Abstract
Objective
Neonatal abstinence syndrome (NAS) is a postnatal drug withdrawal syndrome that may last for months. Our objective was to determine if infants with NAS are at increased risk for hospital readmission compared with uncomplicated term and late preterm newborns.
Methods
In this longitudinal retrospective cohort study, administrative data were used for all births from 2006 to 2009 in the New York State Inpatient Database. We identified infants with NAS, born late preterm or uncomplicated term, as independent groups using diagnostic codes and determined readmission rates. We fit a multivariable logistic regression model with 30-day readmission after discharge as the outcome and infant characteristics, clinical morbidities, insurance type, and length of birth hospitalization as predictors.
Results
From 2006 to 2009 in New York State, 700613 infants were classified as uncomplicated term, 51748 were born late preterm, and 1643 infants were diagnosed with NAS. After adjusting for confounders, infants with NAS (odds ratio [OR] 2.49, 95% confidence interval [CI] 1.75–3.55) were more likely than uncomplicated term infants to be readmitted within 30 days of birth hospitalizations. The risk of readmission was similar to late preterm infants (OR 2.26, 95% CI 2.09–2.45). Length of birth hospitalization in days was inversely related to odds of being readmitted within 30 days of birth hospitalization (OR 0.94 95% CI 0.92–0.96).
Conclusions
When compared with uncomplicated term infants, infants diagnosed with NAS were more than twice as likely to be readmitted to the hospital. Future research and state-level policies should investigate means to mitigate risk of hospital readmission for infants with NAS.
Neonatal abstinence syndrome (NAS) is a drug withdrawal syndrome experienced by some opioid-exposed infants shortly after birth.1,2 The syndrome is characterized by a constellation of gastrointestinal, neurologic, and autonomic signs, including poor feeding, tachypnea, and seizures.1,3 Over the past decade as the rate of opioid use grew among the US population,4 the number of infants diagnosed with NAS nearly tripled.3 Infants with NAS experience longer more complicated initial hospitalizations when compared with other hospital births. In 2009, infants with NAS in the United States had mean length of stay (LOS) of 16 days, with mean hospital charges of $53 000.3
Despite the recent increase in the number of infants with NAS and their associated health care utilization, little is known about infants with NAS after their initial hospitalization. Protracted signs of opioid withdrawal are well described in adults5–7 and have been reported in neonates,1,8 potentially increasing their risk of hospital readmission. Understanding readmission risk is critical to informing safe hospital discharge processes, quality improvement (QI) efforts, and improving health system efficiency for infants with NAS.
Readmission rates are commonly used as a measure of hospital quality among hospitalized adults,9 and although controversial,10 similar policies have been suggested for children. Data are emerging for rates of readmission among hospitalized children11 and high-risk neonates, such as late preterm infants,12 but have not been described among infants with NAS. Our objectives were to determine if infants with NAS were at an increased risk of all-cause hospital readmission compared with “low-risk” (uncomplicated term) and “high-risk” (late preterm) infants and to understand factors associated with hospital readmission in a large population-based cohort.
Methods
Study Design and Setting
In this longitudinal retrospective cohort study, we identified infants by using the New York State Inpatient Database (SID) compiled by the Agency for Healthcare Research and Quality as part of their Healthcare Cost and Utilization project. We included data for births occurring between 2006 and 2009 and health care utilization was followed through 2010. The New York SID includes all hospital discharge records from all nonfederal hospitals in the state and is inclusive of all payers. SID provides clinical and nonclinical data, including principal and secondary diagnoses, patient characteristics (gender, age, race), payment source, and length of hospitalization. The New York SID also includes revisit variables that link multiple hospitalizations together to enable analyses of hospital readmissions.13 These data have been used in previous studies evaluating trends in hospital readmissions among children14 and adults.15,16 We obtained data for live births in New York State from the New York Department of Health (http://www.health.ny.gov/statistics/vital_statistics/). Because the analysis was of de-identified data, it was considered exempt for human subjects review by the Vanderbilt University School of Medicine Institutional Review Board.
