Abstract
Acute subdural hematoma is a serious complication following traumatic brain injury. Large volume hematomas or those with underlying brain injury can cause mass effect, midline shift, and eventually herniation of the brain. Acute subdural hematomas in the young are associated with high-energy trauma and often have underlying contusions, while acute subdural hematomas in the elderly are associated with minor trauma and an absence of underlying contusions, even though the elderly are more likely to be on anticoagulants or anti-platelet therapy. In the young patients with high impact injuries the hematomas tend to be small and the underlying brain injury and swelling is responsible for the increased intracranial pressure and midline shift. In the elderly, the injuries are low impact (e.g fall from standing), the underlying brain is intact, and the volume of the hematoma itself produces symptoms. In addition the use of anticoagulants and antiplatelet agents in the elderly population has been thought to be a poor prognostic indicator and is considered to be responsible for larger hematomas and poor outcome. When managed conservatively, acute subdural hematomas can sometimes progress to chronic subdural hematoma formation, further enlargement, seizures, and progressive midline shift. Another potential difference in the young and the elderly is brain atrophy, which increases the potential space to accommodate a larger hematoma. It is not known if these two groups differ in other ways that might have implications for treatment or prognosis. In this paper, we investigate the clinical course of 80 patients admitted to our institution with acute subdural hematomas, to identify differences in patients above or below the age of 65 years. The natural progression/resolution of acute subdural hematomas was mapped by measuring volume expansion/regression over time. In this retrospective chart review, we investigated clinical baseline metrics and subsequent volumetric expansion outcomes between patients < 65 years old (N=44) and those > 65 years old (N=36). Volume was estimated by the ABC/2 method. We observed a statistically significant difference between groups in use of anticoagulants χ2 =40.305 with p < 0.001, corrective platelet administration χ2 =19.380 with p < 0.001, gender χ2 =14.573 with p < 0.001, and Glasgow Coma Scale with χ2 =23.125 (p=0.026). Overall outcomes were similar in the two groups. Younger patients on average had worse presenting GCS scores, but recovered comparable to older patients. No significant difference in rate of volume expansion, resolution time, or need for surgical treatment was seen between these two groups. We conclude that the initial volume, size, and severity of subdural hematoma determined by the Glasgow Coma Scale score is more likely to predict surgery or future expansion than age of the patient. Patients on oral anti-coagulants that are given appropriate medical reversal agents early do quite well and no impact on the eventual outcome could be demonstrated. Further work is needed to establish better predictors of future volume expansion, and progression to chronic subdural hematoma based on improved severity scales.
Keywords: Acute subdural hematoma, Natural progression, Age comparison, Anti-coagulant use, Injury severity
INTRODUCTION
A common complication of traumatic brain injury (TBI) is subdural hematoma. ~22% of TBI patients can present to the emergency department with subdural hematoma, which contributes to the extensive morbidity and mortality associated with TBI [1,2]. Common complications of subdural hematomas include mass effect resulting in altered cerebral blood flow and increase intracranial pressure [3]. Furthermore, subdural hematomas result in an elevated risk of seizure disorders. The causes of subdural hematomas are variable and consist of falls, particularly in the elderly, assault, other forms of trauma, and child abuse [4–6]. Notably, use of anticoagulants or antiplatelet agents may predispose to subdural hematomas or potentially worsen outcome. Management of subdural hematomas is varied, frequently employing either medical or surgical approaches [7]. The indications for surgery include a hematoma more than 10 mm thick and/or a midline shift of 5 mm or more. In addition any hematoma with a GCS score of 8 or below or an ICP of more than 20 mmHg is surgically explored. The size indication is more relevant for the elderly where the underlying brain is intact and the cause is usually bleeding from a cortical bleeder. The Glasgow Coma Score is more important for the young with high impact injuries and underlying brain injury. When patients are managed conservatively, the incidence of progression to chronic subdural hematoma is not fully known [8]. Ahmed and colleagues found that 5 out of 27 patients with acute subdural hematoma progressed to chronic subdural hematoma [9]. Patients on anticoagulants prior to admission, however, were excluded from their study. In some studies anti-coagulation therapy has been shown to increase mortality from large acute subdural hematomas and limit spontaneous resolution [10]. Additionally, elderly patients on anti-coagulant therapy are more likely to get acute subdural hematomas if they fall and receive head trauma [11]. It has been suggested that resolution of acute subdural hematomas may be more common for patients not on anti-coagulants [12]. Resolution is also thought to be dependent on the size of the initial subdural hematoma [13].
