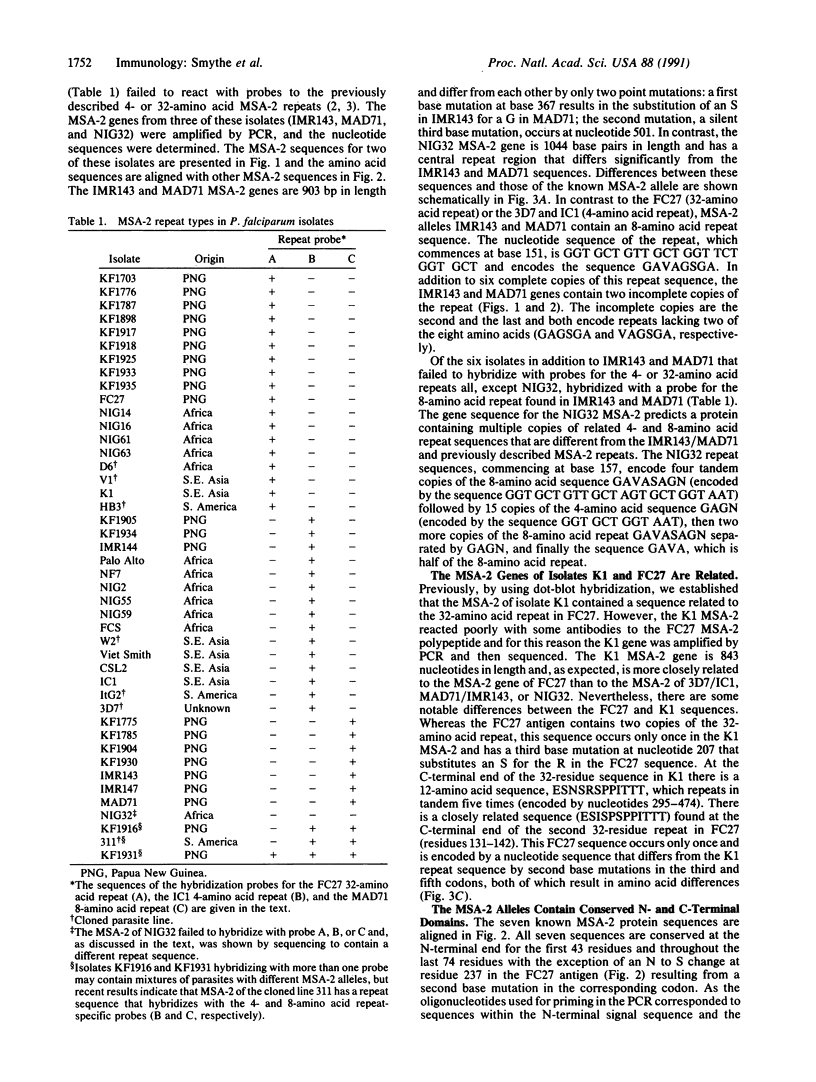
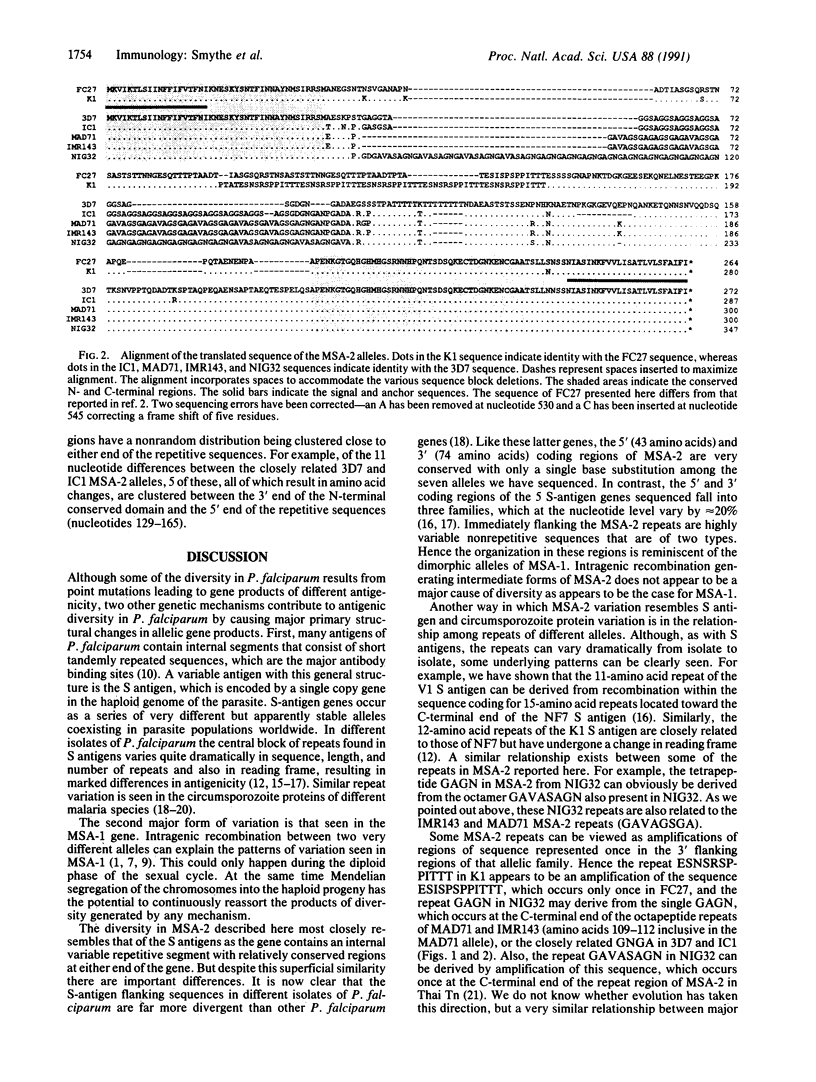
Antigens associated with the surface of merozoites of the malaria parasite Plasmodium falciparum are directly accessible to immune attack and therefore are prime vaccine candidates. We have previously shown that one of the two known merozoite surface antigens (merozoite surface antigen 2; MSA-2) exhibits considerable sequence and antigenic diversity in different isolates. The sequences of MSA-2 from three isolates revealed a central domain composed of repeats that vary in number, length, and sequence, flanked in turn by nonrepetitive variable sequences and by conserved N- and C-terminal domains. We report here the sequences of a further four MSA-2 alleles, containing repetitive sequences that are related but not identical to each other. The seven alleles of MSA-2 can be divided into two distinct allele families on the basis of nonrepetitive sequences. Hybridization studies with repeat probes indicated that all of the 44 P. falciparum isolates examined contained repeat regions similar to those defined in known MSA-2 sequences.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnot D. E., Barnwell J. W., Stewart M. J. Does biased gene conversion influence polymorphism in the circumsporozoite protein-encoding gene of Plasmodium vivax? Proc Natl Acad Sci U S A. 1988 Nov;85(21):8102–8106. doi: 10.1073/pnas.85.21.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H., Kemp D. J., Barzaga N., Brown G. V., Anders R. F., Coppel R. L. Sequence variation in S-antigen genes of Plasmodium falciparum. Mol Biol Med. 1987 Dec;4(6):365–376. [PubMed] [Google Scholar]
- Certa U., Rotmann D., Matile H., Reber-Liske R. A naturally occurring gene encoding the major surface antigen precursor p190 of Plasmodium falciparum lacks tripeptide repeats. EMBO J. 1987 Dec 20;6(13):4137–4142. doi: 10.1002/j.1460-2075.1987.tb02759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. T., Donachie S., Anand R., Wilson C. F., Heidrich H. G., McBride J. S. 46-53 kilodalton glycoprotein from the surface of Plasmodium falciparum merozoites. Mol Biochem Parasitol. 1989 Jan 1;32(1):15–24. doi: 10.1016/0166-6851(89)90125-4. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Saint R. B., Coppel R. L., Brown G. V., Anders R. F., Kemp D. J. Conserved sequences flank variable tandem repeats in two S-antigen genes of Plasmodium falciparum. Cell. 1985 Apr;40(4):775–783. doi: 10.1016/0092-8674(85)90337-x. [DOI] [PubMed] [Google Scholar]
