Abstract
IMPORTANCE
Giant cell arteritis (GCA) is the most common systemic vasculitis in elderly individuals. Diagnosis is confirmed by temporal artery (TA) biopsy, although biopsy results are often negative. Despite the use of corticosteroids, disease may progress. Identification of causal agents will improve outcomes. Biopsy-positive GCA is associated with TA infection by varicella-zoster virus (VZV).
OBJECTIVE
To analyze VZV infection in TAs of patients with clinically suspected GCA whose TAs were histopathologically negative and in normal TAs removed post mortem from age-matched individuals.
DESIGN, SETTING, AND PARTICIPANTS
A cross-sectional study for VZV antigen was performed from January 2013 to March 2015 using archived, deidentified, formalin-fixed, paraffin-embedded GCA-negative, GCA-positive, and normal TAs (50 sections/TA) collected during the past 30 years. Regions adjacent to those containing VZV were examined by hematoxylin-eosin staining. Immunohistochemistry identified inflammatory cells and cell types around nerve bundles containing VZV. A combination of 17 tertiary referral centers and private practices worldwide contributed archived TAs from individuals older than 50 years.
MAIN OUTCOMES AND MEASURES
Presence and distribution of VZV antigen in TAs and histopathological changes in sections adjacent to those containing VZV were confirmed by 2 independent readers.
RESULTS
Varicella-zoster virus antigen was found in 45 of 70 GCA-negative TAs (64%), compared with 11 of 49 normal TAs (22%) (relative risk [RR] = 2.86; 95% CI, 1.75–5.31; P < .001). Extension of our earlier study revealed VZV antigen in 68 of 93 GCA-positive TAs (73%), compared with 11 of 49 normal TAs (22%) (RR = 3.26; 95% CI, 2.03–5.98; P < .001). Compared with normal TAs, VZV antigen was more likely to be present in the adventitia of both GCA-negative TAs (RR = 2.43; 95% CI, 1.82–3.41; P < .001) and GCA-positive TAs (RR = 2.03; 95% CI, 1.52–2.86; P < .001). Varicella-zoster virus antigen was frequently found in perineurial cells expressing claudin-1 around nerve bundles. Of 45 GCA-negative participants whose TAs contained VZV antigen, 1 had histopathological features characteristic of GCA, and 16 (36%) showed adventitial inflammation adjacent to viral antigen; no inflammation was seen in normal TAs.
CONCLUSIONS AND RELEVANCE
In patients with clinically suspected GCA, prevalence of VZV in their TAs is similar independent of whether biopsy results are negative or positive pathologically. Antiviral treatment may confer additional benefit to patients with biopsy-negative GCA treated with corticosteroids, although the optimal antiviral regimen remains to be determined.
Giant cell arteritis (GCA) is a disease occurring in elderly individuals and is characterized by severe headache/head pain and scalp tenderness. Many patients have a history of jaw claudication, polymyalgia rheumatica, fever, night sweats, weight loss, fatigue, elevated erythrocyte sedimentation rate, and elevated C-reactive protein level. Prompt corticosteroid treatment provides symptomatic relief and prevents vision loss. Temporal artery (TA) biopsy reveals inflammation and necrosis in the arterial media, with multinucleated giant cells and/or epithelioid macrophages. Skip lesions are common. Results on TA biopsy are pathologically negative in many clinically suspected cases.
Recently, varicella-zoster virus (VZV) was found in 61 of 82 GCA-positive TAs (74%)1 as well as in TAs from patients with clinically suspected GCA but pathologically negative biopsy results.2–5 Furthermore, histopathological reexamination of sections adjacent to those containing VZV antigen in a GCA-negative TA revealed classic GCA pathology, resulting in a change of diagnosis from GCA negative to GCA positive.4,6 In another GCA-negative TA from a patient with clinical GCA and ipsilateral ophthalmic-distribution zoster, followed 2 weeks later by VZV encephalitis and 2 months later by ischemic optic neuropathy, VZV antigen and VZV DNA were found in multiple noncontiguous (skip) areas.7
To further test the possibility of a causal link between VZV and GCA, we searched for VZV antigen in archived TAs from individuals with clinically suspected GCA whose biopsy results were pathologically negative.
