Abstract
Summary
Based on a population age 50+, significant excess costs relative to matched controls exist for patients with incident fractures that are similar in relative magnitude to other chronic diseases such as stroke or heart disease. Prevalent fractures also have significant excess costs that are similar in relative magnitude to asthma/chronic obstructive pulmonary disease.
Introduction
Cost of illness studies for osteoporosis that only include incident fractures may ignore the long-term cost of prevalent fractures and primary preventive care. We estimated the excess costs for patients with incident fractures, prevalent fractures, and nonfracture osteoporosis relative to matched controls.
Methods
Men and women age 50+ were selected from administrative records in the province of Manitoba, Canada for the fiscal year 2007–2008. Three types of cases were identified: (1) patients with incident fractures in the current year (2007–2008), (2) patients with prevalent fractures in previous years (1995–2007), and (3) nonfracture osteoporosis patients identified by specific pharmacotherapy or low bone mineral density. Excess resource utilization and costs were estimated by subtracting control means from case means.
Results
Seventy-three percent of provincial population age 50+ (52 % of all men and 91 % of all women) were included (121,937 cases, 162,171 controls). There were 3,776 cases with incident fracture (1,273 men and 2,503 women), 43,406 cases with prevalent fractures (15,784 men and 27,622 women) and 74,755 nonfracture osteoporosis cases (7,705 men and 67,050 women). All incident fractures had significant excess costs. Incident hip fractures had the highest excess cost: men $44,963 (95 % CI: $38,498–51,428) and women $45,715 (95 % CI: $36,998–54,433). Prevalent fractures (other than miscellaneous or wrist fractures) also had significant excess costs. No significant excess costs existed for nonfracture osteoporosis.
Conclusion
Significant excess costs exist for patients with incident fractures and with prevalent hip, vertebral, humerus, multiple, and traumatic fractures. Ignoring prevalent fractures underestimate the true cost of osteoporosis.
Keywords: Cost of illness, Excess cost, Fracture, Osteoporosis
Introduction
An estimate of the cost of illness of osteoporosis and fractures is required to forecast the current and future healthcare burden for patients, payers, and society. This is essential because of the high prevalence of osteoporosis and consequent fractures. The current estimate of osteoporosis prevalence in Canada is 26 % of women and 7 % of men age 50 years and older [1]. Moreover, the number of cases with osteoporosis will escalate rapidly as the Canadian population over age 50 years is estimated to increase by 6.2 % per year until the year 2041 [2].
Estimating the cost of illness is not straightforward and there are various costing and methodological assumptions that can produce different results. For example, the extent of attribution to healthcare costs is uncertain. While the acute care admission for a fracture is logically attributable to the fracture, the attribution of postfracture care is not as straightforward [3]. Specifically, for a patient who has dementia and suffers a fall resulting in a hip fracture, it may be inappropriate to attribute the cost of a subsequent transfer to a nursing home only to the fracture, thus disregarding dementia and other comorbidities [4]. Similar arguments can be made for the attribution of other resource utilization such as physician visits and assisted daily living. One approach is to use adjudication to identify the attribution of costs [5]. However, without the certainty of attribution to one disease, cost of illness studies may be biased [4].
To reduce the possible bias due to inexact attribution of resource utilization and costs for osteoporosis and fractures, matching methods have been used. One type of matching method is pre–post designs where the patient serves as their own control. For example, the incremental costs (post minus pre) are attributed to the fracture, allowing for adjustment of factors such as total costs in prior year, number of comorbidities, or prior nursing home use [6]. A limitation with using pre–post incremental costs is that the pre and post period for costing must be specified, such as 1 year. The long-term costs of fractures, such as the need for permanent assistance in daily living, is not captured [7]. Similarly, including prefracture costs that are disease related, such as taking bisphosphonates to reduce the risk of fracture, would underestimate the incremental costs.
A different matching method is to estimate the excess cost of a patient with a fracture versus a patient without a fracture [8]. For example, some studies have estimated the excess cost of fractures in cases with fracture and osteoporosis to matched controls with osteoporosis without fracture [9–11]. A key difference in this method from a pre–post design is that the excess costs are focussed on the patient and not the clinical event which may reduce attribution bias [3, 12]. However, this method also limits the estimates of cost to a defined period, such as the first year following a fracture.
An important gap in the estimation of the cost of fractures and osteoporosis with matching methods is the exclusion of multiyear costs after a fracture. Studies that look only at the first year after an incident fracture exclude the possibility of costs for prolonged care which may be fracture related. In addition, cost of illness studies in osteoporosis may also exclude the costs of preventive therapy in patients who have not incurred a fracture.
Our objective was to use matching methods to estimate the excess cost of illness of osteoporosis and fractures that included prolonged care and nonfracture care. First, we estimated the average resource utilization and costs for each of three types of cases (incident fracture, prevalent fracture, and nonfracture osteoporosis) versus matched controls. The analysis was conducted across subgroups divided by age, sex, and fracture type. The results of the subgroups were pooled to estimate the excess resource utilization and excess costs of incident fractures, prevalent fractures, and nonfracture osteoporosis compared with nonfracture non-osteoporosis controls. In addition, we assessed the factors that were associated with higher excess costs with metaregression techniques.
Methods
The description of the methods and results follows the suggested reporting standard based on the Strengthening the Reporting of Observational studies in Epidemiology statement for observational studies [13]. The analysis was conducted with residents in the province of Manitoba for men and women who were aged 50 years and over in fiscal year (FY) 2007/2008 (i.e., April 1, 2007 to March 31 2008.). This subset represents 389,440 potential cases and controls, which was 3.5 % of Canadians age 50 and over (11.0 million in the year 2008) [2]. The sampling frame included all residents who had used the healthcare system in the province at any time during the FY 2007/2008. For all patients, any record of hospital admission, physician billing, outpatient pharmacy drug dispensations, or results of bone mineral density (BMD) testing were linked by a unique anonymous patient identifier within the population-based Manitoba Centre for Health Policy Research Data Repository to identify cases and controls [14]. Residents in Manitoba without the use of health care with zero costs were not available in the database. The data were obtained in an aggregated format, i.e., without variance estimates and a number of methods discussed later in this section were assessed to impute the variance estimates.
Cases and control definition
Cases and controls were identified from: (a) BMDs recorded in the provincial bone densitometry database, (b) hospital admissions (International Classification of Diseases 10th revision, Canadian version ICD-10-CA coding) and physician billings (ICD-9th Revision Clinical Modification ICD-9-CM coding) 1995–2008, or (c) retail pharmacy dispensations for osteoporosis pharmacotherapy in the previous 12 months. Low BMD levels were defined as a minimum T-score (lumbar spine or hip) of 2.5 or more standard deviation below white female peak BMD. For a hospital admission, a hospital abstract is completed when a patient is discharged from an acute care facility with diagnoses coded using the ICD-10-CA. The ICD-10CA code that corresponded to the greatest portion of the patient’s length of stay or cost (the most responsible diagnosis) was taken from the first diagnosis field. Similarly, physicians submit billing claims to the provincial Ministry of Health for almost all services, including office visits, outpatient and inpatient services; these claims contain a single three-digit ICD-9-CM diagnosis code and optional procedure codes.
Use of osteoporosis medications was obtained by linkage to the Manitoba Drug Programs Information Network database with drugs classified according to the Anatomical Therapeutic Chemical system of the World Health Organization [15]. A computerized record of all retail pharmacy dispensations is available since April 1, 1995. The pharmacy database is accurate both for capture of drugs dispensed as well as most prescription details [16]. Each prescription record contains the date of dispensation and an exact identification of the dispensed drug, including substance, strength, route and dosage form. For purposes of the current analysis, osteoporosis pharmacotherapy was defined as any use of oral bisphosphonates, calcitonin, raloxifene, or teriparatide. This excluded secondary osteoporosis who received intravenous bisphosphonates administered in clinics.
