Abstract
Summary
Based on a systematic review of the literature, only low body weight and menopausal status can be considered with confidence, as important risk factors for low BMD in healthy 40–60 year old women. The use of body weight to identify high risk women may reduce unnecessary BMD testing in this age group.
Introduction
BMD testing of perimenopausal women is increasing but may be unnecessary as fracture risk is low. Appropriate assessment among younger women requires identification of risk factors for low BMD specific to this population.
Methods
We conducted a systematic literature review of risk factors for low BMD in healthy women aged 40–60 years. Articles were retrieved from six databases and reviewed for eligibility and methodological quality. A grade for overall strength of evidence for each risk factor was assigned.
Results
There was good evidence that low body weight and post-menopausal status are risk factors for low BMD. There was good or fair evidence that alcohol and caffeine intake, and reproductive history are not risk factors. There was inconsistent or insufficient evidence for the effect of calcium intake, physical activity, smoking, age at menarche, history of amenorrhea, family history of OP, race and current age on BMD.
Conclusions
Based on current evidence in Caucasians, we suggest that, in healthy women aged 40–60 years, only those with a low body weight (< 70 kg) be selected for BMD testing. Further research is necessary to determine optimal race-specific discriminatory weight cut-offs and to evaluate the risk factors for which there was inconclusive evidence.
Keywords: BMD, Bone density, Osteoporosis, Risk factors, Systematic review
Introduction
Osteoporosis (OP) is a systemic skeletal disease characterized by low bone mass and deterioration of bone tissue resulting in compromised bone strength and increased risk of fragility fractures [1, 2]. OP is recognized as a major health problem worldwide. It affects more than 75 million people in Europe, Japan and the United States. It is a cause of 2.3 million fractures in Europe and the United States annually [3]. Bone loss occurs as estrogen levels decline, and while the risk of OP is low before menopause, evidence from Canadian data demonstrates an increase in bone mineral density (BMD) testing rates among women aged 40–44 years [4]. This may indicate a growing concern about OP among this age group, increased availability of BMD testing or changes in fee schedules for BMD testing, but may also indicate unnecessary testing of individuals not at risk [5].
Current risk assessment for low BMD is based primarily on data from older women, largely ≥ 65 years of age [6], which does not directly incorporate risk factors for low peak bone mass or accelerated perimenopausal bone loss. Risk factors identified in older women may not be relevant to, or highly prevalent among, younger women. Appropriate BMD testing among younger women first requires the identification of risk factors for low BMD in this population. Although the absolute risk of fragility fracture is low in younger women, detection of individuals with significantly reduced BMD will assist with implementation of preventive measures and closer surveillance of those who may benefit from early intervention.
No systematic reviews have been performed to identify risk factors for low BMD in healthy younger women approaching the menopause or recently menopausal. The aim of this study is to review the scientific evidence to identify risk factors for low BMD in healthy women aged 40–60 years. The underlying assumption of this review is that women with co-morbidities known to be associated with low BMD or fracture would receive BMD testing and appropriate management. Thus the focus of this review is on healthy women with none of these co-morbidities.
Materials and methods
Search strategy
We performed a systematic literature search to identify factors associated with low BMD among women age 40–60 years using: Medline, Embase, Cinahl, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews and Effects (DARE) and HealthSTAR (searched from 1990 to January 2006). Search terms “bone density”, “bone mineral content”, “bone loss”, “BMD” or “bone density testing” were used to identify articles on BMD, and search terms “densitometry”, “x-ray densitometry” or “x-ray absorptiometry” were used to identify studies which used dual-energy x-ray absorptiometry (DXA). To include studies that examined associations between risk factors and low BMD, search terms “Exp risk”, “relative risk”, “causation” or “odds and ratio” were used. Reference lists of key articles were hand-searched. Experts in the field were consulted to identify additional studies that might contain relevant data. The search was limited to English-language articles.
Eligibility
We included observational studies using cohort (retrospective or prospective cohort), case-control, and cross-sectional designs, that involved women aged 40–60 years or that contained a subgroup analysis of this age range. Articles evaluating clinical risk assessment tools were also considered. Studies were included only if BMD was measured with dual energy x-ray absorptiometry (DXA). BMD measurement sites were the lumbar spine, proximal femur (total hip, femoral neck or greater trochanter), total body or radius (mid or distal). Randomized controlled drug trials (RCDTs) or cohort studies that were an extension of prior RCDTs where trial case selection was based on BMD status were excluded as were case series, case reports, letters, editorials or narrative reviews. Studies investigating OP associated with diseases or medications known to affect bone metabolism were excluded. Finally, risk factors not easily assessed in primary practice (e.g., genetic markers) or where description of risk factor measurement was inadequate were also excluded.
Quality assessment
Titles and abstracts were screened for eligibility. When eligibility was uncertain, the full-text article was retrieved. Two reviewers independently reviewed the articles for eligibility and methodological quality. The internal validity of each study was assessed using the following criteria: appropriate study design, standardized bone densitometry technique, BMD assessment blinded to exposure (risk factor) status, valid risk factor measurement, minimization of bias (selection bias; recall bias), duration of follow-up, loss to follow-up, appropriate statistical analysis, and control for confounding variables [7]. Studies were assigned quality grades based on the U.S. Preventive Services Task Force (USPSTF) guidelines [8]: ‘good’ if all criteria were met, ‘fair’ if most criteria were met without fatal flaws and ‘poor’ if the study contained one or more methodological fatal flaws. Poor studies were subsequently excluded from the review. Twenty percent of the articles were randomly chosen for duplicate review to assess agreement on assigned quality grades. There was perfect agreement on all duplicate reviews (data not shown).
Data abstraction
Two reviewers independently abstracted both descriptive information and study results; evidence tables were prepared in a standardized format. The data abstracted included a description of the patient population (inclusion and exclusion criteria, mean age, race, country, sample size), study design, duration of follow-up, risk factors assessed, precision of DXA technique, BMD measurement site(s), and results (odds ratios, regression beta-coefficients and/or R2 values) reported after adjustment for key confounding variables. When odds ratios for OP were reported, OP was defined as T-score ≤ − 2.5 unless otherwise specified.
Data synthesis: rating overall strength of the evidence
Two reviewers independently rated the strength of the evidence and differences were resolved by a third reviewer. The strength of the evidence for an association between each risk factor and BMD was graded as good, fair, inconsistent or insufficient (Table 1). Grades were assigned using three criteria: quality, quantity and consistency. Quality was assessed based on the study’s internal validity graded according to USPSTF guidelines as described above. Quantity was assessed based on the number of studies that evaluated each risk factor. Consistency was assessed based on similarity of findings reported across a range of study populations and study designs.
Table 1.
Grading the strength of the evidence
| Grade | Definition |
|---|---|
| Good | There is good evidence for or against an association between the risk factor and BMD Determined by: consistent results across studies; > three studies; at least one study graded as ‘good’ quality |
| Fair | There is fair evidence for or against an association between the risk factor and BMD Determined by: consistent results across studies but limited by quantity (≤ three studies) or quality (no studies graded as ‘good’) |
| Inconsistent | There is inconsistent evidence for or against an association between the risk factor and BMD Determined by: studies had conflicting results |
| Insufficient | There is insufficient evidence for or against an association between the risk factor and BMD Determined by: inadequate number of studies evaluating the risk factor (< three studies) |
Results
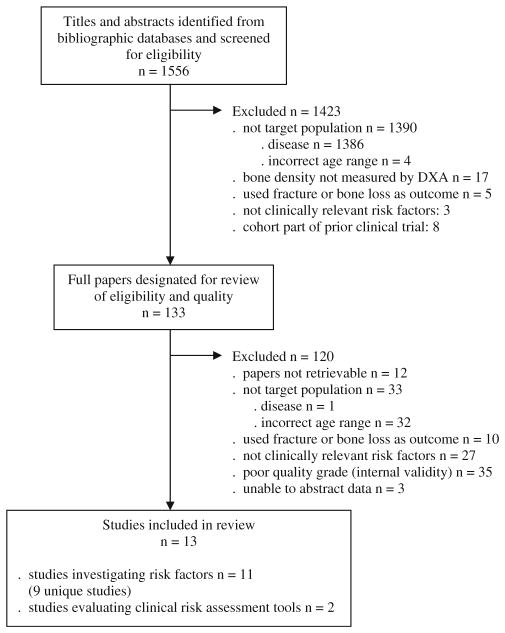
A flow diagram detailing the selection of articles is shown in Fig. 1. Of the initial 1,556 titles and abstracts identified through our search strategy, 1,543 were subsequently excluded. The most common reasons for exclusion were study populations with diseases known to affect bone metabolism (n=1,387), incorrect age range or no subgroup analyses of women aged 40–60 years (n=36) and risk factors not easily measured in primary practice (n=30). Of those meeting inclusion criteria n=35 were excluded for poor methodological quality. Thirteen studies met all criteria and were included in this review; 11 evaluated risk factors and two evaluated the use of risk assessment tools originally developed in older populations. Two articles [9, 10] reported results from analyses of different risk factors in the same study population [11, 12]; therefore a total of nine studies are represented by the 11 risk factor articles.