Identification of Cohort
To facilitate meaningful comparisons of readmission risk of infants with NAS, our cohort included infants diagnosed with NAS, a low-risk group of “uncomplicated term newborns” and a high-risk group of “late preterm” infants. Infants with NAS were identified if the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM)17 code 779.5 (drug withdrawal syndrome in newborn) appeared in any diagnostic field. As previously described,3 we excluded infants with presumed iatrogenic withdrawal by excluding infants with diagnoses of chronic lung disease (770.7), any intraventricular hemorrhage (772.1×) or periventricular leukomalacia (779.7), necrotizing enterocolitis (777.5×) or spontaneous bowel perforation (777.6), or birth weight <1500 g. Then, uncomplicated term infants were identified by using the Diagnosis-Related Group for “Normal Newborn” (version 24, group 391). Last, late preterm infants were identified by using ICD-9-CM codes for gestational ages of 33 1/7 to 36 6/7 weeks (765.27, 765.28). Each infant group was considered to be mutually exclusive (ie, infants with NAS could not be categorized as late preterm or uncomplicated term).
Descriptive Variables
Clinical comorbidities observed during a birth hospitalization may be associated with risk of hospital readmission. To account for the potential confounding effect of infant clinical birth characteristics, we identified common comorbidities associated with late preterm and NAS infants. Both late preterm and NAS infants are commonly born low birth weight (<2500 g), with respiratory complications, feeding problems, and higher risk of sepsis,1,3,18 and infants with NAS are more likely than other infants to have seizures.3 Low birth weight was identified by using a combination of the provided birth weight variable in the SID, ICD-9-CM codes that indicate birthweights and Diagnosis Related Groups.3 Other clinical conditions were identified by using ICD-9-CM codes including, respiratory diagnoses (769.×, 770.×), feeding difficulties (779.3×), neonatal sepsis (771.81), and seizure (779.0, 780.3).1,3,19 Data for gender, insurance type, and length of hospital stay were provided by the SID.
Outcome Variable
Our primary outcome was hospital readmission within 30 days of birth hospitalization discharge with a secondary outcome of hospital readmission within 365 days of birth hospitalization discharge.
Data Analysis
We generated descriptive statistics for each study group (NAS, late preterm, and uncomplicated term). For analyses of hospital readmission, observations were dropped where revisit links were missing (for NAS 4.0%, late preterm 3.3%, and uncomplicated term 1.9% at 30 days after discharge). We calculated rates of hospital readmission within 30 and 365 days of birth hospitalization. We fit a logistic regression model with 30-day hospital readmission after discharge as the outcome and study group (NAS, late preterm, and uncomplicated term), gender, low birth weight, clinical comorbidities, insurance type, and length of birth hospitalization as predictor variables. In a second model, we accounted for a potential nonlinear relationship between readmission risk and length of birth hospitalization by modeling LOS as a categorical variable (≤7, 8–14, 15–21, 22–28, >8 days). We completed a sensitivity analysis excluding any diagnosis of congenital diaphragmatic hernia (756.6), persistent fetal circulation (747.83), gastroschisis (756.73), tracheoesophageal fistula and esophageal fistula (750.3), and congenital heart disease (746.× and 747.×, excluding 747.0 and 747.83). Results from this sensitivity analysis did not differ from our main analysis and are presented in Supplemental Table 4. We completed statistical analyses by using Stata 13.0 (Stata Corp, College Station, TX). All testing was 2-sided and P < .05 was considered statistically significant.
Results
From 2006 to 2009 in New York State, 700 613 uncomplicated term and 51 748 late preterm infants were born, compared with 1643 infants diagnosed with NAS. Infants with NAS and born late preterm had similar clinical characteristics: respiratory diagnoses (36.0% vs 39.6%), possible sepsis (13.8% vs 11.1%), and feeding problems (11.1% vs 10.7%). Late preterm infants were more likely than NAS or uncomplicated term infants to be born low birth weight (57.9% vs 25.1% vs 2.3%). Infants with NAS were more likely than those born as late preterm or uncomplicated term to be insured by Medicaid (73.7% vs 40.4% vs 41.4%; Table 1).
Table 1.
