Abstract
Purpose
Primary open angle glaucoma–associated mutations in myocilin (MYOC) cause protein “nonsecretion,” rendering secreted MYOC difficult to detect using conventional techniques. This study focused on developing and using an assay that can quickly and easily detect mutant MYOC secretion.
Methods
We fused Gaussia luciferase (eGLuc2) to MYOC variants and expressed the constructs in HEK-293T and NTM-5 cells. Secreted and intracellular levels of MYOC eGLuc2 variants were evaluated by Western blotting and compared to untagged and FLAG-tagged MYOC constructs. Secreted and soluble intracellular MYOC eGLuc2 were measured by a GLuc assay. The secretion of nine additional MYOC mutants was assayed in conditioned media from transfected cells to test the applicability of the assay for monitoring other MYOC variants.
Results
Myocilin eGLuc2 behaved similarly to untagged and FLAG-tagged MYOC with respect to secretion, soluble intracellular levels, and in response to drug treatment. The GLuc assay could sensitively detect Y437H MYOC secretion 30 minutes after media change. Gaussia luciferase fused variants followed anticipated trends; nonpathogenic variants (D208E, G244V) were secreted at wild-type (WT) levels, whereas predicted disease-causing variants (C245Y, G246R, E300K, Y437H, I477N) demonstrated substantial secretion defects. Secretion defects caused by the C245Y, G246R, and Y437H mutations were partially rescued by permissive growth temperature. Interestingly, however, this increase in secretion was independent of newly synthesized protein.
Conclusions
Fusion of eGLuc2 to MYOC does not significantly change the behavior of MYOC. This newly developed MYOC reporter system can be used to study engineered MYOC variants and potentially to identify modulators of MYOC secretion and function.
Keywords: Gaussia luciferase, protein folding, permissive growth temperature, nonsecretion, myocilin
Glaucoma is a chronic blinding disease characterized by gradual, irreversible loss of vision from retinal ganglion cell (RGC) death. This form of optic neuropathy currently is the second leading cause of bilateral blindness worldwide, and is projected to affect approximately 80 million people worldwide by 2020.1 The majority of glaucoma cases are comprised of primary open angle glaucoma (POAG), a condition associated with ocular hypertension.1,2 The inherited nature of this glaucoma subtype was established with the identification of a number of genes linked to monogenic POAG (reviewed previously3). Myocilin (MYOC), also known as the trabecular meshwork (TM)–inducible glucocorticoid response (TIGR) gene, was the first of these genes to be linked to inherited POAG.4 Mutations in MYOC are thought to be responsible for 3% to 4% of the total POAG cases.5,6 The MYOC gene encodes for a 57 kDa, secreted glycoprotein7 of unknown function, which is expressed in numerous tissues, including the brain, skeletal muscle, heart, and the eye, with the highest levels occurring within the TM.8–11 Over 100 glaucoma-causing MYOC mutations lead to an autosomal-dominant, gain-of-toxic-function, inherited form of POAG.12 Heterozygous missense mutations in MYOC, primarily located within the olfactomedin (OLF) domain in the C-terminal portion of the protein (Fig. 1A), account for the greatest number of genetically defined autosomal dominant glaucoma cases (reviewed previously13). Studies have illustrated that single point mutations in MYOC are adequate to compromise folding of MYOC and cause a substantial defect in the protein's secretion efficiency (also referred to as “MYOC nonsecretion”) – typically leading to the production of insoluble intracellular protein aggregates,9,14–17 and potentially, amyloid.18 While the mechanism by which MYOC causes POAG still is under contention, one proposed mechanism of POAG pathogenesis involves the formation of MYOC aggregates in the endoplasmic reticulum (ER) of TM cells.14,19,20 This accumulation may cause ER stress, activation of the unfolded protein response (UPR) and trigger TM cell death.21 In turn, this stress ultimately may cause dysfunctional aqueous humor outflow, increased intraocular pressure, RGC death and optic nerve damage (reviewed previously22).
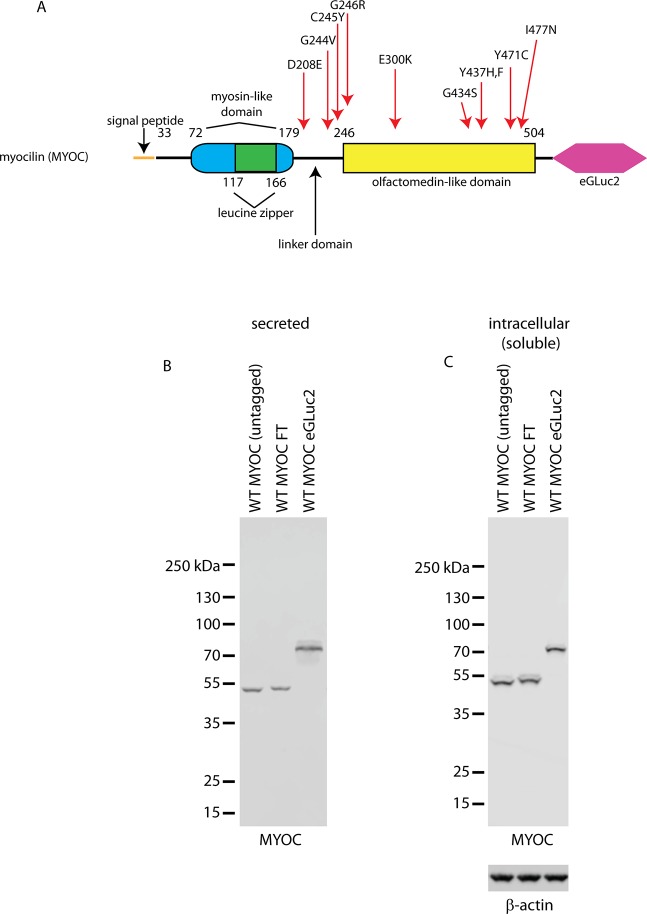
Figure 1.
Schematic of the MYOC eGLuc2 reporter fusion construct. (A) Myocilin is composed of 504 amino acids with a coiled coil myosin (blue/green oval) and OLF-like domains (yellow rectangle) located at the amino and carboxy terminus, respectively. eGLuc2 (pink hexagon) was appended to the C-terminus of MYOC to enable luminescence-based detection. The predicted molecular weight of the construct is 76.5 kDa. Mutants of MYOC used in this study are indicated and their position within MYOC is designated with a red arrow. The vast majority of known MYOC missense mutations occur within the OLF domain. (B, C) HEK-293T cells were transfected with the indicated constructs, and 48 hours after transfection, the conditioned media (B) or soluble cell lysates (C) were analyzed by Western blotting.
Defects in mutant MYOC secretion have been partially rescued by either growth temperature reduction9,17,21 or administration of chemical chaperones such as phenyl butyric acid (PBA).23,24 These treatments have been shown to correlate with a reduction in MYOC-mediated TM cell death21 and a reduction in glaucoma-associated phenotypes in mice,23,25 respectively. Thus, the extent of MYOC secretion appears to be inversely correlated with disease severity and follows a strong genotype–phenotype correlation.12 Nonetheless, the primary function of MYOC, and how alterations in MYOC ultimately cause POAG remain elusive (reviewed previously13,26). Based on these collective observations, being able to sensitively and quantitatively identify cellular conditions or small molecule compounds that can alter mutant MYOC misfolding and restore secretion, albeit partially, could serve as much-needed treatments for MYOC-associated glaucoma.
A number of studies have relied on a dot blot or SDS-PAGE/Western blotting approach to detect mutant MYOC secretion.9,17,21 While SDS-PAGE/Western blotting is arguably the gold standard for detection of MYOC since it provides abundance and molecular weight information, it is time-consuming, costly, and in many instances does not detect small amounts of protein. In previous experiments, we have used the small (19.8 kDa), highly sensitive, and naturally secreted Gaussia luciferase (GLuc) to follow the secreted and intracellular levels of fibulin-3 and fibulin-5.27–30 One major advantage of using GLuc as a reporter protein is that it yields an extremely bright signal, which makes it easy to measure small amounts of the protein.31–33 Since there are a number of biochemical similarities between the fibulin proteins and MYOC (e.g., molecular weight, disulfide formation, and N-linked glycosylation), we reasoned that GLuc could also be used to quantitatively monitor the secretion and intracellular levels of wild-type (WT) and mutant MYOC.
