Abstract
Syncope is a common and challenging presenting complaint to the Emergency Department (ED). Despite substantial research efforts, there is still considerable uncertainty about the optimal ED management of syncope. There is continued interest among clinicians and researchers in improving diagnostic algorithms and optimizing resource utilization. In this paper, we discuss 4 strategies to improve the emergency care of syncope patients: (1) Development of accurate and consistent risk-stratification, (2) Increased use of syncope observation protocols, (3) Evaluation of a discharge with ambulatory monitoring pathway, (4) Use of shared decision--making for disposition decisions.
Since current risk-stratification tools have fallen short with regard to subsequent validation and implementation into clinical practice, we outline key factors for future risk-stratification research. We propose that observation units have the potential to safely decrease length-of-stay and hospital costs for hemodynamically stable, intermediate risk patients without adversely affecting clinical outcomes. For appropriate patients with a negative ED evaluation, we recommend consideration of direct discharge, with ambulatory monitoring and expedited follow-up, as a means of decreasing costs and reducing iatrogenic harms. Finally, we advocate for the use of shared decision-making regarding the ultimate disposition of select, intermediate risk patients who have not had a serious condition revealed in the ED. If properly implemented, these four strategies could significantly improve the care of ED syncope patients by helping clinicians identify truly high-risk patients, decreasing unnecessary hospitalizations, and increasing patient satisfaction.
Keywords: syncope, emergency medicine, clinical management, observation, risk-stratification
Introduction
Syncope is vexing presenting complaint to Emergency Departments (EDs) around the world. Visits for syncope are both common — 740,000 annual ED visits in the United States [1], and costly — $2.4 billion in hospital costs yearly [2]. Despite many years of research on this topic [3–7], substantial variability in clinical management and uncertainty regarding best practice still persist. Since syncope can be precipitated by a dangerous condition that may remain undiagnosed during the ED evaluation, many patients are admitted to the hospital for further investigation. However, diagnostic hospitalization is associated with minimal diagnostic and therapeutic yield [3, 7], and there is no evidence that current clinical practice improves quality-of-life or long-term survival [6]. Accordingly, there is great interest within the international medical community to improve diagnostic algorithms and optimize resource utilization for syncope. We discuss 4 potential avenues for improving the ED evaluation of syncope: risk stratification tools; ED observation units; discharge with ambulatory monitoring; and shared decision-making (SDM).
Risk stratification
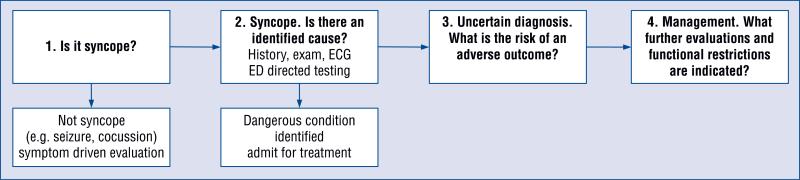
Figure 1 illustrates a conceptual model of the ED management of syncope [8]. A careful history and examination will help distinguish syncope from other symptom complexes with alternative diagnostic pathways, including seizure, concussion, and hypoglycemia. The ED workup includes selective testing guided by the history, exam, and electrocardiogram (ECG). In a minority of patients, this evaluation will reveal a dangerous medical condition such as symptomatic arrhythmia, pulmonary embolus, or severe anemia (Table 1).
Figure 1.
Conceptual model of the Emergency Department (ED) management of syncope; ECG — electrocardiogram.
Table 1.