Clinical Characteristics and Insurance Type for Infants Diagnosed With NAS, Born Late Preterm and as an Uncomplicated Term Infant, New York State 2006–2009.
| NAS | Late Preterm | Uncomplicated Term | P | |
|---|---|---|---|---|
|
|
||||
| n = 1643 | n = 51748 | n = 700613 | ||
|
|
||||
| n (%) | n (%) | n (%) | ||
| Female | 768 (46.7) | 27 686 (47.4) | 348 439 (49.7) | <.001 |
| Clinical comorbidities | ||||
| Respiratory diagnosis | 591 (36.0) | 20 504 (39.6) | 0 (0.0) | <.001 |
| Low birth weight | 413 (25.1) | 29 947 (57.9) | 16 157 (2.3) | <.001 |
| Sepsis | 227 (13.8) | 5728 (11.1) | 0 (0.0) | <.001 |
| Feeding problems | 183 (11.1) | 5514 (10.7) | 2411 (0.3) | <.001 |
| Seizure | 30 (1.8) | 171 (0.3) | 0 (0.0) | <.001 |
| Insurance | ||||
| Medicaid | 1211 (73.7) | 20 908 (40.4) | 289 921 (41.4) | <.001 |
| Private insurance | 264 (16.1) | 26 094 (50.4) | 340 414 (48.6) | <.001 |
| Self-pay | 140 (8.5) | 3612 (7.0) | 55 272 (7.9) | <.001 |
| Other | 28 (1.7) | 1134 (2.2) | 15 006 (2.1) | <.001 |
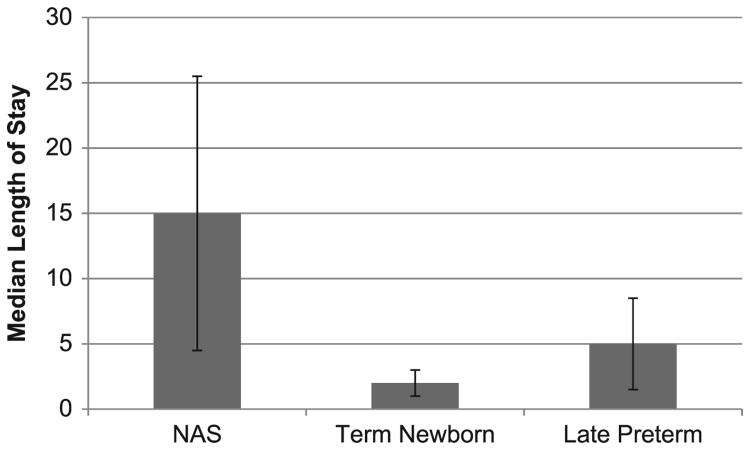
The incidence of NAS in New York State grew 72% during our study period from 1.1 to 2.0 per 1000 live births. Infants with NAS had substantially longer median (interquartile range) birth hospitalizations of 15 days (4.5–25.5) compared with late preterm infants of 5 days (1.5–8.5) and uncomplicated term infants of 2 days (1–3; Fig 1).
Figure 1.
Median length and interquartile range of birth hospitalization for infants with NAS, born late preterm and uncomplicated term, New York 2006–2009.
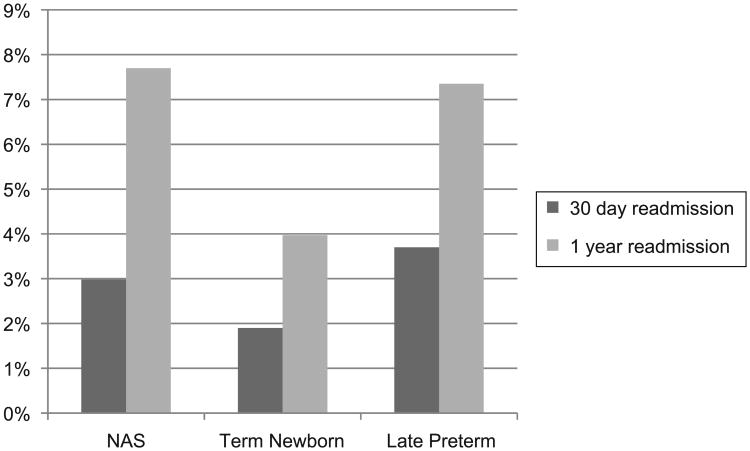
In unadjusted comparisons of hospital readmission at 30 and 365 days after birth hospitalization discharge, infants with NAS had readmission rates of 3.0% and 7.7%, late preterm of 3.7% and 7.4%, and uncomplicated term infants of 1.9% and 4.0%, respectively (P < .001; Fig 2). The most common primary diagnoses for 30-day hospital readmission was drug withdrawal for infants with NAS (26.8%). Jaundice was the most frequent primary diagnosis for both late preterm infants (34.5%) and uncomplicated term infants (51.2%).