- Epping R. J., Goldstone S. D., Ingram L. T., Upcroft J. A., Ramasamy R., Cooper J. A., Bushell G. R., Geysen H. M. An epitope recognised by inhibitory monoclonal antibodies that react with a 51 kilodalton merozoite surface antigen in Plasmodium falciparum. Mol Biochem Parasitol. 1988 Feb;28(1):1–10. doi: 10.1016/0166-6851(88)90173-9. [DOI] [PubMed] [Google Scholar]
- Fenton B., Clark J. T., Wilson C. F., McBride J. S., Walliker D. Polymorphism of a 35-48 kDa Plasmodium falciparum merozoite surface antigen. Mol Biochem Parasitol. 1989 Apr;34(1):79–86. doi: 10.1016/0166-6851(89)90022-4. [DOI] [PubMed] [Google Scholar]
- Foote S. J., Kyle D. E., Martin R. K., Oduola A. M., Forsyth K., Kemp D. J., Cowman A. F. Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum. Nature. 1990 May 17;345(6272):255–258. doi: 10.1038/345255a0. [DOI] [PubMed] [Google Scholar]
- Forsyth K. P., Philip G., Smith T., Kum E., Southwell B., Brown G. V. Diversity of antigens expressed on the surface of erythrocytes infected with mature Plasmodium falciparum parasites in Papua New Guinea. Am J Trop Med Hyg. 1989 Sep;41(3):259–265. [PubMed] [Google Scholar]
- Galinski M. R., Arnot D. E., Cochrane A. H., Barnwell J. W., Nussenzweig R. S., Enea V. The circumsporozoite gene of the Plasmodium cynomolgi complex. Cell. 1987 Jan 30;48(2):311–319. doi: 10.1016/0092-8674(87)90434-x. [DOI] [PubMed] [Google Scholar]
- Holder A. A. The precursor to major merozoite surface antigens: structure and role in immunity. Prog Allergy. 1988;41:72–97. [PubMed] [Google Scholar]
- Jeffreys A. J., Neumann R., Wilson V. Repeat unit sequence variation in minisatellites: a novel source of DNA polymorphism for studying variation and mutation by single molecule analysis. Cell. 1990 Feb 9;60(3):473–485. doi: 10.1016/0092-8674(90)90598-9. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Coppel R. L., Anders R. F. Repetitive proteins and genes of malaria. Annu Rev Microbiol. 1987;41:181–208. doi: 10.1146/annurev.mi.41.100187.001145. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., de la Cruz V. F., Good M. F., Wellems T. E. Antigenic diversity in Plasmodium falciparum. Prog Allergy. 1988;41:173–192. doi: 10.1159/000415223. [DOI] [PubMed] [Google Scholar]