Fig. 1.
Results of literature search to identify studies that evaluated risk factors for low bone density in healthy women aged 40–60 years
Study characteristics
The characteristics of the studies are summarized in Table 2. Sample sizes ranged from 112 to 2835, with mean n=1121. Study populations were predominantly white with the exception of three studies in which participants were Asian [13–15] and one in which 20% of participants were black [16]. One of the 11 risk factor studies and both studies evaluating risk assessment tools were graded as ‘good’ quality based on our criteria; the remaining ten were graded as ‘fair’.
Table 2.
Characteristics of included studies
| A. Studies evaluating specific risk factors | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study (reference) | Study design/type of cohort | N | Age yr, mean (± SD) | Country of recruitment | Ethnic background, % of participants | Skeletal sites measured by DXA | Risk factors considered | Comments |
| Ho et al. 2005 [13] | Cross-sectional Population-based (cluster sampling) |
685 | 55 (± 3.5) | China (Hong Kong) | Asian: 100.0 | LSP (L1-L4), FN, total hip, GT, inter-trochanter, TB | Education level Weight Height Age Years since menopause Age at menarche Physical activity Current job type Pregnancy, lactation Diet (calcium, protein, fruit intake) |
|
| Holm et al. 2002 [16] | Prospective (2-yr) Community-based |
374 (baseline) 306 (Year 2) |
46.7 (± 7.2) | United States | White: 74.3 Black: 21.1 Asian: 2.4 Hispanic: 2.2 |
LSP (L2-L4) | Weight Height Age Menopausal status Race Physical activity Calcium Caffeine Alcohol Smoking Physical activity |
|
| Coupland et al. 1999 [9] | Cross-sectional Population-based (random sampling for a clinical trial) |
580 | 53.2 (± 3.8) | England | Not reported | LSP (L1-L4), FN, GT, TB, total radius | Same study population as Grainge et al. [11]; used baseline data from EPIC clinical trial and data from women not willing/not eligible for trial | |
| Keen et al. 1999 [19] | Cross-sectional case-control Clinical cohort from single general practice |
1003 | 54.2 (± 6.0) | England | White: 98.0 Other: 2.0 |
LSP (L1-L4), FN | Family history of osteoporotic fracture | |
| Grainge et al. 1998 [11] | Cross-sectional Population-based (random sampling for a clinical trial) |
580 | 53.2 (± 3.8) | England | Not reported | LSP (L1-L4), FN, greater trochanter, TB, total radius | Smoking Alcohol Caffeine |
Same study population as Coupland et al. [9]; used base-line data from EPIC clinical trial and data from women not willing/not eligible for trial |
| New et al. 1997 [17] | Cross-sectional Population-based (random sampling from Community Health Index for OP screening program) |
994 | 47.1 (± 1.4) | Scotland | Not reported | LSP (L2-L4), FN, GT, Ward’s triangle | Current diet (protein, calcium, fiber, pot-assium, magnesium, zinc, Vitamin C) Past diet (milk, fruits vegetables) Smoking Physical activity Alcohol Weight Height Age |
|
| Takada et al. 1997 [15] | Retrospective chart review Clinical sample from one health centre |
2835 (sub-group of women aged 37–55) | 44.7 (± 4.3) | Japan | Not reported | distal 1/3 radius | Alcohol Smoking Physical activity at job Menopausal status Lean mass Fat mass |
|
| Mizuno et al. 1995 [14] | Cross-sectional Clinical sample (consecutive patients attending gynecological clinic) |
112 | 53.3 (± 4.9) | Japan | Asian: 100.0 | LSP (L2-L4), TB | BMI Years since menopause Age at menarche Pregnancy |
|
| Tuppurainen et al. 1995 [10] | Cross-sectional (baseline of prospective study) Population-based (random stratified sampling) |
1605 | 53.4 (± 2.9) | Finland | Not reported | LSP (L2-L4), FN | Age at menarche Parity and lactation History of amenorrhea Hysterectomy or oophorectomy Weight Height Age Menopausal status |
Sub-sample of the Kuopio OSTPRE cohort; same study as Kroger et al. [12] |
| Kroger et al. 1994 [12] | Cross-sectional (baseline of prospective study) Population-based (random stratified sampling) |
1600 | 53.2 (± 2.8) | Finland | Not reported | LSP (L2-L4), FN | Age BMI Menopausal status Parity Calcium Caffeine Smoking Alcohol Physical activity Grip strength |
Sub-sample of the Kuopio OSTPRE cohort; same study as Tuppurainen et al. [10] |
| Ryan et al. 1992 [18] | Cross-sectional Clinical sample (consecutive patients referred for OP risk assessment) |
627 | 53 (SD not reported) age range: 40–60 |
England | White: 100.0 | LSP (L1-L4), FN | Weight Age Age at menarche Years since menopause Oral contraceptive use Breastfeeding Smoking Alcohol Miles walked daily Family history of OP or fracture Social class |
|
| B. Studies evaluating clinical risk assessment tools | ||||||||
| Study | Study design/type of cohort | N | Age yr, mean (± SD) | Country of recruitment | Ethnic background, % of participants | Skeletal sites measured by DXA | Clinical risk assessment tools evaluated | Comments |
| Gourlay et al. 2005 [21] | Cross-sectional Clinical cohort (consecutive patients at OP clinic) |
2539 (sub-group of women aged 45–64) | 56.0 (± 5.4) | Belgium | White: 100.0 | FN | OST (age, weight) SCORE (age, weight, race, rheumatoid arthritis, personal low trauma fracture, estrogen therapy) ORAI (age, weight, estrogen use) |
|
| Cadarette et al. 2001 [20] | Cross-sectional (baseline of prospective study) Population-based (stratified random sampling) |
978 (sub-group of women aged 45–64) | Not reported by sub-group Whole cohort: 66.4 (± 8.8) |
Canada | Not reported for sub-group Whole cohort: White: 96.6 Asian: 1.8 Black: 0.3 Other: 1.3 |
FN | SCORE (see Gourlay) ORAI (see Gourlay) ABONE (age ≥ 65 y, weight <63.5 kg), estrogen use) NOF (age ≥ 65 y, weight < 57.6 kg, personal low trauma fracture, family history of fracture, smoker) Body weight criterion (weight <70 kg) |
|
Note: N = number of participants, SD = standard deviation, DXA = dual-energy x-ray absorptiometry, LSP = lumbar spine, FN = femoral neck, GT = greater trochanter, TB = total body, OP = osteoporosis, EPIC = early postmenopausal interventional cohort, BMI = body mass index (kg/m2 ), OSTPRE = Osteoporosis Risk Factor and Prevention Study, OST = Osteoporosis Self-Assessment Tool, SCORE = Simple Calculated Osteoporosis Risk Estimation, ORAI = Osteoporosis Risk Assessment Instrument, ABONE = age, body zize, no estrogen, NOF = National Osteoporosis Foundation
Risk factors for low BMD
Thirteen clinically relevant risk factors for low BMD in healthy women aged 40–60 years were identified (Table 3).
Table 3.