If large acute subdural hematomas are not managed early, the volume can expand acutely, leading to progressive midline shift and possible brain herniation [14]. Often these subdural hematomas have an underlying cortical bleeder, which needs to be controlled. The risk for rapid deterioration is highest within the first 4 hours after severe head trauma [15]. Large acute subdural hematomas are more likely to require surgical intervention [16]. Poorly managed subdural hematomas can lead to spreading cortical depression as well, which is a risk factor for seizure [17]. Okumura and colleagues found that 10 out of 96 patients progressed from mild acute subdural hematoma to chronic subdural hematoma. The mean age of the patients who had progressive subdural hematoma was 63.1 years [18]. Severity of initial injury may account for worse outcome over time [19]. Magnetic resonance imaging has been useful in monitoring subdural hematoma expansion or resolution [20,21]. ABC/2 volumetric calculation with computed tomography images has been verified as an accurate estimation approach for measuring subdural progression over time [22]. In this study, we look at the natural course of subdural hematomas over time using the ABC/2 volumetric calculation comparing young (<65 years old) and older (>65 years old) patients as well as by use of anti-coagulant therapy. The age range for young patients was 23–64 years old where the age range for the older patients was 66–91 years old.
METHODS
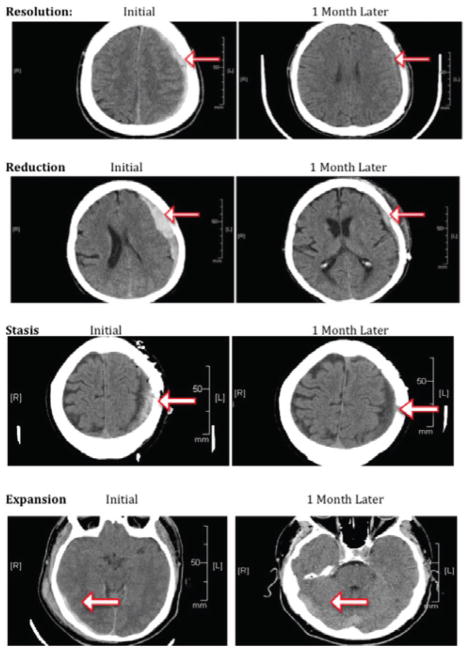
The West Virginia University Institutional Review Board approved the protocol for this study, and the study was performed in accordance with The Code of Ethics of the World Medical Association. We collected retrospective data on 80 patients with acute subdural hematoma that presented to Ruby Memorial Hospital from 2010 to 2014 and were managed by the Department of Neurosurgery. For this type of study, formal consent is not required. Statistical consultation and a power analysis revealed that 30 patients were needed per group to determine statistical significance. Only patients with a CT scan at presentation, confirming acute SDH, as well as follow-up imaging between 3 and 6 weeks, were included for analysis. Data collected included: patient age, sex, presence of anti-coagulant or anti-platelet therapy on admission, Glasgow coma scale score at presentation, and surgical or procedural intervention. Other important variables gathered were presenting symptoms, laterality of convexity subdural hematoma, medical interventions given, midline shift, and clinical values for prothrombin time and international normalized ratio. The cohort was divided into those < 65 years old (N=44) and those >65 years old (N=36). Subdural volume was calculated at presentation and at a 1-month follow-up visit using AB(C*0.5)/2 (Figure 1). A is the width in cm, B is the length in cm, and C is the number of slices. ABC result was multiplied by 0.5 to account for the predetermined slice imaging thickness of 0.5 cm. The entire multiplied product was divided by 2 to account for the crescent volumetric distribution of the subdural hematoma. Volume progression was grouped into four categories: 0–30% of original = resolution, 30–65% of original = reduction, 65–100% of original = stasis, and > 100% = expansion (Figure 2). Volume progression was compared based on age category and separately according to anti-coagulant use. We furthermore excluded patients requiring surgery from the volumetric measurements. 34 conservatively managed patients < 65 years old and 27 conservatively managed patients > 65 years old had scans that were evaluated for volume expansion.
Figure 1.