Thus, the focus of this study was to develop, characterize, and use the GLuc assay as a rapid, sensitive, and inexpensive method to quantify secreted levels of WT and mutant MYOC based on GLuc luminescence readouts in human embryonic kidney (HEK-293T) and normal TM (NTM-5) cells. The development of such a system could serve as an effective platform to study MYOC in more depth and for identifying new drugs to potentially treat MYOC-associated POAG.
Materials and Methods
Plasmid Generation
Untagged and C-terminal FLAG-tagged (FT) WT and Y437H MYOC pcDNA3 plasmids were kind gifts of Charles Searsby and Val Sheffield, University of Iowa. The pcDNA3 WT MYOC FT construct was used as a template to generate the MYOC enhanced Gaussia luciferase version 2 (eGLuc2) fusion construct. The eGLuc2 fusion protein containing the M43I and M110I mutations was described previously.28,30,34,35 Briefly, WT MYOC FT was amplified by PCR using primers that eliminated the FLAG tag and stop codon and generated a 5′ DraI and 3′ BstBI restriction site. This PCR product then was inserted into a pENTR1A (Life Technologies, Carlsbad, CA, USA) eGLuc2 plasmid to generate the WT MYOC eGLuc2 fusion construct. The resulting construct encodes for amino acids 1-504 of MYOC followed by a 16 amino acid linker (FEGSAGSAAGSGEFEA) and eGLuc2 (without a signal sequence) with a predicted molecular weight of 76.5 kDa. The fusion construct then was shuttled into the pTRex-DEST30 vector (Life Technologies) by an LR Clonase II (Life Technologies) reaction. All subsequent MYOC mutants were generated in the pTRex vector using the Q5 mutagenesis kit (New England Biolabs, Ipswich, MA, USA). All constructs were constitutively expressed due to the absence of the tet-repressor in the cell lines used (see below). Constructs were verified by sequencing.
Cell Culture
Human embryonic kidney (HEK-293T; Life Technologies) and normal TM (NTM-5; kind gift from Abbot Clark, University of North Texas Health Science Center) cells were cultured in low-glucose (1 g/L) Dulbecco's modified Eagle's medium (DMEM; Cellgro, Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS; Omega Scientific, Tarzana, CA, USA) and 1% penicillin/streptomycin/L-glutamine (Cellgro). All cells were incubated at typical growth conditions (37°C at 5% CO2) unless otherwise indicated and passaged routinely to propagate cells and maintain appropriate confluency.
Transfection
Myocilin eGLuc2 variants (WT, D208E, G244V, C245Y, G246R, E300K, G434S, Y437H, Y437F, Y471C, I477N) were introduced into HEK-293T or NTM-5 cells by transient transfection using X-tremeGENE HP (Roche, Nutley, NJ, USA). Constructs of pEGFP-N1 (Clontech, Mountain View, CA, USA) or pTRex eGFP were used in separate wells to estimate transfection efficiency for each experiment. HEK-293T and NTM-5 cells were plated at a density of either 50,000 to 55,000 cells/well of a 24-well plate or 100,000 to 110,000 cells/well of a 12-well plate and allowed to attach overnight. Cells in 24-well plates then were transfected overnight with 500 ng/well DNA using either 0.5 μL (NTM-5) or 1 μL (HEK-293T) of X-tremeGENE HP. Cells in 12-well plates were transfected with twice the amount of DNA/transfection reagent as in a 24-well plate. Green fluorescent protein (eGFP) imaging determined transfection efficiencies of approximately 70% to 80% for HEK-293T cells and approximately 10% to 20% for NTM-5 cells. Samples were replated or analyzed 48 to 96 hours after transfection by the GLuc assay to measure secretion.
Preparation of Soluble and Insoluble MYOC
Conditioned media was removed from the tissue culture plate wells and the transfected cells were lysed in PBS (BioRad, Hercules, CA, USA) supplemented with 0.1% Triton X-100 (Fisher, Waltham, MA, USA) and Halt protease inhibitors (Pierce Thermo Fisher, Rockford, IL, USA) with rocking for 30 minutes at 4°C. Insoluble MYOC and cell debris was pelleted at 21,000g at 4°C. The soluble and insoluble portions were saved for subsequent analysis. The soluble portion of the cell lysate was used for Western blotting and the GLuc assay,34 whereas the insoluble portion was processed further to extract insoluble MYOC.
After quantifying the total soluble protein content by a bicinchoninic acid (BCA) assay (Pierce Thermo Fisher), the insoluble pellet was washed in ice-cold Hanks buffered salt solution (HBSS, Sigma-Aldrich Corp., St. Louis, MO, USA) and resuspended in an appropriate amount of ×1 reducing Laemmli SDS PAGE buffer containing 8 M urea (based on the soluble protein content). Samples then were sonicated, followed by denaturation at 100°C for 10 minutes.
GLuc Assay to Measure MYOC Secretion and Soluble Levels
Secretion of MYOC eGLuc2 was monitored by taking 20 to 50 μL aliquots of conditioned media and placing them in a 96-well polystyrene flat-bottomed black assay microplate (Greiner Bio-One, Monroe, NC, USA). Fifty nL of GAR2/GAR2B substrate diluted in 10 μL of GAR2/GAR2B Gaussia luciferase assay buffer (Targeting Systems, El Cajon, CA, USA) then was added and the luminescence was measured immediately with a BioTek Synergy 2 plate reader (BioTek, Winooski, VT, USA) using a 100-ms integration time. Measurement of soluble MYOC eGLuc2 levels was achieved by lysing the cells as described above and using equal volumes (40 μL) of soluble protein.
Temperature-Sensitive MYOC Secretion
HEK-293T and NTM-5 cells were transfected for 24 hours, replated at equal densities, and allowed to attach at 37°C on poly-D-lysine (PDL)-coated (MP Biomedicals, Santa Ana, CA, USA) tissue culture dishes. Cell media was replaced with low-glucose DMEM containing 2% FBS 24 hours after replating, and cells then were grown for up to 48 hours at either 37°C or 30°C. Secretion levels were assessed 3 to 48 hours after temperature shift using the GLuc assay.
Western Blotting
For all Western blot-related experiments on secreted MYOC, 2% FBS containing media was used to minimize gel warping and epitope masking due to excessive FBS/BSA. Conditioned media (20 μL) was denatured in either reducing or nonreducing Laemmli buffer at 95°C for 5 minutes and loaded onto a Tris-Gly SDS-PAGE gel. Proteins were separated and transferred onto a nitrocellulose membrane using a Pierce G2 Fast Blotter (Thermo Scientific). Membranes then were blocked in Odyssey blocking buffer (LI-COR, Lincoln, NE, USA), and probed with either a goat anti-MYOC antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), a rabbit anti-GLuc antibody (1:2000; NEB), a mouse anti-FLAG M2 antibody (1:2000, Sigma-Aldrich Corp.), a rabbit anti-GRP94 (1:1500, GeneTex, Irvine, CA, USA), or a mouse anti-β-actin (1:10,000, Sigma-Aldrich Corp.) primary antibody followed by an appropriate near-infrared secondary antibody (1:10,000; LI-COR). A LI-COR Odyssey Fc infrared imager (LI-COR) was used to image the blots, and the Image Quant software was used to quantify protein bands.