Incident fracture cases included patients who had a fracture in FY 2007/2008, regardless of previous fractures, osteoporosis medication use, or BMD result. Fractures that were included were categorized by type: hip, humerus, vertebral, multiple, trauma, and miscellaneous where miscellaneous included femur, lower leg, lower arm, ribs/sternum, shoulder, clavicle, pelvis, or patella.
Prevalent fracture cases included patients who had a previous fracture 1995–2007 without a fracture in the index FY 2007/2008, regardless of medication use or BMD result. Nonfracture osteoporosis cases included patients with no history of fractures (incident or prevalent), but with use of pharmacotherapy for osteoporosis in the previous 12 months or low BMD result.
Nondisease controls included residents of Manitoba who did not have an incident fracture in FY 2007/2008, a prevalent fracture in 1995–2007, low BMD, or dispensation of an osteoporosis medication in the previous 12 months. Potential controls were also required to have at least one healthcare claim (drug dispensation, physician billing, or hospital admission) identified in the healthcare administration records. This excludes residents without a health claim. For each case, up to three controls (if available) were matched based on age, sex, and area of residence according to 11 healthcare regions referred to as regional health authorities.
Resource utilization and costs
Resource utilization that was captured for cases and controls included: the number of acute care, non-acute care or rehabilitation hospital admissions (and length of stay), number and types of physicians consulted (general practice, internal medicine, imaging specialists, and other specialists), retail pharmacy dispensations, rates of home care admission (and duration of care), and rate of admission to permanent resident nursing-assisted personal care homes (and length of stay). Unit costs specific to Manitoba were applied to the resources identified to estimate costs that included costs for physician services and drugs [6]. In addition, unit costs specific to fracture care as part of home care service are available from Ontario at $24 per day [17] and Ontario had a fixed daily cost of $148 for long-term care [18]. These unit costs were multiplied by the number of days in home care and personal care homes, respectively, to estimate home care and personal care home costs.
National average prices for hospital admission were applied based on resource intensity weights. The resource intensity weights for each hospital admission were estimated by the Canadian Institute for Health Information, where the value of the one unit represents the average resource intensity for all national hospital admissions. The value of the resource intensity weight is adjusted relative to the average value for: (1) the case mix group, (2) five other factors that affect resource utilization and length of stay (age, comorbidity levels, flagged interventions, number of intervention events, and out-of-hospital interventions), and (3) atypical length of stay or level of care. The national average cost per resource intensity weight ($5,399 per unit), was multiplied by the resource intensity weight to estimate the cost of each hospital admission [19].
Excess resource utilization and excess cost analysis
We estimated the average resource utilization and average costs stratified by 10-year age groups, sex, fracture history (prevalent or incident), and fracture type, which resulted in 150 possible subgroups. The number of possible subgroups were (sex (2)×age (5)×fracture history (2)×fracture type (7)=140, plus nonfracture (1) times sex (2)×age (5)=10). Similarly, we estimated the average resource utilization and average costs for the matched controls. For each matched subgroup, we then estimated the excess by subtracting the mean resource utilization and mean costs of the controls from the cases. That is, for each subgroup, we generated excess costs for patients with incident fractures versus controls, excess costs for prevalent fractures versus controls, and excess costs for nonfracture osteoporosis versus controls. To provide an estimate of the average excess cost for patients with incident fractures, prevalent fractures or non-fracture osteoporosis regardless of age, we produced a weighted mean of the excess costs weighted by the frequency of the cases in each subgroup.
Uncertainty in evaluating magnitude and significance of excess costs
The data were obtained in aggregated form and no access to patient level data was available to determine the variances for the estimated means. The data for the present analysis were obtained under a previous project grant and it was not feasible to re-extract the data in order to obtain variance estimates. In the absence of variances for aggregated data, there are 25 different methods for handling missing variances [20]. Of these possible methods, four are possible in this analysis; (1) substitute the arithmetic mean for the standard deviation, (2) assume a value for the coefficient of variation (standard deviation/mean), (3) use external data sources that provide estimates of the standard deviation, and (4) acknowledge the missing data and provide a narrative review of the magnitude of the estimates of the excess costs.
Following all available methods, we generated 95 % confidence intervals by assuming that the coefficient of variation was one (mean = standard deviation) which is a suggested solution to missing variance [21] and is consistent with similar work on the excess costs of diabetes [22].
Second, to assess the impact that the assumed standard deviation had on the significance of the estimates, we estimated the coefficient of variation for the cases and controls where the lower 95 % confidence interval was zero. For example, if the mean cost for a subgroup was $10,000 and if coefficient of variation was 6 that set the 95 % confidence interval to include zero, this implies that the standard deviation for the cases and the controls must be six times larger than the mean.
Third, we compared our derived coefficient of variation that created a significant excess cost to external data estimates. One estimate suggested that the coefficient of variation for cases of nonvertebral fractures for Medicare recipients was 1.15 [10], while other estimates provided lower estimates of the coefficient of variation. We selected the higher value to be more conservative.
Finally, we simply looked at the magnitude of the estimates of excess costs and ignored whether the estimates may be significant. For this, we compared the magnitude of the excess costs to the excess costs of other diseases in the province of Manitoba [23]. This analysis was conducted with the same dataset using the same costing methodology for the years 2006–2007, but also included aged 19 and over. The excess costs for arthritis, asthma/chronic obstructive pulmonary disease (COPD), diabetes, coronary heart disease, and stroke were available from the years 2006 and 2007. These excess costs were estimated using the general population as the control group and represented excess costs over a 2-year period. To be included as a case, there must have been either two physician visits or one hospital admission where the reason for the visit or the admission was the disease. In addition to the narrative review of the cost ratios (cases/controls), we also estimated the total provincial excess costs for incident and prevalent fractures. Furthermore, we projected our provincial estimate to the national level and then compared our estimate with a national estimate of the cost of osteoporosis by Tarride et al. using similar costing methods for the same year, who estimated the cost of illness for Canada for incident fractures, and included a sensitivity analysis from adding the cost of prevalent fractures that required long-term care.
Assessment of predictors of excess cost
Methods for the analysis of predictors for aggregate cost data are not well established but random effects metaregression has been suggested [24]. In the random effects model, the assumption is that the excess costs of different subgroups have a common random distribution component. To evaluate factors that predicted changes in the average excess costs, metaregression was conducted with subgroup mean excess cost as the dependent variable. The independent variables included sex (women), fracture type (hip, humerus, multiple, miscellaneous, traumatic, vertebral, and wrist) separated by fracture history (incident and prevalent fracture), and five age subgroups (from 50–59 to 90+ years), and the average cost of osteoporosis drugs for each subgroup. Data were available for 148 of 150 possible subgroups because two age categories of men did not have multiple fractures and the base case for the regression was for nonfracture osteoporosis in men age 50–59 years. The metaregression was conducted with STATA 11.0 SE using the command metareg [25], with the assumption that the standard deviation of the excess costs was equal to the mean costs for each subgroup.
Assessing the effect of the assumption of normality
The assumption of normality was evaluated by performing a metaregression with the natural log transform of costs and variance. Regression coefficients generated by the log-normal metaregression were retransformed to the original linear scale. Regression coefficients after transformation represent the geometric mean effect of the covariates, while the linear regression coefficients represent the arithmetic mean of effect of the covariates. The criterion for statistical significance was set at alpha=0.05. All costs are reported in 2010 Canadian dollars.
Results
For FY 2007/2008, we identified 284,108 individuals in Manitoba meeting the inclusion criteria (73 % of the provincial population age 50 years and over, 52 % of all men and 91 % of all women 50 years and over). In total, 121,937 cases and 162,171 controls were selected, which averaged 1.33 controls for every case. There were 3,776 patients with incident fractures (66 % women, 34 % men), 43,406 patients with prevalent fractures (68 % women and 32 % men) and 74,755 nonfracture osteoporosis cases (90 % women and 10 % men; see Table 1). For men, the median age occurred in the subgroup 60–69 years for incident, prevalent, and nonfracture osteoporosis cases. For women, the median age occurred in the subgroup 70–79 years for incident and prevalent fractures and for the nonfracture osteoporosis cases in the subgroup 60–69 years. The ratio of controls to cases was lowest in elderly women, due to the majority of elderly women satisfying one of the case definitions (e.g., having incurred a fracture or taking osteoporosis medications).
