Abstract
Background
A novel series of structurally divergent 1,5-diaryl-3-oxo-1,4-pentadiene analogues 1-10 displayed marked cytotoxic potencies towards a number of human leukemia/lymphoma cells.
Objective
To identify novel selective cytotoxic compounds that induce apoptosis.
Methods
The Differential Nuclear Staining (DNS) screening protocol was utilized to measure the cytotoxicity of all experimental dienones on several cancerous cells. Additionally, the selective cytotoxicity index was calculated by comparing the dienone’s cytotoxicity between leukemia/lymphoma cells vs. non-cancerous cells. Furthermore, to discern whether a selected dienone induced cell death via apoptosis or necrosis on T-lymphocyte leukemia cells, diverse approaches were utilized to detect individual biochemical facets of apoptosis.
Results
The dienones were tested for their anti-neoplastic efficiency on human leukemia/lymphoma-derived cell lines. Special emphasis was applied on dienone 1, on the basis of its sub-micromolar cytotoxicity (CC50=0.43+0.02 μM) and high selective cytotoxicity index (11.1) exerted on T-leukemia cells. In general, dienone 1 showed the most potent cytotoxic properties as compared to other dienones and a related reference cytotoxin curcumin as well as the EF-24 curcumin analogue. Dienone 1 caused cell death by apoptosis in Jurkat cells as evidenced by inducing phosphatidylserine externalization, mitochondrial depolarization and caspase-3/7. These effects were mainly attributed to the induction of apoptotic pathways.
Conclusion
The novel dienone 1 was found to exhibit potent anti-leukemia activity by inducing programmed cell death/apoptosis. Consequently, dionone 1 should be developed further to examine its potential efficacy to combat malignancies in a pre-clinical animal model.
Keywords: Apoptosis, Cancer, Caspase 3, Cytotoxins, Curcumin analogue, Leukemia, Mitochondria
Introduction
During 2016, a total of 171,550 people are projected to be clinically diagnosed with leukemia, lymphoma or myeloma disorders in the United States [1, 2]. Moreover during the same year, new cases of combined leukemia, lymphoma and myeloma were estimated to account for 10.2 percent of the 1,685,210 total new cancer cases [1, 2]. Given the large number of new projected and existing cases, it is vital to continue searching for effective treatments against blood malignancies. Approximately 81,080 and 60,140 new cases of lymphoma and leukemia, respectively, will be diagnosed in the US this year [2]. Non-Hodgkin lymphoma (NLH), which is the most common type of lymphoma, affects Caucasians at a highest rate followed closely by people of Hispanic descent [2, 3]. Hodgkin’s lymphoma is rarer and those affected have a higher overall survival rate that those with NLH [2]. Chronic lymphocytic leukemia (CLL), which is of B-lymphocyte origin, is one of the most common forms of leukemia in the U.S. with approximately 15,000 new cases and over 4,000 deaths per year [2]. Although therapies have been developed for CLL, it is still considered an incurable disease since most patients eventually relapse [4]. There are many genetic and environmental factors that not only result in health disparities but also affect cancer incidence and mortality [reviewed in [5]. Several anti-lymphoma therapies have been developed in recent years that have improved survival, which include combination chemotherapy and the use of immune modulators (monoclonal antibodies to specific receptors), but in many cases resistance to these therapies still occurs [4, 6–14]. In addition to resistance, side effects may curtail the use of effective drug doses; thus there is a continuous need for novel drugs with higher potency and lower side effects. Based on the aforementioned distressing statistics, the identification of novel anti-cancer drugs is of paramount importance. The main goal of our current research is to discover novel anti-cancer drugs that can eventually be used as effective therapeutics.
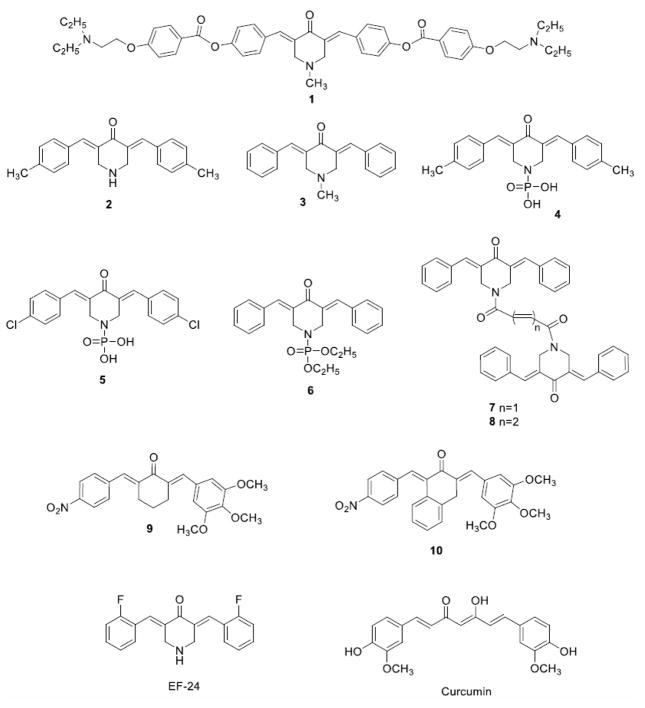
A major interest in our laboratories is the design and development of curcumin related analogues containing the 1,5-diaryl-3-oxo-1,4-pentadienyl pharmacophore [15] as cytotoxic agents. In these compounds, alkylation of cellular constituents can take place sequentially at the olefinic carbon atoms that are adjacent to the aryl rings. This successive attack may lead to tumor-selective toxicity since studies have shown that after an initial chemical insult, some tumors are more vulnerable to a further toxic effect than are non-malignant cells [16, 17]. The 1,5-diaryl-3-oxo-1,4-pentadienyl pharmacophore has been mounted on piperidyl and cycloaliphatic scaffolds [18, 19]. Additional interactions with cellular components at auxiliary binding sites may occur when phosphoryl groups are placed on the nitrogen atom of the piperidyl rings as illustrated by the enones 4–6 [20](Figure 1). In recent years, we have identified and characterized various dienone molecules with potent anti-cancer activities that also induce apoptosis [21–23].