Controlling for Differences in Analyzed Protein Content
While all intracellular Western blots were normalized according to a BCA assay, and protein loading was verified by Ponceau S, and eventually the house-keeping protein, β-actin, demonstrating similar levels of total secreted protein in each analysis is a more difficult task. To ensure that similar amounts of secreted protein were used for Western blots and GLuc assays, we plated the same number of cells in all transfected wells, monitored viability in particular experiments to ensure similar ultimate cell numbers before the assays, performed the experiments up to 8 times, and periodically switched the orientation of the transfected wells so that the trends we observed were not due simply to higher amounts of evaporation in wells along the edge of the plate. Furthermore, previously, we have performed cotransfection experiments with a secreted control protein, humanized GLuc (hGLuc), in an identical manner to the transfection experiments described herein, and observed no significant differences in secreted levels of the control protein across different transfected samples.36
Pathogenic Versus NonPathogenic Prediction of MYOC Mutations
We based the designation of polymorphic versus pathogenic on criteria established previously (available in the public domain at www.myocilin.com12). This designation takes into account the type of mutation (i.e., frame shift versus nonsense), sequence frequency in control populations, location of the mutation within the MYOC domain structure, evidence of partial segregation within a family, and the results of any solubility studies.
Statistical Analysis
To determine statistical significance, samples were compared using either a 1-sample t-test or 2-sample t-test, where appropriate. Significance was set at *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
Fusion of a Naturally Secreted Luciferase to MYOC Does Not Substantially Alter Its Secretion Profile or Soluble Intracellular Levels
Initially, to assess for potential differences in how eGLuc2-tagged MYOC is secreted and handled within the cell, we compared the secretion and intracellular profile of untagged WT MYOC and WT MYOC FLAG (FT, used extensively in past experiments2,37,38) to WT MYOC eGLuc2. We found that indeed, all three variants are readily secreted from transfected cells and detectable in the media 48 hours after initial transfection (Fig. 1B), and that all three variants have similar soluble, intracellular steady-state levels (Fig. 1C). A more detailed assay validation analysis in which we compared and contrasted the behavior of MYOC FT and MYOC eGLuc2 variants under different chemical and biochemical conditions can be found in the Supplementary Material (Supplementary Figs. S1–S4).
Y437H MYOC eGLuc2 Behaves Similarly to Y437H MYOC FT
We further characterized the use of the GLuc reporter assay for monitoring the secretion and intracellular levels of POAG-associated mutant MYOC variants. Initially, we chose the Y437H mutation due to its prevalence (7.2% of all MYOC disease-causing variants, www.myocilin.com) and prior characterization in cell culture and mouse models.9,14,23–25 At 24 hours after media change, a minimal amount of secreted Y437H was detectable in eGLuc2 and FT-tagged conditioned media under reducing conditions (Fig. 2A), but was not observable under nonreducing conditions (Fig. 2A), hinting at the presence of disulfide-bonded aggregates incapable of penetrating the SDS-PAGE gel. On the contrary, WT MYOC was observable under reducing and nonreducing conditions (Fig. 2A). Furthermore, under nonreducing conditions, WT MYOC eGLuc2 displayed near identical apparent disulfide bonding patterns as MYOC FT, consistent with previous findings,39,40 albeit shifted to a slightly higher molecular weight due to the addition of eGLuc2 (Fig. 2A).
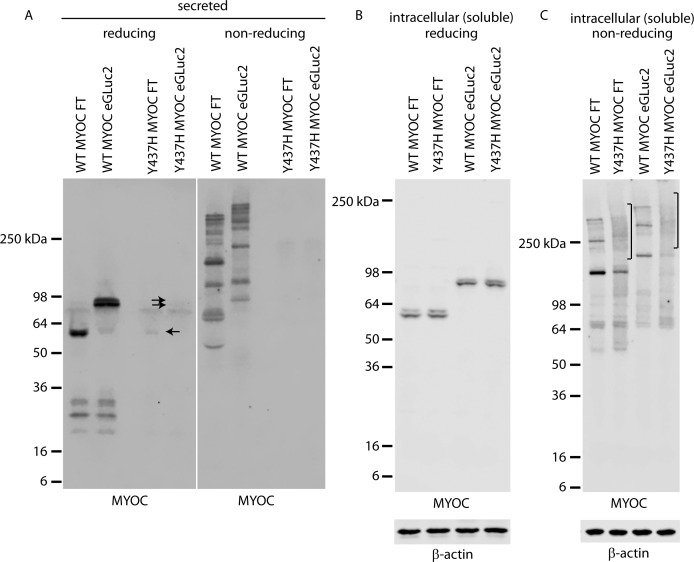
Figure 2.
Comparison of WT and Y437H MYOC FT and eGLuc2 fusion variants. (A–C) HEK-293T cells were transfected for 48 hours followed by a media change (2% FBS media) and incubated for an additional 24 hours before Western blotting the conditioned media (A) or soluble cell lysates (B, C). Single arrow indicates secreted Y437H MYOC FT, whereas the double arrow indicates Y437H MYOC eGLuc2, both of which are difficult to observe in this experiment. Conditioned media and soluble cell lysates were run under reducing and nonreducing conditions to compare disulfide bonding ability of the analyzed variants. Mixed disulfide intermediates are denoted by a bracket. Representative images of three independent experiments.
Triton-X–soluble intracellular levels were similar for WT and Y437H tagged with either FLAG or eGLuc2 (Fig. 2B). Running the same lysates under nonreducing conditions demonstrated that Y437H MYOC has an increased likelihood of forming what appear to be mixed disulfide intermediates as noted by protein smears (Fig. 2C). We did note, however, that under nonreducing conditions, WT and Y437H MYOC eGLuc2 variants showed less soluble immunoreactivity than FLAG-tagged MYOC variants, potentially indicating that MYOC eGLuc2 fusions have a higher likelihood of forming disulfide-linked aggregates which are incapable of penetrating the SDS-PAGE gel (Fig. 2C).
Triton-X–insoluble levels of MYOC have been shown to be an indicative characteristic of a pathogenic, glaucoma-causing mutation.16 Therefore, we also monitored the formation of insoluble MYOC. Consistent with prior observations, the Y437H MYOC variants had a higher likelihood of being insoluble (Supplementary Figs. S3A, S3B). However, we did note that WT MYOC eGLuc2 displayed higher intracellular insoluble aggregates than WT MYOC FT (Supplementary Figs. S3A, S3B). While the reasons behind this phenomenon currently are unclear, the amount of insoluble, aggregated MYOC does not correspond to substantial increased UPR activation as evidenced by GRP94 levels or CHOP induction (Supplementary Figs. S3C–S3E). While a trend indicates that Y437H MYOC FT and Y437H MYOC eGLuc2 likely cause higher levels of ER stress than their WT MYOC counterparts, these levels did not reach statistical significance.