Table 1.
Number of incident fractures, prevalent fractures, non-fracture osteoporosis cases and controls by age and sex in Manitoba
| Total | Hip | Humerus | Multiple | Miscellaneous | Traumatic | Vertebral | Wrist | |
|---|---|---|---|---|---|---|---|---|
| Men | ||||||||
| Incident fracture | 1,273 | 244 | 96 | 6 | 584 | 14 | 120 | 209 |
| Prevalent Fracture | 15,784 | 1,172 | 991 | 85 | 8,167 | 1,277 | 1,201 | 2,891 |
| Non-fracture osteoporosis | 7,705 | |||||||
| Total cases | 24,762 | |||||||
| Number of matched controls (N) | 71,093 | |||||||
| Ratio controls: cases | 2.87 | |||||||
| Provincial population (% captured) | 183,137 (52 %) | |||||||
| Women | ||||||||
| Incident fracture | 2,503 | 507 | 310 | 20 | 795 | 70 | 148 | 653 |
| Prevalent fracture | 27,622 | 3,154 | 2,867 | 191 | 9,035 | 1,302 | 2,075 | 8,998 |
| Nonfracture osteoporosis | 67,050 | |||||||
| Total cases | 97,175 | |||||||
| Number of matched controls (N) | 91,078 | |||||||
| Ratio controls: cases | 0.94 | |||||||
| Provincial population (% captured) | 206,310 (91 %) | |||||||
| Both sexes | ||||||||
| Total Cases | 121,937 | |||||||
| Number of matched controls (N) | 162,171 | |||||||
| Ratio controls: cases | 1.33 | |||||||
| Provincial population (% captured) | 389,447 (73 %) | |||||||
For incident fractures, the most common type was miscellaneous with 37 %, followed by wrist 20 %, hip 20 %, and humerus 11 %; vertebral, multiple, and trauma each contributed less than 10 %. For prevalent fractures, the most common type was miscellaneous with 40 %, followed by wrist 27 % and hip 10 %; humerus, trauma, multiple, and vertebral fractures each contributed less than 10 %.
Resource utilization
The resource utilization associated with the controls increased with advancing age. For men in the control group, the annual number of physician visits rose from 7.4 per year for age 50–59 years to 15.3 per year at age 90 years and over. In addition, home care use rose from 1.1 % for men ages 50–59 years to 38.1 % for age 90 years and over, and the use of personal care homes rose from 0.2 % for men ages 50–59 years to 38.1 % for ages 90 years and over.
The excess resource utilization for incident hip fractures involved an extra 1.8 hospital admissions for men and 1.6 for women (Table 2) which includes the acute care admission, and transfers to non-acute beds or rehabilitation beds. There were also excess hospital admissions for incident humerus fractures (0.8 admissions for men and 0.6 admissions for women), but low excess hospital admissions for incident wrist fractures (0.2 for men and 0.3 for women). For all incident fracture sites combined there was excess mean length of stay, physician visits, home care, and personal care home use. Excess personal care home use was greatest for incident hip fractures (30.3 % excess use for men and 34.9 % for women). For men, nonhip incident fractures had less than 10 % excess use of personal care homes while for women the excess personal care home use was higher for multiple fractures (29.2 %), traumatic fractures (34.7 %), and vertebral fractures (10.9 %). Incident wrist fractures for women and traumatic fractures for men both resulted in higher personal care home use than for the controls.
Table 2.
Excess healthcare resource utilization by sex for incident fracture, prevalent fracture and nonfracture osteoporosis cases
| Men
|
Female
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Hospital admissions (LOS) | Physician (visits) | Home care use %(days) | Personal care homes use % (days) | Hospital admissions (LOS) | Physician (visits) | Home care use % (days) | Personal care homes use % (days) | |
| Incident Fractures | ||||||||
| Hip | 1.8 (34.2) | 25.0 | 35.2 % (36.7) | 30.3 % (47.4) | 1.6 (36.2) | 21.9 | 33.3 % (38.0) | 34.9 % (50.3) |
| Humerus | 0.8 (13.2) | 15.4 | 21.8 % (37.7) | 6.6 % (16.1) | 0.6 (8.3) | 10.3 | 21.8 % (31.8) | 6.9 % (5.1) |
| Multiple | 0.7 (19.8) | 11.2 | 9.9 % (7.9) | 7.8 % (14.1) | 1.5 (38.7) | 29.4 | 36.1 % (22.0) | 29.2 % (40.9) |
| Miscellaneous | 0.6 (7.8) | 11.2 | 9.4 % (15.0) | 2.7 % (2.7) | 0.6 (12.5) | 13.3 | 21.1 % (32.9) | 6.1 % (7.4) |
| Traumatic | 1.3 (26.2) | 14.6 | 40.0 % (60.8) | −1.0 % (−6.2) | 1.7 (53.6) | 24.1 | 37.9 % (43.9) | 34.7 % (22.2) |
| Vertebral | 0.8 (10.3) | 13.3 | 16.1 % (22.9) | 1.6 % (−2.6) | 0.7 (13.3) | 15.3 | 27.0 % (48.2) | 10.9 % (9.5) |
| Wrist | 0.2 (2.7) | 6.0 | 5.4 % (11.5) | 1.5 % (3.8) | 0.3 (2.0) | 32.2 | 8.7 % (15.8) | −0.1 % (−0.7) |
| Prevalent fractures | ||||||||
| Hip | 0.2 (7.2) | 5.2 | 12.7 % (37.6) | 18.4 % (42.6) | 0.1 (95.8) | 5.5 | 11.8 % (36.1) | 25.7 % (71.3) |
| Humerus | 0.1 (4.2) | 4.5 | 7.0 % (16.0) | 5.6 % (12.8) | 0.2 (2.9) | 4.4 | 10.0 % (24.2) | 7.5 % (17.6) |
| Multiple | 0.2 (2.4) | 3.2 | 5.9 % (15.7) | 2.5 % (6.8) | 0.2 (3.8) | 15.0 | 14.1 % (46.2) | 6.9 % (19.7) |
| Miscellaneous | 0.2 (1.5) | 3.3 | 3.6 % (8.1) | 2.3 % (4.9) | 0.3 (2.5) | 5.4 | 7.6 % (21.0) | 6.1 % (15.3) |
| Traumatic | 0.2 (4.5) | 3.9 | 5.7 % (12.6) | 4.6 % (6.2) | 0.2 (5.3) | 5.9 | 11.8 % (33.7) | 12.2 % (26.1) |
| Vertebral | 0.1 (2.0) | 3.5 | 4.0 % (9.3) | 4.4 % (9.0) | 0.2 (4.4) | 7.1 | 12.2 % (33.5) | 8.6 % (20.7) |
| Wrist | 0.1 (1.5) | 2.6 | 1.7 % (4.8) | 1.9 % (5.1) | 0.2 (1.0) | 3.1 | 3.9 % (9.7) | 2.4 % (6.0) |
| Non-fracture osteoporosis | ||||||||
| Non fracture | 0.2 (1.6) | 5.4 | 3.5 % (9.6) | 2.2 % (5.0) | 0.1 (0.0) | 3.4 | 0.9 % (2.7) | −0.2 % (−0.9) |
For example, the excess number of hospital admissions (excess average length of stay) per case minus control for men for hospital admissions was 1.8 admissions (34.2 days)
For all fracture types, the excess resource utilization for prevalent fractures was smaller than for incident fractures. For prevalent fractures, there were still excess rates of hospital admissions, days in hospital, physician visits, drug use, home care and personal care home use. The excess rates were higher in women than for men for physician visits, drug use, home care and personal care home use, while rates of hospital admission were similar between men and women.
