Abstract
Background
Oxidative DNA damage is associated with male infertility. The aim of this study was to evaluate the oxidative DNA damage of sperm cells and blood leukocytes and to determine the levels of MDA and NO levels in seminal and blood plasma of idiopathic infertile men.
Material/Methods
The study enrolled 52 patients, including 30 infertile and 22 fertile men. MDA, NO, and 8-OHdG/106dG were estimated using spectrophotometry and high-pressure liquid chromatography (HPLC)-based methods in seminal and blood plasma. The association with the sperm parameters was assessed, particularly sperm counts and motility.
Results
The mean sperm concentration and sperm motility of the fertile men were significantly higher than that of the infertile men. The mean MDA and NO concentration in the seminal and blood samples of the infertile men were higher than that of fertile men. Also, the mean numbers of sperm cells and leukocytes 8-OHdG/106dG of the infertile men were significantly higher than that of fertile men (p=0.04 and p<0.001, respectively). Sperm motility and sperm count were negatively correlated with leukocyte and sperm cell 8-OHdG/106dG ratio. However, progressive motility was significantly negatively correlated with sperm cell and leukocyte 8-OHdG/106dG ratio (R=−0.357, p=0.026; R=−0.388, p=0.024, respectively).
Conclusions
Oxidative stress is an important factor in male infertility. Therefore, biochemical detection of 8-OHdG/106dG in sperm cells and blood leukocytes may be an additional tool in the diagnosis of male infertility.
MeSH Keywords: DNA Damage; Infertility, Male; Oxidative Stress; Sperm Motility
Background
Infertility is usually defined as the inability to conceive after 1 year of regular unprotected intercourse [1]. It has been reported that nearly 40% of the issues involved with infertility are attributable to a male factor, another 40% due to a female factor, and 20% result from combined male and female factors [2].
Excessive production of reactive oxygen and nitrogen species (ROS and RNS) (i.e., oxidative stress) is involved in the etiology of infertility, especially male infertility [3,4]. All of these reports have associated ROS/RNS, generated both exogenously and endogenously, with several aspects of male infertility. Although ROS/RNS are important for various physiologic functions, excessive amounts contribute to oxidative stress and this may exert pathologic effects on the male reproductive system [5,6]. The mechanism of action of ROS and RNS involves lipid peroxidation of the sperm plasma membrane, which is highly susceptible to oxidative damage due to large amounts of polyunsaturated fatty acids, by impairing membrane fluidity, and mobility [4]. In addition, ROS and RNS can damage sperm axoneme, and impair mitochondrial function, DNA, RNA, and proteins [7].
In the present study, we aimed to evaluate levels of malondialdehyde (MDA) as a marker of oxidative lipid peroxidation and nitric oxide (NO) as a marker of RNS concentrations in blood and seminal plasma samples of infertile men in comparison with fertile men, and to investigate whether there were correlations with semen parameters. In addition, we determined oxidative DNA damage of blood leukocytes and sperm cells in fertile and infertile men.
Material and Methods
Study population
This study was a prospective case-control study of 52 patients (30 infertile men aged 22–40 years, and 22 healthy volunteers aged 22–44 years) in the Urology Department of Yüzüncü Yıl University Hospital (Van, Turkey). In this study, healthy fertile men were considered as those who had no chronic clinical illness and had had a baby within 1 year of unprotected sexual intercourse. All infertile men included in this study had had a minimum of 1 year of regular unprotected intercourse with their respective female partner. Physical examination and laboratory tests was performed to exclude men with known factors such as varicocele, cryptorchidism, and endocrine disorders. Patients who had taken antioxidant supplements in the last 3 months were also excluded. The female partners of the men had no history of untreated female-factor infertility and had a normal reproductive and sexual history, as well as a normal investigation results.
All participants were given an explanation of the nature of the study and informed consent was obtained. This study was approved by the Ethics Committee of Van Yüzüncü Yıl University.
Sample collection
Semen samples were obtained from April 2015 through September 2015. All the samples were collected in a sterile container by masturbation, after a period of 48 to 72 h of sexual abstinence. After liquefaction, aliquots of semen were centrifuged and semen plasma was isolated and stored at −80°C until required for the assay of oxidative stress markers.