Summary of results of studies evaluating specific risk factors for low bone mineral density (BMD)
| Study | Quality | Measurement/categorization of factor | Outcome measures | Summary of results | Model covariate adjustment |
|---|---|---|---|---|---|
| 1. Calcium | |||||
Strength of evidence:
| |||||
| Ho et al. 2005 [13] | Good | current calcium: 100 mg/d units | OP at TB, LSP, total hip, FN, GT, inter-trochanter | Significant at one site only – inter-trochanter OR=0.85, 95% CI 0.74–0.97, R2 2.2, p=0.017 |
Education level, weight, YSM, age at menarche, PA, job type |
| Holm et al. 2002 [16] | Fair | current calcium: mg/d | BMD (g/cm2) at LSP, baseline and Year 2 2-yr change inBMD |
NS at baseline and 2-yr change in BMD Significant at Year 2, p=0.003, direction and magnitude of association not reported |
Age, weight, race, FSH, smoking, alcohol, caffeine, PA |
| Kroger et al. 1994 [12] | Fair | current calcium: mg/d | BMD (g/cm2) at LSP, FN | NS after adjusting for covariates | Menopausal status, age, weight, height, alcohol, smoking, caffeine, parity, PA, OCP use, grip strength |
| New et al. 1997 [17] | Fair | Current calcium: mg/d and upper/lower quartiles Past intake during childhood (≤12 y) or adulthood (20–30 y): low/medium/high milk intake |
BMD (g/cm2) at LSP, FN, GT, Ward’s triangle | Current intake: NS after adjusting for covariates Past childhood intake: NS Past adulthood intake: lower LSP BMD in low intake group (1.04±0.15 g/cm2) vs. medium intake (1.08±0.17 g/cm2) vs. high intake (1.07±0.17 g/cm2), p<0.03 |
Age, weight, height, PA, smoking, social status |
| 2. Physical activity | |||||
Strength of evidence:
| |||||
| Ho et al. 2005 [13] | Good | Hours per day:standing Walking Weight bearing load (carrying load >5 lbs) Mild activity vigorous activity |
OP at TB, LSP, total hip, FN, GT, inter-trochanter | Vigorous activity significant at TB OR=0.93, 95% CI 0.87–1.00, R2 1.1, p=0.044 Weight bearing load significant at inter-trochanter OR=0.62, 95% CI 0.41–0.96, R2 1.5, p=0.031 |
Education level, weight, age, YSM, age at menarche, calcium, job type, fruit and protein intake (TB only) |
| Coupland et al. 1999 [9] | Fair | Weight bearing activity: high vs. low intensity Allied Dunbar Fitness Survey:six levels Housework, gardening, cycling, swimming: h/wk Walking: h/wk, slow/average/brisk/fast pace, total number of walks Stair-climbing: # flights/d Total PA: h/wk Total sports PA: h/wk Peer comparison: much less active/slightly less/average/slightly more/much more |
BMD (g/cm2) at LSP, FN, GT, TB, total radius | Significant association between # flights of stairs climbed daily and BMD at TB (reg. coeff. 0.0001, p=0.012) and GT (reg. coeff. 0.001, p=0.016) Significantly higher GT BMD in walkers with brisk or fast pace (0.683 g/cm2) vs. average pace (0.678 g/cm2) vs. slow pace (0.630 g/cm2), p=0.013 |
Age, height, weight, YSM, duration of HRT, smoking, corticosteroid use, diagnosis of osteoarthritis, personal fracture history, family fracture history |
| Kroger et al. 1994 [12] | Fair | Total PA (regular exercise, daily walking or running, work): three levels | BMD (g/cm2) at LSP, FN | NS at LSP Significantly higher mean FN in high PA group (0.941 g/cm2) vs. moderate PA group (0.931 g/cm2) vs. low PA group (0.921 g/cm2), p<0.001 |
Age, menopausal status, weight, height |
| Holm et al. 2002 [16] | Fair | Prior 12 months PA (leisure, occupation, household): kcal/h Lifetime PA (from age of 15): categorization Unclear |
BMD (g/cm2) at LSP, baseline and Year 2 2-yr change in BMD |
NS | Age, weight, race, FSH, smoking, alcohol, caffeine |
| Ryan et al. 1992 [18] | Fair | Miles walked/d | BMD (g/cm2) at LSP, FN | NS | Age, weight, YSM, age of menarche, breastfeeding, smoking, alcohol, oral contraceptive use |
| New et al. 1997 [17] | Fair | Light, moderate and active PA: total # hours/d in each category | BMD (g/cm2) at LSP, FN, GT, Ward’s triangle | NS | Age, weight, height |
| 3. Smoking | |||||
| Strength of evidence: Inconsistent evidence for an association between current or past smoking and low BMD | |||||
| Takada et al. 1997 [15] | Fair | Current smoker: yes/no | low BMD (bottom 20th %ile) at distal 1/3 radius | NS | Age, menopausal status, lean mass, fat mass, alcohol, job type |
| Ryan et al. 1992 [18] | Fair | Current smoking: cigarettes/d | BMD (g/cm2) at LSP, FN | NS | Age, weight, YSM, age of menarche, breastfeeding, alcohol, # miles walked/d, oral contraceptive use |
| New et al. 1997 [17] | Fair | Current smoker: yes/no | BMD (g/cm2) at LSP, FN, GT, Ward’s triangle | LS BMD significantly lower in smokers (1.04±0.16 g/cm2) vs. non-smokers (1.07±0.16 g/cm2), p=0.02 (Note: unclear if significant after covariate adjustment) | Unclear |
| Holm et al. 2002 [16] | Fair | Lifetime smoking: total years | BMD (g/cm2) at LSP, baseline and Year 2 2-yr change in BMD |
Significant at baseline, p=0.018, direction and magnitude of effect not reported Significant at Year 2, p-value not reported Not associated with 2-yr change in BMD |
Age, weight, race, FSH, alcohol, caffeine, PA, |
| Grainge et al. 1998 [11] | Fair | Lifetime smoking: total months, pack-years, smoker vs. non-smoker at ages 20, 30, 40 | BMD (g/cm2) at LSP, FN, GT, TB, total radius | Negative association between total months smoking and all sites except FN LSP (reg. coeff. −0.0075, R2=0.8, p=0.046), GT (reg. coeff. −0.0072, R2=1.1, p=0.008), TB (reg. coeff. −0.0067, R2=1.2, p=0.005), total radius (−0.0027, R2=0.7, p=0.039) |
Age, YSM, weight, height, duration of HRT, PA, alcohol, caffeine, family history of fracture |
| Kroger et al. 1994 [12] | Fair | Lifetime smoking: pack-years | BMD (g/cm2) at LSP, FN | NS | Menopausal status, age, weight, height, parity, calcium, alcohol, caffeine, PA, OCP use, grip strength |
| 4. Alcohol consumption | |||||
Strength of evidence:
| |||||
| Holm et al. 2002 [16] | Fair | Current intake: ounces/d | BMD (g/cm2) at LSP, baseline and Year 2 2-yr change in BMD |
NS | Age, weight, race, FSH, PA, smoking, caffeine |
| Grainge et al. 1998 [11] | Fair | Current intake: g/wk Lifetime intake: g/wk at ages 20, 30, 40 |
BMD (g/cm2) at LSP, FN, GT, TB, total radius | NS | Age, YSM, weight, height, duration of HRT, PA, smoking, caffeine, family history of fracture |
| Takada et al. 1997 [15] | Fair | Current intake: yes/no for alcohol > 3d/wk | low BMD (bottom 20th %ile) at distal 1/3 radius | NS | Age, menopausal status, lean mass, fat mass, smoking, job type |
| Ryan et al. 1992 [18] | Fair | Current intake: units/wk | BMD (g/cm2) at LSP, FN | NS | Age, weight, YSM, age of menarche, breastfeeding, smoking, # miles walked/d, oral contraceptive use |
| New et al. 1997 [17] | Fair | Current intake: g/d | BMD (g/cm2) at LSP, FN, GT, Ward’s triangle | NS at FN, GT and Ward’s triangle Positive association with LSP (reg. coeff. 0.0014, R2 and p-value not reported) |
Weight, height, magnesium intake |
| Kroger et al. 1994 [12] | Fair | Current intake: g/wk | BMD (g/cm2) at LSP, FN | NS at FN Positive association with LSP (regression coefficient 0.00008, p=0.016) |