For the nonfracture osteoporosis cases, there were still positive excess rates of healthcare utilization for men. However, for women, the excess numbers of hospital admissions was 0.1 with zero excess length of stay, with slightly negative excess rates of admission to personal care homes (i.e., use of personal care homes was higher in the nonfracture non-osteoporosis controls than in the nonfracture osteoporosis cases).
Excess costs
The average cost by sex and fracture type for the cases and the controls are provided in Tables 6 and 7 of the “Appendix” section. The average total costs of the controls varied by the age composition of the matched cases, with values ranging from $4,730 to 13,146. The average costs of the cases were higher for incident fractures than prevalent fractures and non-fracture osteoporosis. The highest costs for women were incident traumatic fractures ($69,189) and for men was incident multiple fractures ($60,515).
Table 6.
Average cost of matched controls by sex for incident fracture, prevalent fracture, and nonfracture osteoporosis
| Men
|
Women
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control costs ($)
|
Control costs ($)
|
|||||||||||
| Hospital admissions | Physician | Drugs | Home care | Personal care homes | Total costs | Hospital admissions | Physician | Drugs | Home care | Personal care homes | Total costs | |
| Incident fractures | ||||||||||||
| Hip | 4,670 | 726 | 1,403 | 838 | 2,921 | 10,558 | 4,080 | 613 | 1,338 | 1,382 | 5,335 | 12,748 |
| Humerus | 2,792 | 555 | 1,142 | 400 | 1,263 | 6,152 | 2,561 | 520 | 1,126 | 665 | 2,119 | 6,991 |
| Multiple | 2,683 | 491 | 1,020 | 421 | 1,510 | 6,125 | 4,139 | 628 | 1,373 | 1,389 | 5,131 | 12,661 |
| Other | 2,527 | 533 | 1,110 | 338 | 994 | 5,503 | 2,578 | 514 | 1,105 | 701 | 2,307 | 7,204 |
| Traumatic | 4,278 | 664 | 1,287 | 762 | 2,871 | 9,862 | 3,811 | 606 | 1,324 | 1,242 | 4,450 | 11,434 |
| Vertebral | 3,284 | 617 | 1,247 | 502 | 1,553 | 7,203 | 2,930 | 549 | 1,193 | 831 | 2,669 | 8,172 |
| Wrist | 2,404 | 518 | 1,082 | 314 | 920 | 5,237 | 2,330 | 503 | 1,082 | 571 | 1,682 | 6,168 |
| Prevalent fractures | ||||||||||||
| Hip | 4,325 | 697 | 1,359 | 753 | 2,627 | 9,762 | 4,156 | 614 | 1,340 | 1,425 | 5,611 | 13,146 |
| Humerus | 2,746 | 563 | 1,160 | 382 | 1,119 | 5,970 | 2,960 | 550 | 1,193 | 847 | 2,801 | 8,351 |
| Multiple | 2,541 | 540 | 1,120 | 338 | 970 | 5,510 | 3,141 | 563 | 1,223 | 927 | 3,124 | 8,978 |
| Other | 2,369 | 518 | 1,086 | 303 | 856 | 5,132 | 2,606 | 516 | 1,114 | 711 | 2,326 | 7,273 |
| Traumatic | 2,250 | 509 | 1,073 | 274 | 736 | 4,842 | 2,988 | 539 | 1,164 | 894 | 3,170 | 8,753 |
| Vertebral | 3,045 | 585 | 1,189 | 455 | 1,433 | 6,706 | 3,169 | 559 | 1,214 | 957 | 3,296 | 9,196 |
| Wrist | 2,285 | 510 | 1,073 | 284 | 783 | 4,934 | 2,556 | 521 | 1,126 | 666 | 2,055 | 6,923 |
| Nonfracture osteoporosis | ||||||||||||
| Nonfracture | 2,552 | 542 | 1,127 | 339 | 967 | 5,527 | 1,875 | 462 | 988 | 389 | 1,016 | 4,730 |
Average cost of controls varies by fracture type due to matching fractures by age groups
Table 7.
Average cost of cases by sex for incident fracture, prevalent fracture, and nonfracture osteoporosis
| Men
|
Women
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average costs for cases ($)
|
Average costs for cases ($)
|
|||||||||||
| Hospital admissions | Physician | Drugs | Home care | Personal care homes | Total costs | Hospital admissions | Physician | Drugs | Home care | Personal care homes | Total costs | |
| Incident fractures | ||||||||||||
| Hip | 38,466 | 3,409 | 1,920 | 2,200 | 9,527 | 55,521 | 38,986 | 3,054 | 1,778 | 2,295 | 12,349 | 58,463 |
| Humerus | 11,871 | 1,655 | 1,642 | 1,304 | 3,511 | 19,983 | 10,515 | 1,232 | 1,899 | 1,428 | 2,832 | 17,905 |
| Multiple | 41,290 | 5,146 | 2,237 | 1,062 | 10,781 | 60,515 | 35,611 | 3,880 | 1,859 | 1,917 | 10,832 | 54,100 |
| Other | 10,946 | 1,353 | 1,661 | 636 | 1,530 | 16,126 | 13,393 | 1,485 | 1,834 | 1,490 | 3,343 | 21,545 |
| Traumatic | 39,953 | 2,732 | 3,878 | 2,206 | 2,134 | 50,903 | 54,090 | 3,155 | 2,098 | 2,297 | 7,548 | 69,189 |
| Vertebral | 15,768 | 2,088 | 1,885 | 1,405 | 1,297 | 22,442 | 16,335 | 1,773 | 2,447 | 1,989 | 3,994 | 26,537 |
| Wrist | 8,743 | 1,179 | 1,362 | 627 | 1,157 | 13,068 | 4,238 | 2,151 | 1,373 | 950 | 1,589 | 10,301 |
| Prevalent fractures | ||||||||||||
| Hip | 10,070 | 999 | 1,844 | 1,742 | 9,209 | 23,864 | 9,469 | 920 | 1,814 | 2,291 | 15,545 | 30,040 |
| Humerus | 7,601 | 877 | 1,594 | 772 | 3,107 | 13,951 | 5,382 | 823 | 1,804 | 1,427 | 5,251 | 14,687 |
| Multiple | 8,019 | 757 | 1,135 | 452 | 2,633 | 12,996 | 6,900 | 1,488 | 2,320 | 2,035 | 5,872 | 18,614 |
| Other | 4,001 | 702 | 1,398 | 539 | 1,583 | 8,222 | 4,899 | 815 | 1,729 | 1,214 | 4,461 | 13,118 |
| Traumatic | 5,291 | 700 | 1,455 | 534 | 1,278 | 9,259 | 8,645 | 876 | 1,816 | 1,703 | 6,805 | 19,844 |
| Vertebral | 5,705 | 855 | 1,679 | 775 | 3,000 | 12,013 | 6,717 | 957 | 2,111 | 1,761 | 6,181 | 17,727 |
| Wrist | 3,877 | 647 | 1,218 | 389 | 1,571 | 7,701 | 3,641 | 679 | 1,435 | 897 | 2,888 | 9,541 |
| Nonfracture osteoporosis | ||||||||||||
| None | 4,158 | 822 | 1,806 | 550 | 1,418 | 8,754 | 2,004 | 605 | 1,466 | 453 | 891 | 5,419 |
There were excess costs associated with incident fractures, prevalent, and nonfracture osteoporosis (Table 3). For men, the mean excess total cost ranged from $7,831 for an incident wrist fracture up to $44,963 for an incident hip fracture. Similarly, for women, the excess total cost ranged from $4,132 for an incident wrist fracture up to $45,715 for an incident hip fracture. Of the total excess costs, about 70 % were associated with the costs of admissions to hospitals.
Table 3.