Standard semen analysis
Following liquefaction, semen samples were evaluated for assessment of volume, appearance, pH, and viscosity. Routine semen analysis was performed in accordance with the World Health Organization (2010) guidelines; patients who had ≥15 ×106/mL sperm concentration, ≥40% motility or ≥32% progressive motility, and over 4% with normal morphology were considered to have normal semen parameters.
Assay of oxidative stress
MDA analysis
Analysis of semen plasma and blood plasma MDA levels were performed using the same high-performance liquid chromatograph (HPLC) with fluorescence detection (HPLC-FLD) as described by Khoschsorur et al. [8]. In brief, 50 μL of a sample was mixed with 0.44 mol/L H3PO4 and 42 mmol/L thiobarbituric acid (TBA), and incubated for 30 minutes in a boiling water bath. After cooling rapidly on ice, an equal volume of alkaline methanol was added to the sample, shaken vigorously, centrifuged (3000 r/min for 3 min), and the aqueous layer removed. We then analyzed 20 μL of supernatant using HPLC (HP, Agilent 1260 modular systems with FLD detector); reverse-phase chromatography column, RP-C18 (5 μm, 4.6×150 mm) (EclipseVDB-C18; Agilent); elution, methanol (40: 60, v/v) containing 50 mmol/L KH2PO4 buffer (pH6.8); flow rate, 0.8mL/min. Fluorometric detection was performed using excitation at 527 nm and emission at 551 nm. The peak of the MDA-TBA adduct was calibrated as a 1,1,3,3 tetra ethoxy propane standard solution, which was performed simultaneously with the plasma sample. MDA levels are expressed as μM.
NO analysis
The measurement of NO in biologic fluids is difficult because this molecule is poorly soluble in water and has a short half-life; therefore, NO concentration was assessed by monitoring seminal and blood plasma concentration of stable oxidation products of NO metabolites (NO2−/NO3−). Semen plasma and blood plasma total nitrite+nitrate level were measured using Griess reagent, as previously described [9,10].
The method is based on a 2-step process. The first step is the conversion of nitrate to nitrite using nitrate reductase. The second step is the addition of Griess reagent, which converts nitrite into a deep-purple azo compound; photometric measurement of the absorbance at 540 nm of this azo chromophore accurately determines nitrite concentrations.
DNA Isolation and hydrolyzation
The sperm cells were first washed with sperm wash buffer (SWB; 10 mM Tris-HCl, 10 mM EDTA, 1 M NaCl, pH 7.0) and then resuspended in SWB and lysed at 55°C for 1 h in the presence of 0.9% SDS, 0.5 mg/mL proteinase K, and 0.04 M dithiothreitol (DTT). DNA extraction was performed using chloroform-isoamyl alcohol (12: 1, v: v). The precipitated DNA was dissolved in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.0) and digested with ribonuclease A (10 units/mL, 37°C × 1 h) [11]. The blood leukocyte DNA isolation was performed with a commercially available DNA isolation kit (Cat. no. CS21110-96, Invitrogen, USA). The DNA aliquots were hydrolyzed using Kaur and Halliwell’s method [12]. Before analysis by HPLC, they were dissolved in the eluent (final volume, 1 ml). DNA isolation from whole blood plasma was performed using a commercially available DNA isolation kit (Vivantis GF-1 DNA Extraction Kit Cat. No. GF-BD-100, China).
In the hydrolyzed DNA samples, 8-OHdG and dG levels were measured using HPLC with electrochemical detection (HPLC-ECD) and variable wave-length detector (HPLC-UV) systems, as described previously [13,14]. Before HPLC analysis, the hydrolized DNA samples were redissolved in the HPLC eluent (final volume, 1 mL). Twenty μL of final hydrolysate were analyzed using HPLC-ECD (Waters 2065 electrochemical detector) and a reverse-phase chromatography column (RP-C18) (250×4.6 mm ×4.0 μm, Phenomenex, CA). The mobile phase consisted of 0.05 M potassium phosphate buffer [pH 5.5] containing acetonitrile (97: 3, v/v) with a flow rate of 1 mL/min. The dG concentration was monitored based on absorbance (245 nm) and 8-OHdG based on the electrochemical reading (600 mV). Levels of dG and 8-OHdG were quantified using the standards of dG and 8-OHdG from Sigma; the level of 8-OHdG level was expressed as 8-OHdG molecules per 106dG.