Methods
Human TAs
A total of 75 deidentified formalin-fixed, paraffin-embedded (FFPE) TA biopsy specimens collected during the past 30 years and studied from January 2013 to March 2015 from individuals older than 50 years with clinically suspected GCA whose TAs were histopathologically negative for GCA were obtained from 12 institutions: the University of Colorado Hospital, Aurora; Henry Ford Health System, Detroit, Michigan; Fort Wayne Neurological Center, Fort Wayne, Indiana; Johns Hopkins Hospital, Baltimore, Maryland; Emory University Hospital, Atlanta, Georgia; Mount Sinai Medical Center, Miami Beach, Florida; Hospital of the University of Pennsylvania, Philadelphia; the Center for Oculoplastic Surgery, Austin, Texas; Mount Sinai Medical Center, New York, New York; University of Würzburg Hospital, Würzburg, Germany; University Hospital, Essen, Germany; and the Assaf Harofeh Medical Center, Zerifin, Israel. Of the 75 individuals whose TAs were analyzed, sex and age data were available for 73 (28 men [38%] and 45 women [62%]; age range, 50–90 years; mean [SD] age, 71.7 [9.6] years). The number of GCA-positive TAs previously examined for VZV1 was increased to 93, with TAs obtained from the following institutions: University of Colorado Hospital, Aurora; Exempla Lutheran Medical Center, Wheat Ridge, Colorado; Oregon Health and Science University, Portland; Landspitali University Hospital, Reykjavik, Iceland; and University of Würzburg Hospital, Würzburg, Germany. Finally, 53 control TAs were removed post mortem from age-matched individuals older than 50 years at the University of Colorado Hospital and Office of the Medical Examiner, Denver, and the Arapahoe County Coroner’s Office, Centennial, Colorado, with appropriate oral consent of next of kin. No control participants exhibited skin evidence of recent herpes zoster infection. Control TAs were formalin fixed and paraffin embedded for virological analysis.
Deidentified archived TAs containing only noncoded specimen number, age, and sex as well as control TAs obtained at autopsy are not considered human subject research by the Colorado Multiple Institutional Review Board and are therefore deemed exempt from review and requirement for informed consent as defined by its policies and regulations and in accordance with US Office for Human Research Protections and Food and Drug Administration guidelines. Clinical information regarding a history of zoster infection, zoster vaccination, or medication use was not available.
Immunohistochemical Analysis of Human TAs for VZV Antigen
One hundred 5-μm sections of all FFPE TAs were cut and alternate sections were analyzed by immunohistochemistry using mouse monoclonal anti-VZV gE IgG1 antibody (Santa Cruz Biotechnology) and control mouse IgG1 antibody (Dako). Positive controls consisted of VZV-infected cadaveric cerebral arteries maintained for 14 days in vitro followed by immunostaining with mouse anti-VZV gE IgG1 antibody, all as previously described.1
Using light microscopy, 2 readers (D.G. and M.A.N.), blinded to the diagnosis of GCA biopsy–negative or normal TA, examined each section. A TA section was deemed positive or negative for VZV antigen only when both readers agreed; in 5 of 75 GCA-negative TAs and 4 of 53 normal TAs, the readers disagreed and those 9 TAs were excluded from the study.
Distribution of VZV in TAs
The distribution of VZV antigen and number of skip lesions, defined as at least 2 VZV antigen–positive regions flanked by VZV antigen–negative regions, were assessed. The location of viral antigen in each section was recorded as adventitia, media, or intima and the frequency of VZV in each arterial layer was determined. When VZV was detected in the adventitia of a TA, the location of viral antigen in the nerve bundle, vasa vasorum, and adventitial cells was similarly determined.