Figure 2.
Thirty-day and 1-year hospital readmission rates for infants with NAS, born late preterm and uncomplicated term in New York, 2006–2009.
After adjusting for sex, birth weight, clinical comorbidities, and insurance type, infants with NAS (odds ratio [OR] 2.49, 95% confidence interval [CI] 1.75–3.55) and late preterm infants (OR 2.26, 95% CI 2.09–2.45) were more likely than uncomplicated term infants to be readmitted within 30 days of birth hospitalizations. Also in adjusted analyses, length of birth hospitalization in days was inversely related to odds of being readmitted within 30 days of birth hospitalization (OR 0.94, 95% CI 0.92–0.96; Table 2). Applying our model to varying lengths of birth hospitalization, we found that compared with infants discharged within the first week of life, those discharged between 8 and 14 days (OR 0.51, 95% CI 0.45–0.59), 15 and 21 days (OR 0.50, 95% CI 0.41–0.62), and 22 to 28 days of life (OR 0.54, 95% CI 0.38–0.75) have lower rates of readmission. There was no statistically significant difference in readmission risk between those discharged from the hospital within the first week of life and after 28 days of life (Table 3).
Table 2. Multivariable Model of Risk of 30-Day Hospital Readmission Based On Infant Characteristics, Clinical Diagnoses and Health Care Utilization Patterns Among Infants With NAS, Born Late Preterm and Uncomplicated Term.
| OR | P | |
|---|---|---|
|
|
||
| (95% CI) | ||
| Infant characteristics | ||
| Female | 0.75 (0.73–0.78) | <.001 |
| Low birth weight | 1.13 (1.04–1.23) | .004 |
| Uncomplicated term | REF | |
| Late preterm | 2.26 (2.09–2.45) | <.001 |
| NAS | 2.49 (1.75–3.55) | <.001 |
| Clinical comorbidities | ||
| Respiratory diagnoses | 1.00 (0.89–1.13) | .981 |
| Sepsis | 1.19 (1.02–1.39) | .03 |
| Feeding problems | 1.38 (1.20–1.59) | <.001 |
| Seizure | 5.01 (3.02–8.33) | <.001 |
| Health care utilization | ||
| Private insurance | REF | |
| Medicaid | 1.50 (1.45–1.56) | <.001 |
| Uninsured | 1.10 (1.02–1.18) | .01 |
| Other | 1.61 (1.46–1.79) | <.001 |
| Length of birth hospitalization, d | 0.94 (0.92–0.96) | <.001 |
Table 3. Predicted 30-Day Readmission Rates for Infants With NAS by Length of Birth Hospitalization.
| Length of Birth Hospitalization, d | Adjusted Odds of 30-Day Readmissiona (95% CI) |
|---|---|
| ≤7 | REF |
| 8–14 | 0.51 (0.45–0.59) |
| 15–21 | 0.50 (0.41–0.62) |
| 22–28 | 0.54 (0.38–0.75) |
| >28 | 1.20 (0.93–1.55) |
Adjusted for sex, insurance type, birth weight, and clinical comorbidities.
Discussion
Among more than 750 000 infants born in New York, those with NAS are nearly 2.5 times more likely than uncomplicated term infants to be readmitted to a hospital within 30 days of their birth hospitalization discharge. Even after adjusting for potential confounders, we found an inverse relationship between length of birth hospitalization and risk of readmission. Together this suggests that great care should be taken to ensure a safe discharge home to avoid hospital readmission.