Statistical analysis
All results are expressed as mean ±SEM. The statistical analysis of the data was performed using the Statistical Package of Social Sciences, version 16.0, (SPSS Inc, Chicago, Illinois, USA) and Graph Pad Prism version 5.
Our data showed normal distribution; thus, we used the independent samples t-test for comparisons between 2 groups. A 95% confidence interval was used. P values less than 0.05 were considered as statistically significant. The correlation between 2 continuous outcomes among infertile men was evaluated using Pearson’s correlation coefficient.
Results
Characteristics of the study population, semen parameters, and the mean of MDA, NO, and 8-OHdG/106dG levels of the 2 groups are summarized in Table 1. There were no significant differences in the mean of participant age between the 2 groups, but the mean sperm concentration and sperm motility of fertile men were significantly higher than those of the infertile men (p<0.001 and p<0.001, respectively).
Table 1.
Characteristic of the study population, semen parameters and the mean of MDA, NO concentration and 8-OHdG/106dG.
| Fertile (M±SD) | Infertile (M±SD) | p value | |
|---|---|---|---|
| Age (y) | 30.40±4.97 | 29.90±4.28 | .694 |
| Sperm concentration (106/ml) | 62.72±17.84 | 24.0±26.14 | .000 |
| Total motility (%) | 65.00±12.24 | 27.33±22.27 | .000 |
| Progressive motility (%) | 41,82±11,50 | 10,83±9,92 | .000 |
| Seminal plasma MDA (μM) | 6,63±2.99 | 9.68±2.87 | .001 |
| Serum MDA (μM) | 7.70±2.37 | 12.55±3.17 | .000 |
| Seminal plasma NO (μM) | 5.65±1.85 | 6.64±1.66 | .07 |
| Serum NO (μM) | 11.18±5.61 | 19.26±7.81 | .001 |
| Sperm 8-OHdG/106dG | 1.03±1.03 | 1.55±0.61 | .04 |
| Leukocyte (8-OHdG/106dG) | 0.77±0.27 | 1.25±0.37 | .000 |
The mean of MDA concentration in the seminal plasma and blood plasma of infertile men was significantly higher than that of the fertile men (p=0.001, p<0.001, respectively). Also, the mean NO concentration in the seminal plasma of infertile men was higher than that of the fertile men, although there was no a statistically significant difference (p=0.07) However, there was a statistically significant difference between the blood samples of the fertile and infertile men in terms of NO concentration (p=0.001). Also, the mean sperm cell and leukocyte 8-OHdG/106dG of the infertile men were significantly higher than that of fertile men (p=0.04, p<0.001, respectively).
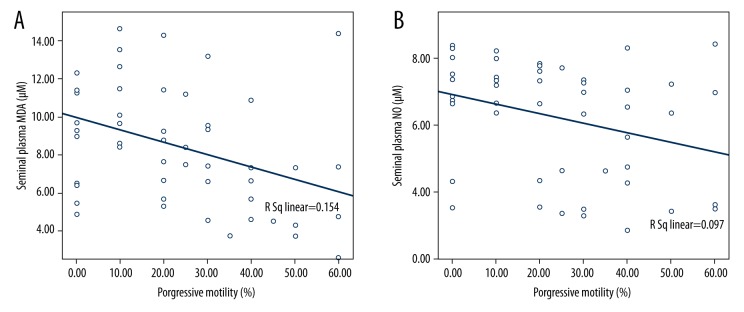
MDA and NO levels of semen were significantly positively correlated with sperm cell 8-OHdG/106dG ratio (R=0.504, p=0.002; R=0.769, p<0.001, respectively). Also, MDA and NO levels of blood plasma were significantly positively correlated with leukocyte 8-OHdG/106dG ratio (R=0.624, p<0.001; R=0.393, p=0.043, respectively). In addition, MDA and NO levels of semen were significantly negatively correlated with progressive motility (Figure 1A, 1B).