Histopathological Analysis of Sections Adjacent to Those Containing VZV Antigen for Pathologic Features of GCA
For each section that contained VZV antigen, an adjacent section (within 5 μm) was stained with hematoxylin-eosin. Slides were examined by standard light microscopy by the neuropathologist (P.J.B.) and neurovirologists (D.G. and M.A.N.) for GCA positivity, defined based on the following: (1) the presence of transmural inflammation; (2) medial necrosis or other damage or disruption; and (3) giant or epithelioid macrophages. A TA section was deemed GCA positive only when all readers agreed that all 3 criteria were met. If a TA section was deemed GCA negative but was suspected to contain inflammatory cells, the hematoxylin-eosin–stained section was destained using acid alcohol and immunostained with rabbit anti-CD45 antibody (1:000 dilution; Abcam) using antigen retrieval as previously described.1 Positive controls consisted of FFPE human lymph nodes obtained post mortem and sections were examined by light microscopy by neurovirologists (D.G. and M.A.N.). A TA section was deemed positive for inflammation when both readers agreed that 10 or more cells expressed CD45 in a field (magnification ×600).
Immunohistochemical Analysis of Cells Surrounding Nerve Bundles in the Arterial Adventitia That Contained VZV Antigen
To identify the cell type containing VZV antigen, sections adjacent to those containing VZV antigen in nerve bundles were immunostained with multiple antibodies as previously described1: rabbit anti-claudin-1 IgG, rabbit anti-S-100 antibody, and mouse antimyelin basic protein antibody (all at 1:1000 dilution; Abcam); mouse anti–βIII tubulin antibody (1: 1000 dilution; StemCell Technologies); and rabbit anti-CD45 antibody (1:100 dilution; Abcam). Positive controls consisted of FFPE human trigeminal ganglia and lymph nodes obtained post mortem.
Polymerase Chain Reaction Amplification of VZV DNA in TA Sections Containing VZV Antigen
Each VZV antigen–positive section from 45 GCA-negative TAs and 11 normal TAs that contained VZV antigen was scraped with a scalpel and pooled, and DNA was extracted and analyzed by polymerase chain reaction for the presence of VZV DNA as previously described.1 Samples were considered positive for VZV DNA if they met the following criteria: (1) no VZV DNA amplification was detected in samples without added DNA; (2) glyceraldehyde-3-phosphate dehydrogenase was detected in wells with TA DNA; and (3) at least 2 of 4 polymerase chain reaction replicates amplified at least 10 copies of target VZV DNA per 5 μL of DNA extracted. The VZV DNA copy number was determined using known concentrations of VZV DNA as polymerase chain reaction standards.8
Results
Presence and Distribution of VZV Antigen in GCA-Negative and Normal TAs
Immunohistochemical analysis of 3500 sections from 70 GCA-negative TAs (50 slides/TA) detected VZV antigen in sections from 45 participants (64%), compared with 11 of 49 participants with normal TAs (22%) (Table 1). Varicella-zoster virus antigen was 2.86 times more likely to be present in GCA-negative TAs than in normal TAs (relative risk = 2.86; 95% CI, 1.75–5.31; P < .001). Table 1 also includes data from our earlier study,1 which have been expanded and show that VZV was present in 68 of 93 GCA-positive TAs (73%); overall, VZV antigen was 3.26 times more likely to be present in GCA-positive TAs than in normal TAs (relative risk = 3.26; 95% CI, 2.03–5.98; P < .001). The frequency of VZV antigen in GCA-positive TAs compared with GCA-negative TAs did not differ significantly (relative risk = 1.14; 95% CI, 0.93–1.43; P = .23).
Table 1.
Frequency of VZV Antigen in TAs Pathologically Positive and Negative for GCA and in Normal TAs
| Variable | TAs | ||
|---|---|---|---|
| GCA Positive | GCA Negative | Normal | |
| Total TAs analyzed, No. | 93 | 70 | 49 |
| VZV antigen–positive TAs, No. (%) | 68 (73) | 45 (64) | 11 (22) |
Abbreviations: GCA, giant cell arteritis; TAs, temporal arteries; VZV, varicella-zoster virus.