Fig. (1).
Structures of compound 1 to 10, EF-24 and Curcumin.
The objectives of the present investigation are as follows. First, in view of the need for new bioactive agents to combat leukemias and lymphomas, a number of structurally divergent 1,5-diaryl-3-oxo-1,4-pentadienes 1-10 were evaluated against a wide range of hematological malignancies in order to identify a lead compound for further development. Second, their toxicity towards non-transformed cells was investigated with the goal of assessing whether these compounds demonstrate tumor-selective toxicity. Third, an investigation of the modes of action of a lead cytotoxin was implemented.
MATERIALS AND METHODS
Chemical Synthesis and Reagents
The synthesis of 1 was accomplished by the reaction of 1-methyl-4-piperidone with 4-hydroxybenzaldehyde to produce 3,5-(4-hydroxybenzylidene)-4-piperidone which was further condensed with 4-(2-diethylaminoethoxy)benzoyl chloride to produce 3,5-bis{4-[4-(2-diethylaminoethoxy)phenylcarbonyloxy]benzylidene}-1-methyl-4-piperidone (1). The structure of this compound was confirmed by 1H NMR (500 MHz), 13C NMR (125 MHz), mass spectrometry and elemental analysis. Details of the synthesis of 1 (and related compounds) will be submitted to a chemically oriented journal in the near future. The synthesis and or methods for preparation of the other compounds used have been previously published to produce 2 [24], 3 [25], 4-6 [20], 7, 8 [26], 9 [27] and 10 [28]. For the bioassays, the compounds were dissolved in DMSO to prepare a 1 mM stock solution and stored at -20°C. The final solvent concentrations on the experimental cultured cells were 1% (v/v), which had negligible effect on any of the parameters studied and was used as the solvent control. Hoechst 33342 (Hoechst; Invitrogen, Eugene, OR); and propidium iodide (PI; MP Biomedicals, Solon, OH) stock solutions were prepared in PBS, diluted with the cell culture medium, and added to each experimental well at a final concentration of 1 μg/ml for each dye. Annexin V-FITC (Beckman Coulter, Miami, FL), JC-1 dye (MitoProbe JC-1 assay kit; Life Technologies, Thermo Fisher Scientific, Grand Island, NY, USA), and NucView 488 Caspase-3/7 enzyme substrate (Biotium, Inc., Hayward, CA) were diluted according to the respective manufacturers’ instructions.
Cell lines and Culture Conditions
Eleven human leukemia/lymphoma cancer cell lines and two normal non-cancerous cell lines were utilized to test the cytotoxic potential of the curcumin analogues. Each lymphoid cancer cell line selected for this study was a representative hematological malignancy with their unique genetic heterogeneity. Unless otherwise specified, all cell lines used in this study were obtained from the American Type Tissue Collection (ATCC, Manassas, VA). The lymphoid cancer cell lines used were the human T–cell lymphomas/ leukemias, Jurkat, SUP-T1, HUT 102, MOLT-3, and CCRF-CEM, the Burkitt’s lymphomas Raji, Ramos and BJAB, pre-B acute lymphoblastic leukemia Nalm-6 (DSMZ, Braunschweig, Germany), the human YT natural killer (NK)-like cell line (kind gift from Dr. Robert A. Kirken from The University of Texas at El Paso;[29]), and mouse T -cell lymphoma EL-4 cell line. The culture medium for the lymphoid cancer cells was Roswell Park Memorial Institute (RPMI; HyClone). The normal human non-transformed cell lines were the CCD-112CoN colon fibroblast, Hs-27 foreskin fibroblasts and MCF-10A mammary epithelium cell lines. Non-transformed cell lines were selected to test the cytotoxicity of the analogues on tissues from normal origin. CCD-112CoN and Hs-27 cells were grown in Dulbecco's Modified Eagle Medium (DMEM; HyClone, Logan, UT) supplemented with 10% Fetal BS. MCF-10A cells were grown in DMEM/F12 media (HyClone, Logan, UT), 10 μg/ml recombinant human insulin (Sigma, Lenexa, KS), 20 ng/ml of epidermal growth factor (Peprotech, Rocky Hill, NJ), 0.5 μg/ml hydrocortisone (Sigma, Saint Louis, MO), 2.5 mM L-glutamine (Life Technologies, Paisley, Scotland, UK), and 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies, Carlsbad, CA). All culture media were supplemented with 10% heat inactivated serum and with 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (Lonza, Walkersville, MD). The incubation conditions for the cells were 37ºC in humidified 5% carbon dioxide (CO2) atmosphere, in a regular water jacketed incubator. To guarantee high cellular viability, Hs-27 and MCF-10A cells were washed with fresh media, as necessary, to eliminate cell debris and floating dead cells, before seeding them on multi-well plates [30]. Hs-27 and MCF-10A cells were harvested from culture flasks by adding 2 ml of 0.25% trypsin solution (Invitrogen, Carlsbad, CA), diluted in serum free DMEM, and incubated for approximately 15 min at 37°C. Trypsin was neutralized by addition of culture media with 10% serum. Cells were then centrifuged to remove the neutralized trypsin and transferred to a new culture flask and replenished with complete growth media. When necessary, the viability of the lymphoid cancer cells was increased by removing dead cells via the use of a Ficoll-Paque™PLUS density gradient [31]. After centrifugation at 400xg for 20 min at 25°C, live cells at the interface between the Ficoll-Paque and the cell suspension media were collected and washed with fresh warm culture media. These lymphoid cancer cells were then expanded by starting a new culture. Only cultures containing cell viabilities of 95% or higher were used for the cytotoxicity studies. Viability was monitored via flow cytometry after staining the cells with PI.