Menopausal status, age, weight, height, parity, calcium, coffee, smoking, PA, oral contraceptive use, grip strength |
| 5. Caffeine intake | |||||
Strength of evidence:
| |||||
| Holm et al. 2002 [16] | Fair | current intake (coffee, tea, cola): cups/d | BMD (g/cm2) at LSP, baseline and Year 2 2-yr change in BMD |
NS | Age, weight, race, FSH, PA, smoking, alcohol, |
| Grainge et al. 1998 [11] | Fair | Current intake (coffee, tea, cola): mg/d Lifetime intake (5-year intervals from age 15): mg/d |
BMD (g/cm2) at LSP, FN, GT, TB, total radius | NS | Age, YSM, weight, height, duration of HRT, PA, smoking, alcohol, family history of fracture |
| Kroger et al. 1994 [12] | Fair | Current intake (coffee): dl/d | BMD (g/cm2) at LSP, FN | NS | Menopausal status, age, weight, height, parity, calcium, alcohol, smoking, PA, OCP use, grip strength |
| 6. Age at menarche | |||||
| Strength of evidence: Inconsistent evidence for an association between older age at menarche and low BMD | |||||
| Ho et al. 2005 [13] | Good | Years | OP at TB, LSP, total hip, FN, GT, inter-trochanter | NS at LSP, FN, TB Older age at menarche associated with higher odds of OP at Total hip: OR=1.20, 95% CI 1.04–1.40, R2=2.6, p=0.016 GT: OR=1.17, 95% CI 1.03–1.34, R2=2.8, p=0.02 Intertrochanter: OR=1.22, 95% CI1/05–1.43, R2=2.7, p=0.01 |
Education level, weight, YSM, PA, protein, calcium, job type |
| Tuppurainen et al. 1995 [10] | Fair | Years | BMD (g/cm2) at LSP, FN | NS at FN Negatively associated with LSP (reg. coeff −0.0069, p=0.011) |
Menopausal status, weight, height, age, hysterectomy, oophorectomy, age at first delivery, lactation premenopausal amenorrhea, |
| Mizuno et al. 1995 [14] | Fair | Years | BMD (g/cm2) at LSP | NS | BMI, YSM, parity |
| Ryan et al. 1992 [18] | Fair | Years | BMD (g/cm2) at LSP, FN | NS | Age, weight, YSM, breastfeeding, smoking, alcohol, # miles walked/d, oral contraceptive use |
| 7. Reproductive history: parity and lactation | |||||
| Strength of evidence: Good evidence that there is no association between parity or lactation and low BMD | |||||
| Ho et al. 2005 [13] | Good | Number of pregnancies Months of lactation | OP at TB, LSP, total hip, FN, GT, inter-trochanter | NS, parity and lactation | Education level, weight, YSM, PA, protein, calcium, job type |
| Mizuno et al. 1995 [14] | Fair | Number of pregnancies | BMD (g/cm2) at LSP | NS | BMI, YSM, age at menarche |
| Tuppurainen et al. 1995 [10] | Fair | Number of pregnancies Age at first delivery Months of lactation |
BMD (g/cm2) at LSP, FN | NS, parity and lactation | Menopausal status, weight, height, age, age at menarche, hysterectomy, oophorectomy, premenopausal amenorrhea |
| Kroger et al. 1994 [12] (same study as [10]) | Fair | Number of deliveries | BMD (g/cm2) at LSP, FN | NS | Menopausal status, age, weight, height, calcium, alcohol, smoking, PA, OCP use, grip strength |
| Ryan et al. 1992 [18] | Fair | Months of lactation | BMD (g/cm2) at LSP, FN | NS | Age, weight, YSM, smoking, alcohol, # miles walked/d, oral contraceptive use |
| 8. History of premenopausal amenorrhea | |||||
| Strength of evidence: Insufficient evidence for an association between a history of amenorrhea and low BMD | |||||
| Tuppurainen et al. 1995 [10] | Fair | Premenopausal amenorrhea for >3 months before the age of 30: no/yes | BMD (g/cm2) at LSP, FN | NS | Menopausal status, weight, height, age, age at menarche, age at first delivery, lactation, hysterectomy, oophorectomy |
| 9. Menopausal status | |||||
| Strength of evidence: Good evidence that being post-menopausal is associated with low BMD | |||||
| Ho et al. 2005 [13] | Good | Total years since menopause | OP at TB, LSP, total hip, FN, GT, inter-trochanter | Significantly associated with higher odds of OP at all sites except GT TB: OR=1.19, 95% CI 1.11–1.27, R2=6.1, p=0.000 LSP: OR=1.18, 95% CI 1.11–1.26, R2=5.5, p=0.000 Total hip: OR=1.15, 95% CI 1.05–1.27, R2=3.3, p=0.004 FN: OR=1.09, 95% CI 1.03–1.16, R2=2.0, p=0.005 Intertrochanter: OR=1.22, 95% CI 1.05–1.43, R2=2.7, p=0.01 |
Education level, age, weight, PA, protein, calcium, job type |
| Mizuno et al. 1995 [14] | Fair | Total years since menopause | BMD (g/cm2) at LSP | Negative association with LSP (regression coefficient −0.014, p=0.0001) | BMI, age at menarche, number of pregnancies |
| Ryan et al. 1992 [18] | Fair | Total years since menopause | BMD (g/cm2) at LSP, FN | Adjusted correlation coefficient at LSP=0.243 and FN=0.216 (direction of association is unclear) | Age, weight, breastfeeding, smoking, alcohol, # miles walked/d, OCP use |
| Takada et al. 1997 [15] | Fair | Postmenopausal: no/yes | Low BMD (bottom 20th %ile) at distal 1/3 radius | Being postmenopausal associated with higher odds of having low BMD OR=1.72, 95% CI 1.37–2.18, p<0.001 |
Age, lean mass, fat mass, smoking, alcohol |
| Tuppurainen et al. 1995 [10] | Fair | Postmenopausal: no/yes | BMD (g/cm2) at LSP, FN | Significantly associated with lower BMD at LSP (reg. coeff. −0.0577, p=0.000) and FN(reg. coff. −0.0281, p=0.002) | Weight, height, age, age at menarche, age at first delivery, lactation, hysterectomy, oophorectomy, premenopausal amenorrhea |
| Kroger et al. 1994 [12] (same study as 10]) | Fair | Postmenopausal: no/yes | BMD (g/cm2) at LSP, FN | Significantly associated with lower BMD at LSP (reg. coeff. −0.0618, p=0.000) and FN(reg. coeff. −0.0307, p=0.000) | Age, weight, height, alcohol, smoking, caffeine, calcium, parity, PA, OCP use, grip strength |
| 10. Family history of osteoporosis or low trauma fracture | |||||
| Strength of evidence: Inconsistent evidence for an association between family history of osteoporosis or low trauma fracture and low BMD | |||||
| Keen et al. 1999 [19] | Fair | Self-reported fracture (wrist, hip) in female first-degree relative after 35 years of age: no/yes | BMD (g/cm2) at LSP, FN OP at LSP, FN |
History of fracture associated with lower BMD at LSP (mean difference of 0.04 g/cm2, 95% CI 0.0–0.08, p=0.02) and FN (mean difference of 0.03 g/cm2, 95% CI 0.0–0.05, p=0.02) Associated with higher odds of OP at LSP but not FN OR=1.82, 95% CI 1.08–3.05, p=0.02 |
No difference between groups in age, BMI, menopause age and duration, smoking status, HRT use |
| Grainge et al. 1998 [11] | Fair | Self-reported fracture in female first-degree relative at any age: no/yes | BMD (g/cm2) at LSP, FN, GT, TB, total radius | Reported R2 values for six major risk factors including family history of fracture but unclear if significant R2: LSP 0.6%, FN 1.6%, GT 1.1%, radius 0.9%, TB 1.5% | Age, YSM, weight, height, HRT use, smoking |
| Ryan et al. 1992 [18] | Fair | Family history of OP or fracture (wrist, spine, femur) in parents, siblings: no/yes | BMD (g/cm2) at LSP, FN | NS | Unclear |
| 11. Race | |||||
| Strength of evidence: Insufficient evidence that race is associated with BMD | |||||
| Holm et al. 2002 [16] | Fair | White vs. black | BMD (g/cm2) at LSP, baseline and Year 2 2-yr change in BMD |
White women had significantly lower BMD at baseline, p=0.001 and at Year 2 (p-value not reported (magnitude of effect not reported) Race not related to 2 year change in BMD |
Age, weight, FSH, calcium, smoking, alcohol, PA |