In GCA-negative TAs, the frequencies of viral antigen detection in the adventitia, media, and intima were 64%, 13%, and 23%, respectively, compared with 26%, 49%, and 25% in normal TAs (Table 2). Table 2 includes data expanded from our earlier study of GCA-positive TAs1 and indicates VZV antigen in the adventitia, media, and intima 53%, 31%, and 16% of the time, respectively. Compared with normal TAs, VZV antigen was more likely to be present in the adventitia of both GCA-negative TAs (relative risk = 2.43; 95% CI, 1.82–3.41; P < .001) and GCA-positive TAs (relative risk = 2.03; 95% CI, 1.52–2.86; P < .001). Varicella-zoster virus antigen was detected less frequently in the adventitia of GCA-positive TAs than in that of GCA-negative TAs (relative risk = 0.84; 95% CI, 0.76–0.92; P = .001).
Table 2.
Distribution of VZV Antigen in TAs Pathologically Positive and Negative for GCA and in Normal TAs
| Site of VZV Antigen | TAs, % | ||
|---|---|---|---|
| GCA Positive | GCA Negative | Normal | |
| Adventitia | 53 | 64 | 26 |
| Media | 31 | 13 | 49 |
| Intima | 16 | 23 | 25 |
Abbreviations: GCA, giant cell arteritis; TAs, temporal arteries; VZV, varicella-zoster virus.
Further analysis of the distribution of VZV within adventitia revealed viral antigen in GCA-negative TAs predominantly around nerve bundles as well as in the vasa vasorum and adventitial cells at frequencies of 37%, 4%, and 59%, respectively, compared with 19%, 9%, and 72% in normal TAs and 25%, 4%, and 72% in GCA-positive TAs (eTable in the Supplement). Varicella-zoster virus antigen was 1.50 times more likely to be present around nerve bundles in GCA-negative TAs than around those of GCA-positive TAs (relative risk = 1.50; 95% CI, 1.22–1.87; P < .001).
In most GCA-negative TAs that contained VZV (36 of 45 [78%]), VZV antigen was detected in 2 to 58 skip areas (defined as ≥2 VZV antigen–positive regions flanked by VZV antigen–negative regions). A total of 370 VZV-positive skip areas were found in the 45 GCA-negative TAs containing VZV antigen. In the 11 normal TAs that contained VZV antigen, VZV was found in 77 skip areas.
VZV Antigen in Skeletal Muscle Adjacent to VZV-Infected GCA-Negative TAs
Skeletal muscle was present in 38 of 70 GCA-negative TAs (54%). Among the 45 GCA-negative TAs that contained VZV antigen, 25 (56%) also contained muscle, and VZV antigen was found in 4 of these 25 samples (16%).
VZV DNA in GCA-Negative and Normal TAs
Of the 45 GCA-negative TAs that contained VZV antigen, 39 contained amplifiable cellular DNA, of which 1 contained VZV DNA. Of the 11 normal TAs that contained VZV antigen, 9 contained amplifiable cellular DNA, and 3 of these contained VZV DNA.
Association of Histopathological Changes With VZV Antigen in GCA-Negative and Normal TAs
In GCA-negative TAs, histopathological analysis of 462 sections adjacent to those containing VZV antigen revealed 1 section with GCA pathology (Figure 1). In another 118 sections of GCA-negative TAs adjacent to those with detectable viral antigen, inflammation was suspected by light microscopy. Immunohistochemical analysis of these sections with anti-CD45 antibody confirmed the presence of inflammatory cells in 50 sections (42%), always in the adventitia (Figure 2). Cadaveric lymph nodes serving as controls for CD45 were positive (not shown). Inflammation was seen in 11% of GCA-negative TA sections that contained viral antigen, but not in any of 73 sections adjacent to those containing VZV antigen in normal TAs. Overall, of 45 GCA-negative participants whose TAs contained VZV antigen, histological reexamination of sections adjacent to those containing VZV antigen revealed that 1 TA had histopathological features characteristic of GCA, and 16 (36%) showed adventitial inflammation adjacent to viral antigen.