Dangerous causes of syncope.
| Cardiac | Circulatory | Neurological |
|---|---|---|
| Bradydysrhythmias | Aortic dissection | Subarachnoid hemorrhage |
| Tachydysrhythmias | Pulmonary embolism | Transient ischemic attack/stroke |
| Structural heart disease | Gastrointestinal bleeding | |
| Myocardial infarction | Ruptured abdominal aortic aneurysm | |
| Valvular disease | Ruptured ectopic pregnancy | |
| Other significant hemorrhage |
In all other patients with unknown or presumptive diagnoses, risk stratification is the fundamental task for the ED provider. Patients at low risk for serious cardiovascular events or sudden death may be candidates for discharge, whereas observation or hospitalization may be considered for non-low risk patients. Persistent practice patterns suggest that physicians ‘over-triage’ patients with syncope [9]. Patients at high-risk for serious cardiovascular events (based on age, symptomatology, co-morbidities, ECG, or serious ED diagnosis) should be admitted for prolonged cardiac monitoring to detect potentially significant arrhythmias and to undergo targeted investigations, (guided by the history, physical exam and initial testing) such as echocardiography, provocative cardiac testing, electrophysiological evaluation to uncover any serious conditions amenable to intervention.
For example, few ED physicians would disagree that a 90-year-old male with a history of ventricular tachycardia and implanted cardiac device presenting to the ED with syncope should be admitted. On the other end of the spectrum, most low-risk patients, such as young healthy adults with a clear benign diagnosis (e.g. situational syncope), should be discharged with out-patient follow-up. Patients who are in neither of the above categories, and therefore are intermediate risk, such as a 60-year-old male with one or 2 comorbidities and no diagnosis identified in the ED but may be at risk for an occult cardiac cause such as transient arrhythmia, create a more challenging situation for the ED physician. It is precisely in this cohort that accurate risk-stratification becomes so important to guide further management.
To address this gap, multiple syncope risk--stratification tools have been developed with a goal of identifying patients who can safely be discharged from the ED [5, 10–14]. However, most of the studies have suffered from methodological weaknesses, small sample sizes, and variation in study variable definition. The initially promising sensitivity and specificity of these clinical decision instruments have not been borne out in validation studies [15, 16]. This is likely due, in part, to the inherent challenges present in studying such a complex clinical entity with a wide variety of benign and malignant etiologies. As a result, the dissemination and implementation of these tools among emergency physicians has been limited at best and thus they have not actually impacted clinical practice.
Future research aimed at risk-stratification must take into consideration the following key principles [8, 17].
-
(1)
There must be standardization in study eligibility and key parameters such as predictor variables and adverse outcomes, as well as a uniform outcome time frame. Although the most clinically relevant time frame is debatable, a recent international expert panel recommended the measurement of 30-day outcomes [18].
-
(2)
Future risk-stratification studies should exclude patients who have a serious cause of syncope that is identified in the ED. Such tools are of little utility to the clinician if a dangerous diagnosis has already been uncovered. For example, consider a patient with syncope and who exhibits complete heart block on ECG or melena on exam with a significant decrease in hemoglobin. The management of this patient is not likely to be enhanced by the administration of a risk-stratification tool since the necessary intervention (i.e. implanted cardiac device or endoscopy) is rather obvious. Inclusion of such patients in risk--stratification studies also biases results towards the identification of “obvious” problems.
-
(3)
Clinically coherent and specific outcome categories should be used as opposed to general adverse event outcomes. Some of the reported syncope risk-stratification tools combine disparate conditions, including arrhythmia, strokes, and gastrointestinal bleed, into 1 study endpoint, and it is unlikely that the resulting prediction tool will be valid for uncommon events. The use of related outcomes (e.g. arrhythmias and sudden death) would have greater face validity and likely result in more robust tools.
-
(4)
Future studies should strive to enroll large sample sizes to mitigate the issue of rare adverse events and lead to more stable risk-prediction models. This may be more realistically achieved with the help of external funding.
-
(5)
The role of novel ED tests, such as high--sensitivity troponins, natriuretic peptides, and bedside cardiac ultrasound could provide objective, valuable information to improve risk stratification.
-
(6)
Future risk-stratification tools should generate continuous, rather than binary risk assessments. In the population where the most clinical uncertainty exists i.e. older adults with a negative ED work-up, it is unlikely that a “zero--risk” group can be reliably identified. A continuous or categorical risk assessment, (e.g. low-medium--high) is likely to generate more nuanced, actionable and realistic information for the ED clinician.