Excess costs by source of costs by sex for incident fracture, prevalent fracture, and nonfracture osteoporosis
| Men
|
Women
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excess costs ($)
|
Excess costs ($)
|
|||||||||||
| Hospital admissions | Physician | Drugs | Home care | Personal care homes | Total costs | Hospital admissions | Physician | Drugs | Home care | Personal care homes | Total costs | |
| Incident fractures | ||||||||||||
| Hip | 33,796 | 2,683 | 517 | 1,362 | 6,606 | 44,963 | 34,906 | 2,441 | 440 | 913 | 7,015 | 45,715 |
| Humerus | 9,080 | 1,100 | 499 | 904 | 2,249 | 13,832 | 7,954 | 712 | 773 | 763 | 714 | 10,914 |
| Multiple | 38,607 | 4,654 | 1,217 | 641 | 9,271 | 54,390 | 31,472 | 3,252 | 486 | 528 | 5,702 | 41,439 |
| Miscellaneous | 8,420 | 820 | 550 | 359 | 379 | 9,862 | 10,815 | 971 | 729 | 789 | 1,037 | 14,341 |
| Traumatic | 35,675 | 2,068 | 2,591 | 1,444 | −737 | 41,042 | 50,279 | 2,550 | 774 | 1,055 | 3,098 | 57,755 |
| Vertebral | 12,485 | 1,471 | 637 | 903 | −256 | 15,240 | 13,405 | 1,223 | 1,255 | 1,158 | 1,325 | 18,365 |
| Wrist | 6,339 | 662 | 280 | 313 | 237 | 7,831 | 1,908 | 1,648 | 291 | 378 | −93 | 4,132 |
| Prevalent fractures | ||||||||||||
| Hip | 5,745 | 301 | 485 | 990 | 6,582 | 14,103 | 5,313 | 306 | 474 | 866 | 9,934 | 16,894 |
| Humerus | 4,855 | 314 | 434 | 390 | 1,988 | 7,982 | 2,422 | 273 | 611 | 580 | 2,449 | 6,336 |
| Multiple | 5,478 | 217 | 15 | 114 | 1,663 | 7,486 | 3,760 | 925 | 1,096 | 1,108 | 2,748 | 9,636 |
| Miscellaneous | 1,631 | 184 | 312 | 236 | 727 | 3,090 | 2,293 | 299 | 615 | 503 | 2,135 | 5,845 |
| Traumatic | 3,041 | 191 | 382 | 260 | 543 | 4,416 | 5,657 | 338 | 652 | 809 | 3,635 | 11,091 |
| Vertebral | 2,660 | 270 | 491 | 319 | 1,567 | 5,307 | 3,547 | 398 | 897 | 804 | 2,885 | 8,531 |
| Wrist | 1,378 | 114 | 142 | 73 | 788 | 2,767 | 1,086 | 159 | 309 | 232 | 833 | 2,618 |
| Nonfracture osteoporosis | ||||||||||||
| Nonfracture | 1,606 | 280 | 679 | 211 | 451 | 3,227 | 129 | 143 | 478 | 64 | −124 | 689 |
For prevalent fractures, the average excess total costs for men ranged from $2,767 for a wrist fracture up to $14,103 for a hip fracture. The excess costs for women were higher, ranging from $2,618 for a wrist fracture up to $16,894 for a hip fracture. For men, the excess cost of prevalent fracture largely came from hospital admissions (47 %) followed by long-term care (38 %), while for women the excess costs mostly came from long-term care (48 %) followed by hospital admissions (34 %).
For the nonfracture osteoporosis, there was an average excess total cost of $3,227 for men, which were made up of hospital admissions (48 %), drugs (20 %), and personal care homes (17 %). For women with nonfracture osteoporosis, the average excess cost was $689 of which drugs ($504) was the largest contributing factor.
The excess cost estimates were significantly greater than zero for all incident fracture sites, some prevalent fracture sites, but not for nonfracture osteoporosis (Table 4). Both men and women with prevalent fracture had significant excess costs for hip, humerus, multiple, and traumatic fractures, but these were not significant for miscellaneous or wrist fractures.
Table 4.
Average cost per case, average cost per control, and average excess cost with an assessment of uncertainty of cost distribution
| Men
|
Women
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Case average cost | Control average costs | Excess costs (95 % CI) | CV | n | Case average cost | Control average costs | Excess costs (95 % CI) | CV | |
| Incident fractures | ||||||||||
| Hip | 244 | $55,521 | $10,558 | $44,963 ($38,498; $51,428) | 7.0 | 507 | $58,463 | $12,748 | $45,715 ($36,998; $54,433) | 5.2 |
| Humerus | 101 | $19,983 | $6,152 | $13,832 ($5,676; $21,987) | 9.0 | 310 | $17,905 | $6,991 | $10,914 ($8,724; $13,105) | 5.0 |
| Multiple | 12 | $60,515 | $6,125 | $54,390 ($52,098; $55,873) | 36.7 | 27 | $54,100 | $12,661 | $41,439 ($39,600; $43,279) | 22.5 |
| Miscellaneous | 584 | $16,126 | $5,503 | $10,624 ($7,612; $13,616) | 3.5 | 795 | $21,545 | $7,204 | $14,341 ($10,202; $18,481) | 3.5 |
| Traumatic | 29 | $50,903 | $9,862 | $41,042 ($39,025; $43,058) | 20.4 | 75 | $69,189 | $11,434 | $57,755 ($53,839; $61,672) | 14.7 |
| Vertebral | 125 | $22,442 | $7,203 | $15,240 ($13,312; $17,167) | 7.9 | 148 | $26,537 | $8,172 | $18,365 ($16,180; $20,550) | 8.4 |
| Wrist | 209 | $13,068 | $5,237 | $7,831 ($6,341; $9,321) | 5.3 | 653 | $10,301 | $6,168 | $4,132 ($2,148; $6,116) | 2.1 |
| Prevalent fractures | ||||||||||
| Hip | 1,172 | $23,864 | $9,762 | $14,103 ($7,670; $20,536) | 2.2 | 3,154 | $30,040 | $13,146 | $16,894 ($5,138; $28,650) | 1.4 |
| Humerus | 991 | $13,951 | $5,970 | $7,982 ($4,496; $11,467) | 2.3 | 2,867 | $14,687 | $8,351 | $6,336 ($552; $12,120) | 1.1 |
| Multiple | 87 | $12,996 | $5,510 | $7,486 ($6,524; $8,448) | 7.8 | 191 | $18,614 | $8,978 | $9,636 ($7,788; $11,484) | 5.2 |
| Miscellaneous | 8,167 | $8,222 | $5,132 | $3,090 (−$3,008; $9,188) | 0.5 | 9,035 | $13,118 | $7,273 | $5,845 (−$2,986; $14,676) | 0.7 |
| Traumatic | 1,277 | $9,259 | $4,842 | $4,416 ($1,697; $7,136) | 1.6 | 1,302 | $19,844 | $8,753 | $11,091 ($6,046; $16,136) | 2.2 |
| Vertebral | 1,201 | $12,013 | $6,706 | $5,307 ($1,883; $8,781) | 1.5 | 2,075 | $17,727 | $9,196 | $8,531 ($2,691; $14,371) | 1.5 |
| Wrist | 2,891 | $7,701 | $4,934 | $2,767 (−$776; $6,310) | 0.8 | 8,998 | $9,541 | $6,923 | $2,618 (−$4,309; $9,546) | 0.4 |
| Nonfracture osteoporosis | ||||||||||
| Nonfracture | 7,705 | $8,754 | $5,527 | $3,227 (−$3,118, $9,572) | 0.5 | 67,050 | $5,419 | $4,730 | $689 (−$8,492, $9,870) | 0.1 |
95 % confidence intervals estimated assuming the standard deviation equal to the mean
CV coefficient of variation (standard deviation/mean) where 95 % confidence interval includes zero
Uncertainty in evaluating magnitude and significance of excess costs
Adjusting the coefficient of variation for the cases and controls until the 95 % confidence interval included zero produced large values for incidence fractures ranging from a coefficient of variation of 2.1 for incident multiple fractures in women to 36.7 for multiple fractures in men. For prevalent fractures, the coefficient of variation was above 1.15 for prevalent fractures except for miscellaneous and wrist fractures. The coefficient of variation for men with nonfracture osteoporosis was 0.5 and for women was 0.1, which indicated the unlikely occurrence that the standard deviation must be half the mean for men and one tenth the value of the mean excess costs before the excess costs for nonfracture osteoporosis would be significant.