Figure 1. Identification of Giant Cell Arteritis Pathology in the Temporal Artery Adjacent to a Section Containing Varicella-Zoster Virus (VZV) Antigen in a Temporal Artery Signed Out as Pathologically Negative for Giant Cell Arteritis.
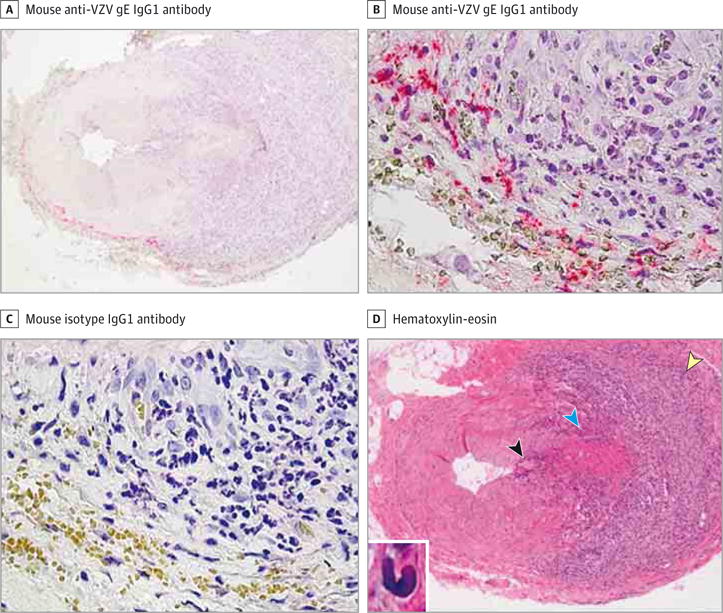
A-C, Immunohistochemical analysis using mouse anti-VZV gE IgG1 antibody revealed VZV antigen at the border of the adventitia and media of a temporal artery that was originally signed out as negative for giant cell arteritis (A and B, pink), but not when mouse isotype IgG1 antibody was substituted for mouse anti-VZV gE IgG1 antibody (C) on adjacent sections (original magnification ×100 [A] and ×600 [B and C]). D, Hematoxylin-eosin staining of a temporal artery section adjacent to that containing VZV antigen in A and B revealed giant cell arteritis pathology, with extensive inflammation in the adventitia (yellow arrowhead) and lesser inflammation in the media (blue arrowhead) and intima (black arrowhead) (original magnification ×100). Inset, Medial damage as well as numerous epithelioid cells were seen throughout the artery (hematoxylin-eosin, original magnification ×600).
Figure 2. Identification of Inflammatory Cells in a Section of the Temporal Artery Adjacent to Varicella-Zoster Virus (VZV) Antigen.
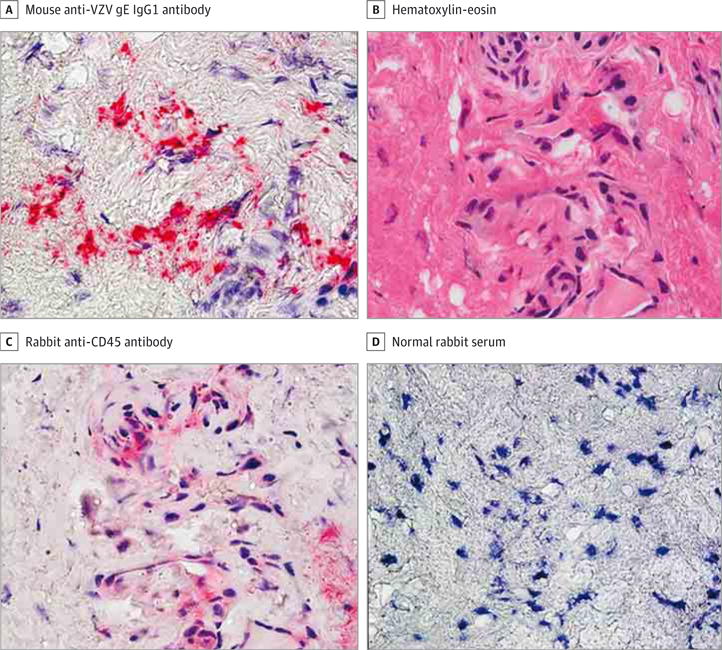
A, Immunohistochemical analysis using mouse anti-VZV gE IgG1 antibody revealed VZV antigen in the adventitia of a giant cell arteritis–negative temporal artery (bright pink) (original magnification ×600). B–D, Hematoxylin-eosin staining of a temporal artery section adjacent to that containing VZV antigen in A revealed inflammatory cells (B) expressing CD45 antigen with rabbit anti-CD45 antibody (C, pink) not detected when normal rabbit serum was substituted for rabbit anti-CD45 antibody (D) (original magnification ×600).