The GLuc Assay Can Sensitively Monitor Y437H MYOC Secretion Within 30 Minutes of Media Change
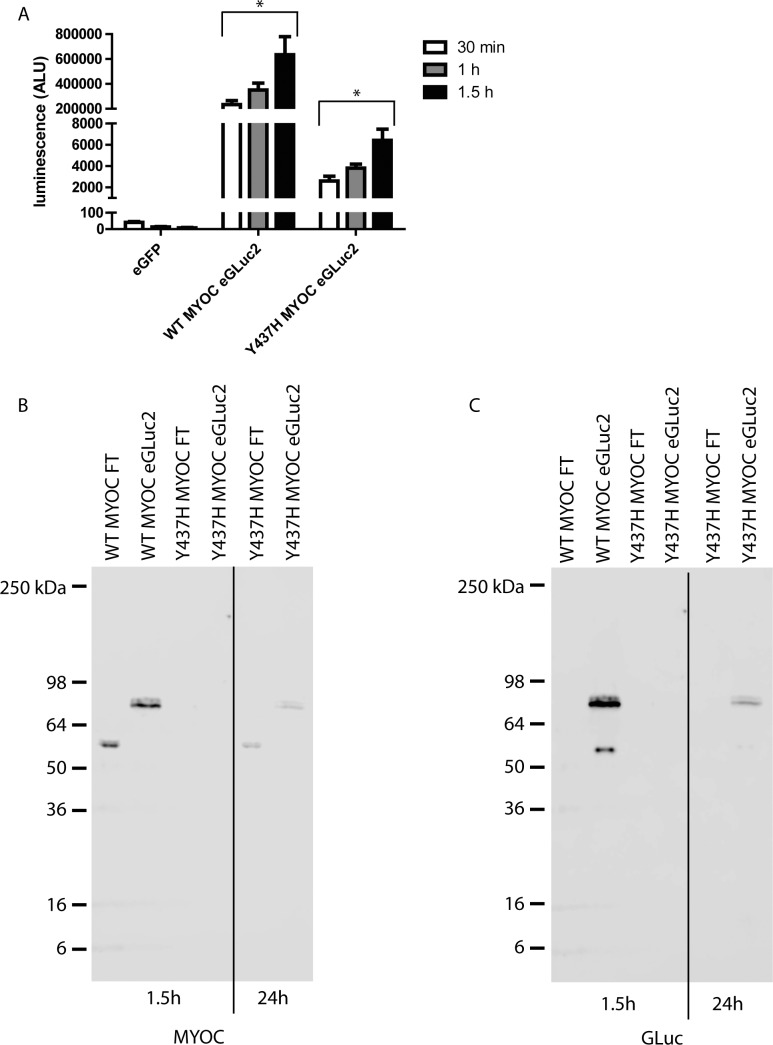
One major strength of the GLuc assay is its sensitivity32; it is one of the brightest known luciferases. Thus, even small amounts of the GLuc protein that are not detectable by Western blotting or other conventional techniques still can be detected by the GLuc assay.29 The amount of secreted Y437H MYOC 24 hours after a media change varies between minimal and nondetectable as assessed by Western blotting (Fig. 2A). Given the sensitivity of the GLuc assay, we decided to determine how soon after a media change we could reliably detect Y437H MYOC eGLuc2 secretion via the GLuc luminescence assay. Within 30 minutes of media change, we were able to easily detect Y437H MYOC eGLuc2 secretion (2599 ± 432 ALU, Fig. 3A). This value was nearly 65 times higher than the luminescence background signal originating from media collected from eGFP-expressing control cells (40 ± 4.9 ALU, Fig. 3A), and represented only 1.1% of the WT MYOC eGLuc2 signal at the same time point (233,784 ± 32,290 ALU, Fig. 3A). The luminescence values from the MYOC eGLuc2 signals increased with increasing media incubation time, but Y437H MYOC eGLuc2 still was secreted at approximately 1% of WT MYOC eGLuc2 levels across the time points (Fig. 3A). As expected, WT MYOC (FT or eGLuc2-tagged) was efficiently secreted and was easily detectable by Western blotting 1.5 hours after media change (Figs. 3B, 3C). In contrast, 1.5 hours after media change, Y437H MYOC (FT or eGLuc2-tagged) was not detectable by Western blotting (Figs. 3B, 3C). A more detailed, direct comparison between the readouts of the GLuc assay versus quantification via Western blotting for WT and Y437H MYOC can be found in Supplementary Figure S4.
Figure 3.
The GLuc assay can easily measure Y437H MYOC eGLuc2 levels before they are detectable by Western blotting. (A) HEK-293T cells were transfected for 48 hours followed by a media change with 2% FBS media. Thirty minutes to 1.5 hours after media change, conditioned media aliquots (20 μL) were taken and analyzed by the GLuc assay (n = 3 independent experiments, ±SEM, *P < 0.05 versus eGFP). (B, C) One and a half hours after media change conditioned media was analyzed by Western blotting. A 24-hour conditioned media sample from Y437H MYOC expressing cells was included as a positive control. Representative images of three independent experiments.
The GLuc Assay Can Be Extended to Measure Additional POAG-Associated MYOC Mutants
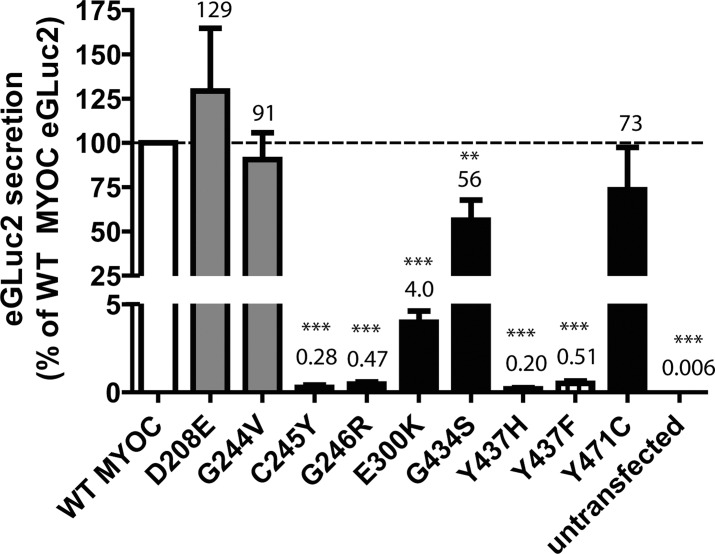
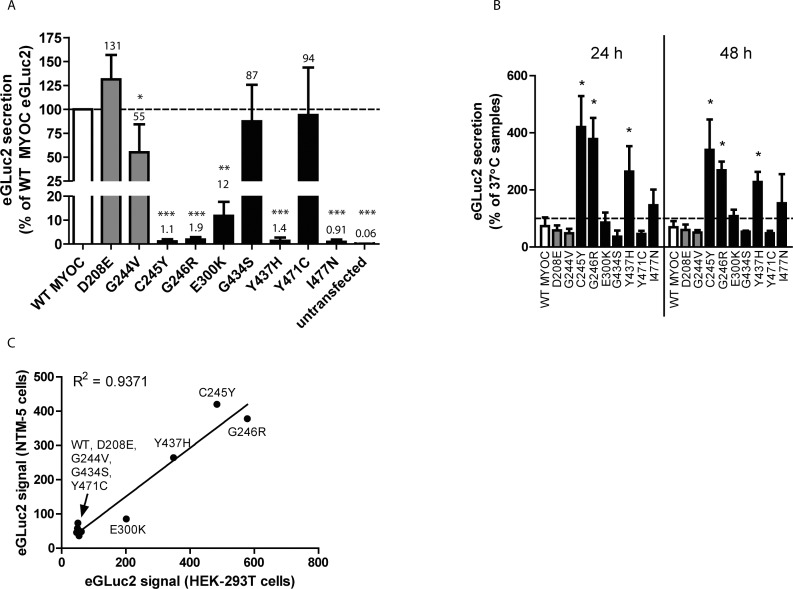
After validation that the FLAG tagged and eGLuc2-tagged versions of MYOC behave similarly with respect to soluble intracellular and secreted levels, we asked whether the GLuc assay could be used to sensitively monitor the secretion of additional variants that have been speculated to cause POAG. Wild type and nine additional mutant MYOC eGLuc2 fusion constructs were transfected into HEK-293T and conditioned media aliquots were assayed for GLuc activity (Fig. 4). As expected, mutants that were predicted to be nondisease–causing polymorphisms (D208E and G244V) were secreted within error of WT MYOC levels (129% ± 35% and 93% ± 15% of WT MYOC levels, respectively, Fig. 4). Of the six predicted pathogenic mutants assayed in HEK-293T cells, four variants (C245Y, G246R, E300K, and Y437H) exhibited nominal (≤4%) secretion compared to WT MYOC, in accordance with previous studies,9,21 although to our knowledge, the E300K mutation has not been previously characterized. Surprisingly, two predicted pathogenic mutants, G434S and Y471C, did not cause substantial secretion defects (59% ± 11% and 73% ± 24% of WT MYOC levels, respectively, Fig. 4).
Figure 4.
Analysis of pathogenic and nonpathogenic MYOC eGLuc2 mutants. (A) GLuc luminescence assay of secreted WT MYOC (white bar), predicted polymorphic (nonpathogenic) MYOC mutants (gray bars, D208E, G244V), predicted pathogenic MYOC mutants (black bars, C245Y, G246R, E300K, G434S, Y437H, Y471C) and an engineered mutant (striped bar, Y437F), from transfected HEK-293T cells. At 48 hours after transfection, 50 μL of conditioned media was assayed for the MYOC eGLuc2 fusion protein. Media from untransfected cells was used as a control (n ≥ 3 independent experiments, ±SD, **P < 0.01, ***P < 0.001 vs. hypothetical mean value of 100).