Figure 1.
The correlation of progressive motility with (A) seminal plasma MDA, (B) seminal plasma NO.
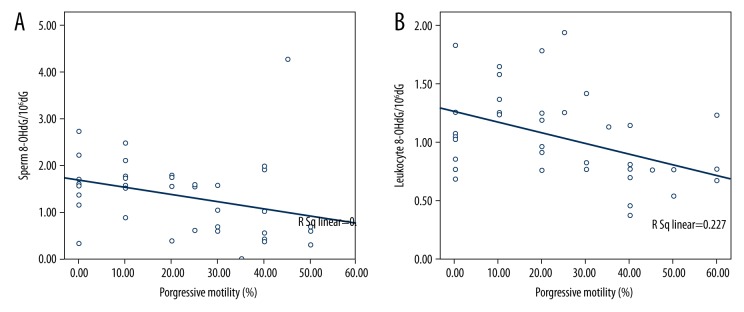
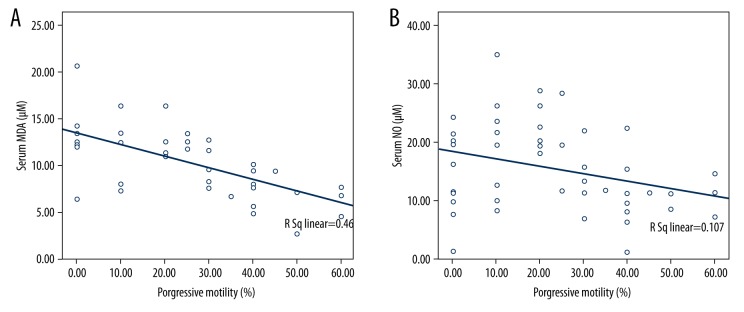
Sperm motility and sperm count were negatively correlated with leukocyte and sperm cell 8-OHdG/106dG ratio. However, progressive motility was significant negatively correlated with sperm cell and leukocyte 8-OHdG/106dG ratio (R=−0.357, p=0.026; R=−0.388, p=0.024, respectively) (Figure 2A, 2B). MDA and NO levels of serum were significantly negatively correlated with progressive motility (Figure 3A, 3B). The correlations among all parameters are presented in Table 2.
Figure 2.
The correlation of progressive motility with (A) sperm 8-OHdG/106dG, (B) leukocyte 8-OHdG/106dG.
Figure 3.
The correlation of progressive motility with (A) serum MDA, (B) serum NO.
Table 2.
The correlations of seminal and blood plasma levels of MDA, NO, 8-OHdG/106dG and sperm parameters.
| Volume (ml) | Sperm concentration (106/ml) | Total motility % | Progresive motility % | Seminal plasma MDA (μM) | Seminal plasma NO (μM) | Sperm 8-OHdG/106dG | Leukocyte 8-OHdG/ 106dG | Serum MDA (μM) | Serum NO (μM) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Volume (ml) | Pearson Correlation | 1 | |||||||||
| Sig. (2-tailed) | |||||||||||
| N | 52 | ||||||||||
| Sperm concentration (106/ml) | Pearson Correlation | −.147 | 1 | ||||||||
| Sig. (2-tailed) | .299 | ||||||||||
| N | 52 | 52 | |||||||||
| Total motility % | Pearson Correlation | −.137 | .786(**) | 1 | |||||||
| Sig. (2-tailed) | .334 | .000 | |||||||||
| N | 52 | 52 | 52 | ||||||||
| Progresive motility % | Pearson Correlation | −.136 | .792(**) | .935(**) | 1 | ||||||
| Sig. (2-tailed) | .338 | .000 | .000 | ||||||||
| N | 52 | 52 | 52 | 52 | |||||||
| Seminal plasma MDA (μM) | Pearson Correlation | .061 | −.276 | −.266 | −.376(**) | 1 | |||||
| Sig. (2-tailed) | .689 | .063 | .074 | .010 | |||||||
| N | 52 | 52 | 52 | 52 | 52 | ||||||
| Seminal plasma NO (μM) | Pearson Correlation | .015 | −.214 | −.267 | −.301(*) | .510(**) | |||||
| Sig. (2-tailed) | .925 | .163 | .080 | .047 | .001 | ||||||
| N | 52 | 52 | 52 | 52 | 52 | 44 | |||||
| Sperm 8-OHdG/106dG |
Pearson Correlation | .126 | −.242 | −.317(*) | −.357(*) | .504(**) | .769(**) | 1 | |||
| Sig. (2-tailed) | .446 | .138 | .049 | .026 | .002 | .000 | |||||
| N | 52 | 52 | 52 | 52 | 52 | 52 | 52 | ||||
| Leukocyte 8-OHdG/106dG |
Pearson Correlation | .105 | −.166 | −.197 | −.388(*) | .545(**) | .324 | .351 | 1 | ||
| Sig. (2-tailed) | .556 | .348 | .265 | .024 | .002 | .087 | .057 | ||||
| N | 52 | 52 | 52 | 52 | 52 | 52 | 52 | 52 | |||
| Serum MDA (μM) | Pearson Correlation | .267 | −.416(*) | −.559(**) | −.657(**) | .663(**) | .389(*) | .536(**) | .624(**) | 1 | |