Live-cell Cytotoxicity Analysis via a Image-based High Throughput Screening Protocol
The Differential Nuclear Staining (DNS) assay that was previously validated for high-throughput screening was used to monitor the cytotoxicity of the compounds [30]. In this process, Hoechst 33342 stains all cells and PI stains dead or dying cells [30]. Hs-27, MCF-10A, and the lymphoid cancer cells were seeded in black flat-bottomed plastic 96-well assay plates (BD Biosciences, Rockville, MD) at densities of 5,000 cells/well for adherent cell lines and 10,000 cells/well for suspension cells in 100 μl of culture media/well. 1 was tested at the final concentration of 1 μM (diluted in 1% v/v DMSO) per well according to the CC50 values previously examined by cytotoxicity assays. The compound was dispensed into the wells via a robotic pipette (epMotion 5070, Eppendorf, New York, NY). As a positive control for cytotoxicity, cells were treated with 300 μM final concentration of H2O2. This was required to achieve constancy in every cytotoxicity assay, since images are segmented based on controls. As solvent control and for normalization purposes, cells were treated with 1% v/v DMSO. Untreated cells were also included as negative controls and as an indicator of cell viability during the incubation period. Cells exposed to the experimental compounds, plus their controls were incubated for a total of 20 h under the conditions described above, and immediately followed by image acquisition. One hour prior to imaging, the mixture of Hoechst 33342 and PI was added. All screening was carried out in triplicate. The BD Pathway 855 Bioimager system and its associated AttoVision v1.6.2 software (BD Biosciences, Rockville, MD) were utilized for image collection and cytotoxic analysis, as previously described [30]. Briefly, after the Hoechst and PI dye mixture addition and incubation, images were captured with selected filter sets of 380/535 nm for Hoechst and 555/645 nm for PI, excitation/emission wavelengths, respectively. Images from each well were acquired using a 20x/ NA 0.75 dry objective. To include an adequate amount of regions of interest (ROI=cell numbers) for statistical purposes, images from nine (3x3 montage) contiguous fields were captured per well. Under these settings the images were subsequently analyzed using the AttoVision software. To define nuclei as individual units or ROIs, pre-processing filters and intensity thresholds were applied for image segmentation. Segmented images were subjected to data classification by the use of the AttoVision software. The percentage of dead cells was calculated from the total number of ROIs per well. Cell nuclei emitting fluorescence signals from both Hoechst and PI (fluorescence co-localization) were considered as dead cells, while cells emitting only the Hoechst signal were counted as live cells. Heat maps were constructed using the MeV Multiexperiment Viewer Software v.4.9.
Generation of Dose-Response Curves and Determination of CC50 Values
The CC50 value was defined as the concentration of compound that causes 50% of cell death as compared to solvent treated cells after 20 h of incubation. The cancerous lymphoid cell lines and non-transformed cell lines were plated into black bottom 96-well plates at the same densities and conditions used in the DNS assay. The CC50 values were obtained using the linear regression equation as previously described [32, 33]. To create dose-response curves and determine the CC50 values, each lead compound was tested at several concentrations. Data was normalized by subtracting from each experimental value the average percentage of dead cells from six wells treated with 1% v/v DMSO.
Selective Cytotoxicity Index
The selective cytotoxicity index (SCI) for 1 was obtained by using an equation as previously described: SCI=CC50 of non-cancerous cells/CC50 of cancer cells [21]. SCI denotes the capacity of an experimental chemical compound or compounds (e.g. plant extracts) to efficiently kill a specific cancer cells with minimal toxicity on the non-cancerous cells [21]. SCI values of a compound which are greater than 1 indicate greater toxicity to neoplasms than non-malignant cells and high SCI values are preferred for any potential candidate anti-cancer drug [21].
Apoptosis Assay via Annexin V-FITC/PI Staining
Apoptosis assays were performed on the cancerous cell lines to test whether or not 1 induced apoptosis or necrosis. Apoptosis detection was determined by flow cytometry (Cytomics FC 500 Series Beckman Coulter) using annexin V-FITC/PI kit (Beckman Coulter, Miami, FL) in combination with PI (essentially as previously described [32]. Untreated cells and cells treated with 1μM curcumin, 1μM EF-24 (Sigma, 300 μM H2O2, and 1% v/v DMSO (all reagents from Sigma-Aldrich, St. Louis, MO, USA) were included as controls and incubated for a total of 24 h. FITC (excitation/emission maxima of 495/519 nm) and PI (excitation/emission maxima of 493/636 nm) emitting green and red signals, respectively, were excited with a 20 mW argon ion laser operation at 488 nm and their ensuing fluorescence signals were captured by using FL1 and FL2 detectors, respectively. Data acquisition and analysis was performed by using CXP software (Beckman Coulter).
Mitochondrial Membrane Potential Assay
The JC-1 dye (MitoProbe JC-1 assay kit) was used to study the involvement of mitochondria during the early stages of apoptosis [23]. Jurkat cells were seeded in 24-well plates at a density of 3x104 cells in 1 mL of media per well. Cells treated with 1 μM of 1, 50 μM carbonyl cyanide 3-chlorophenylhydrazone (CCCP), 1% v/v DMSO, and untreated cells were incubated for 7 h. At the end of the incubation period, cells were transferred to flow cytometry tubes and 1.5μL (200 μM) of JC-1 stock solution were added to each tube. After 30 min incubation at 37ºC, the JC-1 stained cells were analyzed via flow cytometry (Cytomics FC 500 Series Beckman Coulter).