-
(7)
Finally, we believe that future clinical decision tools should be compared to existing physician performance. Such tools should outperform, on average, existing physician practice, which would justify widespread implementation.
We believe that improved risk stratification is a foundational step for improving ED management of syncope; at least 2 major multicenter studies are ongoing to further this objective [19, 20]. However, improved risk assessment must be matched to specific actions. For example, these tools should identify patients where further hospital-based evaluation is unlikely to be of benefit. Conversely, ‘high’ risk patients should be admitted for further evaluation. The determination of such ‘discharge/admit’ thresholds is likely to be highly dependent on provider risk tolerance and will require further research and consensus.
Observation units/protocols
For non-low risk patients without an obvious cause for syncope, the goal of extended hospital--based evaluation is to uncover a dangerous cause that may not have been evident during the initial ED evaluation. This may involve hospital-based cardiac monitoring to capture intermittent arrhythmia, echocardiogram to identify structural heart conditions, or serial cardiac biomarkers to ‘rule--out’ myocardial infarction. Hospital-based testing may be costly and result in low diagnostic yield [21–23].
Structured observation unit protocols offer a safe and less costly alternative to unstructured inpatient admission for evaluation of intermediate--risk patients presenting with syncope. Two randomized trials of observation unit care vs. ‘routine’ diagnostic admission have been reported [9, 24]. In the Syncope Evaluation in the Emergency Department Study (SEEDS), 103 patients at a single academic center ED were randomized and followed for up to 2 years [24]. The observation unit protocol included at least 6 h of cardiac monitoring, echocardiogram for patients with abnormal cardiovascular exam or an abnormal ECG, and tilt-table testing in selected patients. Electrophysiology consultation and follow-up were available to observation unit providers. The SEEDS study reported a 57% decrease in admissions in the observation group, and there were no differences in 2-year mortality or syncope recurrence between the two groups.
More recently, the ED Observation Syncope Protocol (EDOSP) study randomized 124 intermediate-risk patients presenting with syncope at 5 ED to structured observation vs. routine admission [9]. The EDOSP required at least 12 h of cardiac monitoring, 2 serial troponins separated by a minimum of 6 h, and echocardiogram for patients with abnormal heart sounds. There were otherwise no restrictions on diagnostic testing or consultations that could be ordered by the treating observation providers. Compared to routine admission, EDOSP reduced length-of-stay by 18 h and hospital costs by $629, with no differences in 30-day safety events, quality-of-life scores, or patient satisfaction. The EDOSP study was performed at sites with a diversity of hospital characteristics, geography, and patient populations, suggesting that a structured observation protocol is generalizable to most settings with an observation unit.
Both the SEEDS and EDOSP studies used semi-structured criteria and physician judgment to identify ‘intermediate’ risk patients eligible for observation. Physicians are likely to be highly conservative; for example, almost half of screened patients for the EDOSP study were excluded for being ‘high risk.’ Improved and objective risk-stratification may increase the proportion of eligible patients for an observation protocol. In addition, changes in payer policies may increase the use of structured observation protocols. Since the SEEDS and EDOSP studies have been published, payers in the United States have increased scrutiny of hospitalizations for syncope (e.g. Medicare Recovery Audit Contractors). Proprietary screening tools for inpatient eligibility may be less conservative than published guidelines and expand use of structured syncope observation protocols to even ‘high’ risk patients.
In summary, a structured observation protocol for syncope was found to be as safe as unstructured hospitalization while resulting in lower length--of-stay and costs. The presence of an existing observation unit, the use of a structured protocol, and careful screening for observation eligibility may safely expedite the ED evaluation of syncope. It should be noted that both of these trials had relatively small sample sizes and thus, if the health care economic environment were to allow it, larger, multicenter trials comparing observation protocols to admission should be conducted.