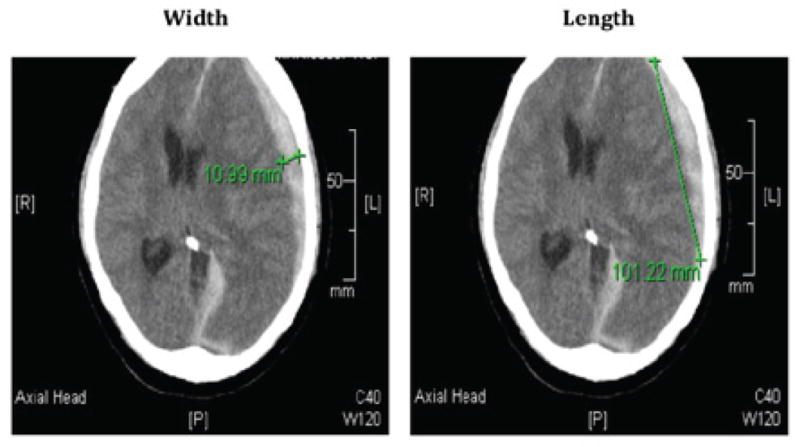
Calculating Volume. Volume is calculated by ABC*5/2 where A is the width in cm, B is the length in cm, and C is the number of slices where the subdural hematoma is present. ABC is multiplied by 5 based on the predetermined set thickness of the CT slices. The total value is divided by 2 to account for the crescent volumetric shape of the subdural hematoma.
Figure 2.
Volume Progression. Representative images showing examples of volume resolution, reduction, stasis, and expansion.
Statistical analysis
All statistical analysis was performed using JMP 9.0. Categorical variables were analyzed by χ2 analysis. Dichotomous variables were analyzed by a Fischer’s exact test with a 2-tailed distribution. A t-test was used to compare clinical test values between groups. Mean ± standard error is reported with a mean difference ± standard error of the mean. P < 0.05 is considered statistically significant for all groups.
RESULTS
Metrics by age
A significant difference in gender was observed between patients > 65 years old and < 65 years old χ2 =14.573 with p < 0.001. Of those patients > 65 years old, 69% were female, whereas only 27% were female in those patients < 65 years old. No significant differences between groups were observed for the five reported symptoms: headache, altered mental status, paresis, seizure, and none. A significant difference in mechanism of injury was observed between patients > 65 years old and < 65 years old χ2 =31.946 with p < 0.001. Minor falls, i.e. from standing, accounted for 80% of the subdural hematomas in those > 65 years old, whereas only 41% of subdural hematomas were from falls in those < 65 years old. Motor vehicle collision (41%) and assault (7%) caused a significant proportion of subdural hematomas in those < 65 years old, but were minimal contributors for subdural hematomas in those > 65 years old (6% and 2% respectively). The laterality of subdural hematoma was not statistically significant between groups (Table 1).
Table 1. Metrics by Age.
| Gender χ2 =14.573 P < 0.001*** |
Male | Female |
|---|---|---|
| Younger (< 65 years old) | N=32 (73%) | N=12 (27%) |
| Older (> 65 years old) | N=11 (31%) | N=25 (69%) |
| Symptoms χ2 =20.859 p=0.1414 |
Altered Mental Status | Headache | None | Paresis | Seizure |
|---|---|---|---|---|---|
| Younger (< 65 years old) | N=13 (30%) | N=18 (41%) | N=2 (4%) | N=4 (9%) | N=7 (16%) |
| Older (> 65 years old) | N=10 (28%) | N=13 (36%) | N=7 (20%) | N=3 (8%) | N=3 (8%) |
| Mechanism χ2 =31.946 p<0.01** |
Fall | Motor Vehicle Collision | Hypertensive | Assault | Unknown |
|---|---|---|---|---|---|
| Younger (< 65 years old) | N=18 (41%) | N=18 (41%) | N=0 (0%) | N=3 (7%) | N=5 (11%) |
| Older (> 65 years old) | N=29 (80%) | N=2 (6%) | N=1 (6%) | N=2 (2%) | N=2 (6%) |
| Convexity SDH Laterality χ2 =19.501 p=0.1919 |
Left | Right | Both |
|---|---|---|---|
| Younger (< 65 years old) | N=19 (43%) | N=18 (41%) | N=7 (16%) |
| Older (> 65 2years old) | N=15 (42%) | N=14 (39%) | N=7 (19%) |
Clinical variables by age
A significant difference in the use of anti-coagulants was seen between those patients > 65 years old and those < 65 years old, χ2 =40.305 with p < 0.001. In those < 65 years old, 87% were not using any anti-coagulant or antiplatelet medication on admission. In those > 65 years old, 39% were on aspirin, 17% were on aspirin + clopidogrel, and 8% were on aspirin + warfarin. A significant difference was also seen between groups for the medical interventions: prothrombin complex concentrate and platelet infusion. 5 patients > 65 years old were given prothrombin complex concentrate, where only 1 patient < 65 years old was given this intervention (Fischer’s Exact test P < 0.01). Patients given prothrombin complex concentrate (PCC) had a −.33 correlation with time to complete subdural resolution (95% CI −.571 to −0.037). This indicates faster recovery when PCC was given. 19 patients > 65 years old were given platelets, where 4 patients < 65 years old were given platelets (Fisher’s Exact test P < 0.001). No significant difference between groups was observed for percentages treated surgically, or type of surgery (Table 2). Patients requiring surgery were not analyzed in the serial volume expansion/regression measurements. Only patients managed conservatively were evaluated for volume expansion/regression.