VZV Antigen Colocalized With Cells Expressing Claudin-1 at the Periphery of Nerve Bundles in TAs Pathologically Negative for GCA
When VZV antigen was detected in nerve bundles in the adventitia by immunohistochemical analysis using mouse anti-VZV gE IgG1 antibody, viral antigen was seen predominantly in cells at the periphery of nerve bundles (Figure 3A and B), but not in adjacent sections when mouse isotype IgG1 antibody was substituted for mouse anti-VZV gE IgG1 antibody (Figure 3E and F). Immunohistochemical analysis of sections adjacent to those containing VZV antigen with rabbit anti-claudin-1 antibody showed that VZV antigen was present in perineurial cells expressing claudin-1 (Figure 3C and D), but not when normal rabbit serum was substituted for rabbit anti–claudin-1 antibody (Figure 3G and H). No immunostaining of cells at the periphery of nerve bundles was seen using antibodies directed against Schwann cells (anti-S-100 and anti-myelin basic protein), nerve fibers (anti–βIII tubulin), and inflammatory cells (anti-CD45) (not shown).
Figure 3. Colocalization of Varicella-Zoster Virus (VZV) Antigen and Claudin-1 in Cells in the Perineurium of Nerve Bundles in Temporal Arteries Pathologically Negative for Giant Cell Arteritis.
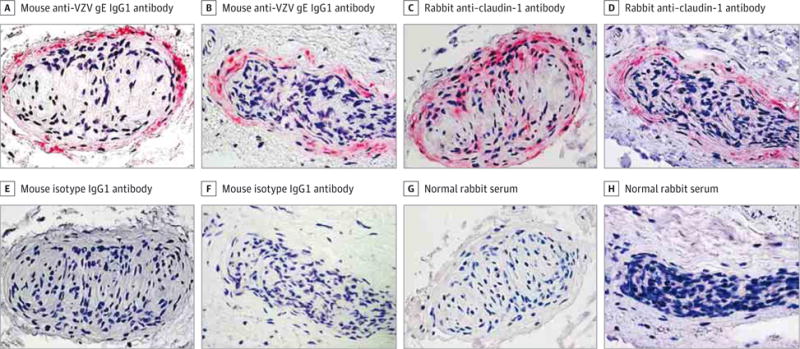
A, B, E, and F, Immunohistochemical analysis using mouse anti-VZV gE IgG1 antibody revealed VZV antigen predominantly in association with the perineurium of nerves in 2 representative temporal arteries (A and B, pink), but not in adjacent sections in which mouse isotype IgG1 antibody was substituted for mouse anti-VZV gE IgG1 antibody (E and F) (original magnification ×600). C, D, G, and H, Immunohistochemical analysis of sections adjacent to those containing VZV antigen with rabbit anti–claudin-1 antibody confirmed that the VZV antigen was located in association with perineurial cells expressing claudin-1 (C and D, pink), but not when normal rabbit serum was substituted for rabbit anti–claudin-1 antibody (G and H) (original magnification ×600).