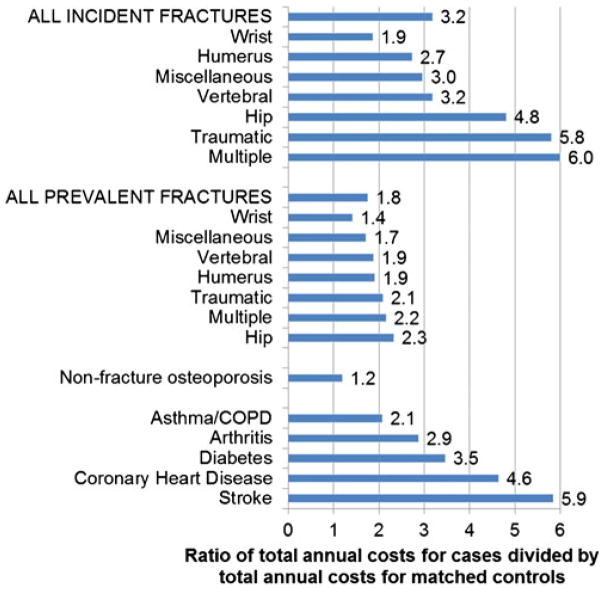
If we ignore the issue of significance and only look at the magnitude of the excess costs in comparisons to other disease, we see that incident and prevalent fractures have high excess costs (Fig. 1). The cost ratio (cases/controls) for all incident fractures was 3.2, which was higher than episodes of asthma/COPD (2.1) and arthritis (2.9) but less than diabetes (3.9). The cost ratio for incident hip fracture (4.8), multiple fracture (6.0), and traumatic fractures (5.8) are similar to the cost ratio for episodes of coronary heart disease (4.6) and stroke (5.9).
Fig. 1.
Comparison of the ratio of costs to controls for incident fractures, prevalent fractures, and nonfracture osteoporosis in comparison to ratios of cost to controls for other chronic diseases for the province of Manitoba. COPD chronic obstructive pulmonary disease
Prevalent fractures have an average cost ratio of 1.8, which is lower than the cost ratios for other chronic diseases. However, prevalent hip fractures (2.3), multiple fracture (2.2), traumatic fracture (2.1) had higher cost ratios than asthma/COPD (2.1). In addition, prevalent humerus (1.9), miscellaneous (1.7), and vertebral (1.9) were also high. The cost ratio for nonfracture osteoporosis was negligibly small (1.2).
The provincial burden for excess costs for incident fractures was $24.0 million for men and $48.8 million for women, for a total excess cost of incident fractures for both sexes of $72.8 million. The provincial burden for excess costs for prevalent fractures was $70.3 million for men (2.9 times incident fractures), $181.8 million for women (3.7 times incident fractures), for a total excess costs of prevalent fractures for both sexes of $252.1 million (3.5 times incident fractures).
Using our provincial estimates, we projected the national cost of incident fractures as $2.1 billion, and the costs of major prevalent fractures (hip, multiple, traumatic, and vertebral fractures) as $3.3 billion (including long term care of prevalent fractures $1.3 billion and excess costs of other services $2.0 billion), for a total burden of $5.4 billion (2.6 times incident costs), or $9.2 billion if we include all prevalent fractures. We compared our projected national estimate to concurrent national estimates obtained by Tarride, and found good agreement although the two sets of data had minimal overlap (Manitoba contains only 3.6 % of the Canadian population). Tarride et al. reported the national burden of incident fractures to be $2.3 billion, which is very similar to our national projection of $2.1 billion. The sensitivity analysis from Tarride et al. reported $1.6 billion for long-term care of major prevalent fractures, again similar to our national projection of $1.3 billion. Thus, the provincial population estimates appear to be representative of the national average.
Assessment of predictors of excess cost
Based on the random effects metaregression, the base case male aged 50–59 years with nonfracture osteoporosis had a nonsignificant excess cost of $145 (P=0.872; Table 5). After adjusting for all other factors, the excess costs for women was not significantly different from men (P=0.706), and there were no significant differences by age group. Having an incident hip fracture resulted in significant mean excess costs of $40,302 (95 % CI: $14,435–65,630; P=0.002). Having an incident fracture other than vertebral, traumatic, or wrist also gave significant excess costs. Prevalent fractures of the hip ($11,945; 95%CI $4,065–19,825; P=0.003) also resulted in significant excess costs.
Table 5.
Metaregression of subgroup level predictors of average excess cost
| Linear metaregression model
|
Log transform metaregression model
|
|||
|---|---|---|---|---|
| Independent variables | Estimate (95 % CI) | P value | Estimate (95 % CI) | P value |
| Women | $368 (−$1,544; $2,280) | 0.706 | $2,251 ($583; $8,687) | 0.960 |
| Incident—hip | $40,032 ($14,435; $65,630) | 0.002 | $27,417 ($2,434; $308,883) | 0.040 |
| Incident—humerus | $7,341 ($1,712; $12,971) | 0.011 | $6,980 ($687; $70,943) | 0.324 |
| Incident—multiple | $21,626 ($3,504; $39,748) | 0.019 | $29,173 ($2,351; $361,959) | 0.043 |
| Incident—miscellaneous | $9,432 ($2,444; $16,420) | 0.008 | $9,053 ($903; $90,799) | 0.225 |
| Incident—traumatic | $9,172 (−$231; $18,576) | 0.056 | $22,747 ($2,118; $244,259) | 0.053 |
| Incident—vertebral | $338 (−$1,571; $2,247) | 0.728 | $10,742 ($858; $134,441) | 0.215 |
| Incident—wrist | $1,339 (−$535; $3,213) | 0.161 | $1,435 ($168; $12,274) | 0.704 |
| Prevalent—hip | $11,945 ($4,065; $19,825) | 0.003 | $9,127 ($916; $90,963) | 0.221 |
| Prevalent—humerus | $3,503 ($573; $6,433) | 0.019 | $4,018 ($428; $37,696) | 0.591 |
| Prevalent—multiple | −$163 (−$1,920; $1,595) | 0.856 | $5,860 ($555; $61,860) | 0.410 |
| Prevalent—miscellaneous | $2,375 (−$33; $4,783) | 0.053 | $3,117 ($340; $28,568) | 0.750 |
| Prevalent—traumatic | $2,527 (−$164; $5,219) | 0.066 | $4,595 ($489; $43,219) | 0.513 |
| Prevalent—vertebral | $4,262 ($979; $7,544) | 0.011 | $4,281 ($421; $43,487) | 0.567 |
| Prevalent—wrist | $820 (−$853; $2,494) | 0.337 | N.A. | N.A. |
| Age 60–69 years | $1,016 (−$505; $2,537) | 0.190 | $2,672 ($581; $12,281) | 0.791 |
| Age 70–79 years | $910 (−$1,632; $3,452) | 0.483 | $3,943 ($716; $21,717) | 0.494 |
| Age 80–89 years | $3,269 (−$444; $6,982) | 0.084 | $5,094 ($899; $28,862) | 0.336 |
| Age 90+ years | −$15 (−$2,366; $2,336) | 0.990 | $4,140 ($818; $20,946) | 0.436 |
| Osteoporosis drug costs | −$17 (−$55; $21) | 0.378 | $2,170 ($2,128; $2,213) | 0.852 |
| Constant | $145 (−$1,612; $1,902) | 0.872 | $2,174 ($409; $11,563) | <0.001 |
Dependent variable is excess cost. The constant represents the base care result for men aged 50–59 years with nonfracture osteoporosis
N.A. Prevalent—wrist was dropped in regression due to collinearity with other predictors. All predictors are binary except osteoporosis drugs, which is in the linear metaregression is interpreted as 1$ extra in osteoporosis drugs, on average, is associated with a drop of $17 in excess cost. All other coefficients are interpreted as the change in the additional excess cost with the addition of the independent variable
Sensitivity analysis on assumption of normality
When we assessed the impact the assumption of non-normality had on the metaregression estimates, two important differences occurred. First, using log-normal transformation the level of excess cost for the base case analysis, men aged 50–59 years with nonfracture osteoporosis, was now significant ($2,174; 95%CI: $409–11,563; P<0.001). Secondly, prevalent fractures were not a contributing factor in increasing excess costs relative to the base case, and the incident fractures of humerus and miscellaneous were not significant although the coefficients had similar values to the linear coefficients.