Table 2. Clinical Variables by Age.
| Anticoagulation χ2 =40.305 P < 0.001*** | Aspirin | Warfarin | Aspirin + Clopidogrel | Aspirin + Warfarin | Aspirin + Clopid. + Warfarin | Other | None |
|---|---|---|---|---|---|---|---|
| Younger (< 65 years old) | N=1 (2%) | N=0 (0%) | N=1 (2%) | N=3 (7%) | N=0 (0%) | N=1 (2%) | N=38 (87%) |
| Older (> 65 years old) | N=14 (39%) | N=1 (3%) | N=6 (17%) | N=3 (8%) | N=2 (6%) | N=0 (0%) | N=10 (27%) |
| Medical Intervention | Fresh Frozen Plasma | Prothrombin Complex Concentrate | Vitamin K | Platelets |
|---|---|---|---|---|
| Younger (< 65 years old) | N=3 Yes N=41 No |
N=1 Yes N=43 No |
N=3 Yes N=41 No |
N=4 Yes N=40 No |
| Older (> 65 years old) | N=4 Yes N=32 No |
N=5 Yes N=31 No |
N=5 Yes N=31 No |
N=19 Yes N=17 No |
| Fischer’s Exact Test:2-tail difference between groups p=0.69 | Fischer’s Exact Test:2-tail difference between groups p<0.01* | Fischer’s Exact Test:2-tail difference between groups p=0.45 | Fischer’s Exact Test:2-tail difference between groups p< 0.001*** |
| Surgery Required Fischer’s Exact Test:2-tail difference between groups p=1 | Yes | No |
|---|---|---|
| Younger (< 65 years old) | N=10 | N=34 |
| Older (> 65 years old) | N=9 | N=27 |
| Type of Surgery χ2 =4.226 p=0.238 | Bur-Hole Drainage | Craniotomy | Twist-drill Craniostomy |
|---|---|---|---|
| Younger (< 65 years old) | N=1 (11%) | N=8 (89%) | N=0 (0%) |
| Older (> 65 years old) | N=0 (0%) | N=7 (78%) | N=2 (22%) |
Clinical tests by age
No significant difference between groups was observed for prothrombin time −0.99 ± 1.01 (p=0.16). Those > 65 years old had a mean of 12.64 ± 0.79 compared to 11.65 ± 0.63 for those < 65 years old. No significant difference between groups was also found for the international normalized ratio −0.15 ± 0.12 (p=0.11). Those > 65 years old had a mean of 1.28 ± 0.09 compared to 1.13 ± 0.07 for those < 65 years old. Presenting Glasgow Coma Scale Scores were significantly different −2.68 ± 0.74 (p < 0.001). Those > 65 years old had a mean of 14.24 ± 0.6 compared to 11.56 0.54 for those < 65 years old. No significant difference in initial subdural hematoma volume was observed across age groups, despite the difference in other clinical variables (Table 3). The mean volume in cm3 ± SE for the younger group is 30.3 ± 15.08 and for the older group 13.99 ± 2.27. The standard deviation for the younger group is 100.02 and for the older group 13.6.
Table 3. Clinical Tests by Age.