Quantitative real-time PCR (qPCR) and viability assays were conducted on transfected cells to verify that secretion differences between WT and mutant MYOC were not simply due to differences in transfection efficiency, transcriptional changes or cell death. Expression levels of the 3 most severely misfolded MYOC variants (C245Y, G246R, Y437H) were within error of WT MYOC, verifying that compromised mutant MYOC secretion is not due to transcriptional or transfection differences (Supplementary Fig. S5A). In addition, a resazurin mitochondrial reduction potential assay was used to analyze the metabolic activity of transfected cells and ensure cell viability. No MYOC variant caused any apparent toxicity in HEK-293T cells after 72 hours of expression (Supplementary Fig. S5B).
We hypothesized that the GLuc assay also could be used to probe the necessary intramolecular interactions required for MYOC secretion. A recent study by Donegan et al.41 showed a number of intramolecular interactions within MYOC (e.g., calcium coordination sites, ligand binding pockets, hydrogen bonding, and so forth) which appear to be critical for maintaining MYOC folding, structure, and eventual secretion. One particular aspect that we decided to probe was whether hydrogen bonding between water and the hydroxyl group of Tyr437 was necessary for stabilizing the region of MYOC near its disulfide bond.41 We reasoned that simply mutating Tyr437 to Phe (which is physically identical to Tyr but lacks the hydroxyl group) would eliminate this hydrogen bonding and compromise MYOC folding/secretion. Indeed, we found this to be the case. The Tyr437Phe (Y437F) mutation severely compromised MYOC secretion (0.44% ± 0.13% of WT MYOC levels, Fig. 4), most likely due to a lack of ability to form hydrogen bonding within the Cys245/Gly246/Leu248 – Ile432/Cys433/Tyr437 region.41
Compromised Secretion of MYOC Mutants Is Improved by Growth at Permissive Temperature
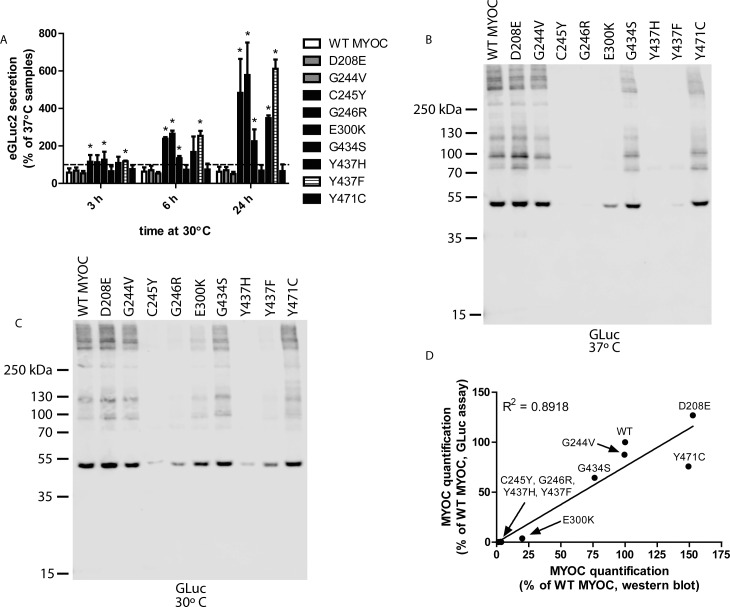
Previous research has shown that permissive temperature (e.g., 30°C) can stimulate the secretion of the majority of secretion-deficient, glaucoma-associated MYOC mutants.9,21 To verify that the MYOC eGLuc2 fusion proteins were behaving similarly to previous reports on MYOC, we also used permissive growth temperature (30°C) to enhance the secretion of mutant MYOC (Fig. 5A). The secretion of five MYOC mutants was significantly enhanced after shifting the growth temperature from 37°C to 30°C for up to 24 hours (Fig. 5A). We observed an approximately 2- to 6-fold increase in E300K (225% ± 63%), Y437H (349% ± 14%), C245Y (483% ± 181%), G246R (579% ± 173%), and Y437F MYOC (612% ± 49%) MYOC variants, respectively, after 24 hours of permissive temperature. The secretion of the majority of these variants (C245Y, G246R, E300K, and Y437F) was enhanced even by 6 hours of permissive growth temperature (Fig. 5A).
Figure 5.
Temperature-sensitive secretion of MYOC mutants in HEK-293T cells. (A) HEK-293T cells were transfected for 48 hours at 37°C. Media then was changed and transfected cells were incubated at either 37°C or 30°C for up to 24 hours. At 3, 6, and 24 hours post-temperature shift, aliquots were taken and assayed for eGLuc2 secretion. Results are presented as a percentage of the 37°C sample secretion (n ≥ 3 independent experiments, ±SD, *P < 0.05 vs. WT MYOC). (B) Western blot analysis of eGLuc2-tagged MYOC variants in HEK-293T cell culture media under nonreducing conditions at 37°C. (C) Western blot analysis of MYOC eGLuc2 secretion from HEK-293T cells grown at permissive (30°C) temperature. Lower growth temperature enhances secretion of numerous MYOC mutants as suggested by the presence of detectable bands. Representative blots of three independent experiments. (D) Western blotting for the GLuc antigen parallels GLuc assay results. MYOC (from the 37°C expt) was quantified by a luminescence assay (y axis, average of two independent experiments) or Western blotting (signal from the entire lane, x axis, average of two independent experiments) and plotted against each other.
The GLuc luciferase assay results were verified by Western blot analysis (performed under nonreducing conditions to mimic the conditions used for the GLuc assay) of media from transfected cells incubated at either 37°C (Fig. 5B) or 30°C (Fig. 5C) for 24 hours. The poorly secreted variants, C245Y, G246R, Y437H, and Y437F MYOC were barely detectable by Western blotting in the conditioned media from cells grown at 37°C, whereas the polymorphic variants, D208E and G244V, and the potentially pathogenic mutants, E300K, G434S, and Y471C MYOC were readily observable (Fig. 5B). The prominent band at approximately 56 kDa (actually running slightly less than 55 kDa in these instances) represents MYOC eGLuc2 which has been cleaved by calpain II and subsequently secreted from the cells,42 whereas the high molecular weight species originate from MYOC oligomerization under nonreducing conditions.39,43 The predominant bands of the four poorly secreted mutants (and the E300K variant) were greatly enhanced by growth at 30°C (Fig. 5C). Interestingly, the band corresponding to C245Y MYOC after temperature reduction also migrated slightly higher than the rest of the variants (Fig. 5C), in accordance with previous studies.43
To determine exactly how well the GLuc assay corresponded to the signals we obtained by Western blotting, we next quantified the intensity of each Western blot lane (Fig. 5B) and plotted it against the values we obtained for the same variants using the GLuc assay (Fig. 4). We found a strong positive correlation and an R2 value of 0.8918 (Fig. 5D), indicating that the GLuc assay values can be directly correlated to the Western blotting results.
Secretion of MYOC eGLuc2 Variants From NTM-5 Cells Parallels Secretion From HEK-293T Cells
The HEK-293T cell-based system, while robust and easily transfectable, is arguably not the most physiologically relevant system to test the secretion of POAG-associated MYOC proteins. The pathogenic effects of MYOC mutants primarily originate from the TM. Therefore, we also validated our GLuc assay in normal TM (NTM-5) cells. Even though the transfection efficiency of the NTM-5 cells was much lower than that of HEK-293T cells (10%–20% vs. 70%–80%), the GLuc assay still was sensitive enough to easily detect the “nonsecreted” MYOC proteins (Fig. 6A). The results from the NTM-5 cells compare well with those from HEK-293T cells (cf. Figs. 4–6A). However, in general, the MYOC variants were better tolerated in NTM-5 cells versus HEK-293T cells, potentially reflecting a cell-type difference in MYOC folding or processing, as noted previously.21 A clear example of this is the E300K variant, which was secreted at 4.0% of WT levels in HEK-293Tcells (Fig. 4) versus 12% of WT levels in NTM-5 cells (Fig. 6A). Nonetheless, the secretion of the folding defective variants, C245Y, C246R, and Y437H was significantly enhanced at permissive growth temperature for 24 to 48 hours in NTM-5 cells (Fig. 6B). Intriguingly, throughout our studies in HEK-293T and NTM-5 cells, the G434S and Y471C MYOC variants acted consistently like WT MYOC at physiologic or permissive temperature, perhaps suggesting that these mutants are characteristically different from the other pathogenic MYOC mutants. Finally, we directly compared the enhanced secretion at 30°C in HEK-293T versus NTM-5 cells to evaluate whether these cell lines in fact handled MYOC similarly. We found a strong positive relationship between the two sets of values, with a correlation coefficient of 0.9371, indicating that while the lineages of the two cell lines are drastically different (i.e., kidney versus TM), the general effects of growth temperature on MYOC secretion in each of the cells is nearly identical (Fig. 6C).