| Sig. (2-tailed) | .111 | .010 | .000 | .000 | .000 | .023 | .002 | .000 | |||
| N | 52 | 52 | 52 | 52 | 52 | 52 | 52 | 52 | 52 | ||
| Serum NO (μM) | Pearson Correlation | .154 | −.242 | −.286 | −.384(*) | .477(**) | .225 | .312 | .393(*) | .542(**) | 1 |
| Sig. (2-tailed) | .355 | .144 | .082 | .017 | .004 | .201 | .094 | .043 | .002 | ||
| N | 52 | 52 | 52 | 52 | 52 | 52 | 52 | 52 | 52 | 38 |
Correlation is significant at the 0.01 level (2-tailed);
Correlation is significant at the 0.05 level (2-tailed).
Discussion
Semen analysis has traditionally been the cornerstone of evaluation of male infertility and remains the initial test of choice. However, basic semen analysis does not adequately demonstrate all parameters of semen quality and function, which are required for an optimum determination of fertility status [15].
There is a consensus about the clinical use of seminal oxidative stress testing in infertility clinics [5,16]. Studies have shown that a balance between ROS/RNS and antioxidants is undoubtedly important for a variety of functions in the male reproductive system, such as cell signaling, tight junction regulation, hormone production, capacitation, acrosomal reaction, sperm motility, and zona pellucida binding [16,17]. In addition, some studies have shown that evaluation of seminal oxidative biomarkers (for example MDA and 8-OHdG) could be a valuable diagnostic tool for defining sperm fertilization potential [18,19].
The sources of oxidative stress in seminal plasma of infertile men are unknown, but some studies suggest that they may be produced by induced genital tract cells such as Leydig cells, epididymal or vas deferens epithelial cells, or spermatozoa itself, and in some conditions such as subinfectious or inflammatory disease of the male genital tract; induced leukocytes are the source of high concentrations of ROS/RNS in seminal plasma [20].
MDA is the end product of lipid peroxidation and is, therefore, an indirect indicator of the intensity of the process in the cell [21]. MDA has been used as a marker of oxidative stress in several studies [22,23]. The impairment of the antioxidant defence system in infertile men was associated with a significant accumulation of seminal plasma MDA as a biomarker of lipid peroxidation, which was negatively correlated, in turn, with sperm motility and morphology.
Examples of RNS include peroxynitrite anion, nitroxyl ion, nitrosyl-containing compounds, and NO. A moderate amount of NO in seminal plasma is essential for acrosome reaction, sperm motility, and capacitation [24]. NO increases the motility of spermatozoa by increasing energy production originating in the mitochondrial compartment [25]. It has also been reported that low concentrations of NO increase human sperm capacitation without affecting sperm motility [26].