We found that the incidence of NAS in New York grew 72% during our study period, reaching 2.0 per 1000 live births in 2009. This increase was smaller than the national incidence of NAS that grew from 1.2 to 3.4 per 1000 hospital births from 2000 to 2009.3 The rise nationally in NAS occurred at the same time as a rise in opioid prescribing and opioid-related overdose deaths in the United States.20 Recent literature suggests that the rise in opioid prescribing in the United States occurred in a wide range of the population, including pregnant women,21,22 and exposure to opioid pain relievers in pregnancy is associated with an increased risk of NAS.23 In 2012, 59.5 opioid prescriptions were written for every 100 New York residents.4 New York's opioid-prescribing rate, while high, is below the national rate of 82.5,4 perhaps explaining New York's relatively low rate of NAS compared with the nation.
Beginning in the late 1990s, pediatricians turned their attention to late preterm infants as a group vulnerable to adverse outcomes, including hospital readmission.12,24,25 In our study, infants with NAS were similar to late preterm infants in rates of respiratory complications, feeding difficulty, concern for sepsis, and hospital readmission. Similar to our findings, some studies of late preterm infants suggested that shorter birth hospitalizations increase risk of readmission,12 although other studies have not replicated this finding.26 In response to the vulnerability of infants born late preterm, the American Academy of Pediatrics27 and the National Perinatal Association25 created discharge guidelines to improve the national standard of care in transitioning this group of high-risk infants home. However, comprehensive guidelines for safe transition home for infants diagnosed with NAS have not been developed, despite their similar high-risk characteristics.
Interestingly, we found that infants discharged within the first week of life and those discharged after 28 days of life have the highest risk of hospital readmission even after controlling for gender, insurance type, and clinical comorbidities. Importantly, the threshold for hospital readmission is lower for infants discharged within the first 28 days of life, particularly for those with clinical signs of sepsis.28 This suggests that some infants may be being discharged from the hospital too quickly. Last, we hypothesize that infants with initial hospitalizations lasting more than 28 days represent more complicated or severe signs of withdrawal, placing them at an increased risk of readmission.
The most common indication for readmission in this population was drug withdrawal, as opposed to late preterm and uncomplicated term infants who are most commonly readmitted for jaundice. This is likely a result of longer LOS among infants with NAS and their discharge home after the onset of peak newborn physiologic hyperbilirubinemia. Importantly, however, it demonstrates that ongoing signs of postacute withdrawal may be problematic for some infants with NAS.
Hospital readmission subjects patients and families to the inherent disruption, stress, and cost of a hospitalization.29–32 Further, as many as a third of unplanned pediatric readmissions are potentially preventable.33 Readmissions are also costly for payers, resulting in policymakers focusing on reducing readmission to optimize resource utilization. Medicare reduces reimbursement to adult facilities with high readmission rates,34 and in some states Medicaid, including the New York, financially penalizes institutions with more than expected readmissions.35–37
As rates of NAS have increased throughout the country, hospitals and providers are faced with the challenge of standardizing care19,38 through QI efforts. A frequent outcome for QI efforts has been a reduction in LOS.39 As efforts to improve the care delivered to infants with NAS progress, it will be important to recognize that they are at an increased risk of hospital readmission. Whenever possible, QI efforts aimed at reducing LOS should include 30-day hospital readmission as a counterbalancing measure.
Infants with NAS are at significant risk of readmission when compared with uncomplicated term infants; however, it merits addressing that the absolute risk of readmission for infants with NAS is still low when compared with some other pediatric conditions.11 However, given the rise in NAS incidence, readmission for this group merits further monitoring. High-risk infants may benefit from additional postdischarge resources. In our study, as in other population-based studies of NAS,3,19 we found that >70% of infants with the syndrome were enrolled in state Medicaid programs. This suggests that Medicaid can play a role in improving the transition home for this high-risk population, possibly through home visitation or case management. Among preterm infants, home visitation has been shown to improve some clinical outcomes, including neurocognitive development,40 and decrease health care utilization.41 Effective interventions for infants with NAS that focus on early detection and mitigation of withdrawal symptoms after discharge merit further study.