Caspase-3/7 Activity Assays
Jurkat cells were seeded at a density of 100 cells/μL in 2.5 mL of media in 24-well-plates. 1 was tested at a final concentration of 1 μM. As positive controls, 2 μg/ml camptothecin and 10 μM curcumin were used according to their above determined CC50 values [23]. As negative controls, 1% v/v DMSO-treated and untreated cells were included. Intracellular active caspase-3/7 in response to dienone 1 was measured after 20 h of incubation. For live cell detection of caspase-3/7 activity, NucView™ 488 apoptosis assay kit was used containing a membrane-permeable fluorogenic caspase-3 substrate (Biotium, Inc., Hayward, CA). Caspase-3/7 and-8 activity was analyzed by flow cytometry (Cytomics FC 500 Series Beckman Coulter) and data was analyzed with CXP software (Beckman Coulter).
Statistical Analysis
All experiments were performed in triplicate. Averages and standard deviations were calculated in Excel (Microsoft). Outliers, Student’s t-test, and P values were calculated using Graph Pad software. To designate whether comparisons between two sample groups have statistical significance, a P < 0.05 value was deemed significant.
RESULTS
A Novel Curcumin Analogue 1 Shows Potent Toxicity Against Leukemia/Lymphoma Cells
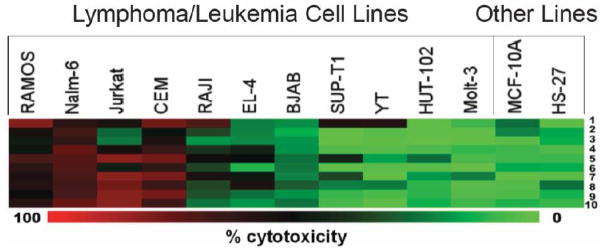
The evaluation of 1-10 against leukemia and lymphoma neoplasms as well as two non-malignant cells are presented in (Fig. 2). Compound 1 shows good potencies towards the malignant cell lines except against EL-4, BJAB, HUT102 and Molt-3 cells and low toxicity to the non-malignant MCF-10A and Hs-27 cell lines indicating that in general the compound demonstrates tumor-specific toxicity. The 4-piperidones 2 and 3 are like 1 in possessing a basic center and have similar cytotoxicity. In general the phosphoramides 4-6 have diminished potency against the neoplasms compared to 1. The dimer 7 has a wide spectrum of antineoplastic properties and is more potent than the analog 8 which has an additional vinylene (−CH=CH−) group. The dienones in which the 1,5-diaryl-3-oxo-1,4-pentadienyl pharmacophore is located on cyclohexyl (9) and 1, 2, 3, 4-tetrahydronaphthyl (10) rings have the weakest potencies among the compounds 1-10. In summary, the 1-10 dienones have promising potencies towards a range of leukemia and lymphoma cells while demonstrating less growth-inhibiting properties towards non-malignant MCF-10A and Hs-27 cells. Overall, the Jurkat (acute T-cell leukemia), Nalm-6 (pre-B acute lymphoblastic leukemia), CEM (acute T-lymphoblastic leukemia) and Ramos (Burkitt’s lymphoma) were found to be the most sensitive cell lines to the analogues as indicated by red and black squares which represent 50–100% cytotoxicity (Fig. 2). The more resistant cells to the experimental compounds were BJAB, Sup-T1, Molt-3, HUT-102 and the YT leukemia/lymphoma and the non-transformed MCF-10A and Hs-27 cell lines (Fig. 2). Among the curcumin analogues tested, 1 was selected since it exhibited potent cytotoxic properties towards human leukemia/lymphoma cell lines, at sub-micromolar concentrations, and also, showed high selective cytotoxicity as compared to non-cancerous cells (Table 1).
Fig. (2).
Analysis of the cytotoxic properties of various 1,5-diaryl-3-oxo-1,4-pentadienes 1–10 against hematological malignancies. The cytotoxic potential of 10 curcumin analogues was studied in 13 cell lines by DNS. The compounds were tested at a final concentration of 1 μM and incubated for 20 h. For ease of presentation, a heat map was used to depict the results that shows a gradient from green (no toxicity) to red (100% toxicity) with black representing ~50% cytotoxicity. The most affected cell lines (red squares) were Ramos, Nalm-6, Jurkat, and CEM. The two non-transformed cell lines tested, MCF-10A and Hs-27, were not significantly affected by the test compound after 20 h of exposure. Note that 1 at the top of the heat map was selected for further characterization as it exhibited cytotoxic activity against the majority of leukemia/lymphoma cell lines but not the non-transformed cell lines. The MeV heat-map generating software was used to construct the heat maps.
Table 1.
Cytotoxic concentration 50% (CC50) and selective cytotoxicity index (SCI) values of 1, EF 24 and curcumin.
| Cell Line | 1 | EF-24a | Curcumina |
|---|---|---|---|
| CEM | 0.59 ± 0.03b (8.1)c | 0.93 ± 0.06 (3.1) | 10.6 ±0.06 (1.4) |
| Jurkat | 0.43 ± 0.02 (11.1) | 0.60 ± 0.04 (4.9) | 10.86 ± 0.04 (1.4) |
| Nalm 6 | 0.86 ± 0.03 (5.6) | 0.69 ± 0.04 (4.3) | 11.0 ± 0.20 (1.4) |
| Ramos | 0.71 ± 0.01 (6.7) | 3.94 ±0.74 (0.7) | 9.25 ± 0.37 (1.7) |
| CCD-112coNd | 4.68 ± 0.83 | 0.50 ± 0.42 | 15.8 ±0.13 |
| Hs-27d | 4.78 ± 0.03 | 2.95 ± 0.25 | 15.27 ± 0.38 |
| MCF-10Ad | 15.6 ±0.14 | 5.00 ±0.40 | 14.06 ±0.66 |
Curcumin and its EF-J4 analogue were used as reference drugs.