| Clinical Tests | Prothrombin Time | International Normalized Ratio | Glascow Coma Scale | Initial Subdural Hematoma Volume |
|---|---|---|---|---|
| Younger (< 65 years old) | Mean: 11.65 SE: 0.63 |
Mean: 1.13 SE: 0.07 |
Mean: 11.56 SE: 0.54 |
Mean: 30.3 SE: 15.08 |
| Older (> 65 years old) | Mean: 12.64 SE: 0.79 |
Mean: 1.28 SE: 0.09 |
Mean: 14.24 SE: 0.60 |
Mean 13.99 SE: 2.27 |
| Mean Difference: −0.99 ± 1.01 t=0.98 p=0.16 |
Mean Difference: −0.15 ± 0.12 t=1.25 p=0.11 |
Mean Difference: −2.68 ± 0.74 t=3.61 p < 0.001* |
Mean Difference: 16.32 ± 15.25 t=1.07 p=0.855 |
|

| ||||
Outcomes
No patients suffered acute mortality following subdural hematoma. No significant difference between groups was observed on volume change at the 1-month follow up (χ2 =1.514, p=0.68). In those > 65 years old, 40.74% had resolution, 14, 81% had reduction, 14.81% had stasis, and 29.63% had expansion. Of those patients that had expansion, 75% progressed to chronic subdural hematoma. In those < 65 years old, 52.94% had resolution, 17.65% had reduction, 8.82% had stasis, and 20.59% had expansion. Of those patients that had expansion, 71% progressed to chronic subdural hematoma. No significant differences between groups were seen in midline shift (mean difference 0.101 ± 0.19, p=0.703). Mean size difference of subdural hematoma at baseline and the 1-month follow up appointment were also not statistically significant between groups. We also compared volume changes according to the use of anti-coagulants. No significant differences were seen between groups. Of patients with no anti-coagulant use, 56.41% had resolution, 12.82% had reduction, 7.69% had stasis, and 23.08% had expansion. For those on aspirin, 23.08% had resolution, 23.08% had reduction, 15.38% had reduction, and 38.46% had expansion. For those on aspirin + clopidogrel, 50% had resolution, 0% had reduction, 50% had stasis, and 0% had expansion. For those on aspirin + warfarin, 33.33% had resolution, 33.33% had reduction, 0% had stasis, and 33.33% had expansion (Table 4). When patients that required surgery were excluded, the χ2 statistic was 0.51.
Table 4. Outcomes.
| Volume Change by Age χ2 =1.514 p=0.68 | Resolution | Reduction | Stasis | Expansion |
|---|---|---|---|---|
| Younger (< 65 years old) | N=18 (52.94%) | N=6 (17.65%) | N=3 (8.82%) | N=7 (20.59%) |
| Older (> 65 years old) | N=11 (40.74%) | N=4 (14.81%) | N=4 (14.81%) | N=8 (29.63%) |
| Volume Change by Anticoagulant χ2 =20.17 p=0.51 | Resolution | Reduction | Stasis | Expansion |
|---|---|---|---|---|
| Aspirin | N=3 (23.08%) | N=3 (23.08%) | N=2 (15.38%) | N=5 (38.46%) |
| Plavix | N=1 (100%) | N=0 (0%) | N=0 (0%) | N=0 (0%) |
| Warfarin | N=0 (0%) | N=0 (0%) | N=1 (100%) | N=0 (0%) |
| Aspirin + Plavix | N=1 (50%) | N=0 (0%) | N=1 (50%) | N=0 (0%) |
| Aspirin + Warfarin | N=1 (33.33%) | N=1 (33.33%) | N=0 (0%) | N=1 (33.33%) |
| Aspirin + Plavix + Warfarin | N=1 (100%) | N=0 (0%) | N=0 (0%) | N=0 (0%) |
| Other | N=0 (0%) | N=1 (100%) | N=0 (0%) | N=0 (0%) |
| None | N=22 (56.41%) | N=5 (12.82%) | N=3 (7.69%) | N=9 (23.08%) |
DISCUSSION
Patients with subdural hematoma and underlying comorbidities often require advanced care from tertiary care centers. Most of these patients can be successfully managed conservatively, but a subset requires surgical intervention [19]. Surgical intervention is often necessary when the subdural hematoma is large, expands over time, or is associated with a significant midline shift [14]. Appropriate clinical evaluation is critical for determining when to manage conservatively vs. surgically in these cases [23]. In our cohort, 61 patients were managed conservatively and 19 required surgery for chronic subdural hematoma. The surgical procedures utilized for hematoma evacuation were Bur-hole drainage, craniotomy, or twist drill craniotomy. Craniotomy and evacuation of acute subdural hematoma was performed early in the admission for acute subdural hematomas. For chronic enlarging hematomas either a twist drill craniostomy (bedside placement of a subdural drain with a 5 mm drill to access the hematoma) or a burr-hole drainage (one or more14 mm opening/openings in the skull to allow washout of the hematoma and placement of a drain) in the operating room, were utilized.
Ahmed and colleagues reported that 19% of patients managed conservatively progress to chronic subdural hematoma [9]. We show that 25% of patients in our study had volume expansion at a 1-month follow-up visit with 18.25% progressing to chronic subdural hematoma. We sought to investigate the natural progression of conservatively managed subdural hematomas in a younger cohort (< 65 years old) and an older cohort (> 65 years old). We hypothesized that older patients would have a greater likelihood for subdural hematoma expansion.