| 12. Chronological age | |||||
| Strength of evidence: Inconsistent evidence that older age is associated with lower BMD | |||||
| Ho et al. 2005 [13] | Good | Age: years | OP at TB, LSP, total hip, FN, GT, inter-trochanter | Older age associated with higher odds of OP at GT only OR=1.09, 95% CI 1.02–1.17, R2=2.0, p=0.017 | Education level, weight, YSM, age at menarche, PA, protein, calcium |
| Takada et al. 1997 [15] | Fair | Age: years | low BMD (bottom 20th %ile) at distal 1/3 radius | Associated with higher odds of having low BMD OR=1.15, 95% CI 1.12–1.18, p<0.001 | Menopausal status, lean mass, fat mass |
| Tuppurainen et al. 1995 [10] | Fair | Age: years | BMD (g/cm2) at LSP, FN | Negatively associated with BMD at LSP (reg. coeff. −0.009, p=0.000) and FN (reg. coeff. −0.007, p=0.000) | Menopausal status, weight, height, age at menarche, hysterectomy, oophorectomy, parity, premenopausal amenorrhea, lactation |
| Kroger et al. 1994 [12] (same study [10]) | Fair | Age: years | BMD (g/cm2) at LSP, FN | Negatively associated with BMD at LSP (reg. coeff. −0.006, p=0.000) and FN (reg. coeff. −0.0032, p=0.005) | Menopausal status, weight, height, alcohol, smoking, caffeine, calcium, parity, PA, OCP use, grip strength |
| Holm et al. 2002 [16] | Fair | Age: years | BMD (g/cm2) at LSP, baseline and Year 2 2-yr change in BMD |
Borderline associated with BMD at baseline, p=0.045 (direction and magnitude of effect not reported) Unclear if associated with Year 2 BMD. NS: 2 year change in BMD |
Weight, race, FSH, smoking, alcohol, PA, caffeine |
| Mizuno et al. 1995 [14] | Fair | Age: years | low (t-score < 2) vs. high (t-score>1) BMD at LSP |
Significantly older mean age in low BMD group (56.3±5.0) vs. high BMD group (50.8±3.5), p<0.0001 | Unadjusted |
| New et al. 1997 [17] | Fair | Age: years | BMD (g/cm2) at LSP, FN, GT, Ward’s triangle | NS | Unclear |
| Ryan et al. 1992 [18] | Fair | Age: years | BMD (g/cm2) at LSP, FN | NS | Age at menarche, YSM, weight, lactation, smoking, alcohol, miles walked /d, OCP use |
| 13. Body weight | |||||
| Strength of evidence: Good evidence for an association between low body weight and low BMD | |||||
| Ho et al. 2005 [13] | Good | Weight: 10 kg bands | OP at TB, LSP, total hip, FN, GT, inter-trochanter | Negative association with odds of OP at all sites TB: OR=0.52, 95% CI 0.40–0.68, R2=5.7, p=0.000 LSP: OR=0.43, 95% CI 0.34–0.55, R2=9.1, p=0.000 Total hip: OR=0.23, 95% CI 0.15–0.36, R2=16.5, p=0.000 FN: OR=0.31, 95% CI 0.24–0.40, R2=18.9, p=0.000 GT: OR=0.32, 95% CI 0.22–0.45, R2=12.3, p=0.000 Intertrochanter: OR=0.23, 95% CI 0.15–0.35, R2=16.1 |
Education level, age or YSM, age at menarche, calcium, protein, fruit intake, PA, job type |
| Holm et al. 2002 [16] | Fair | Weight: kg | BMD (g/cm2) at LSP, baseline and Year 2 2-yr change in BMD |
Significant at baseline and year 2, p=0.0001 (direction and magnitude of effect not reported) NS: 2-year change in BMD |
Age, race, FSH, calcium, smoking, alcohol, caffeine, PA |
| New et al. 1997 [17] | Fair | Weight: kg | BMD (g/cm2) at LSP, FN, GT, Ward’s triangle | Positively associated with LSP BMD (reg. coeff. 0.004 (p-value not reported) No data reported for regression analysis at other sites Weight positively correlated with BMD at all sites LSP: r=0.36; FN: r=0.41; GT: 0.39; Ward’s: r=0.49, all p<0.001, simple correlations - unadjusted |
Age, height, smoking, PA, calcium, magnesium, potassium, social status Correlation analysis, unadjusted |
| Mizuno et al. 1995 [14] | Fair | BMI: kg/m2 | BMD (g/cm2) at LSP | Positively associated with LSP BMD (reg. coeff. 0.012, p=0.02) | YSM, age at menarche, number of pregnancies |
| Tuppurainen et al. 1995 [10] | Fair | Weight: kg | BMD (g/cm2) at LSP, FN | Positively associated with BMD at LSP (reg. coeff. 0.004, p=0.000) and FN (reg. coeff. 0.005, p=0.000) | Menopausal status, age, age at menarche, hysterectomy, oophorectomy, parity, lactation, premenopausal amenorrhea |
| Kroger et al. 1994 [12] (same study [10]) | Fair | Weight: kg | BMD (g/cm2) at LSP, FN | Positively associated with BMD at LSP (reg. coeff. 0.004, p=0.000) and FN (reg. coeff. 0.005, p=0.000) | Menopausal status, age, height, alcohol, smoking, caffeine, calcium, parity, PA, OCP use, grip strength |
| Ryan et al. 1992 [18] | Fair | Weight: 5 kg bands | BMD (g/cm2) at LSP, FN | Adjusted correlation coefficient at LSP=0.22 and FN=0.20 Unadjusted reg. coeff. LSP (0.005) and FN (0.004) |
YSM, age, age at menarche, lactation, smoking, # miles walked/d, OCP use Regression analysis unadjusted |
| Clinical risk assessment tools | |||||
| Gourlay et al. 2005 [21] | Good | OST (age, weight) SCORE (age, weight, race, RA, fracture, estrogen) ORAI (age, weight, estrogen) |
OP at FN | OST (score ≤ 1): Sensitivity: 89.2%, Specificity: 45.0% SCORE (score ≥ 7): Sensitivity: 88.5%, Specificity: 46.2% ORAI (score ≥ 8): Sensitivity: 88.5%, Specificity: 46.2% |
|
| Cadarette et al. 2001 [20] | Good | SCORE (a/a) ORAI (a/a) NOF (age, weight, personal and family history of fracture, smoker) Weight only criterion |
OP at FN | SCORE (score ≥ 6): Sensitivity: 97.8%, Specificity: 32.9% ORAI (score ≥ 9): Sensitivity: 87.0%, Specificity: 63.0% ABONE (score≤2): Sensitivity 50.0%, Specificity: 79.0% NOF (score ≥1): Sensitivity: 80.4%, Specificity: 40.6% Body weight <70 kg: Sensitivity: 89.1%, Specificity: 49.4% |
|
Note: OR = odds ratio, 95% CI = 95% confidence interval, reg.coeff. = regression coefficient generated by linear regression analysis, OP = osteoporosis, defined as having a T-score ≤ −2.5, YSM = years since menopause, LSP = lumbar spine, FN = femoral neck, GT = greater trochanter, TB = total body, PA = physical activity, BMI = body mass index (kg/m2 ), NS = not statistically significant (p>0.05), FSH = follicle stimulating hormone, OCP = oral contraceptive, HRT = hormone replacement therapy, OST = Osteoporosis Self-Assessment Tool, SCORE = Simple Calculated Osteoporosis Risk Estimation, ORAI = Osteoporosis Risk Assessment Instrument, NOF = National Osteoporosis Foundation, RA = rheumatoid arthritis
Calcium intake
There is inconsistent evidence for an association between current calcium intake and BMD and insufficient evidence for an association between past calcium intake and BMD. Four studies investigated the relationship between self-reported current calcium intake and BMD [12, 13, 16, 17]. No association was demonstrated between current calcium intake and BMD in two studies [12, 17]. A third study measured BMD at six sites and reported decreased odds of OP with increased calcium intake at the inter-trochanteric site only [13]. The fourth study [16] did not find a relationship at baseline or with 2-year change in BMD, but reported a significant association at Year 2 and noted that calcium intake increased significantly over the 2 years. The direction and magnitude of this association was not reported. Only one study also evaluated past calcium intake and demonstrated an association between lower spinal BMD and low milk consumption during early adulthood, but not with low milk consumption during childhood [17].