NO reacts rapidly with superoxide to form highly toxic peroxy nitrite (ONOO−). Both NO and ONOO− have demonstrated the ability to directly damage DNA [27]. Several studies suggested that ROS attacks the integrity of DNA in the sperm nucleus by causing nucleotide modifications, DNA strand breaks, and chromatin cross-linking [28–30]. ROS leads to oxidative modifications in the nucleic acids of DNA. There are about 20 oxidized nucleic acids found in the human body. The most abundant among these nucleic acids is 8-OHdG [31,32]. 8-OHdG is used as a biomarker in the determination of oxidative DNA damage [33]. Spermatozoa have limited defense mechanisms against oxidative free radical attack on their DNA; therefore, 8-OHdG is produced during oxidative DNA damage. In addition, it was reported that sperm DNA damage was closely related to male infertility and 8-OHdG was a sensitive marker of oxidative DNA damage caused by ROS in human sperm [11]. Intact human sperm DNA is an essential prerequisite for successful fertilization and embryo development. Thus, sperm DNA fragmentation decreases pregnancy rates in assisted reproductive techniques [28].
The blood antioxidant profile in relation to the semen antioxidant profile and semen quality in infertile subjects has been less investigated. An evaluation of the blood plasma antioxidant profile revealed significant differences between fertile and infertile subjects [34,35]. Together with seminal oxidative stress assessment, blood oxidative status determination has recently been proposed as a valuable tool to evaluate sperm reproductive capacity and functional competence.
Kodama et al. reported that the levels of 8-hydroxy-2-deoxyguanosine in sperm DNA were significantly higher in infertile male patients than in control patients and were correlated with sperm concentrations in ejaculates [36]. Hosen et al. showed that sperm motility, count, and morphology were negatively correlated with oxidant status and 8-OHdG, and positively correlated with antioxidant status. They also found a positive correlation of 8-OHdG with oxidant status and a negative correlation with total antioxidant status [19].
Guz et al. demonstrated that 8-oxodG level was highly and significantly correlated with sperm count, motility, and morphology and indicated highly significant elevated level of 8-oxodG in sperm DNA compared with DNA of surrogate tissue (leukocytes) in infertile men as well as in the healthy control group [37]. In the present study, the levels of sperm cell and leukocyte 8-OHdG/106dG had statistically significant differences between infertile and fertile men. Leukocyte 8-OHdG/106dG levels were positively correlated with sperm cell 8-OHdG/106dG levels and negatively correlated with sperm count and motility, especially progressive motility.
In this study, NO and MDA levels were detected at significantly higher levels in seminal plasma and blood plasma of idiopathic infertile patients than in normozoospermic fertile men. MDA concentrations in particular had statistically significant differences in blood and seminal plasma between infertile and fertile men (p=0.01 and p=0.001, respectively). NO levels in serum were significantly different, while there were no statistically significant differences in the seminal plasma. Seminal MDA and NO levels were negatively correlated with sperm parameters, and positively correlated with MDA and NO levels in blood plasma. A positive correlation of sperm cell 8-OHdG/106dG with seminal MDA and NO levels and an inverse correlation of 8-OHdG/106dG with motility and sperm count was found, which clearly indicated the effect of oxidative stress on fertility potentials. In particular, we observed that oxidative stress has a greater effect on sperm progressive motility than on other sperm parameters.
Conclusions
The findings of the present study suggest that oxidative conditions have a potential pathogenetic role in the reduction of sperm motility and count. We found high concentrations of NO, MDA, and 8-OHdG/106dG in blood and semen samples from a group of infertile men. Therefore, biochemical evaluation of the blood oxidative stress profile may be a useful additional tool in the diagnosis of male infertility.
Footnotes
Conflict of interest
None declared.