We found that volume changes however were not statistically different between the two groups. Despite having a statistically significant difference in the mechanisms of injury, the initial volume and volume changes were similar between groups. Surgical intervention and type of surgery were also not statistically significant between groups. The younger cohort of patients had a mean Glasgow Coma Scale score of 11.56 compared to 14.24 in the older cohort. This suggests that severity of injury may have been higher in younger cohort patients, especially considering that the older cohort patients had a statistically significant increase in use of anticoagulants and medical interventions. The mechanisms of injury suggest that the younger cohort had more motor vehicle collisions and assault (high energy trauma), where the older cohort had minor falls (low energy trauma).
The use of anti-coagulants may account for why falls were higher in the older cohort. If medical reversal interventions are given early to counteract the oral anticoagulants, patients will often do quite well [10]. Recent evidence suggests that anticoagulant use in those > 65 years old with small subdural hematomas is not a significant determinant of volume expansion [24]. Rapid resolution of subdural hematoma across age groups may be more common than previously thought [12]. Case series have reported rapid resolution of subdural hematomas in young patients [25], but we did not observe rapid resolution in any of our cases. It has been noted that the severity of the traumatic brain injury causing the subdural hematoma is more important than the age of the patient in determining the susceptibility for delayed deterioration [26]. Gerard and colleagues suggest that prothrombin time and the international normalized ratio should be quickly evaluated to determine the necessity of reversal agents and medical management [27]. If anticoagulants are not reversed in subdural hematomas quickly, they can accentuate the severity of injury and bleeding duration [28]. Our data shows that when reversal agents are given rapidly and the coagulation defect corrected, there were no significant adverse effects of anticoagulant therapy for subdural hematoma.
It has been hypothesized that an increase in intracerebral pressure following a tear of a cortical bridging vein accounts for subdural hematoma expansion [29]. The increase in intracerebral pressure elevates cerebral venous pressure thereby causing an increased risk for re-bleeding and higher levels of morbidity [30]. If the re-bleeding is associated with a potentially increased subdural space, then acute subdural hematomas can progress to chronic subdural hematomas [31]. Most subdural hematomas however spontaneously resolve with pre-existing subdural hygromas being more likely to progress to chronic subdural hematoma. Brain atrophy in the elderly may also allow stasis of the acute hematoma and favor conversion to a chronic hematoma. Our data shows that 55.55% of patients > 65 years old and 68.59% of patients < 65 years old had reduction or resolution of the subdural hematoma at 1 month when managed conservatively, while 14.91% of the younger cohort and 22.22% of the older cohort went on to develop chronic subdural hematoma. This supports the finding of older patients with pre-existing brain atrophy and enlarged spaces as being more likely to develop chronic subdural hematomas, but the presence of an acute subdural hematoma does not always imply progression to chronic subdural hematoma.
CONCLUSION
We failed to observe a statistically significant difference between groups for progression of acute subdural hematoma to chronic subdural hematomas based on age. The use of surgical intervention was also not statistically significant between groups. The severity of initial injury measured by GCS is more likely to affect the overall outcome with our data showing young patients are more likely to develop subdural hematoma following high energy trauma and older patients often present after minor falls. Among conservatively managed acute subdural hematomas, the risk of conversion to chronic SDH was 14.91% in the younger cohort and 22.22% in the older cohort. Volume expansion may depend on the mechanism and severity of injury correlated with the age of the patient. Although the younger cohort had worse GCS scores at admission, the resolution of subdural hematomas was comparable to the older cohort with better GCS scores. The use of anti-coagulants should be determined early and treated rapidly with reversal agents to prevent expansion of subdural hematoma. Appropriate management also includes the use of imaging to determine volume expansion, careful clinical exams, and the use of surgical intervention where warranted. Further work is needed to establish a better clinical scale for the evaluation of subdural hematoma severity. The progression to chronic subdural hematoma is not uncommon, ~18–19%, but must be considered, diagnosed and managed appropriately in order to reduce overall morbidity and mortality.
Acknowledgments
SOURCES OF SUPPORT
Brandon Lucke-Wold received funding from the American Foundation of Pharmaceutical Education predoctoral grant, American Medical Association Foundation seed grant, and Neurosurgery Research and Education Foundation Medical Student Summer Research Fellowship
FUNDING
Brandon Lucke-Wold received funding support in the amount of $10000 from an American Foundation of Pharmaceutical Education pre-doctoral grant, $2500 from a Neurosurgery Research and Education Foundation Medical Student Summer Research Fellowship, $500 from a Sigma Xi Grants in Aid of Research, and $2500 from an American Medical Association Foundation Seed Grant.