Discharge with ambulatory cardiac monitoring
The use of an observation protocol rather than unstructured hospitalization begs a more fundamental question — do most patients require any hospital--based evaluation after a normal ED evaluation? The potential benefit of hospital-based evaluation must be weighed against the possibility of hospital--acquired infections, medication errors, and out-of--pocket costs to the patient. One important reason to admit patients is to identify those who might require cardiac device placement for potentially dangerous arrhythmias. We previously reviewed the medical charts of 2,584 older adults (≥ 60 years), who had an unrevealing ED evaluation for syncope [25]. Fewer than 7% experienced a subsequent serious medical event within 30 days. We found that it was often difficult to determine whether such events were related to the index syncope. The most common condition uncovered at 30 days was an arrhythmia (4%) and half of those patients received a pacemaker or implantable defibrillator. We conclude that patients with a normal ED evaluation rarely require a cardiac device intervention; admitting the majority of such patients may result in early diagnosis for a few but expose the rest to iatrogenic harm and costs.
One possible ED management approach mimics the outpatient clinic approach: instead of keeping patients in the hospital for observation or inpatient based testing, carefully selected patients could be discharged directly from the ED with an ambulatory heart monitor. Barriers to such an approach may include lack of ready access to Holter/event monitors in the ED, lack of follow-up for monitor results, and patient/physician discomfort with ED discharge. However, a pilot study demonstrates that such barriers can be overcome [26]. There were 174 patients with suspected arrhythmia (symptoms of palpitations, syncope, or dizziness) who were discharged from a single ED with a single use, continuous cardiac monitoring device. There were no safety events and the diagnostic yield was 63%.
How would patients be selected for an outpatient, ambulatory approach? One factor would be careful risk assessment, for example, ‘high’ risk patients should not be sent home. For ‘intermediate’ risk patients, outpatient cardiac monitoring may be an alternative to continued hospital-based evaluation. Table 2 describes a previously used guideline for categorical risk stratification [9]. The next section describes a possible approach for selecting between these alternatives.
Table 2.
Risk-stratification for Emergency Department (ED) syncope patients.
|
High risk criteria
|
| Serious condition identified in the ED |
| History of ventricular arrhythmia |
| Cardiac device with dysfunction |
| Exertional syncope |
| Presentation concerning for acute coronary syndrome |
| Severe cardiac valve disease (e.g., aortic stenosis < 1 cm2) |
| Known cardiac ejection faction < 40% |
| Electrocardiogram findings of QTc > 500 ms, pre-excitation, non-sustained ventricular tachycardia |
| Emergency physician judgment |
|
Intermediate risk criteria |
| No high risk features AND |
| No low risk features AND |
| Clinical judgment by emergency physician that patient requires further diagnostic evaluation |
|
Low risk |
| Symptoms consistent with orthostatic or vasovagal syncope |
| Emergency physician judgment that no further diagnostic evaluation is needed |
Shared decision-making
SDM is defined as a “process by which patients and providers consider outcome probabilities and patient preferences and reach a health care decision based on mutual agreement” [27]. The Institute of Medicine defined patient-centered care as “care that is respectful of and responsive to individual patient preferences, needs, and values” and that ensures “that patient values guide all clinical decisions” [28]. Thus, SDM is an ideal vehicle through which to deliver patient centered-care. It should not, however, be confused with informed consent, as it goes much farther. Notably, SDM is featured in the United States Affordable Care Act (Section 3506) and SDM research has been supported by the United States Patient-Centered Outcomes Research Institute (PCORI) [29].
SDM is appropriate in clinical scenarios in which there are 2 or more reasonable and appropriate management options. It involves several different components. The physician must express to the patient that a decision regarding his/her medical care needs to be made. Then, the physician should explain to the patient, in a manner that is clear and understandable, the nature of the decision, the different options that exist, and the potential harms and benefits of each. Finally, through a collaborative process, the physician and patient come to a mutually agreed upon course of action that is both medically sound and consistent with the patient's values and preferences.