Our study does have several important limitations to consider. Similar to other studies relying on hospital administrative data, errors of omission and commission are possible, leading to misclassification bias. Opioid-exposed infants who do not develop signs of withdrawal also may be at risk for hospital readmission; however, our data do not allow for linkage to maternal records to assess opioid-exposure and its risk to infants independent of NAS. Further, hospital administrative data when compared with clinical data may lead to underidentification of cases of NAS.42 Infant pharmaceutical treatment data were not available in the New York SID and differences in pharmacotherapy for NAS may influence clinical outcomes, including birth hospitalization LOS.19 Our definition of late preterm infants was overly inclusive because ICD-9-CM diagnostic codes include infants from 33 to 36 weeks' gestation. Inclusion of infants at 33 weeks' gestation may lead to a biased estimate toward being at an increased risk of hospital readmission. Although the literature supports that most infants with NAS were exposed to opioids in utero,1 other substances, including benzodiazepines43 and selective serotonin reuptake inhibitors44 also have been implicated. We did not have access to maternal records and were not able to account for differences in drug exposure in our cohort. Last, revisit links were missing for some infants, possibly due to moving out of the state. However, for our main analysis of hospital readmission within 30 days of birth hospitalization discharge, nearly the entire cohort had revisit links (>97%).
Conclusions
Infants with NAS were 2.5 times as likely as uncomplicated term infants to be readmitted to a hospital within 30 days of discharge. Infants with NAS were similar to late preterm infants in characteristics (eg, respiratory diagnoses, feeding difficulties) and in probability of readmission. QI efforts aimed at reducing LOS for infants with NAS should consider trade-offs between LOS and readmission risk. Last, future policy development and research should focus on efforts to reduce hospital readmission for this vulnerable population by ensuring a safe transition home and should explore strategies to address postdischarge complications in both primary care and community-based settings.
Supplementary Material
Acknowledgments
Funding: Supported by Clinical and Translational Science Awards (CTSA) award KL2TR000446 from the National Center for Advancing Translational Sciences and by the National Institute on Drug Abuse through the award 1K23DA038720–01 (Dr Patrick). The sponsor had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript or the decision to submit.
Footnotes
Financial Disclosure: The authors have indicated they have no financial relationships relevant to this article to disclose.
Potential Conflict of Interest: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Hudak ML, Tan RC Committee on Drugs; Committee on Fetus and Newborn. American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics. 2012;129(2) Available at: www.pediatrics.org/cgi/content/full/129/2/e540. [Google Scholar]
- 2.Kocherlakota P. Neonatal abstinence syndrome. Pediatrics. 2014;134(2) doi: 10.1542/peds.2013-3524. Available at: www.pediatrics.org/cgi/content/full/134/2/e547. [DOI] [PubMed] [Google Scholar]
- 3.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307(18):1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 4.Paulozzi LJ, Mack KA, Hockenberry JM Division of Unintentional Injury Prevention, National Center for Injury Prevention and Control, CDC. Vital signs: variation among states in prescribing of opioid pain relievers and benzodiazepines—United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63(26):563–568. [PMC free article] [PubMed] [Google Scholar]
- 5.Satel SL, Kosten TR, Schuckit MA, Fischman MW. Should protracted withdrawal from drugs be included in DSM-IV? Am J Psychiatry. 1993;150(5):695–704. doi: 10.1176/ajp.150.5.695. [DOI] [PubMed] [Google Scholar]
- 6.Martin WR, Jasinski DR. Physiological parameters of morphine dependence in man—tolerance, early abstinence, protracted abstinence. J Psychiatr Res. 1969;7(1):9–17. doi: 10.1016/0022-3956(69)90007-7. [DOI] [PubMed] [Google Scholar]
- 7.Eisenman AJ, Sloan JW, Martin WR, Jasinski DR, Brooks JW. Catecholamine and 17-hydroxycorticosteroid excretion during a cycle of morphine dependence in man. J Psychiatr Res. 1969;7(1):19–28. doi: 10.1016/0022-3956(69)90008-9. [DOI] [PubMed] [Google Scholar]
- 8.Desmond MM, Wilson GS. Neonatal abstinence syndrome: recognition and diagnosis. Addict Dis. 1975;2(1–2):113–121. [PubMed] [Google Scholar]
- 9.Centers for Medicare and Medicaid Services. 30-day unplanned readmission and death measures. [Accessed October 1, 2014];2014 Available at: www.medicare.gov/hospitalcompare/Data/30-day-measures.html?AspxAutoDetectCookieSupport=1.