Figure 6.
MYOC secretion from NTM-5 cells parallels secretion from HEK-293T cells (A) GLuc luminescence assay of secreted MYOC eGLuc2 variants (same color designation as described in Fig. 6). Conditioned media aliquots (50 μL) from NTM-5 cells 48 hours post-transfection were assayed for the MYOC eGLuc2 fusion protein (n = 3 independent experiments, ±SD, *P < 0.05, **P < 0.01, ***P < 0.001 versus hypothetical mean value of 100). (B) Temperature-sensitive secretion of MYOC variants in NTM-5 cells. NTM-5 cells were transfected for 48 hours, followed by a media change and grown at 37°C or 30°C for up to 48 hours. At 24 and 48 hours after temperature shift media aliquots were taken and assayed for eGLuc2. Results are presented as a percentage of the 37°C samples' secretion (n = 3 independent experiments, ±SD, *P < 0.05 versus WT MYOC). (C) Temperature-sensitive secretion of MYOC between HEK-293T and NTM-5 cells 24 hours after temperature shift. An average of three independent temperature-shift experiments (performed 24 hours post growth temperature reduction) in HEK-293T and NTM-5 cells were plotted against each other.
The Pool of Mutant MYOC Protein Secreted During Permissive Temperature Originates From Intracellular Stores, and Is Not Newly Synthesized
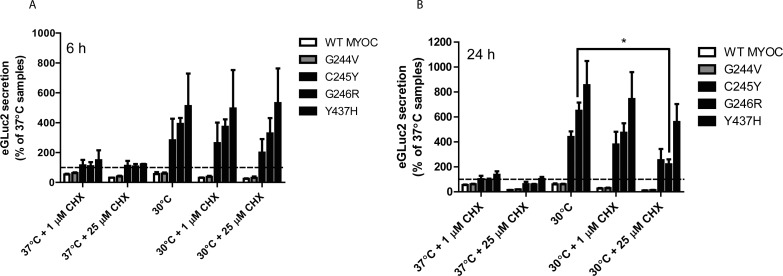
At lower growth temperature, a number of cellular changes occur that may contribute to enhanced secretion of misfolded proteins such as MYOC. One key physiologic change at lowered temperature is translational attenuation.44 We were curious to determine the origins of the secreted mutant MYOC protein under permissive temperature. Does MYOC originate as newly synthesized protein at the permissive temperature, or does it originate from the pool of mutant MYOC that accumulates intracellularly due to protein misfolding and inefficient secretion? To determine the answer to this question, we treated transfected cells with cycloheximide (CHX) which nonspecifically prevents translational elongation45 and incubated the cells at either 37°C or 30°C. We followed the secretion of a subset of variants described in Figure 4 for either 6 or 24 hours and found that CHX had no effect on inhibiting mutant MYOC secretion (Figs. 7A, 7B). These results indicate that even if translation is prevented, secretion of the severely misfolded MYOC protein proceeds in a translation-independent manner and is not derived from newly synthesized protein. These results were verified in parallel by Western blotting (Supplementary Fig. S5C). Notably, there was a significant reduction in the secretion of G246R MYOC after 24 hours of 25 μM CHX treatment (Fig. 7B), however this general reduction in secreted protein is likely due to substantial reductions in cell viability after prolonged treatment with high levels of CHX (Supplementary Fig. S5D).
Figure 7.
Enhanced MYOC secretion occurs independent of translation. (A, B) HEK-293T cells were transfected with WT, G244V, C245Y, G246R, or Y437H MYOC eGLuc2 for 48 hours at 37°C. The media was changed and cells were treated with 1 or 25 μM cycloheximide (CHX) at 37°C or 30°C for up to 24 hours. Conditioned media aliquots (50 μL) were assayed for MYOC eGLuc2 by the GLuc assay 6 (A) or 24 (B) hours after the temperature shift/CHX treatment (n ≥ 3 independent experiments, ± SD, *P < 0.05).
Discussion
We have demonstrated that WT and mutant MYOC eGLuc2 variants closely resemble more conventionally tagged forms of MYOC (i.e., MYOC FT) in terms of their responsiveness to ER stressors (see Supplementary Material), secretion levels, soluble intracellular levels, and apparent disulfide bonding ability. The major advantages of the GLuc assay are its speed (routinely completed within 5 minutes), its sensitivity (it can detect “nonsecreted” Y437H MYOC within 30 minutes of media change), and its cost (a single assay in a 96-well plate costs approximately 5 cents and can be scaled down to 384 well or 1586 well plates). We envision that the true use of this luciferase-based MYOC assay system is twofold: (1) for performing kinetic experiments on mutant MYOC which requires a quick measurement approach and sensitivity, and (2) for use in chemical or genetic high-throughput screens which aim to identify compounds or genes that modulate the secretion of MYOC. Such small molecules or genes could subsequently be used as possible therapeutic candidates for MYOC-associated POAG.
While we think that this luciferase-based assay has promise, there still are a number of limitations of the GLuc assay. As it currently stands, the GLuc assay cannot monitor insoluble MYOC since denaturing chaotropes or ionic detergents are used to solublize aggregated MYOC and compromise GLuc activity. Additionally, the GLuc assay cannot be easily used for determining subcellular localization, in contrast to other reporters like GFP/RFP. Thirdly, based on our observations described herein, it appears that the GLuc assay is able to detect only a portion (approximately 60%) of the full amount of intracellular, misfolded mutant MYOC. Finally, as with all reporter assays, all findings using the eGLuc2-tagged MYOC must be validated in orthogonal assays using untagged or well-characterized tagged forms of MYOC.
Nonetheless, using this assay, we were able to glean insight into a number of new facets of MYOC folding/secretion. Specifically, to our knowledge, our report is the first to demonstrate the secretion propensities of D208E, G244V, E300K, G434S, and Y471C MYOC. We found that four of these variants (D208E, G244V, G434S, and Y471C) were secreted at similar levels compared to WT MYOC. Since the D208E mutation has been found in control and POAG patients,46,47 it is highly likely that it is a polymorphism, in agreement with the prediction made previously (available in the public domain at www.myocilin.com).12 The G244V variant of MYOC was found in a single patient with mild POAG who was compound heterozygous for a G252R MYOC mutation.48 Therefore, it is very possible that the G244V mutation really is a polymorphic variant that does not affect MYOC secretion, while the true disease-causing mutation is the G252R mutation. In contrast to D208E and G244V, prediction software suggested that G434S and Y471C actually are pathogenic. Our secretion data in HEK-293T and NTM-5 cells would suggest otherwise. These variants are well tolerated in HEK-293T and NTM-5 cells (>56% of WT MYOC secretion in all instances). Whereas G434S MYOC has been found in one POAG patient,49 Y471C MYOC has been found in two POAG patients46,47 and is listed as having “unknown pathologic significance” on the UniProt website (available in the public domain at www.uniprot.org). The combination of small patient cohorts combined with our secretion results, begs the question if Y471C and G434S MYOC are truly pathogenic. Nonetheless, we cannot rule out that these mutations may abrogate MYOC function in some manner, but do not necessarily substantially affect secretion.