Cytotoxic concentration 50% (in μM) is defined as the concentration of drug required to disrupt the integrity of the cellular membrane of 50% of the cell population as compared to DMSO treated cells, after 24 h of incubation, quantified via the DNS assay.
Each number in parenthesis refers to the SCI, which was obtained as follow: SCI=CC50 of non-cancerous cells/CC50 of cancer cells; for this purpose Hs-27 cells were used as the non-cancerous cell line control.
Human non-cancerous cell lines used as controls.
Compound 1 Exhibits Selective Cytotoxicity Index (SCI) on Leukemia/Lymphoma Cells
Jurkat cells were the most sensitive to 1 toxicity, as displayed by its sub-micromolar CC50 value (0.43 μM; Table 1), and for this reason, this cell line was used to calculate the SCI values. After comparing the efficiency of 1 in killing Jurkat cells, with the three non-malignant cells included in this study, its SCI exhibited a consistent significant values greater than 10, even considering that the non-malignant cell lines were isolated from diverse tissue origin; skin, breast and colon, respectively (Table 2). Thus, 1 displayed considerable selectivity in killing Jurkat cells.
Table 2.
Selective cytotoxicity index (SCI) of 1 on human T-leukemia Jurkat cells as compared with three non-cancerous cell lines; Hs-27, MCF-10A and CCD-112CoN.
| Jurkat vs. | SCI |
|---|---|
| Hs-27 | 11.1 |
| MCF-10A | 36.3 |
| CCD-112CoN | 10.9 |
Compound 1 Induces Cytotoxicity via Apoptosis
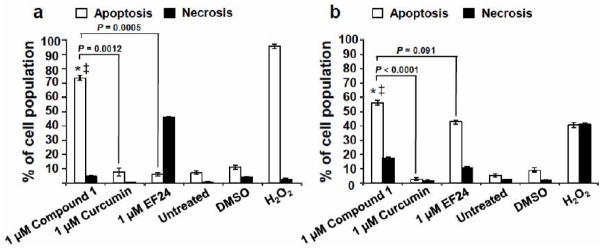
As shown in (Table 1), the lead compound 1 showed potent activity against a variety of leukemia/lymphoma cell lines with sub-micromolar CC50 values that in general were significantly lower than the values obtained with curcumin and EF-24 (Table 1). Due to the strong induction of cell death by 1 at sub-micromolar CC50 values, this compound was tested for induction of apoptosis or necrosis, based on its ability to induce phosphatidylserine (PS) externalization on the Jurkat and Nalm-6 cell lines. These assays revealed that 1 induced strong PS externalization after 24 h of incubation in both cell lines (Fig. 3). In particular, incubation of Jurkat and Nalm-6 cells with 1 μM concentration of the analogue induced ~70% of apoptosis in Jurkat and ~50% in Nalm-6 cells (Fig. 3a & 3b) while EF-24 did not induce PS turnover in Jurkat and only ~40% in Nalm-6. 1 exhibited significantly increased apoptotic activity on both Jurkat and Nalm-6 cells as compared with curcumin, being 10 (P = 0.0012) and 22.3 (P < 0.0001) fold more effective, respectively, after both compounds were tested at the same concentration (1 μM; Figure 3). Similarly, 1 was more efficient than EF-24 in inducing cell death on Jurkat cells (P = 0.0005; Fig. 3). In addition, curcumin did not induce significant PS externalization in either the Jurkat or Nalm-6 cell lines at the time period tested.
Fig. (3).
Compound 1 induced significant phosphatidylserine (PS) externalization on Jurkat and Nalm-6 leukemia cells after 24 h of treatment. PS exposure on the cell surface was monitored after treatment of two distinct lymphoma lines, Jurkat (a) and Nalm-6 (b). Apoptosis and necrosis were examined after incubation of the cell lines and co-staining with annexin V-FITC and PI and analyzed via flow cytometry assay. Each bar represents the average of three replicas with standard deviations. The total percentage of apoptotic cells is displayed as the sum of annexin V-FITC positive cells at early and late stages of apoptosis (white bars). PI-positive cells, but negative for annexin V-FITC, are considered undergoing necrosis (black bars). The following controls were included in these assays: treatment with the reference drugs curcumin and the EF-24 analogue (both at 1 μM); untreated cells as a negative control; DMSO as the solvent control; and H2O2-treated cells as a positive control for cytotoxicity. For both cell lines, a comparison of 1-treated cells with untreated (*) and DMSO (‡) controls, consistently resulted in P-values lower than 0.001. P-values comparing the percentage of apoptosis induced by 1 as compared with curcumin are annotated in the graphs. Data acquisition, processing and analysis were achieved by utilizing CXP software (Beckman Coulter).