- Nicholls S. C., Hillman Y., Lockyer M. J., Odink K. G., Holder A. A. An S antigen gene from Plasmodium falciparum contains a novel repetitive sequence. Mol Biochem Parasitol. 1988 Feb;28(1):11–19. doi: 10.1016/0166-6851(88)90174-0. [DOI] [PubMed] [Google Scholar]
- Peterson M. G., Coppel R. L., Moloney M. B., Kemp D. J. Third form of the precursor to the major merozoite surface antigens of Plasmodium falciparum. Mol Cell Biol. 1988 Jun;8(6):2664–2667. doi: 10.1128/mcb.8.6.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy R. Studies on glycoproteins in the human malaria parasite Plasmodium falciparum. Identification of a myristilated 45kDa merozoite membrane glycoprotein. Immunol Cell Biol. 1987 Oct;65(Pt 5):419–424. doi: 10.1038/icb.1987.48. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saint R. B., Coppel R. L., Cowman A. F., Brown G. V., Shi P. T., Barzaga N., Kemp D. J., Anders R. F. Changes in repeat number, sequence, and reading frame in S-antigen genes of Plasmodium falciparum. Mol Cell Biol. 1987 Aug;7(8):2968–2973. doi: 10.1128/mcb.7.8.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe J. A., Coppel R. L., Brown G. V., Ramasamy R., Kemp D. J., Anders R. F. Identification of two integral membrane proteins of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5195–5199. doi: 10.1073/pnas.85.14.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe J. A., Peterson M. G., Coppel R. L., Saul A. J., Kemp D. J., Anders R. F. Structural diversity in the 45-kilodalton merozoite surface antigen of Plasmodium falciparum. Mol Biochem Parasitol. 1990 Mar;39(2):227–234. doi: 10.1016/0166-6851(90)90061-p. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Mackay M., Goman M., Scaife J. G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987 May 20;195(2):273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- Thomas A. W., Carr D. A., Carter J. M., Lyon J. A. Sequence comparison of allelic forms of the Plasmodium falciparum merozoite surface antigen MSA2. Mol Biochem Parasitol. 1990 Dec;43(2):211–220. doi: 10.1016/0166-6851(90)90146-d. [DOI] [PubMed] [Google Scholar]