Physical activity
There is inconsistent evidence of an association between current physical activity (PA) and BMD and insufficient evidence of an association between past PA and BMD. Six studies investigated the effect of various types of PA on bone mass [9, 12, 13, 16–18] of which three studies reported a significant positive effect [9, 12, 13]. Ho et al. [13] reported decreased odds of OP at the total body (TB) associated with ‘vigorous activity’ and at the inter-trochanter site associated with ‘weight-bearing activity’. Neither factor explained >1.5% of total variability in the odds of having OP. Coupland et al. [9] used six measures of PA and found that only stair climbing was associated with increased TB and trochanteric BMD and that fast walkers had higher trochanteric BMD than slow walkers. The strength of these associations was not reported. Kroger et al. [12] reported that overall PA (regular exercise, daily walking, occupational activity) predicted BMD at the FN but not at the LSP. In contrast to these reports, three studies found no association with BMD and either lifetime or current PA [16], miles walked per day [18] or the number of daily hours of work and leisure time PA [17].
Smoking
There is inconsistent evidence that smoking is associated with low BMD. Six studies examined smoking and BMD—three evaluated lifetime smoking history [11, 12, 16], while three considered only current smoking [15, 17, 18]. Three studies did not demonstrate a significant relationship [12, 15, 18]. One prospective study documented an association between lifetime number of years smoked and baseline and year 2 spinal BMD (the direction and magnitude of the associations were not reported); smoking was not related to 2-year change in BMD [16]. Grainge et al. [11] identified a negative association between total lifetime months of smoking and BMD at LSP, TB, greater trochanter and radius but not FN. However, smoking explained only ≤ 1.2% of the total variability in bone density at these sites. Finally, one study reported that spinal BMD was lower in current smokers than non-smokers (p<0.02), but it was unclear whether this association remained significant after adjusting for confounding factors [17].
Alcohol consumption
There is fair evidence that moderate alcohol consumption (< 150 g/wk or < 12 drinks/wk) is not associated with lower BMD and insufficient evidence for an association between past consumption or high consumption and BMD. Six studies investigated the relationship between current alcohol intake and BMD [11, 12, 15–18] and one also considered lifetime consumption [11]. Four studies reported no association between alcohol consumption and BMD [11, 15, 16, 18] while two demonstrated a small positive relationship with spinal BMD [12, 17].
Caffeine intake
There is fair evidence that current caffeine intake is not associated with BMD and insufficient evidence for an association between past intake and BMD. Three studies evaluated the relationship between current caffeine intake and BMD [11, 12, 16], and one also examined lifetime intake [11]. An association between caffeine intake and BMD was not detected in any study.
Age at menarche
There is inconsistent evidence that older age at menarche is associated with lower BMD. Four studies investigated the association between older age at menarche and BMD [10, 13, 14, 18]. Two studies found no association [14, 18]. Ho et al [13] reported increased odds of OP for each year of age at menarche at the total hip, trochanter and intertro-chanter but not FN. In this study, age at menarche contributed 2.6 to 2.8% of the total variance of BMD values after adjusting for body weight and years since menopause. Tuppurainen et al. [10] also reported a negative association between older age at menarche and spinal BMD but not FN, after adjusting for weight, age and menopausal status.
Reproductive history: parity and lactation
There is good evidence that there is no association between parity or lactation and lower BMD. Four studies investigated the relationship between parity and BMD and none reported a significant association [10, 12–14]. Two of these studies [10, 13] and one additional study [18] examined the relationship between breastfeeding and BMD and found no association.
History of amenorrhea
There is insufficient evidence of an association between a history of amenorrhea and lower BMD. Tuppurainen et al. [10] investigated the effect of three or more consecutive months of amenorrhea before the age of 30 on BMD and did not find a relationship.
Menopausal status or years post-menopause
There is good evidence that menopausal status is associated with BMD. This factor was evaluated in six studies and all demonstrated a negative association between either menopausal status or years since menopause and BMD [10, 12–15, 18]. Takada et al. [15] found that being post-menopausal increased the odds of being below the 20th percentile of BMD at the radius while Tuppurainen et al. [10] reported that post-menopausal status was associated with a decrease in both spinal and FN BMD. Kroger et al. [12] concur with these findings in the same study population. Ho et al. [13] reported increased odds of OP at the spine and FN with each year post-menopause, accounting for 5.5% and 2.0% of total variability at these sites, respectively, after adjusting for weight and age. Mizuno et al. [14] reported a decrease in LSP BMD for each year post-menopause and Ryan et al. [18] noted a correlation between years since menopause and BMD at both the spine and FN, although the direction of the correlation was unclear. Two additional studies [9, 11] adjusted for menopausal status but did not provide specific data.
Family history of OP or low trauma fracture
There is inconsistent evidence that either a family history of OP or low trauma fracture is associated with lower BMD. Only three studies addressed this factor [11, 18, 19]. Keen et al. [19] reported that having a family history of low trauma fracture in female first-degree relatives after the age of 35, was associated with increased odds of spinal OP but not OP at FN. Grainge et al. [11] noted that a history of low trauma fracture sustained at any age by a mother or sister was one of six major risk factors for lower BMD at five skeletal sites, but it is unclear whether it was statistically significant. After adjusting for other risk factors, family history contributed ≤ 1.6% of total variability in BMD at any site. The third study [18] reported no association between family history and BMD, but the methodology used to ascertain family history was unclear.
Race
There is insufficient evidence that race is associated with lower BMD. Only one study had a racially mixed study population with 74% white, 21% black and 4.5% listed as other [16]. In this study, white women had significantly lower BMD at baseline and Year 2 after adjusting for age, weight, menstrual status, calcium and smoking history, but race was not significantly related to 2-year change in density. Results were not provided regarding the strength of this association.
Older age
There is inconsistent evidence that within the 40–60 year old age group, older age is associated with lower BMD after adjustment for menopausal status. Eight studies evaluated the relationship between age and BMD [10, 12–18]. Four of these studies reported a negative association [10, 12, 13, 15]. Ho et al. [13] reported an increase in odds of OP at the trochanter for each yearly increase in age with age contributing 2% to the total variance in BMD values. Age was not significantly associated with BMD at five other skeletal sites after adjusting for years since menopause. Takada et al. [15] found that each yearly increase in age was associated with increased odds of low BMD (below the 20th percentile) at the radius. Kroger et al. [12] reported that each year of age was associated with a decrease in BMD at both the LSP and FN; Tuppurainen et al. [10] reporting similar results in the same study population. In a fifth study, the association between age and BMD was only borderline significant (p=0.045) [16]. Finally, a sixth study noted that women with BMD T-scores < − 2 were significantly older than those with T-scores > −1 (p<0.0001) but they did not report results for age adjusted for other risk factors [14]. Two studies found no relationship between age and BMD [17, 18].
Body weight
There is good evidence that lower body weight is associated with lower BMD. Seven studies demonstrated a positive association between lower body weight and/or BMI and BMD at one or more skeletal sites [10, 12–14, 16–18]. The remaining studies either adjusted for lean mass [15] or weight [9, 11, 19]. but did not provide data to indicate its statistical significance. One of the largest studies (n=1600) [12], reported that each kg increase in weight corresponded to an increase in BMD at the LSP by 0.004 g/cm2 and FN by 0.005 g/cm2. Ho et al. [13] found that weight was the best predictor of OP at each of six skeletal sites measured and accounted for 18.9% of the total variability in the odds of having OP at the FN, 9.1% at the LSP and 5.7% at the TB.
Clinical risk assessment tools
Two studies evaluated risk assessment tools, developed in postmenopausal women aged 60 years or greater, to determine whether they were predictive in younger women aged 45–65 years [20, 21]. Gourlay et al. [21] compared three tools, each including age and weight as risk factors: the Osteoporosis Risk Assessment Tool (OST); the Osteoporosis Risk Assessment Index (ORAI), which also includes estrogen use; and the Simple Calculated Osteoporosis Risk Estimation (SCORE), which includes rheumatoid arthritis diagnosis, low trauma fracture after age 45 years, race, and estrogen therapy. Sensitivity and specificity was similar across tools and were reported, respectively, as: 89.2% and 45% for the OST (cut-point <2); 88.5% and 46.2%, for the ORAI (cut-point ≥ 8); and 88.5% and 39.8%, for the SCORE (cut-point ≥ 7).