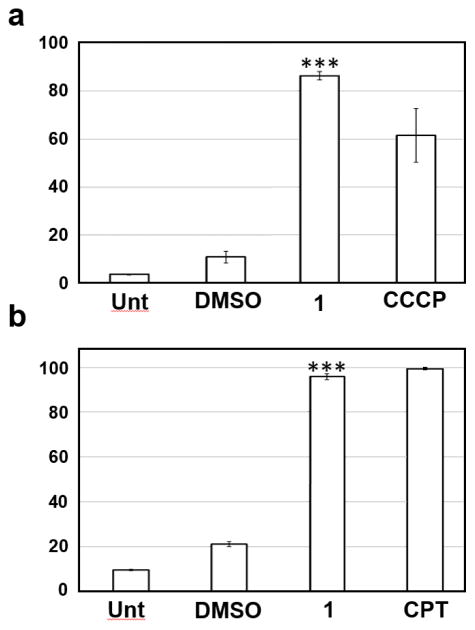
To further corroborate the induction of apoptotic cell death by 1, additional assays were performed by monitoring the induction of pro-apoptotic biochemical signals. As depicted in (Fig. 4a), 1 was found to strongly induce mitochondrial depolarization which is a hallmark feature of apoptosis. Incubation of the cells for 7 h with 1 μM of the 1 analogue was enough to induce ~80% of mitochondrial depolarization in Jurkat cells (Fig. 4a). In addition, the compound was found to activate (cleave) caspase-3/7 (>90%) in treated cells (Fig. 4b). In summary, these findings implicate the apoptosis pathway as the main cell death mechanism induced by 1 through the induction mitochondrial depolarization, and ultimately, caspase-3 activation.
Fig. (4).
Compound 1 induced mitochondrial depolarization and capase-3/7 activation in Jurkat cells. All assays were performed by flow cytometry and approximately 10,000 events (cells) were acquired for each sample. DMSO (1% v/v)-treated and untreated (Unt) cells were included in all three assays as controls. (a) JC-1 polychromatic fluorophore was used as a mitochondrial transmembrane potential indicator, after 7 h of exposure. Cells treated with the proton ionophore, 50 μM carbonyl cyanide m-chlorophenyl hydrazone (CCCP), were used as the positive control for mitochondria depolarization. (b) Intracellular active caspase-3/7 was identified after 20 h of incubation via NucView 488 fluorogenic substrate; the percentage of active caspase 3/7-positive cells, exhibiting green fluorescence signal, is indicated on the y-axis. Camptothecin (CPT; 2 μg/ml) was used as positive control. P values (***) were consistently below 0.001 when 1-treated cells were compared to DMSO-treated cells as evaluated by using Student’s t-test in all three assays. Data acquisition, processing and analysis were performed using CXP software (Beckman Coulter). Each bar represents the average of triplicates and the error bars represent the standard deviation of the mean.
DISCUSSION
In a recent study of similar curcumin-like compounds, we determined that several of these agents exhibited potent toxicity to a diverse array of human tumors [22]. The evaluation of these compounds against a wide range of tumors revealed that leukemias, lymphomas, and colon cancers were the most sensitive to these compounds while breast and prostate cancers and non-cancerous cell lines were more refractory to the treatments [22]. Further analyses revealed that the cytotoxicity of representative dienones on a lymphoma cell line (CEM) was mediated via apoptosis [22]. In this report, a new dienone was developed and characterized that exhibits potent and selective cytotoxic activity towards hematological malignancies.
After exposure to a toxic agent, cells can mainly undergo either apoptosis or necrosis; the two most widely recognized forms of cell death [34]. Additionally, apoptosis can be initiated via the intrinsic and/or extrinsic biochemical pathways [35]. The intrinsic pathway primarily involves mitochondrial depolarization leading to caspase 3 activation [36]. Thus, our results suggest that 1 can induce its cytotoxic effect on Jurkat cells by activating the intrinsic apoptotic pathway, as evidenced by mitochondrial depolarization and caspase-3 activation [37]. The intrinsic and extrinsic apoptotic signaling pathways converge upon caspase-3 activation and once this caspase is activated, cell death is irreversible [38]. Our results show that 1 elicited significant (>90%) caspase-3 activation on Jurkat cells, corroborating that indeed 1 induces cell death via apoptosis.
The efficiency of 1 in killing leukemia/lymphoma cells was in general more efficient than curcumin and the analogue EF-24. Moreover, both 1 and EF-24 had more potent anti-leukemia/lymphoma activity than curcumin. Interesting, we have noticed that 1 possesses selective cytotoxicity towards cells derived from males. The CC50 values obtained with 1 from female (CEM, CCD112 and MCF-10A cells) and male (Jurkat, Ramos, Nalm-6 and Hs-27 cells) donor-derived cells were grouped and an average was calculated as previously detailed [39]. The calculated cytotoxicity value was 6.9 μM for cells derived from female donors, whereas the average CC50 values obtained with male-derived cells was 1.7 μM. These observations are in agreement with previous findings, supporting the existence of gender differences in cell sensitivity to cytotoxic compounds [39–41].
CONCLUSION
Our findings suggest that 1 offers advantages over curcumin as evidenced by its potent cytotoxic activity towards all the human leukemia/lymphoma cell lines tested. The compound exhibited sub-micromolar CC50 values, and displayed high selective cytotoxicity towards malignant cells, as compared with non-cancerous cells. The in vitro cytotoxic concentration of 1 was approximately ten times more effective than curcumin, and on the basis of its SCI values, 1 is an attractive candidate as an anti-leukemia/lymphoma drug. Additionally, findings from four independent experiments for the apoptosis assessment indicate that 1 provoked phosphatidylserine externalization, mitochondrial depolarization, and caspase-3/7 activation; suggesting that it activates the intrinsic apoptotic pathway. Discovery of compounds with novel structures and cytotoxic pathways can be an opportunistic way of targeting cancerous cells that escape from death. Future directions on this research will be to study the molecular mechanisms of induction of pro-apoptotic genes.