The E300K MYOC mutation also has been found in only one patient,47 and is considered as a mutation with “unknown pathologic significance” (available in the public domain at www.uniprot.org). Our studies suggest that this mutant is unique in comparison to the other MYOC mutants we tested. Whereas most mutants showed either minimal secretion (<0.5% of WT MYOC secretion in HEK-293T cells, or <2% of WT MYOC secretion in NTM-5 cells) or WT-like secretion (>55% of WT MYOC levels), E300K MYOC was secreted at an intermediate level – 4% of WT MYOC levels in HEK-293T cells and 12% of WT MYOC levels in NTM-5 cells. These results would suggest that this mutation only moderately compromises MYOC folding and secretion. The secretion of this mutant was slightly (but significantly) increased at permissive temperature in HEK-293T cells, but not in NTM-5 cells, potentially indicating that these two cell lines handle this specific variant in a different fashion.
The C245Y MYOC mutation, which abolishes the ability of MYOC to form an intramolecular disulfide bond,50 results in an aggressive juvenile form of POAG.43 We found that indeed, this mutation significantly compromised MYOC secretion (0.28% of WT MYOC levels in HEK-293T cells and 1.1% of WT MYOC levels in NTM-5 cells). Yet, the secretion of this variant was rescued to the greatest extent in NTM-5 cells after growth at permissive temperature. Previous groups also have shown that the aggregation and secretion of the C245Y mutation can be partially rescued with a different approach using a small molecule pharmacologic chaperone, PBA,24 but no group has shown that permissive temperature can partially alleviate C245Y secretion defects. These data indicated that the MYOC intramolecular disulfide bond is not absolutely necessary for MYOC secretion, but is a requirement for efficient MYOC secretion.
According to our data, enhanced mutant MYOC secretion at permissive temperature is independent of newly translated protein. These results suggest that the amount of protein secreted at lower temperature originates from intracellular stores, possibly arising from protein trapped in the ER, or exosomes.51,52 Previous work has suggested that the ER-associated degradation machinery is unable to effectively degrade misfolded, mutant MYOC which accumulates intracellularly, thus creating a buildup of MYOC.53 We speculate that some of the ER protein folding machinery may be able to reengage and fold this pool of mutant MYOC under permissive temperature. However, as of yet, we cannot rule out the possibility that lowered growth temperature directly affects MYOC folding and secretion, independent of engagement with cellular folding machinery.
One surprising aspect of our studies was that we did not detect any overt activation of the UPR or notice any differences in cell viability after expression of mutant MYOC. These results are in accordance with at least one study,54 but in contrast to others.9,14,20,21,23 There are at least two potential reasons for these observations. It is possible that the extent to which we expressed MYOC by transient transfection is minimal compared to other studies that used adenovirus or cell lines specifically selected for high levels of MYOC expression. Secondly, we performed the majority of these experiments in HEK-293T cells, not human TM cells. It is possible that mutant MYOC expression selectively kills TM cells, and not other cell types, like kidney cells. Due to the poor transfection efficiency in NTM-5 cells (10%–20%), we were unlikely to detect any cell death in the TM cells after transfection.
To summarize, the GLuc-based MYOC assay we describe herein can be used to quickly and quantitatively determine secreted (and soluble intracellular) MYOC levels nearly in real-time. Use of this assay could assist in easily identifying the secretion propensities and kinetics of newly identified MYOC mutations, assessing the consequences of rationally-designed mutations (like the Y437F mutation), and could serve as a stand-alone or complementary assay for use in high-throughput screening.28,55 Future efforts will be directed toward miniaturization of the MYOC eGLuc2 assay in a physiologically relevant, inducible cell-based model, which will serve as an ideal proving ground for identifying chemical and/or genetic modulators of MYOC folding and secretion in an unbiased manner.
Supplementary Material
Acknowledgments
Supported in part by an endowment from the Roger and Dorothy Hirl Research Fund (JDH), a Career Development Award from Research to Prevent Blindness (JDH), a National Eye Institute Visual Science Core Grant (EY020799), an unrestricted grant from Research to Prevent Blindness, funding from the UT Southwestern Summer Medical Student Research Program (SZ), and a National Eye Institute R00 Grant EY022077 (GZ).
Disclosure: S. Zadoo, None; A. Nguyen, None; G. Zode, None; J.D. Hulleman, None
References
- 1. Quigley HA,, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacobson N,, Andrews M,, Shepard AR,, et al. Nonsecretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet. 2001; 10: 117–125. [DOI] [PubMed] [Google Scholar]
- 3. Fingert JH. Primary open-angle glaucoma genes. Eye (Lond). 2011; 25: 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stone EM,, Fingert JH,, Alward WL,, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997; 275: 668–670. [DOI] [PubMed] [Google Scholar]
- 5. Fingert JH,, Heon E,, Liebmann JM,, et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999; 8: 899–905. [DOI] [PubMed] [Google Scholar]
- 6. Fingert JH,, Stone EM,, Sheffield VC,, Alward WL. Myocilin glaucoma. Surv Ophthalmol. 2002; 47: 547–561. [DOI] [PubMed] [Google Scholar]
- 7. Caballero M,, Borras T. Inefficient processing of an olfactomedin-deficient myocilin mutant: potential physiological relevance to glaucoma. Biochem Biophys Res Commun. 2001; 282: 662–670. [DOI] [PubMed] [Google Scholar]
- 8. Swiderski RE,, Ying L,, Cassell MD,, Alward WL,, Stone EM,, Sheffield VC. Expression pattern and in situ localization of the mouse homologue of the human MYOC (GLC1A) gene in adult brain. Brain Res Mol Brain Res. 1999; 68: 64–72. [DOI] [PubMed] [Google Scholar]
- 9. Vollrath D,, Liu Y. Temperature sensitive secretion of mutant myocilins. Exp Eye Res. 2006; 82: 1030–1036. [DOI] [PubMed] [Google Scholar]
- 10. Karali A,, Russell P,, Stefani FH,, Tamm ER. Localization of myocilin/trabecular meshwork--inducible glucocorticoid response protein in the human eye. Invest Ophthalmol Vis Sci. 2000; 41: 729–740. [PubMed] [Google Scholar]
- 11. Swiderski RE,, Ross JL,, Fingert JH,, et al. Localization of MYOC transcripts in human eye and optic nerve by in situ hybridization. Invest Ophthalmol Vis Sci. 2000; 41: 3420–3428. [PubMed] [Google Scholar]
- 12. Hewitt AW,, Mackey DA,, Craig JE. Myocilin allele-specific glaucoma phenotype database. Hum Mutat. 2008; 29: 207–211. [DOI] [PubMed] [Google Scholar]
- 13. Resch ZT,, Fautsch MP. Glaucoma-associated myocilin: a better understanding but much more to learn. Exp Eye Res. 2009; 88: 704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joe MK,, Sohn S,, Hur W,, Moon Y,, Choi YR,, Kee C. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003; 312: 592–600. [DOI] [PubMed] [Google Scholar]
- 15. Burns JN,, Turnage KC,, Walker CA,, Lieberman RL. The stability of myocilin olfactomedin domain variants provides new insight into glaucoma as a protein misfolding disorder. Biochemistry. 2011; 50: 5824–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou Z,, Vollrath D. A cellular assay distinguishes normal and mutant TIGR/myocilin protein. Hum Mol Genet. 1999; 8: 2221–2228. [DOI] [PubMed] [Google Scholar]
- 17. Gobeil S,, Letartre L,, Raymond V. Functional analysis of the glaucoma-causing TIGR/myocilin protein: integrity of amino-terminal coiled-coil regions and olfactomedin homology domain is essential for extracellular adhesion and secretion. Exp Eye Res. 2006; 82: 1017–1029. [DOI] [PubMed] [Google Scholar]