ABBREVIATIONS
- CT
Computed Tomography
- FFP
Fresh Frozen Plasma
- GCS
Glasgow Coma Scale
- INR
International Normalized Ration
- PCC
Prothrombin Complex Concentrate
- PT
Prothrombin Time
- TBI
Traumatic Brain Injury
Footnotes
CONFLICTS OF INTEREST
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
For this retrospective study, formal informed consent is not required.
References
- 1.Agrawal A, Coronado VG, Bell JM, Baisakhiya N, Kakani A, Galwankar S, et al. Characteristics of patients who died from traumatic brain injury in two rural hospital emergency departments in Maharashtra, India, 2007–2009. Int J Crit Illn Inj Sci. 2014;4:293–297. doi: 10.4103/2229-5151.147521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross AG. Prudent care of head trauma in the elderly: a case report. J Med Case Rep. 2014;8:448. doi: 10.1186/1752-1947-8-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karibe H, Hayashi T, Hirano T, Kameyama M, Nakagawa A, Tominaga T. Surgical management of traumatic acute subdural hematoma in adults: a review. Neurol Med Chir (Tokyo) 2014;54:887–894. doi: 10.2176/nmc.ra.2014-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hitosugi M, Murayama H, Motozawa Y, Ishii K, Ogino M, Koyama K. Biomechanical analysis of acute subdural hematoma resulting from judo. Biomed Res. 2014;35:339–344. doi: 10.2220/biomedres.35.339. [DOI] [PubMed] [Google Scholar]
- 5.Inoue H, Hyodoh H, Watanabe S, Okazaki S, Mizuo K. Acute enlargement of subdural hygroma due to subdural hemorrhage in a victim of child abuse. Leg Med (Tokyo) 2015;17:116–119. doi: 10.1016/j.legalmed.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Satpathy PK, Diggikar PM, Chahal HS, Kakrani AL, Rupnar PB. Subdural haematoma in an adult due to hypernatraemia. J Ass Phys Ind. 2014;62:342–344. [PubMed] [Google Scholar]
- 7.Fujimoto K, Otsuka T, Yoshizato K, Kuratsu J. Predictors of rapid spontaneous resolution of acute subdural hematoma. Clin Neurol Neurosurg. 2014;118:94–97. doi: 10.1016/j.clineuro.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Son S, Yoo CJ, Lee SG, Kim EY, Park CW, Kim WK. Natural course of initially non-operated cases of acute subdural hematoma : the risk factors of hematoma progression. J Korean Neurosurg Soc. 2013;54:211–219. doi: 10.3340/jkns.2013.54.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed E, Aurangzeb A, Khan SA, Maqbool S, Ali A, Zadran KK, et al. Frequency of conservatively managed traumatic acute subdural haematoma changing into chronic subdural haematoma. J Ayub Med Coll Abbottabad. 2012;24:71–74. [PubMed] [Google Scholar]
- 10.Senft C, Schuster T, Forster MT, Seifert V, Gerlach R. Management and outcome of patients with acute traumatic subdural hematomas and pre-injury oral anticoagulation therapy. Neurol Res. 2009;31:1012–1018. doi: 10.1179/174313209X409034. [DOI] [PubMed] [Google Scholar]
- 11.Gaetani P, Revay M, Sciacca S, Pessina F, Aimar E, Levi D, et al. Traumatic brain injury in the elderly: considerations in a series of 103 patients older than 70. J Neurosurg Sci. 2012;56:231–237. [PubMed] [Google Scholar]
- 12.Wen L, Liu WG, Ma L, Zhan RY, Li G, Yang XF. Spontaneous rapid resolution of acute subdural hematoma after head trauma: is it truly rare? Case report and relevant review of the literature. Ir J Med Sci. 2009;178:367–371. doi: 10.1007/s11845-008-0168-5. [DOI] [PubMed] [Google Scholar]
- 13.Cosar M, Eser O, Aslan A, Ela Y. Rapid resolution of acute subdural hematoma and effects on the size of existent subdural hygroma: a case report. Turk Neurosurg. 2007;17:224–227. [PubMed] [Google Scholar]
- 14.Kim BJ, Park KJ, Park DH, Lim DJ, Kwon TH, Chung YG, et al. Risk factors of delayed surgical evacuation for initially nonoperative acute subdural hematomas following mild head injury. Acta Neurochir. 2014;156:1605–1613. doi: 10.1007/s00701-014-2151-4. [DOI] [PubMed] [Google Scholar]