Acknowledgments
This project was funded by the National Institute of General Medical Sciences-Support of Competitive Research Excellence Grant 1SC3GM103713-3 to RJA, as well as by Canadian Institutes of Health Research (CIHR)-Regional Partnerships Program Saskatchewan grant to JRD and UD. The authors thank the Saudi Arabia Cultural Association for providing a stipend to Alaa Baryyan and grants to JRD. The authors also thank the staff of the Cytometry, Screening and Imaging, the Biomolecules Analysis, and the Genomic Analysis Core Facilities at UTEP for services and facilities provided. All these Core Facilities are supported by grant 5G12MD007592-22 to the Border Biomedical Research Center (BBRC) from Research Centers in Minority Institutions (RCMI) program funded by the National Institute on Minority Health and Health Disparities (NIMHD). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of UTEP, CIHR, NIMHD or NIH. Special thanks to Drs. Robert A. Kirken and Zsuzsanna S. Nagy for the kind gift of YT cells and also, to Ruben I. Calderon and Gladys Almodovar for excellent technical assistance; all at UTEP. YS-V and YA-M were supported by NIGMS RISE training grant R25 GM069621-12.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article’s content has no conflict of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures. 2016 Data posted at the following website: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, et al. SEER Cancer Statistics Review. National Cancer Institute; Apr, 2016. http://seer.cancer.gov/statfacts/ posted to the SEER website. [Google Scholar]
- 3.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung HC. Deaths: Preliminary Data for 2009. Natl Vital Stat Rep. 2011;59(4) [PubMed] [Google Scholar]
- 4.Masood A, Sher T, Paulus A, Miller KC, Chitta KS, Chanan-Khan A. Targeted treatment for chronic lymphocytic leukemia. Onco Targets Ther. 2011;4:169–83. doi: 10.2147/OTT.S7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace TA, Martin DN, Ambs S. Interactions among genes, tumor biology and the environment in cancer health disparities: examining the evidence on a national and global scale. Carcinogenesis. 2011;32(8):1107–21. doi: 10.1093/carcin/bgr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrondeau J, Gan HK, Razak AR, Paoletti X, Le TC. Development of anti-cancer drugs. Discov Med. 2010;10(53):355–62. [PubMed] [Google Scholar]
- 7.Burington B, Yue P, Shi X, et al. CD40 pathway activation status predicts response to CD40 therapy in diffuse large B cell lymphoma. Sci Transl Med. 2011;3(74):74ra22. doi: 10.1126/scitranslmed.3001620. [DOI] [PubMed] [Google Scholar]
- 8.Diaz T, Navarro A, Ferrer G, et al. Lestaurtinib inhibition of the Jak/STAT signaling pathway in hodgkin lymphoma inhibits proliferation and induces apoptosis. PLoS One. 2011;6(4):e18856. doi: 10.1371/journal.pone.0018856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart S, Goh KC, Novotny-Diermayr V, et al. SB1518, a novel macrocyclic pyrimidine-based JAK2 inhibitor for the treatment of myeloid and lymphoid malignancies. Leukemia. 2011;25:1751–9. doi: 10.1038/leu.2011.148. [DOI] [PubMed] [Google Scholar]
- 10.Hegemann L, Toso SM, Lahijani KI, Webster GF, Uitto J. Direct interaction of antifungal azole-derivatives with calmodulin: a possible mechanism for their therapeutic activity. J Invest Dermatol. 1993;100(3):343–6. doi: 10.1111/1523-1747.ep12470043. [DOI] [PubMed] [Google Scholar]
- 11.Jain S, Diefenbach C, Zain J, O'Connor OA. Emerging role of carfilzomib in treatment of relapsed and refractory lymphoid neoplasms and multiple myeloma. Core Evid. 2011;6:43–57. doi: 10.2147/CE.S13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musto P, Pagano L, Petrucci MT, et al. Primary plasma cell leukemia in the era of new drugs: Has something changed? Crit Rev Oncol Hematol. 2012;82(2):141–9. doi: 10.1016/j.critrevonc.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Naqvi K, Verstovsek S, Kantarjian H, Ravandi F. A potential role of ruxolitinib in leukemia. Expert Opin Investig Drugs. 2011;20(8):1159–66. doi: 10.1517/13543784.2011.589383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi W, Cooke LS, Liu X, et al. Aurora inhibitor MLN8237 in combination with docetaxel enhances apoptosis and anti-tumor activity in mantle cell lymphoma. Biochem Pharmacol. 2011;81(7):881–90. doi: 10.1016/j.bcp.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das U, Sharma RK, Dimmock JR. 1,5-diaryl-3-oxo-1,4-pentadienes: a case for antineoplastics with multiple targets. Curr Med Chem. 2009;16(16):2001–20. doi: 10.2174/092986709788682218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Waxman DJ. Role of cellular glutathione and glutathione S-transferase in the expression of alkylating agent cytotoxicity in human breast cancer cells. Biochem Pharmacol. 1994;47(6):1079–87. doi: 10.1016/0006-2952(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsui K, Komuro C, Ono K, Nishidai T, Shibamoto Y, Takahashi M, Abe M. Chemosensitization by buthionine sulfoximine in vivo. Int J Radiat Oncol Biol Phys. 1986;12(7):1183–6. doi: 10.1016/0360-3016(86)90254-3. [DOI] [PubMed] [Google Scholar]