Source of support: Departmental sources
References
- 1.Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 2.Sharlip ID, Jarow JP, Belker AM, et al. Best practice policies for male infertility. Fertil Steril. 2002;77:873–82. doi: 10.1016/s0015-0282(02)03105-9. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Allamaneni SS. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod Biomed Online. 2004;9:338–47. doi: 10.1016/s1472-6483(10)62151-7. [DOI] [PubMed] [Google Scholar]
- 4.Doshi SB, Khullar K, Sharma RK, Agarwal A. Role of reactive nitrogen species in male infertility. Reprod Biol Endocrinol. 2012;10:109. doi: 10.1186/1477-7827-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buzadzic B, Vucetic M, Jankovic A, et al. New insights into male (in)fertility: The importance of NO. Br J Pharmacol. 2015;172:1455–67. doi: 10.1111/bph.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sikka SC, Rajasekaran M, Hellstrom WJ. Role of oxidative stress and antioxidants in male infertility. J Androl. 1995;16:464–68. [PubMed] [Google Scholar]
- 7.Agarwal A, Prabakaran SA. Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology. Indian J Exp Biol. 2005;43:963–74. [PubMed] [Google Scholar]
- 8.Khoschsorur GA W-RB, Rabl H, Auer T, et al. Evaluation of a sensitive HPLC method for the determination of malondialdehyde, and application of the method to different biological materials. Chromatographia. 2000;52:181–84. [Google Scholar]
- 9.Bories PN, Bories C. Nitrate determination in biological fluids by an enzymatic one-step assay with nitrate reductase. Clin Chem. 1995;41:904–7. [PubMed] [Google Scholar]
- 10.Moshage H, Kok B, Huizenga JR, Jansen PL. Nitrite and nitrate determinations in plasma: A critical evaluation. Clin Chem. 1995;41:892–96. [PubMed] [Google Scholar]
- 11.Shen H, Ong C. Detection of oxidative DNA damage in human sperm and its association with sperm function and male infertility. Free Radic Biol Med. 2000;28:529–36. doi: 10.1016/s0891-5849(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 12.Kaur H, Halliwell B. Measurement of oxidized and methylated DNA bases by HPLC with electrochemical detection. Biochem J. 1996;318( Pt 1):21–23. doi: 10.1042/bj3180021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong D. Free radical and antioxidant protocols. Introduction. Methods Mol Biol. 1998;108:v–viii. [PubMed] [Google Scholar]
- 14.Shigenaga MK, Aboujaoude EN, Chen Q, Ames BN. Assays of oxidative DNA damage biomarkers 8-oxo-2′-deoxyguanosine and 8-oxoguanine in nuclear DNA and biological fluids by high-performance liquid chromatography with electrochemical detection. Methods Enzymol. 1994;234:16–33. doi: 10.1016/0076-6879(94)34073-0. [DOI] [PubMed] [Google Scholar]
- 15.Samplaski MK, Agarwal A, Sharma R, Sabanegh E. New generation of diagnostic tests for infertility: review of specialized semen tests. Int J Urol. 2010;17:839–47. doi: 10.1111/j.1442-2042.2010.02619.x. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: An update. Am J Reprod Immunol. 2008;59:2–11. doi: 10.1111/j.1600-0897.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee NP, Cheng CY. Nitric oxide and cyclic nucleotides: Their roles in junction dynamics and spermatogenesis. Oxid Med Cell Longev. 2008;1:25–32. doi: 10.4161/oxim.1.1.6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amiri I, Sheikh N, Najafi R. Nitric oxide level in seminal plasma and its relation with sperm DNA damages. Iran Biomed J. 2007;11:259–64. [PubMed] [Google Scholar]
- 19.Hosen MB, Islam MR, Begum F, et al. Oxidative stress induced sperm DNA damage, a possible reason for male infertility. Iran J Reprod Med. 2015;13:525–32. [PMC free article] [PubMed] [Google Scholar]
- 20.Huang I, Jones J, Khorram O. Human seminal plasma nitric oxide: Correlation with sperm morphology and testosterone. Med Sci Monit. 2006;12(3):CR103–6. [PubMed] [Google Scholar]