Cadarette et al. [20] evaluated the diagnostic properties of the SCORE, the ORAI and three additional risk assessment tools in a population-based cohort of Canadian women. Similar to the SCORE and ORAI, two of the tools –the US National Osteoporosis Foundation (NOF) guidelines and the Age, Body Size, No Estrogen (ABONE) – include both age and weight as risk factors. In addition, the NOF includes personal and family history of low trauma fracture and smoking as risk factors and the ABONE includes estrogen use. The third tool includes only a simple body weight criterion (BWC) of <70 kg. The SCORE had the highest sensitivity (97.8%) for identifying women with a BMD T score at the FN of ≤ −2.5, with a specificity of 32.9%. The high specificity may be due to the inclusion of rheumatoid arthritis in this decision tool, a known cause of secondary osteoporosis. The remaining tools target women at risk for primary osteoporosis; of these tools, the BWC and ORAI had the highest sensitivities (89% and 87%, respectively) with specificities of 49.4% and 63%, respectively. The NOF guidelines had a sensitivity of 80.4% and specificity of 40.6% while the ABONE had a sensitivity of only 50% with specificity of 79%.
Discussion
The purpose of this systematic review was to identify clinical risk factors for low BMD in healthy women aged 40–60 years that could be used to discriminately select appropriate candidates for BMD assessment, thereby potentially reducing unnecessary testing. The current review identified few studies that focused specifically on risk factors for low BMD in this age group.. Those that did were frequently limited by methodological weaknesses, in particular, lack of adjustment for important confounders such as body weight. Consequently, only 13 studies met the eligibility and quality criteria for this systematic review. Among these studies, thirteen potential risk factors for low BMD were identified. Based on the strength of evidence, in addition to menopausal status, only low body weight can be considered, with confidence, as an important risk factor for low BMD in healthy 40–60 year old women. There was good evidence that parity and lactation are not risk factors and fair evidence that neither moderate alcohol consumption or caffeine intake are associated with low BMD. There was insufficient or inconsistent evidence for the association of other risk factors identified, with low BMD. Therefore, with the exception of low body weight, the clinical risk factors used to select high risk older women (≥ 65 years of age) for BMD testing are not useful in this regard for perimenopausal women.
Body weight is undoubtedly a proxy for other factors, including physical activity, age at menarche, nutritional status and lean body mass, and therefore might be considered a simple ‘composite risk factor’ for low BMD, and therefore useful in identifying women at risk, for bone density testing. This is supported by Cadarette et al. [20] who demonstrated that using a simple body weight criterion performed as well or better in discriminating women with significantly reduced BMD than tools that included additional risk factors. The ideal weight cut-point to recommend women for BMD testing was not identified as insufficient data was provided in the studies reviewed. The NOF guidelines recommend BMD testing at a weight <57.7 kg, based on data from older women [22]. In comparison, data from a small study of 175 Caucasian women aged 28 – 74 years recommended BMD testing at a much higher weight cut-point of < 70 kg [23]. In 45–60 year old women, the NOF guidelines selected 80% of cases with low BMD (T score of ≤ −2.5), while 89% of cases were selected using the cut-point of <70 kg [20]. The NOF had a slightly lower specificity, but for screening purposes, we would contend that higher sensitivity takes precedence over lower specificity. Additional research is necessary to ascertain optimal weight cut-point(s) that will discriminate women of different races, age 40–60 years with versus without low BMD. Until such information is available, we would suggest the use of <70 kg as a cut-point to select women in this age group for BMD testing.
Self-reported postmenopausal status or, a greater number of years since menopause, were consistently reported to be associated with low BMD, even after adjustment for body weight. Results were inconsistent regarding the effect of increasing age on BMD, but most studies found that the effect of age was greatly diminished after controlling for menopausal status. Although good evidence exists that post-menopausal status is associated with low BMD, this is a non-discriminatory factor which would result in the testing of all women at some point before the currently recommended age of 65 years [28]. It is therefore not useful for the identification of high risk individuals for BMD testing.
This review found no evidence of an association between moderate alcohol consumption and caffeine intake and lower BMD. Evidence for the effect of other lifestyle factors was either inconsistent (current calcium intake, smoking and current PA) or insufficient (calcium intake and PA during childhood). In a large Canadian cohort of premenopausal women, being physically inactive during adolescence was an independent predictor of lower peak BMD [24], suggesting that prior history of PA may be particularly important to investigate further in women age 40–60 years. The effect of high alcohol consumption on BMD in this age group remains unknown, as there were insufficient numbers of participants with high alcohol intake in the studies in this review.
Good evidence exists that neither parity nor breastfeeding are associated with low BMD, but there is insufficient evidence to draw conclusions regarding other reproductive factors, such as age at menarche or a history of amenorrhea. Two of four studies documented a negative association between age at menarche and low BMD. However, age at menarche explained only 2.5% of the variability in predictive models of OP at the hip, after adjusting for body weight and duration of menopause [13]. This finding, in conjunction with inconsistent results across the studies, suggests that age at menarche may at best serve as a minor risk factor for lower BMD in the age group of interest. Only one study investigated the effect of a past history of amenorrhea on BMD, and found no association [10]. A possible explanation for this negative result is that, while prolonged amenorrhea related to low estrogen levels is well recognized to result in reduced bone mass [25–27], recovery of menstruation may lead to a regain of bone mass, and thus the effect of amenorrhea may not be apparent in later life.
The effect of self-reported family history of OP on low BMD was evaluated in one study and no association was identified [18]. However, the method of determining family history was not described. Two studies investigated whether a fragility fracture sustained by a first degree female relative was a risk factor for low BMD [11, 19]. While both studies reported a positive relationship, one showed that, after adjusting for important confounders, the contribution of a fragility fracture to explaining the variability in BMD was minimal (0.6% at the LSP and 1.6% at the FN) [11]. However, it is unknown whether using other definitions of family history of fracture, including non-clinical spine fractures and fractures in non-female first degree relatives, would generate different results.
Finally, only one study evaluated the relationship between race and BMD, and found that white women had lower BMD than did black women [16]. Although there was insufficient evidence regarding race in this review, normative data collected by makers of DXA machines show clear differences by race, with Caucasians and Asians being most at risk for low BMD. Race may be important not only as a risk factor for low BMD, but as a factor that may modify the effect of other risk factors. In particular, the optimal weight cut-point for discriminating low versus normal BMD may well differ by race. Further evaluation of the relationship of race to BMD, as well as studies that examine risk factors for low BMD within racial subgroups are warranted to determine if differential effects exist.
A limitation to summarizing the results of the 13 studies included in this review was the inconsistency in outcomes used across the studies. The majority of studies used BMD as a continuous variable, while several used a dichotomous outcome of no OP vs. OP (T-score ≤ −2.5) or normal vs. low BMD based on percentiles. First, this prevented us from performing a meta-analysis. Second, the clinical significance of a statistically significant association between a particular risk factor and “lower BMD” (i.e., BMD used as a continuous variable) is difficult to ascertain. We would suggest that future studies report dichotomous outcomes using accepted definitions of normal vs. low BMD to enhance the clinical relevance of the results and enable comparisons across studies.
In summary, this systematic literature review found that there is good evidence that post-menopausal status and lower body weight are risk factors for lower BMD in women aged 40–60 years, and propose that body weight may be useful for selecting high risk individuals for BMD assessment. Many women in this age group undergo BMD testing, perhaps indicating an increasing concern about osteoporosis as they approach the menopause. However, the risk of fragility fracture in this age group is low and many women may be unnecessarily tested. Based on the current evidence, we would suggest that, in the absence of co-morbidities known to be associated with low BMD, only women with a body weight of < 70 kg be selected for testing, while providing reassurance to women without these risk factors that their fracture risk is minimal. As this weight cut-point was determined in a small sample of Caucasian women with a large age range, further research is required to determine ideal race-specific weight cut-points to optimize the discrimination of low versus normal BMD in women aged 40–60 years. Moreover, additional evaluation of the many factors for which there was inconsistent or insufficient evidence is needed. This knowledge will increase our ability to identify healthy young women with asymptomatic low BMD who might benefit from BMD testing and early intervention with the aim of fracture prevention, while eliminating unnecessary testing.