- 18.Das U, Pati HN, Sakagami H, et al. 3,5-Bis(benzylidene)-1-[3-(2-hydroxyethylthio)propanoyl]piperidin-4-ones: a novel cluster of potent tumor-selective cytotoxins. J Med Chem. 2011;54(9):3445–9. doi: 10.1021/jm101595p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das U, Doroudi A, Das S, Bandy B, Balzarini J, De Clercq E, Dimmock JR. E,E-2-Benzylidene-6-(nitrobenzylidene)cyclohexanones: syntheses, cytotoxicity and an examination of some of their electronic, steric, and hydrophobic properties. Bioorg Med Chem. 2008;16(11):6261–8. doi: 10.1016/j.bmc.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das S, Das U, Selvakumar P, et al. 3,5-Bis(benzylidene)-4-oxo-1-phosphonopiperidines and related diethyl esters: Potent cytotoxins with multi-drug-resistance reverting properties. ChemMedChem. 2009;4(11):1831–40. doi: 10.1002/cmdc.200900288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robles-Escajeda E, Das U, Ortega NM, et al. A novel curcumin-like dienone induces apoptosis in triple-negative breast cancer cells. Cell Oncol (Dordrecht) 2016;39(3):265–77. doi: 10.1007/s13402-016-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santiago-Vazquez Y, Das S, Das U, et al. Novel 3,5-bis(arylidene)-4-oxo-1-piperidinyl dimers: structure-activity relationships and potent antileukemic and antilymphoma cytotoxicity. Eur J Med Chem. 2014;77:315–22. doi: 10.1016/j.ejmech.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunes LM, Hossain M, Varela-Ramirez A, Das U, Ayala-Marin YM, Dimmock JR, Aguilera RJ. A novel class of piperidones exhibit potent, selective and pro-apoptotic anti-leukemia properties. Oncol Let. 2016;11(6):3842–8. doi: 10.3892/ol.2016.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das U, Alcorn J, Shrivastav A, Sharma RK, De Clercq E, Balzarini J, Dimmock JR. Design, synthesis and cytotoxic properties of novel 1-[4-(2-alkylaminoethoxy)phenylcarbonyl]-3,5-bis(arylidene)-4-piperidones and related compounds. Eur J Med Chem. 2007;42(1):71–80. doi: 10.1016/j.ejmech.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Pati HN, Das U, Das S, et al. The cytotoxic properties and preferential toxicity to tumour cells displayed by some 2,4-bis(benzylidene)-8-methyl-8-azabicyclo[3. 2. 1] octan-3-ones and 3,5-bis(benzylidene)-1-methyl-4-piperidones. Eur J Med Chem. 2009;44(1):54–62. doi: 10.1016/j.ejmech.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das S, Das U, Varela-Ramirez A, et al. Bis[3,5-bis(benzylidene)-4-oxo-1-piperidinyl]amides: a novel class of potent cytotoxins. Chem Med Chem. 2011;6(10):1892–9. doi: 10.1002/cmdc.201100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das U, Gul HI, Alcorn J, et al. Cytotoxic 5-aryl-1-(4-nitrophenyl)-3-oxo-1,4-pentadienes mounted on alicyclic scaffolds. Eur J Med Chem. 2006;41(5):577–85. doi: 10.1016/j.ejmech.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Dimmock JR, Padmanilyam MP, Zello GA, et al. Cytotoxic 1,3-diarylidene-2-tetralones and related compounds. Eur J Med Chem. 2002;37(10):813–24. doi: 10.1016/s0223-5234(02)01402-2. [DOI] [PubMed] [Google Scholar]
- 29.Nagy ZS, Rui H, Stepkowski SM, Karras J, Kirken RA. A preferential role for STAT5, not constitutively active STAT3, in promoting survival of a human lymphoid tumor. J Immunol. 2006;177(8):5032–40. doi: 10.4049/jimmunol.177.8.5032. [DOI] [PubMed] [Google Scholar]
- 30.Lema C, Varela-Ramirez A, Aguilera RJ. Differential nuclear staining assay for high-throughput screening to identify cytotoxic compounds. Curr Cell Biochem. 2011;1(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- 31.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 32.Elie BT, Levine C, Ubarretxena-Belandia I, Varela-Ramirez A, Aguilera RJ, Ovalle R, Contel M. Water Soluble Phosphane-Gold(I) Complexes. Applications as Recyclable Catalysts in a Three-component Coupling Reaction and as Antimicrobial and Anticancer Agents. Eur J Inorg Chem. 2009;2009(23):3421–30. doi: 10.1002/ejic.200900279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varela-Ramirez A, Costanzo M, Carrasco YP, Pannell KH, Aguilera RJ. Cytotoxic effects of two organotin compounds and their mode of inflicting cell death on four mammalian cancer cells. Cell Biol Toxicol. 2011;27(3):159–68. doi: 10.1007/s10565-010-9178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solary E, Droin N, Bettaieb A, Corcos L, Dimanche-Boitrel MT, Garrido C. Positive and negative regulation of apoptotic pathways by cytotoxic agents in hematological malignancies. Leukemia. 2000;14(10):1833–49. doi: 10.1038/sj.leu.2401902. [DOI] [PubMed] [Google Scholar]
- 35.Schlegel J, Peters I, Orrenius S, Miller DK, Thornberry NA, Yamin TT, Nicholson DW. CPP32/apopain is a key interleukin 1 beta converting enzyme-like protease involved in Fas-mediated apoptosis. J Biol Chem. 1996;271(4):1841–4. doi: 10.1074/jbc.271.4.1841. [DOI] [PubMed] [Google Scholar]
- 36.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798–811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 37.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6(2):99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 38.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nunes LM, Robles-Escajeda E, Santiago-Vazquez Y, et al. The gender of cell lines matters when screening for novel anti-cancer drugs. The AAPS J. 2014;16(4):872–4. doi: 10.1208/s12248-014-9617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollitzer E. Biology: Cell sex matters. Nature. 2013;500(7460):23–4. doi: 10.1038/500023a. [DOI] [PubMed] [Google Scholar]
- 41.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–3. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]