- 18. Orwig SD,, Perry CW,, Kim LY,, et al. Amyloid fibril formation by the glaucoma-associated olfactomedin domain of myocilin. J Mol Biol. 2012; 421: 242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zode GS,, Kuehn MH,, Nishimura DY,, et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2015; 125: 3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peters JC,, Bhattacharya S,, Clark AF,, Zode GS. Increased endoplasmic reticulum stress in human glaucomatous trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2015; 56: 3860–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Y,, Vollrath D. Reversal of mutant myocilin nonsecretion and cell killing: implications for glaucoma. Hum Mol Genet. 2004; 13: 1193–1204. [DOI] [PubMed] [Google Scholar]
- 22. Stothert AR,, Fontaine SN,, Sabbagh JJ,, Dickey CA. Targeting the ER-autophagy system in the trabecular meshwork to treat glaucoma. Exp Eye Res. 2015; 144: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zode GS,, Kuehn MH,, Nishimura DY,, et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2011; 121: 3542–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yam GH,, Gaplovska-Kysela K,, Zuber C,, Roth J. Sodium 4-phenylbutyrate acts as a chemical chaperone on misfolded myocilin to rescue cells from endoplasmic reticulum stress and apoptosis. Invest Ophthalmol Vis Sci. 2007; 48: 1683–1690. [DOI] [PubMed] [Google Scholar]
- 25. Zode GS,, Bugge KE,, Mohan K,, et al. Topical ocular sodium 4-phenylbutyrate rescues glaucoma in a myocilin mouse model of primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamm ER. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res. 2002; 21: 395–428. [DOI] [PubMed] [Google Scholar]
- 27. Nguyen A,, Hulleman JD. Differential tolerance of ‘pseudo-pathogenic' tryptophan residues in calcium-binding EGF domains of short fibulin proteins. Exp Eye Res. 2015; 130: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hulleman JD,, Brown SJ,, Rosen H,, Kelly JW. A high-throughput cell-based Gaussia luciferase reporter assay for identifying modulators of fibulin-3 secretion. J Biomol Screen. 2013; 18: 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hulleman JD,, Kaushal S,, Balch WE,, Kelly JW. Compromised mutant EFEMP1 secretion associated with macular dystrophy remedied by proteostasis network alteration. Mol Biol Cell. 2011; 22: 4765–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hulleman JD,, Balch WE,, Kelly JW. Translational attenuation differentially alters the fate of disease-associated fibulin proteins. FASEB J. 2012; 26: 4548–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tannous BA,, Kim DE,, Fernandez JL,, Weissleder R,, Breakefield XO. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther. 2005; 11: 435–443. [DOI] [PubMed] [Google Scholar]
- 32. Tannous BA. Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat Protoc. 2009; 4: 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Badr CE,, Hewett JW,, Breakefield XO,, Tannous BA. A highly sensitive assay for monitoring the secretory pathway and ER stress. PLoS One. 2007; 2: e571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maguire CA,, Deliolanis NC,, Pike L,, et al. Gaussia luciferase variant for high-throughput functional screening applications. Anal Chem. 2009; 81: 7102–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Welsh JP,, Patel KG,, Manthiram K,, Swartz JR. Multiply mutated Gaussia luciferases provide prolonged and intense bioluminescence. Biochem Biophys Res Commun. 2009; 389: 563–568. [DOI] [PubMed] [Google Scholar]
- 36. Nguyen A,, Hulleman JD. Evidence of alternative cystatin c signal sequence cleavage which is influenced by the A25T polymorphism. PLoS One. 2016; 11: e0147684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yam GH,, Gaplovska-Kysela K,, Zuber C,, Roth J. Aggregated myocilin induces Russell bodies and causes apoptosis: implications for the pathogenesis of myocilin-caused primary open-angle glaucoma. Am J Pathol. 2007; 170: 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Joe MK,, Kwon HS,, Cojocaru R,, Tomarev SI. Myocilin regulates cell proliferation and survival. J Biol Chem. 2014; 289: 10155–10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nguyen TD,, Chen P,, Huang WD,, Chen H,, Johnson D,, Polansky JR. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998; 273: 6341–6350. [DOI] [PubMed] [Google Scholar]
- 40. Fautsch MP,, Vrabel AM,, Peterson SL,, Johnson DH. In vitro and in vivo characterization of disulfide bond use in myocilin complex formation. Mol Vis. 2004; 10: 417–425. [PubMed] [Google Scholar]
- 41. Donegan RK,, Hill SE,, Freeman DM,, et al. Structural basis for misfolding in myocilin-associated glaucoma. Hum Mol Genet. 2015; 24: 2111–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanchez-Sanchez F,, Martinez-Redondo F,, Aroca-Aguilar JD,, Coca-Prados M,, Escribano J. Characterization of the intracellular proteolytic cleavage of myocilin and identification of calpain II as a myocilin-processing protease. J Biol Chem. 2007; 282: 27810–27824. [DOI] [PubMed] [Google Scholar]
- 43. Fan BJ,, Leung DY,, Wang DY,, et al. Novel myocilin mutation in a Chinese family with juvenile-onset open-angle glaucoma. Arch Ophthalmol. 2006; 124: 102–106. [DOI] [PubMed] [Google Scholar]
- 44. Fujita J. Cold shock response in mammalian cells. J Mol Microbiol Biotechnol. 1999; 1: 243–255. [PubMed] [Google Scholar]
- 45. Siegel MR,, Sisler HD. Inhibition of protein synthesis in vitro by cycloheximide. Nature. 1963; 200: 675–676. [DOI] [PubMed] [Google Scholar]
- 46. Fan BJ,, Wang DY,, Fan DS,, et al. SNPs and interaction analyses of myocilin, optineurin, and apolipoprotein E in primary open angle glaucoma patients. Mol Vis. 2005; 11: 625–631. [PubMed] [Google Scholar]
- 47. Pang CP,, Leung YF,, Fan B,, et al. TIGR/MYOC gene sequence alterations in individuals with and without primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2002; 43: 3231–3235. [PubMed] [Google Scholar]
- 48. Hewitt AW,, Bennett SL,, Richards JE,, et al. Myocilin Gly252Arg mutation and glaucoma of intermediate severity in Caucasian individuals. Arch Ophthalmol. 2007; 125: 98–104. [DOI] [PubMed] [Google Scholar]
- 49. Michels-Rautenstrauss K,, Mardin C,, Wakili N,, et al. Novel mutations in the MYOC/GLC1A gene in a large group of glaucoma patients. Hum Mutat. 2002; 20: 479–480. [DOI] [PubMed] [Google Scholar]
- 50. Nagy I,, Trexler M,, Patthy L. Expression and characterization of the olfactomedin domain of human myocilin. Biochem Biophys Res Commun. 2003; 302: 554–561. [DOI] [PubMed] [Google Scholar]
- 51. Hoffman EA,, Perkumas KM,, Highstrom LM,, Stamer WD. Regulation of myocilin-associated exosome release from human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2009; 50: 1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fautsch MP,, Howell KG,, Vrabel AM,, Charlesworth MC,, Muddiman DC,, Johnson DH. Primary trabecular meshwork cells incubated in human aqueous humor differ from cells incubated in serum supplements. Invest Ophthalmol Vis Sci. 2005; 46: 2848–2856. [DOI] [PubMed] [Google Scholar]
- 53. Suntharalingam A,, Abisambra JF,, O'Leary JC,, III, et al. Glucose-regulated protein 94 triage of mutant myocilin through endoplasmic reticulum-associated degradation subverts a more efficient autophagic clearance mechanism. J Biol Chem. 2012; 287: 40661–40669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gould DB,, Reedy M,, Wilson LA,, Smith RS,, Johnson RL,, John SW. Mutant myocilin nonsecretion in vivo is not sufficient to cause glaucoma. Mol Cell Biol. 2006; 26: 8427–8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Orwig SD,, Chi PV,, Du Y,, et al. Ligands for glaucoma-associated myocilin discovered by a generic binding assay. ACS Chem Biol. 2014; 9: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shoulders MD,, Ryno LM,, Genereux JC,, et al. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep. 2013; 3: 1279–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oh RS,, Pan WC,, Yalcin A,, et al. Functional RNA interference (RNAi) screen identifies system A neutral amino acid transporter 2 (SNAT2) as a mediator of arsenic-induced endoplasmic reticulum stress. J Biol Chem. 2012; 287: 6025–6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fawcett TW,, Martindale JL,, Guyton KZ,, Hai T,, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999; 339: 135–141. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.