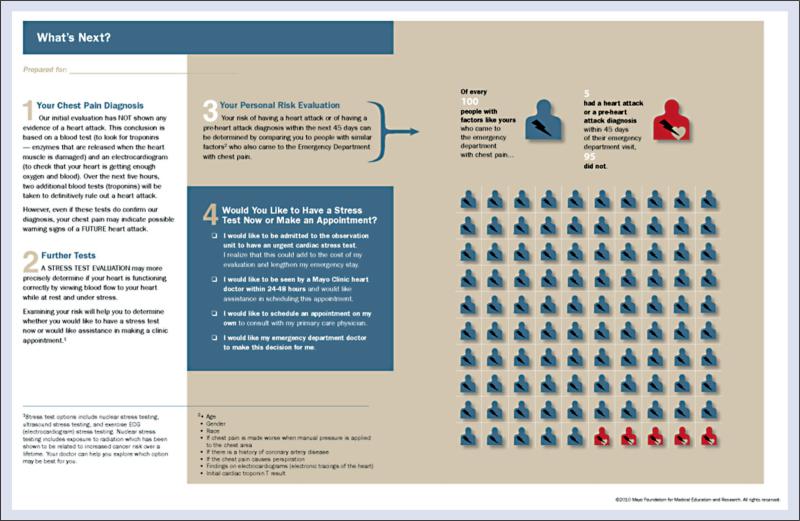
The practice of SDM within the context of emergency care has only recently gained traction. The literature is still in its infancy, yet we believe the potential is huge, specifically with regard to the care of ‘intermediate-risk’ ED patients with syncope. The concept of SDM for low-risk chest pain patients has been described and studied in a randomized fashion [30]. This single center trial, Chest Pain Choice, showed that use of a decision aid (Fig. 2) to facilitate the disposition decision increased patient and provider satisfaction, improved post-visit patient knowledge, decreased hospitalization while having no effect on major clinical outcomes.
Figure 2.
Chest pain choice decision aid.
SDM, by actively engaging the patient and incorporating his/her values and preferences, has the potential to greatly enhance care with regards to both decision quality and patient satisfaction. If the initial ED work-up has been completed without revealing any serious conditions, but the patient is not clearly high or low risk, the ED physician should approach the patient and engage him/her in a discussion starting with what is known (e.g. potential causes of syncope, ED test results, approximate risks of adverse outcomes, risks of unnecessary testing and admission) and progressing towards the decision that needs to be made (i.e. disposition). A key component of this discussion would be an explicit and readily understandable communication of risk with the patient. This could be facilitated by using a personalized print out stating the estimated risk of 30-day serious clinical events based on that particular patient's clinical variables using the best risk stratification model currently available This risk would be displayed as a natural frequency, percent risk, and graphically, using a 100-man pictogram. The different disposition options and potential downstream testing (admission to the hospital, admission to the observation unit, discharge with ambulatory monitor and scheduled follow-up, or discharge with primary care follow-up) for the patients would be listed and described in plain language. A fifth option, whereby the ED physician would make the disposition decision for the patient, would also be offered.
At that point, the patient would be invited to ask for additional information and then he/she would express his/her preferences as to what disposition decision he/she feels is best for them. Different patients, much like physicians, have different levels of risk tolerance as well as varying aversion to hospitalization and variable access to their outpatient physician. Many social factors could conceivably come into play e.g. need to feed pets or care for a dependent family member. If there truly exists more than one reasonable disposition option, and we argue that there often does, it is our responsibility to genuinely engage our patients in this discussion in order to deliver patient-centered care. Thus, we propose an additional step in the below clinical model (Fig. 1), “Shared decision--making”, which would influence step 4 “management” via the process described above.
By bringing the patient into the decision-making process, with an explicit and honest discussion of risks, the moral responsibility for the outcome of the medical encounter could potentially be shared between physician and patient. All of these factors have the potential to mitigate the medical-legal risk of the physician thereby allowing for more emphasis on the medical needs of the patient as rather than engaging in ‘defensive medicine.’
We feel that the potential benefits of SDM in the care of ED syncope patients justify further research in this area. Such an intervention has the potential to enhance patient engagement, while decreasing resource utilization. Most importantly, it promotes the delivery of patient-centered care — a standard that we, as physicians, would expect if we were, one day, to be syncope patients in an ED.