Discussion
Recent evidence that VZV triggers the immunopathology of GCA is based on the detection of VZV antigen in skip areas of TAs that correlated with adjacent GCA pathology.1 Herein, we show that in all patients with clinically suspected GCA, the prevalence of VZV in TAs is comparable whether negative or positive for GCA on biopsy. Specifically, extension of our earlier study1 not only confirmed the presence of VZV antigen in 73% of GCA-positive TAs but also revealed VZV antigen in 45 of 70 GCA-negative TAs (64%). Compared with normal TAs, the presence of VZV in both GCA-positive and GCA-negative TAs was highly significant (P < .001), raising the possibility that biopsy-negative and biopsy-positive GCAs represent a continuum of VZV vasculopathy in the TA. Such a notion is supported by the detection of VZV antigen, mostly in the arterial adventitia and particularly around nerve bundles in both GCA-positive and GCA-negative TAs, reinforcing earlier findings that VZV spreads transaxonally after reactivation from ganglia and initially infects the arterial adventitia.
Importantly, histopathological analysis of sections adjacent to those exhibiting VZV antigen revealed the typical pathological features of GCA (extensive transmural inflammation in all 3 arterial layers, medial necrosis, and epithelioid macrophages) in 1 of the 45 GCA-negative TAs that contained VZV antigen, resulting in a change in pathological diagnosis from GCA negative to GCA positive. Twice before, research-based histopathological examination of sections adjacent to those in which VZV antigen was found in a specimen lacking GCA features on initial pathological examination and signed out as GCA negative revealed classic GCA pathology.4,6 Areas of classic GCA pathology in patients with clinically suspected GCA whose TAs were read as histopathologically negative may have been missed because they were present in skip regions of vasculitis not examined initially, similar to the distribution of VZV antigen in skip regions.
Among the other 44 GCA-negative TAs that contained VZV antigen, inflammation was seen adjacent to viral antigen in 16 (36%), always in the arterial adventitia, whereas no inflammation was detected in any of 11 normal TAs that contained VZV antigen. The presence of adventitial inflammation adjacent to viral antigen in many GCA-negative TAs suggests that GCA-negative TAs may represent early disease in which VZV initially infects the adventitia followed by development of inflammation there and eventual transmural spread of inflammation with accumulation of lymphocytes and giant or epithelioid macrophages and medial damage.
The detection of VZV antigen without associated inflammation or pathology in 22% of control TAs obtained with next-of-kin consent at autopsy from healthy elderly individuals with no cutaneous evidence of recent herpesvirus infection raises the possibility that VZV can reactivate subclinically in elderly individuals, although the percentage of elderly individuals who ultimately develop GCA or any other form of VZV vasculopathy after VZV reactivation remains unknown.
It should be noted that VZV was detected in the adventitia of GCA-negative and GCA-positive TAs 37% and 25% of the time, respectively, in the perineurium of adventitial nerve bundles and colocalized with cells expressing the tight junction protein claudin-1, consistent with a perineurial cell phenotype. Anatomically, adventitial nerve fibers along which VZV likely enters an affected artery are surrounded by Schwann cells and form a portion of the endoneurium, which along with perineurium forms a metabolically active diffusion barrier between the endoneurium and extrafascicular tissue.9 These findings raise the interesting possibility that nerve fibers within the perineurial barrier represent an immunoprivileged site and that the inflammatory cascade leading to GCA is elicited only after breakdown of tight junctions in the perineurium and spread of virus to adjacent adventitial cells.
Our recent demonstration of the presence of VZV in skip areas of most GCA-positive TAs in correlation with adjacent GCA pathology1 has raised the issue of whether these patients should be treated with antiviral agents and corticosteroids, awaiting clinical trials to prove that antiviral agents confer substantial additional benefit to treatment with corticosteroids alone.10 Herein, we show that VZV antigen is present in 64% of GCA-negative TAs, similar to the frequency (73%) in GCA-positive TAs. Overall, the presence of VZV in both GCA-positive and GCA-negative TAs compared with normal TAs is highly significant (P < .001). Given the demonstrated high incidence of ischemic optic neuropathy in GCA-negative patients whose TAs contain VZV antigen,5,10 it appears judicious to treat GCA-negative patients whose TAs contain VZV antigen with both corticosteroids and antiviral agents until studies provide data for or against combined modality therapy.