- 10.Srivastava R, Keren R. Pediatric readmissions as a hospital quality measure. JAMA. 2013;309(4):396–398. doi: 10.1001/jama.2012.217006. [DOI] [PubMed] [Google Scholar]
- 11.Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372–380. doi: 10.1001/jama.2012.188351. published correction appears in JAMA. 2013;309 (10):986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escobar GJ, Joffe S, Gardner MN, Armstrong MA, Folck BF, Carpenter DM. Rehospitalization in the first two weeks after discharge from the neonatal intensive care unit. Pediatrics. 1999;104(1) doi: 10.1542/peds.104.1.e2. Available at: www.pediatrics.org/cgi/content/full/104/1/e2. [DOI] [PubMed] [Google Scholar]
- 13.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) HCUP Supplemental Variables for Revisit Analyses. [Accessed September 30, 2014];2014 Available at: www.hcup-us.ahrq.gov/toolssoftware/revisit/revisit.jsp.
- 14.Schiltz NK, Finkelstein Rosenthal B, Crowley MA, et al. Rehospitalization during the first year of life by insurance status. Clin Pediatr (Phila) 2014;53(9):845–853. doi: 10.1177/0009922814536924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson EE, Simpson KN. Discharge disposition after bariatric surgery. Obes Surg. 2014;24(10):1821–1825. doi: 10.1007/s11695-014-1372-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop Relat Res. 2014;472(8):2317–2324. doi: 10.1007/s11999-014-3613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Classification of Diseases, 9th Revision-Clinical Modification. Chicago, IL: American Medical Association; 2012. [Google Scholar]
- 18.McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111(1):35–41. doi: 10.1097/01.AOG.0000297311.33046.73. [DOI] [PubMed] [Google Scholar]
- 19.Patrick SW, Kaplan HC, Passarella M, Davis MM, Lorch SA. Variation in treatment of neonatal abstinence syndrome in US Children's Hospitals, 2004–2011. J Perinatol. 2014;34(11):867–872. doi: 10.1038/jp.2014.114. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Vital signs: overdoses of prescription opioid pain relievers— United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487–1492. [PubMed] [Google Scholar]
- 21.Epstein RA, Bobo WV, Martin PR, et al. Increasing pregnancy-related use of prescribed opioid analgesics. Ann Epidemiol. 2013;23(8):498–503. doi: 10.1016/j.annepidem.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123(5):997–1002. doi: 10.1097/AOG.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellogg A, Rose CH, Harms RH, Watson WJ. Current trends in narcotic use in pregnancy and neonatal outcomes. Am J Obstet Gynecol. 2011;204(3):259.e1–e4. doi: 10.1016/j.ajog.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 24.Young PC, Korgenski K, Buchi KF. Early readmission of newborns in a large health care system. Pediatrics. 2013;131(5) doi: 10.1542/peds.2012-2634. Available at: www.pediatrics.org/cgi/content/full/131/5/e1538. [DOI] [PubMed] [Google Scholar]
- 25.Phillips RM, Goldstein M, Hougland K, et al. National Perinatal Association. Multidisciplinary guidelines for the care of late preterm infants. J Perinatol. 2013;33(suppl 2):S5–S22. doi: 10.1038/jp.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goyal N, Zubizarreta JR, Small DS, Lorch SA. Length of stay and readmission among late preterm infants: an instrumental variable approach. Hosp Pediatr. 2013;3(1):7–15. doi: 10.1542/hpeds.2012-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engle WA, Tomashek KM, Wallman C Committee on Fetus and and Newborn, American Academy of Pediatrics. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120(6):1390–1401. doi: 10.1542/peds.2007-2952. [DOI] [PubMed] [Google Scholar]