Conclusions
Despite substantial research efforts, there is still pervasive uncertainty about the optimal ED management of syncope. We propose 4 potential avenues to improve the emergency care of syncope patients:
Development of accurate and consistent risk--stratification.
Increased use of syncope observation protocols.
Evaluation of a discharge with ambulatory monitoring pathway.
Use of SDM for disposition decisions.
Since current published risk stratification tools have not been validated for routine use, we outline key principles to guide future risk-stratification efforts. We argue that observation units have the potential to decrease length-of-stay and hospital costs while maintaining patient safety. For select patients with a negative evaluation, the option of direct discharge, with an ambulatory monitor and close follow-up could decrease costs and prevent iatrogenic harms. Finally, we propose a novel approach to disposition decisions, using SDM, for intermediate risk patients presenting with syncope who have not had a serious condition revealed during their ED evaluation. Combined, these 4 avenues have the potential to substantially improve the care of ED syncope patients.
Acknowledgments
Grant funding This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number 5K12 HL109005 (Dr Probst) and Award Number R01 HL111033 (Dr Sun). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
conflicts of interest: There are no other conflicts of interest.
References
- 1.Sun BC, Emond JA, Camargo CA., Jr. Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992–2000. Acad Emerg Med. 2004;11:1029–1034. doi: 10.1197/j.aem.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 2.Sun BC, Emond JA, Camargo CA., Jr. Direct medical costs of syncope-related hospitalizations in the United States. Am J Cardiol. 2005;95:668–671. doi: 10.1016/j.amjcard.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Linzer M, Yang EH, Estes NA, 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 1: Value of history, physical examination, and electrocardiography. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Int Med. 1997;126:989–996. doi: 10.7326/0003-4819-126-12-199706150-00012. [DOI] [PubMed] [Google Scholar]
- 4.Linzer M, Yang EH, Estes NA, 3rd, Wang P, Vorperian VR, Kapoor WN. Diagnosing syncope. Part 2: Unexplained syncope. Clinical Efficacy Assessment Project of the American College of Physicians. Ann Int Med. 1997;127:76–86. doi: 10.7326/0003-4819-127-1-199707010-00014. [DOI] [PubMed] [Google Scholar]
- 5.Sarasin FP, Hanusa BH, Perneger T, Louis-Simonet M, Rajeswaran A, Kapoor WN. A risk score to predict arrhythmias in patients with unexplained syncope. Acad Emerg Med. 2003;10:1312–1317. doi: 10.1111/j.1553-2712.2003.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 6.Crane SD. Risk stratification of patients with syncope in an accident and emergency department. Emerg Med J. 2002;19:23–27. doi: 10.1136/emj.19.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mozes B, Confino-Cohen R, Halkin H. Cost-effectiveness of in-hospital evaluation of patients with syncope. Israel J Med Sciences. 1988;24:302–306. [PubMed] [Google Scholar]
- 8.Sun BC, Costantino G, Barbic F, et al. Priorities for Emergency Department syncope research. Ann Emerg Med. 2014 doi: 10.1016/j.annemergmed.2014.04.014. VOL???? PAGES????? [DOI] [PubMed] [Google Scholar]
- 9.Sun BC, McCreath H, Liang LJ, et al. Randomized Clinical Trial of an Emergency Department Observation Syncope Protocol Versus Routine Inpatient Admission. Ann Emerg Med. 2013 doi: 10.1016/j.annemergmed.2013.10.029. VOL???? PAGES????? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colivicchi F, Ammirati F, Melina D, Guido V, Imperoli G, Santini M. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: The OESIL risk score. Eur Heart J. 2003;24:811–819. doi: 10.1016/s0195-668x(02)00827-8. [DOI] [PubMed] [Google Scholar]
- 11.Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004;43:224–232. doi: 10.1016/s0196-0644(03)00823-0. [DOI] [PubMed] [Google Scholar]