- 15.Sawauchi S, Taya K, Hashimoto T, Ishii T, Otsuka T, Morooka S, Yuhki K, Takao H, Murakami S, Abe T. Progressive brain injury. No shinkei geka Neurological surgery. 2003;31:749–755. [PubMed] [Google Scholar]
- 16.Endo H, Fukawa O, Mashiyama S, Kawase M. Single burr hole surgery for acute spontaneous subdural hematoma in the aged: patient reports of three cases. No shinkei geka. 2004;32:271–276. [PubMed] [Google Scholar]
- 17.Okumura Y, Shimomura T, Park YS. A study of acute subdural hematoma developing into hematoma with capsule formation. No Shinkei Geka. 1998;26:691–698. [PubMed] [Google Scholar]
- 18.Bajsarowicz P, Prakash I, Lamoureux J, Saluja RS, Feyz M, Maleki M, et al. Nonsurgical acute traumatic subdural hematoma: what is the risk? J Neurosurg. 2015;123:1176–1183. doi: 10.3171/2014.10.JNS141728. [DOI] [PubMed] [Google Scholar]
- 19.Duprez T, Grandin C, Malghem J. MRI monitoring of an acute spinal subdural haematoma with spontaneous resolution. Acta neurologica Belg. 1995;95:101–103. [PubMed] [Google Scholar]
- 20.Tomida M, Muraki M, Uemura K, Yamasaki K. Postcontrast magnetic resonance imaging to predict progression of traumatic epidural and subdural hematomas in the acute stage. Neurosurgery. 1998;43:66–70. doi: 10.1097/00006123-199807000-00040. [DOI] [PubMed] [Google Scholar]
- 21.Gebel JM, Sila CA, Sloan MA, Granger CB, Weisenberger JP, Green CL, et al. Comparison of the ABC/2 estimation technique to computer-assisted volumetric analysis of intraparenchymal and subdural hematomas complicating the GUSTO-1 trial. Stroke. 1998;29:1799–1801. doi: 10.1161/01.str.29.9.1799. [DOI] [PubMed] [Google Scholar]
- 22.Towers WS, Kurtom KH. Spontaneous resolution of large acute subdural hematoma and the value of neurological exam in conservative management of high risk patients. Clin Neurol Neurosurg. 2014;118:98–100. doi: 10.1016/j.clineuro.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhary N, Krivosheya D, Small E, Hsia C, Ng W, Leung A. Rapid resolution of acute subdural hematoma in a coagulopathic patient. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2013;40:599–600. doi: 10.1017/s0317167100014748. [DOI] [PubMed] [Google Scholar]
- 24.Wu MC, Liu JX, Luo GC, Zhang ZW, Min J, Yu H, et al. Rapid natural resolution of intracranial hematoma. Chin J Traumatol. 2004;7:96–100. [PubMed] [Google Scholar]
- 25.Carlson AP, Ramirez P, Kennedy G, McLean AR, Murray-Krezan C, Stippler M. Low rate of delayed deterioration requiring surgical treatment in patients transferred to a tertiary care center for mild traumatic brain injury. Neurosurg Focus. 2010;29:3. doi: 10.3171/2010.8.FOCUS10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerard C, Busl KM. Treatment of acute subdural hematoma. Curr Treat Options Neurol. 2014;16:275. doi: 10.1007/s11940-013-0275-0. [DOI] [PubMed] [Google Scholar]
- 27.Cabral KP, Fraser GL, Duprey J, Gibbons BA, Hayes T, Florman JE, et al. Prothrombin complex concentrates to reverse warfarin-induced coagulopathy in patients with intracranial bleeding. Clin Neurol Neurosurg. 2013;115:770–774. doi: 10.1016/j.clineuro.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Miller JD, Nader R. Acute subdural hematoma from bridging vein rupture: a potential mechanism for growth. J Neurosurg. 2014;120:1378–1384. doi: 10.3171/2013.10.JNS13272. [DOI] [PubMed] [Google Scholar]
- 29.Romero JM, Kelly HR, Delgado Almandoz JE, Hernandez-Siman J, Passanese JC, Lev MH, et al. Contrast extravasation on CT angiography predicts hematoma expansion and mortality in acute traumatic subdural hemorrhage. AJNR. 2013;34:1528–1534. doi: 10.3174/ajnr.A3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KS. Natural history of chronic subdural haematoma. Brain Inj. 2004;18:351–358. doi: 10.1080/02699050310001645801. [DOI] [PubMed] [Google Scholar]
- 31.Lee KS, Bae WK, Doh JW, Bae HG, Yun IG. Origin of chronic subdural haematoma and relation to traumatic subdural lesions. Brain Inj. 1998;12:901–910. doi: 10.1080/026990598121972. [DOI] [PubMed] [Google Scholar]