Acknowledgments
The authors gratefully acknowledge Osteoporosis Canada for coordinating this project.
No sources of support were received for this project.
Footnotes
Conflicts of interest: None.
Contributor Information
E. J. Waugh, Osteoporosis Research Program, Women’s College Hospital, Toronto, ON, Canada
M.-A. Lam, Osteoporosis Research Program, Women’s College Hospital, Toronto, ON, Canada
G. A. Hawker, Osteoporosis Research Program and Department of Medicine, Women’s College Hospital, Toronto, ON, Canada
J. McGowan, Institute of Population Health, Ottawa Health Research Institute, Ottawa, ON, Canada. Department of Medicine and Family Medicine, University of Ottawa, Ottawa, ON, Canada
A. Papaioannou, Division of Geriatrics, Department of Medicine, Hamilton Health Sciences and McMaster University, Hamilton, ON, Canada
A. M. Cheung, Department of Medicine, University Health Network and University of Toronto, Toronto, ON, Canada
A. B. Hodsman, Division of Nephrology, Department of Medicine, St. Joseph’s Health Care, London, ON, Canada
W. D. Leslie, Departments of Medicine and Radiology, University of Manitoba, Winnipeg, MB, Canada
K. Siminoski, Department of Radiology and Diagnostic Imaging and Division of Endocrinology and Metabolism, Department of Medicine, University of Alberta, Edmonton, AB, Canada
S. A. Jamal, Osteoporosis Research Program and Department of Medicine, Women’s College Hospital, Toronto, ON, Canada
References
- 1.Consensus development conference. Diagnosis, prophylaxis and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.NIH. Osteoporosis prevention, diagnosis and therapy. NIH consensus statements. 2000;17:1–45. [PubMed] [Google Scholar]
- 3.WHO. Report of a WHO Study Group (ref type: report) World Health Organization; Geneva: 2003. Prevention and management of osteoporosis; pp. 1–165. Technical Report Series 919. [Google Scholar]
- 4.Jagal SB. Bone density testing. In: Stewart DE, Ferris L, Hyman I, et al., editors. Ontario Women’s Health Status Report. 2002. pp. 113–120. ref type: report. [Google Scholar]
- 5.Osteoporosis Action Plan Committee. Osteoporosis Action Plan: an osteoporosis strategy for Ontario. Report of the Osteoporosis Action Plan Committee to the Ministry of Long-term Care. 2003:1–86. ref type: report.
- 6.Brown JP, Josse RG. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ. 2002;167:S1–S34. [PMC free article] [PubMed] [Google Scholar]
- 7.Sackett DL, Haynes RB, Guyatt GH, et al. Clinical epidemiology: a basic science for clinical medicine. Little, Brown and Company; Boston: 1991. [Google Scholar]
- 8.Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prevent Med. 2001;20:S21–S35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 9.Coupland CA, Cliffe SJ, Bassey EJ, et al. Habitual physical activity and bone mineral density in postmenopausal women in England. Int J Epidemiol. 1999;28:241–246. doi: 10.1093/ije/28.2.241. [DOI] [PubMed] [Google Scholar]
- 10.Tuppurainen M, Kroger H, Saarikoski S, et al. The effect of gynecological risk factors on lumbar and femoral bone mineral density in peri-and postmenopausal women. Maturitas. 1995;21:137–145. doi: 10.1016/0378-5122(94)00878-b. [DOI] [PubMed] [Google Scholar]
- 11.Grainge MJ, Coupland CA, Cliffe SJ, et al. Cigarette smoking, alcohol and caffeine consumption, and bone mineral density in postmenopausal women. The Nottingham EPIC Study Group. Osteoporos Int. 1998;8:355–363. doi: 10.1007/s001980050075. [DOI] [PubMed] [Google Scholar]
- 12.Kroger H, Tuppurainen M, Honkanen R, et al. Bone mineral density and risk factors for osteoporosis - a population-based study of 1600 perimenopausal women. Calcif Tissue Int. 1994;55:1–7. doi: 10.1007/BF00310160. [DOI] [PubMed] [Google Scholar]
- 13.Ho SC, Chen YM, Woo JL. Educational level and osteoporosis risk in postmenopausal Chinese women. Am J Epidemiol. 2005;161:680–690. doi: 10.1093/aje/kwi047. [DOI] [PubMed] [Google Scholar]
- 14.Mizuno K, Suzuki A, Ino Y, et al. Postmenopausal bone loss in Japanese women. Int J Gynaecol Obstet. 1995;50:33–39. doi: 10.1016/0020-7292(95)02419-d. [DOI] [PubMed] [Google Scholar]
- 15.Takada H, Washino K, Iwata H. Risk factors for low bone mineral density among females: the effect of lean body mass. Prev Med. 1997;26:633–638. doi: 10.1006/pmed.1997.0170. [DOI] [PubMed] [Google Scholar]
- 16.Holm K, Dan A, Wilbur J, et al. A longitudinal study of bone density in midlife women. Health Care Women Int. 2002;23:678–691. doi: 10.1080/07399330290107421. [DOI] [PubMed] [Google Scholar]
- 17.New SA, Bolton-Smith C, Grubb DA, et al. Nutritional influences on bone mineral density: a cross-sectional study in premenopausal women. Am J Clin Nutr. 1997;65:1831–1839. doi: 10.1093/ajcn/65.6.1831. [DOI] [PubMed] [Google Scholar]
- 18.Ryan PJ, Blake GM, Fogelman I. Postmenopausal screening for osteopenia. Br J Rheumatol. 1992;31:823–828. doi: 10.1093/rheumatology/31.12.823. [DOI] [PubMed] [Google Scholar]
- 19.Keen RW, Hart DJ, Arden NK, et al. Family history of appendicular fracture and risk of osteoporosis: a population-based study. Osteoporos Int. 1999;10:161–166. doi: 10.1007/s001980050211. [DOI] [PubMed] [Google Scholar]
- 20.Cadarette SM, Jaglal SB, Murray TM, et al. Canadian Multicentre Osteoporosis Study. Evaluation of decision rules for referring women for bone densitometry by dual-energy x-ray absorptiometry. JAMA. 2001;286:57–63. doi: 10.1001/jama.286.1.57. [DOI] [PubMed] [Google Scholar]
- 21.Gourlay ML, Miller WC, Richy F, et al. Performance of osteoporosis risk assessment tools in postmenopausal women aged 45–53 years. Osteoporos Int. 2005;16:921–927. doi: 10.1007/s00198-004-1775-2. [DOI] [PubMed] [Google Scholar]
- 22.National Osteoporosis Foundation. Physician’s Guide to Prevention and Treatment of Osteoporosis. Excerpta Medica Inc; Bell Mead, New Jersey: 1999. [Google Scholar]
- 23.Michaelsson K, Bergstrom R, Mallmin H, et al. Screening for osteopenia and osteoporosis: selection by body composition. Osteoporos Int. 1996;6:120–126. doi: 10.1007/BF01623934. [DOI] [PubMed] [Google Scholar]
- 24.Rubin LA, Hawker GA, Peltekova VD, et al. Determinants of peak bone mass: clinical and genetic analyses in a young female Canadian cohort. J Bone Miner Res. 1999;14:633–643. doi: 10.1359/jbmr.1999.14.4.633. [DOI] [PubMed] [Google Scholar]
- 25.Cobb KL, Bachrach LK, Greendale G, et al. Disordered eating, menstrual irregularity and bone mineral density in female runners. Med Sci Sports Exerc. 2003;35:711–719. doi: 10.1249/01.MSS.0000064935.68277.E7. [DOI] [PubMed] [Google Scholar]
- 26.Davies MC, Hall JL, Jacobs HS. Bone mineral loss in young women with amenorrhoea. BMJ. 1990;301:790–793. doi: 10.1136/bmj.301.6755.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon CM, Nelson LM. Amenorrhea and bone health in adolescents and young women. Curr Opin Obstet Gynecol. 2003;15:377–384. doi: 10.1097/00001703-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Brown JP, Josse RG. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ. 2002;167:S1–S34. [PMC free article] [PubMed] [Google Scholar]