Discussion
This analysis demonstrates that there may exist significant excess costs for patients with incident fractures and for some types of prevalent fractures but not for non-fracture osteoporosis cases. For incident hip fractures, we report an excess cost of $44,963 for men and $45,715 for women. The mean costs for controls were 19 % of those for men with incident hip fractures and 22 % of those for women with incident hip fractures. In other work from the same province [6], the costs occurring in the year prior to hip fracture within the same patient represented 38 % of the 12 month costs after the fracture for men and 33 % for women. The higher correlation between pre- and post-fracture costs within patients compared with the correlation in post fracture costs between cases and matched controls is not unexpected, since the former approach partially adjusts for the costs of comorbidities. This has also been reported elsewhere, where closer matching increased the correlation between the controls and cases, thereby reducing the magnitude of the excess costs. For example, in an unadjusted matching analysis, the prefracture costs were 16–17 % of the incident costs [8]. When both the cases and controls are required to have osteoporosis, effectively matching for a greater level of comorbidity, the cost in the pre-fracture period is 31 % of the post fracture period and 41 % in a concurrent control group [11]. When the matching includes employment status, the cost in the controls is 40 % of the incident fracture cases [10].
The different interpretation of the matching methods is important to address the underlying research question and whether we looking at the incremental cost of a fracture to patients or the incremental cost to payers for patients with a disease. Matching pre–post with adjustment for comorbidities estimates the incremental cost of a fracture after adjusting for their previous level of care. The closer the match, the more certain the cost attribution is to the clinical event alone. Matching to the general population provides an estimate of the excess cost related to only one factor, the marker for the disease. This latter method is better suited for measuring the overall burden of disease, while pre–post matching is best used for analysis of clinical events [12]. Our results indicate that the excess cost of fracture alone is a significant cost driver because the excess cost for nonfracture osteoporosis is not significantly increased. This indicates that fracture is largely driving the costs and not the underlying osteoporosis and related comorbidities.
Beyond looking at costs in the 1-year period before and after a fracture, our analysis included prevalent fractures. The magnitude of the excess costs is lower than that of an incident fracture, but our analysis indicates that excess costs may be significant for fractures, other than miscellaneous and wrist fractures. For example, the magnitude of the cost ratios (cases/controls) where higher than other chronic diseases such as asthma/COPD which may have significant excess costs. This suggests that cost of illness studies that evaluate only incident fractures may underestimate the cost of fractures and osteoporosis.
However, the Manitoba analysis used methods that were different in a few ways. First, the analysis included age 19 and over, which is different than our aged 50 and over analysis. Whether this difference leads to different ratios is uncertain because the cost of the cases and controls would both rise with age. Second, the Manitoba report provided three ratios for diseases including asthma/COPD, a gross population analysis of cases versus nondiseases controls (ratio=1.73), an age-and sex-matched analysis (ratio=2.08) and a matched analysis based on age, sex, number of aggregated diagnostic groups (ratio=2.56). This trend in the rise in the ratio with further matching was consistent across all diseases. This trend suggests that the excess cost ratio for an analysis of fractures that included comorbidities would provide an even larger cost ratio.
A limitation to our analysis of the prevalent fractures is that the time dependence for the costs was not established and the results were averaged for fractures occurring from 1995 to 2007. Since the excess costs of prevalent fractures were significant, having a prevalent fracture in past years is predictive of higher future excess costs for up to 15 years (average, 7.5 years), but we cannot claim for which years the post fracture costs were highest. In addition, our analysis did not include cases with osteopenia (BMD between −1 and −2.5 standard deviations below peak reference levels). Also, our analysis looks at the cost of fractures and not necessarily osteoporosis related fractures, such as trauma ICD-10 V codes and E codes (ICD-9) for accidents. The attribution to osteoporosis may vary by age and might be lower in the lower age groups, such as aged 50–59 years.
We also excluded all residents in Manitoba with zero healthcare expenditure. Although more than 90 % of residents aged 50 and over require a physician visit in any given year, we may have excluded from our controls some residents with zero healthcare expenditure. This may have resulted in an underestimation of excess costs by up to 10 %, though we could not calculate this overestimation with any accuracy.
Other limitations to our analysis are that we did not include the cost of emergency room visits because these data were not available in the linkable database repository. In addition, in Manitoba Canada, the codes used for hospitalizations and most institutional services are ICD-10-CA while the ICD codes for physician services are ICD-9-CM. This may have led to discrepancies in diagnostic coding.
Another limitation is the lack of patient level data to estimate variances. However, our analysis indicates that the excess cost of prevalent fractures is likely to be significant over a large range of variances. In addition, when we assumed different shapes of distributions, the results were consistent with the cost estimates based on normality. This finding is supported by simulation analyses of cost differences between two groups each having highly skewed data resulted in a distribution of incremental costs that was still approximately normally distributed, with p values only changing from 0.05 to 0.06 after adjustment for non-normality [26, 27]. However, we encountered a problem with interpretation of the results of our meta-regression of lognormal data where the re-transformed coefficients provided estimates of cost for geometric means instead of arithmetic means. An arithmetic mean is a more important estimate for budget prediction even if other measures such as medians or geometric means fit the data more efficiently [28].
Another limitation is the low level of matching for some of the subgroups where we sought three controls for every case but in some subgroups, we had more controls than cases. This implied that the average cost for all of the controls might be underweighted by some subgroups if we wished to estimate a provincial average for the controls to estimate excess cost of cases versus the general population.
In conclusion, we observed that the highest excess costs were seen for patients with incident fractures. Patients who have prevalent fractures also incur excess costs, and these excess costs, while lower than the excess costs of incident fractures, may be significant. Patients with nonfracture osteoporosis are not different from nonfracture controls. This suggests that cost of illness studies that evaluate only incident fractures and exclude prevalent fractures may underestimate the cost of fractures and osteoporosis.
Acknowledgments
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Population Health Research Data Repository (HIPC file number 2009/2010–09). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred.
Footnotes
Conflicts of interest None.