Conclusions
In patients with clinically suspected GCA, most GCA-negative and GCA-positive TAs contain VZV antigen. Antiviral treatment may confer additional benefit to patients with biopsy-negative GCA treated with corticosteroids, although the optimal antiviral regimen remains to be determined.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grant AG032958 (Drs Gilden and Nagel) and core facility grant EY06360 (Dr Grossniklaus) from the National Institutes of Health.
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplemental content at jamaneurology.com
CME Quiz at jamanetworkcme.com
Author Contributions: Dr Gilden had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Nagel, Gilden. Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Nagel, White, Gilden.
Critical revision of the manuscript for important intellectual content: All authors.
Obtained funding: Nagel, Gilden.
Administrative, technical, or material support: Nagel, Khmeleva, Bennett, Haller, Kandasmy, Amato, Wood, Fogt, Tamhankar, Grossniklaus, Poppiti, Bockelman, Keyvani, Pollak, Mendlovic, Fowkes, Eberhart, Buttmann, Toyka, Meyer-ter-Vehn, Petursdottir, Gilden.
Study supervision: Nagel, Gilden.
Conflict of Interest Disclosures: None reported.
Additional Contributions: Stefan Sillau, PhD, Department of Neurology, University of Colorado School of Medicine, Aurora, provided statistical analysis; Marina Hoffman, BS, private editorial consultant, provided editorial assistance; and Cathy Allen, Department of Neurology, University of Colorado School of Medicine, Aurora, provided word processing and formatting. None of these individuals received compensation from the National Institutes of Health.
References
- 1.Gilden D, White T, Khmeleva N, et al. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology. 2015;84(19):1948–1955. doi: 10.1212/WNL.0000000000001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salazar R, Russman AN, Nagel MA, et al. Varicella zoster virus ischemic optic neuropathy and subclinical temporal artery involvement. Arch Neurol. 2011;68(4):517–520. doi: 10.1001/archneurol.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathias M, Nagel MA, Khmeleva N, et al. VZV multifocal vasculopathy with ischemic optic neuropathy, acute retinal necrosis and temporal artery infection in the absence of zoster rash. J Neurol Sci. 2013;325(1–2):180–182. doi: 10.1016/j.jns.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagel MA, Khmeleva N, Boyer PJ, Choe A, Bert R, Gilden D. Varicella zoster virus in the temporal artery of a patient with giant cell arteritis. J Neurol Sci. 2013;335(1–2):228–230. doi: 10.1016/j.jns.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagel MA, Bennett JL, Khmeleva N, et al. Multifocal VZV vasculopathy with temporal artery infection mimics giant cell arteritis. Neurology. 2013;80(22):2017–2021. doi: 10.1212/WNL.0b013e318294b477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilden D, White TM, Nagae L, Gurdin WH, Boyer PJ, Nagel MA. Successful antiviral treatment of giant cell arteritis and Takayasu arteritis. JAMA Neurol. 2015;72(8):943–946. doi: 10.1001/jamaneurol.2015.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teodoro T, Nagel MA, Geraldes R, et al. Biopsy-negative, varicella zoster virus (VZV)-positive giant cell arteritis, zoster, VZV encephalitis and ischemic optic neuropathy, all in one. J Neurol Sci. 2014;343(1–2):195–197. doi: 10.1016/j.jns.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baird NL, Bowlin JL, Yu X, et al. Varicella zoster virus DNA does not accumulate in infected human neurons. Virology. 2014;458–459:1–3. doi: 10.1016/j.virol.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ubogu EE. The molecular and biophysical characterization of the human blood-nerve barrier: current concepts. J Vasc Res. 2013;50(4):289–303. doi: 10.1159/000353293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golas L, Bennett JL, White TM, et al. Varicella zoster virus in ischemic optic neuropathy [published online June 5, 2015] Ophthalmology. doi: 10.1016/j.ophtha.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