- 28.Aronson PL, Thurm C, Alpern ER, et al. Febrile Young Infant Research Collaborative. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667–677. doi: 10.1542/peds.2014-1382. [DOI] [PubMed] [Google Scholar]
- 29.Diaz-Caneja A, Gledhill J, Weaver T, Nadel S, Garralda E. A child's admission to hospital: a qualitative study examining the experiences of parents. Intensive Care Med. 2005;31(9):1248–1254. doi: 10.1007/s00134-005-2728-8. [DOI] [PubMed] [Google Scholar]
- 30.Lapillonne A, Regnault A, Gournay V, et al. Impact on parents of bronchiolitis hospitalization of full-term, preterm and congenital heart disease infants. BMC Pediatr. 2012;12:171. doi: 10.1186/1471-2431-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leader S, Jacobson P, Marcin J, Vardis R, Sorrentino M, Murray D. A method for identifying the financial burden of hospitalized infants on families. Value Health. 2002;5(1):55–59. doi: 10.1046/j.1524-4733.2002.51076.x. [DOI] [PubMed] [Google Scholar]
- 32.Leidy NK, Margolis MK, Marcin JP, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics. 2005;115(6):1536–1546. doi: 10.1542/peds.2004-1149. [DOI] [PubMed] [Google Scholar]
- 33.Toomey SL, Peltz A, Nakamura MM, et al. Potentially preventable 30-day readmissions at an academic children's hospital. Paper presented at: Pediatric Academic Society Annual Meeting; Vancouver, BC. May 3–6, 2014. [Google Scholar]
- 34.Centers for Medicare and Medicaid Services. Readmissions reduction program. [Accessed November 2, 2013];2013 Available at: www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html.
- 35.Illinois Department of Healthcare and Family Services. Potentially Preventable Readmissions (PPRs) policy and calculations. [Accessed September 27, 2013];2012 Available at: http://www2.illinois.gov/hfs/SiteCollectionDocuments/PPRSlides.pdf.
- 36.New York State Department of Health Division of Quality and Evaluation Office of Health Insurance Programs. Potentially preventable hospital readmissions among Medicaid recipients: New York State, 2007. [Accessed September 27, 2013];2007 Available at: www.health.ny.gov/health_care/managed_care/reports/statistics_data/2hospital_readmissions.pdf.
- 37.Texas Health and Human Services Commission. Potentially preventable readmissions in the Texas Medicaid population, state fiscal year 2011. [Accessed September 27, 2013];2012 Available at: www.hhsc.state.tx.us/reports/2012/PPR-Readmissions-FY2011.pdf.
- 38.Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J Perinatol. 2006;26(1):15–17. doi: 10.1038/sj.jp.7211427. [DOI] [PubMed] [Google Scholar]
- 39.Neonatal Abstinence Syndrome Project. [Accessed October 4, 2004];2014 Available at: https://opqc.net/projects/NAS.
- 40.Goyal NK, Teeters A, Ammerman RT. Home visiting and outcomes of preterm infants: a systematic review. Pediatrics. 2013;132(3):502–516. doi: 10.1542/peds.2013-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitzman H, Olds DL, Henderson CR, Jr, et al. Effect of prenatal and infancy home visitation by nurses on pregnancy outcomes, childhood injuries, and repeated childbearing. A randomized controlled trial. JAMA. 1997;278(8):644–652. [PubMed] [Google Scholar]
- 42.Burns L, Mattick RP. Using population data to examine the prevalence and correlates of neonatal abstinence syndrome. Drug Alcohol Rev. 2007;26(5):487–492. doi: 10.1080/09595230701494416. [DOI] [PubMed] [Google Scholar]
- 43.Rementería JL, Bhatt K. Withdrawal symptoms in neonates from intrauterine exposure to diazepam. J Pediatr. 1977;90(1):123–126. doi: 10.1016/s0022-3476(77)80785-3. [DOI] [PubMed] [Google Scholar]
- 44.Levinson-Castiel R, Merlob P, Linder N, Sirota L, Klinger G. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch Pediatr Adolesc Med. 2006;160(2):173–176. doi: 10.1001/archpedi.160.2.173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.