Contributor Information
R. B. Hopkins, Department of Clinical Epidemiology and Biostatistics, Faculty of Health Science, McMaster University, Hamilton, ON, Canada. Programs for Assessment of Technology in Health, St. Joseph’s Healthcare–Hamilton, Hamilton, ON, Canada
J. E. Tarride, Department of Clinical Epidemiology and Biostatistics, Faculty of Health Science, McMaster University, Hamilton, ON, Canada. Programs for Assessment of Technology in Health, St. Joseph’s Healthcare–Hamilton, Hamilton, ON, Canada. Centre for Evaluation of Medicines, Hamilton, ON, Canada
W. D. Leslie, University of Manitoba, Winnipeg, MB, Canada
C. Metge, University of Manitoba, Winnipeg, MB, Canada
L. M. Lix, School of Public Health, University of Saskatchewan, Saskatoon, SK, Canada
S. Morin, Department of Medicine, McGill University, Montreal, QC, Canada
G. Finlayson, University of Manitoba, Winnipeg, MB, Canada
M. Azimaee, University of Manitoba, Winnipeg, MB, Canada
E. Pullenayegum, Department of Clinical Epidemiology and Biostatistics, Faculty of Health Science, McMaster University, Hamilton, ON, Canada. Centre for Evaluation of Medicines, Hamilton, ON, Canada. Biostatistics Unit, St. Joseph’s Healthcare–Hamilton, Hamilton, ON, Canada
R. Goeree, Department of Clinical Epidemiology and Biostatistics, Faculty of Health Science, McMaster University, Hamilton, ON, Canada. Programs for Assessment of Technology in Health, St. Joseph’s Healthcare–Hamilton, Hamilton, ON, Canada. Centre for Evaluation of Medicines, Hamilton, ON, Canada
J. D. Adachi, Department of Medicine, McMaster University, Hamilton, ON, Canada
A. Papaioannou, Department of Medicine, McMaster University, Hamilton, ON, Canada
L. Thabane, Department of Clinical Epidemiology and Biostatistics, Faculty of Health Science, McMaster University, Hamilton, ON, Canada. Centre for Evaluation of Medicines, Hamilton, ON, Canada. Biostatistics Unit, St. Joseph’s Healthcare–Hamilton, Hamilton, ON, Canada
References
- 1.Leslie WD, Lix LM, Langsetmo L, Berger C, Goltzman D, Hanley DA, Adachi JD, Johansson H, Oden A, McCloskey E, Kanis JA. Construction of a FRAX(R) model for the assessment of fracture probability in Canada and implications for treatment. Osteoporos Int. 2011;22:817–827. doi: 10.1007/s00198-010-1464-2. [DOI] [PubMed] [Google Scholar]
- 2.Statistics Canada. Estimates of population, by age group and sex for July 1, Canada, provinces and territories, annual. 2010 Table 051–0001. [Google Scholar]
- 3.Becker DJ, Kilgore ML, Morrisey MA. The societal burden of osteoporosis. Curr Rheumatol Rep. 2010;12:186–191. doi: 10.1007/s11926-010-0097-y. [DOI] [PubMed] [Google Scholar]
- 4.Akobundu E, Ju J, Blatt L, Mullins CD. Cost-of-illness studies: a review of current methods. PharmacoEconomics. 2006;24:869–890. doi: 10.2165/00019053-200624090-00005. [DOI] [PubMed] [Google Scholar]
- 5.Strom O, Borgstrom F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, Svensson O, Abdon P, Ornstein E, Ceder L, Thorngren KG, Sernbo I, Jonsson B. Long-term cost and effect on quality of life of osteoporosis-related fractures in Sweden. Acta Orthop. 2008;79:269–280. doi: 10.1080/17453670710015094. [DOI] [PubMed] [Google Scholar]
- 6.Leslie WD, Metge CJ, Azimaee M, Lix LM, Finlayson GS, Morin SN, Caetano P. Direct costs of fractures in Canada and trends 1996–2006: a population-based cost-of-illness analysis. J Bone Miner Res. 2011;26(10):2419–2429. doi: 10.1002/jbmr.457. [DOI] [PubMed] [Google Scholar]
- 7.Wiktorowicz ME, Goeree R, Papaioannou A, Adachi JD, Papadimitropoulos E. Economic implications of hip fracture: health service use, institutional care and cost in Canada. Osteoporos Int. 2001;12:271–278. doi: 10.1007/s001980170116. [DOI] [PubMed] [Google Scholar]
- 8.Melton LJ, III, Gabriel SE, Crowson CS, Tosteson AN, Johnell O, Kanis JA. Cost-equivalence of different osteoporotic fractures. Osteoporos Int. 2003;14:383–388. doi: 10.1007/s00198-003-1385-4. [DOI] [PubMed] [Google Scholar]
- 9.Orsini LS, Rousculp MD, Long SR, Wang S. Health care utilization and expenditures in the United States: a study of osteoporosis-related fractures. Osteoporos Int. 2005;16:359–371. doi: 10.1007/s00198-004-1694-2. [DOI] [PubMed] [Google Scholar]
- 10.Pike C, Birnbaum HG, Schiller M, Sharma H, Burge R, Edgell ET. Direct and indirect costs of non-vertebral fracture patients with osteoporosis in the US. PharmacoEconomics. 2010;28:395–409. doi: 10.2165/11531040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Rousculp MD, Long SR, Wang S, Schoenfeld MJ, Meadows ES. Economic burden of osteoporosis-related fractures in Medicaid. Value Health. 2007;10:144–152. doi: 10.1111/j.1524-4733.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 12.Goeree R, O’Reilly D, Hopkins R, Blackhouse G, Tarride JE, Xie F, Lim M. General population versus disease-specific event rate and cost estimates: potential bias for economic appraisals. Expert Rev Pharmacoecon Outcomes Res. 2010;10:379–384. doi: 10.1586/erp.10.41. [DOI] [PubMed] [Google Scholar]
- 13.Knottnerus A, Tugwell P. STROBE—a checklist to strengthen the reporting of observational studies in epidemiology. J Clin Epidemiol. 2008;61:323. doi: 10.1016/j.jclinepi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Manitoba Centre for Health Policy. University of Manitoba; Canada: 2010. [Google Scholar]
- 15.WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment. Oslo: 2010. 2009. [Google Scholar]
- 16.Kozyrskyj AL, Mustard CA. Validation of an electronic, population-based prescription database. Ann Pharmacother. 1998;32:1152–1157. doi: 10.1345/aph.18117. [DOI] [PubMed] [Google Scholar]
- 17.Poss JW, Hirdes JP, Fries BE, McKillop I, Chase M. Validation of Resource Utilization Groups version III for Home Care (RUG-III/HC): evidence from a Canadian home care jurisdiction. Med Care. 2008;46:380–387. doi: 10.1097/MLR.0b013e31815c3b6c. [DOI] [PubMed] [Google Scholar]
- 18.Ontario Ministry of Health and Long Term Care. Long-Term Care Home Financial Policy. Ontario Ministry of Health and Long Term Care; 2010. [Google Scholar]
- 19.Canadian Institute for Health Information. Canadian MIS Database—hospital financial performance indicators, 1999–2000 to 2008–2009. 2010. [Google Scholar]
- 20.Wiebe N, Vandermeer B, Platt RW, Klassen TP, Moher D, Barrowman NJ. A systematic review identifies a lack of standardization in methods for handling missing variance data. J Clin Epidemiol. 2006;59:342–353. doi: 10.1016/j.jclinepi.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Briggs AH, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford University Press; USA: 2006. [Google Scholar]
- 22.Goeree R, O’Reilly D, Hux J, Lim M, Hopkins R, Tarride J, Blackhouse G, Xie F. Total and excess costs of diabetes and related complications in Ontario. Can J Diabetes. 2009;33:35–45. [Google Scholar]
- 23.Finlayson G, Ekuma O, Yogendran M, Burland E, Forget E. The additional cost of chronic disease in Manitoba. Manitoba Centre for Health Policy; Winnipeg: 2010. [Google Scholar]
- 24.Cochrane Handbook for Systematic Reviews of Interventions. 2011. Version 5.1.0. [Google Scholar]
- 25.StataCorp. Stata Statistical Software: Release 11. 2009. [Google Scholar]
- 26.Briggs AH, Mooney CZ, Wonderling DE. Constructing confidence intervals for cost-effectiveness ratios: an evaluation of parametric and non-parametric techniques using Monte Carlo simulation. Stat Med. 1999;18:3245–3262. doi: 10.1002/(sici)1097-0258(19991215)18:23<3245::aid-sim314>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Willan AR, O’Brien BJ. Confidence intervals for cost-effectiveness ratios: an application of Fieller’s theorem. Health Econ. 1996;5:297–305. doi: 10.1002/(SICI)1099-1050(199607)5:4<297::AID-HEC216>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 28.Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ. 2000;320:1197–2000. doi: 10.1136/bmj.320.7243.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]