- 12.Del Rosso A, Ungar A, Maggi R, et al. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to a general hospital: The EGSYS score. Heart (British Cardiac Society) 2008;94:1620–1626. doi: 10.1136/hrt.2008.143123. [DOI] [PubMed] [Google Scholar]
- 13.Costantino G, Perego F, Dipaola F, et al. Short- and long-term prognosis of syncope, risk factors, and role of hospital admission: results from the STePS (Short-Term Prognosis of Syncope) study. J Am Coll Cardiol. 2008;51:276–283. doi: 10.1016/j.jacc.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 14.Reed MJ, Newby DE, Coull AJ, Prescott RJ, Jacques KG, Gray AJ. The ROSE (risk stratification of syncope in the emergency department) study. J Am Coll Cardiol. 2010;55:713–721. doi: 10.1016/j.jacc.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 15.Sun BC, Mangione CM, Merchant G, et al. External validation of the San Francisco Syncope Rule. Ann Emerg Med. 2007;49:420–427. 7.e1–4. doi: 10.1016/j.annemergmed.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Serrano LA, Hess EP, Bellolio MF, et al. Accuracy and quality of clinical decision rules for syncope in the emergency department: A systematic review and meta-analysis. Ann Emerg Med. 2010;56:362–373. e1. doi: 10.1016/j.annemergmed.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun B, Costantino G. Syncope risk stratification in the ED: Directions for future research. Acad Emerg Med. 2013;20:503–506. doi: 10.1111/acem.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun BC, Thiruganasambandamoorthy V, Cruz JD. Standardized reporting guidelines for emergency department syncope risk--stratification research. Acad Emerg Med. 2012;19:694–702. doi: 10.1111/j.1553-2712.2012.01375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thiruganasambandamoorthy V, Stiell IG, Sivilotti ML, et al. Risk stratification of adult emergency department syncope patients to predict short-term serious outcomes after discharge (RiSEDS) study. BMC Emerg Med. 2014;14:8. doi: 10.1186/1471-227X-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Improving Risk Stratification in Older Adults. NCT01802398. National Library of Medicine; Bethesda, MD, US: 2000. [Google Scholar]
- 21.Getchell WS, Larsen GC, Morris CD, McAnulty JH. Epidemiology of syncope in hospitalized patients. J General Internal Med. 1999;14:677–687. doi: 10.1046/j.1525-1497.1999.03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendu ML, McAvay G, Lampert R, Stoehr J, Tinetti ME. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Internal Med. 2009;169:1299–1305. doi: 10.1001/archinternmed.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun BC. Quality-of-life, health service use, and costs associated with syncope. Progress Cardiovasc Diseases. 2013;55:370–375. doi: 10.1016/j.pcad.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Shen WK, Decker WW, Smars PA, et al. Syncope Evaluation in the Emergency Department Study (SEEDS): A multidisciplinary approach to syncope management. Circulation. 2004;110:3636–3645. doi: 10.1161/01.CIR.0000149236.92822.07. [DOI] [PubMed] [Google Scholar]
- 25.Sun BC, Derose SF, Liang LJ, et al. Predictors of 30-day serious events in older patients with syncope. Ann Emerg Med. 2009;54:769–778. e1–5. doi: 10.1016/j.annemergmed.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. Western J Emerg Med. 2014;15:194–198. doi: 10.5811/westjem.2013.11.18973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frosch DL, Kaplan RM. Shared decision making in clinical medicine: past research and future directions. Am J Prev Med. 1999;17:285–294. doi: 10.1016/s0749-3797(99)00097-5. [DOI] [PubMed] [Google Scholar]
- 28.Council NR. Crossing the quality chasm: a new health system for the 21st century. National Academies Press; Washington, DC: 2001. [PubMed] [Google Scholar]
- 29.Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med. 2013;368:6–8. doi: 10.1056/NEJMp1209500. [DOI] [PubMed] [Google Scholar]
- 30.Hess EP, Knoedler MA, Shah ND, et al. The chest pain choice decision aid: aA randomized trial. Circulation Cardiovasc Quality Outcomes. 2012;5:251–259. doi: 10.1161/CIRCOUTCOMES.111.964791. [DOI] [PubMed] [Google Scholar]