- 21.Suleiman SA, Ali ME, Zaki ZM, et al. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl. 1996;17:530–37. [PubMed] [Google Scholar]
- 22.Hsieh YY, Chang CC, Lin CS. Seminal malondialdehyde concentration but not glutathione peroxidase activity is negatively correlated with seminal concentration and motility. Int J Biol Sci. 2006;2:23–29. doi: 10.7150/ijbs.2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasqualotto FF, Sharma RK, Nelson DR, et al. Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil Steril. 2000;73:459–64. doi: 10.1016/s0015-0282(99)00567-1. [DOI] [PubMed] [Google Scholar]
- 24.Balercia G, Moretti S, Vignini A, et al. Role of nitric oxide concentrations on human sperm motility. J Androl. 2004;25:245–49. doi: 10.1002/j.1939-4640.2004.tb02784.x. [DOI] [PubMed] [Google Scholar]
- 25.Otasevic V, Korac A, Vucetic M, et al. Is manganese (II) pentaazamacrocyclic superoxide dismutase mimic beneficial for human sperm mitochondria function and motility? Antioxid Redox Signal. 2013;18:170–78. doi: 10.1089/ars.2012.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zini A, De Lamirande E, Gagnon C. Low levels of nitric oxide promote human sperm capacitation in vitro. J Androl. 1995;16:424–31. [PubMed] [Google Scholar]
- 27.Ishikawa T, Kondo Y, Goda K, Fujisawa M. Overexpression of endothelial nitric oxide synthase in transgenic mice accelerates testicular germ cell apoptosis induced by experimental cryptorchidism. J Androl. 2005;26:281–88. doi: 10.1002/j.1939-4640.2005.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 28.Benchaib M, Braun V, Lornage J, et al. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod. 2003;18:1023–28. doi: 10.1093/humrep/deg228. [DOI] [PubMed] [Google Scholar]
- 29.Saleh RA, Agarwal A, Nelson DR, et al. Increased sperm nuclear DNA damage in normozoospermic infertile men: A prospective study. Fertil Steril. 2002;78:313–18. doi: 10.1016/s0015-0282(02)03219-3. [DOI] [PubMed] [Google Scholar]
- 30.Said TM, Agarwal A, Sharma RK, et al. Impact of sperm morphology on DNA damage caused by oxidative stress induced by beta-nicotinamide adenine dinucleotide phosphate. Fertil Steril. 2005;83:95–103. doi: 10.1016/j.fertnstert.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 31.Mori T, Tano K, Takimoto K, Utsumi H. Formation of 8-hydroxyguanine and 2,6-diamino-4-hydroxy-5-formamidopyrimidine in DNA by riboflavin mediated photosensitization. Biochem Biophys Res Commun. 1998;242:98–101. doi: 10.1006/bbrc.1997.7916. [DOI] [PubMed] [Google Scholar]
- 32.Ravanat JL, Cadet J. Reaction of singlet oxygen with 2′-deoxyguanosine and DNA. Isolation and characterization of the main oxidation products. Chem Res Toxicol. 1995;8:379–88. doi: 10.1021/tx00045a009. [DOI] [PubMed] [Google Scholar]
- 33.Loft S, Fischernielsen A, Jeding IB, et al. 8-hydroxydeoxyguanosine as a urinary biomarker of oxidative DNA-damage. J Toxicol Environ Health. 1993;40:391–404. doi: 10.1080/15287399309531806. [DOI] [PubMed] [Google Scholar]
- 34.Benedetti S, Tagliamonte MC, Catalani S, et al. Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod Biomed Online. 2012;25:300–6. doi: 10.1016/j.rbmo.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Shamsi MB, Venkatesh S, Kumar R, et al. Antioxidant levels in blood and seminal plasma and their impact on sperm parameters in infertile men. Indian J Biochem Biophys. 2010;47:38–43. [PubMed] [Google Scholar]
- 36.Kodama H, Yamaguchi R, Fukuda J, et al. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril. 1997;68:519–24. doi: 10.1016/s0015-0282(97)00236-7. [DOI] [PubMed] [Google Scholar]
- 37.Guz J, Gackowski D, Foksinski M, et al. Comparison of oxidative stress/DNA damage in semen and blood of fertile and infertile men. PLoS One. 2013;8(7):e68490. doi: 10.1371/journal.pone.0068490. [DOI] [PMC free article] [PubMed] [Google Scholar]