Abstract
Objective
To design a Bayesian random effects model for pooling binary outcome data from cluster randomized trials (CRTs) with individually randomized trials (IRTs) and then use this model to determine if hip protectors decrease the risk of hip fracture in elderly nursing home residents.
Study Design and Setting
Eight electronic databases were searched; abstracts and papers were reviewed in duplicate. Randomized controlled trials of hip protectors in nursing homes were included. The pooled mean odds ratio (OR) of a hip fracture in an individual allocated to hip protectors with 95% credibility interval (CRI) was calculated.
Results
We included four trials of 1,922 individuals (including three CRTs). The pooled OR of an elderly nursing home resident sustaining one or more hip fractures with hip protector allocation was 0.40 (95% CRI 0.25, 0.61). The model was robust in multiple sensitivity analyses assuming alternative intracluster correlation coefficient values.
Conclusion
The Bayesian approach may be used in meta-analyses of IRTs and CRTs. Using this approach, we have determined that hip protectors decrease the risk of hip fracture in elderly nursing home residents. Methodologic limitations of the included trials and a possible herd effect in CRTs may have influenced these results.
Keywords: Meta-analysis, Randomized controlled trials, Bayesian, Osteoporosis, Hip fracture, Hip protectors
1. Introduction
Cluster randomized trials (CRTs), in which an intervention is randomized at the level of a cluster rather than the individual [1], have become increasingly popular in the health care literature [2]. Advantages of cluster-randomized designs include administrative convenience (particularly if individual randomization is not feasible) and minimization of treatment contamination between study groups [3]. However, statistical independence of individuals randomized at a cluster level cannot be assumed [1]. An explanation for the lack of independence of individuals in CRTs is that individuals within a cluster may be more similar to each other than to individuals in other clusters; the similarities within a cluster are represented by the intracluster correlation co-efficient (ICC, rho) [1,4]. As first noted by Cornfield in 1978, cluster randomization results in some loss of statistical efficiency compared to individual randomization and this must be accounted for in the analysis [5]. The variance inflation factor (VIF) measures the effect on the variance of an estimate of treatment effect attributed to clustering effect, and this value is related both to the ICC, which measures the correlation within a cluster and the cluster size [3]. In a recently extended CONSORT guideline pertaining to CRTs, it has been recommended that statistical adjustment for cluster randomization be incorporated in sample size calculations and in the analysis of primary outcomes, with the ICC being explicitly reported for each primary outcome in the final analysis [6].
Meta-analysis of CRTs poses many special methodologic challenges [7]. Several alternative approaches to meta-analysis of CRTs have been proposed [7,8]. One issue in meta-analysis of CRTs is the decision on whether to pool results of such trials with results of individually randomized trials (IRTs). A second issue is how to adjust for clustering effect in a pooled analysis. Inclusion of all studies (cluster and individually randomized) with appropriate statistical adjustment for clustering effect would be ideal, in order to maximize use of available information in the pooled analysis. Unfortunately, many clinical researchers have not considered such details in pooling data from CRTs. In a recent systematic review of meta-analyses of CRTs, inappropriate pooled analyses ignoring clustering effect were noted in more than half of studies [9].
Another important issue in meta-analysis of CRTs is the pooling of data from cluster-randomized studies in which the ICC or VIF were not reported or are not possible to estimate due to lack of reported data. One approach is to exclude such methodologically deficient trials from meta-analyses. We used such an approach in a recent meta-analysis of hip protectors (a protective undergarment designed to decrease the risk of hip fracture) [10]. An obvious disadvantage of simply excluding studies with missing information on ICC or VIF is the loss of relevant data from the meta-analysis. Other strategies used in dealing with missing data for ICCs in the meta-analysis of CRTs have included increasing the variance by an arbitrary number (such as 30%), averaging the known ICCs for the included trials, or using an external estimate of an ICC (from other studies in the same field) [7,9]. Such strategies in which design effects are imputed may be subject to inaccuracy or bias, so sensitivity analyses using multiple variations in assumptions have been recommended [7].
Given the inherent subjectivity in imputation of design effects in meta-analysis of CRTs, we consider a Bayesian approach for analysis of such data as an alternative. Bayesian statistical methodology is founded on Bayes’ theorem, a formula in which probability distributions for prior beliefs are modified by new information (the likelihood function) in developing posterior inferences [11]. An advantage of a Bayesian approach in meta-analysis of CRTs is that prior knowledge, beliefs, or assumptions about design effects attributed to cluster randomization can be systematically incorporated in the hierarchical modeling of a meta-analysis. The objective of this paper is to describe a Bayesian model for pooling results of cluster and individually randomized trials with binary outcomes, incorporating a systematic strategy for sensitivity analyses of imputed design effects. We have highlighted the role of the Bayesian approach in quantifying sensitivity analysis in meta-analysis of CRTs. We used a Bayesian model to determine if provision of hip protectors may decrease the risk of hip fracture in elderly nursing home residents. The reason for focusing on fracture outcomes with hip protectors in a population of nursing home residents include the following: a) the availability of published cluster and individually randomized trials in this field, b) the natural units of cluster randomization in such populations (e.g., nursing home or ward), c) the consistency of populations of nursing home residents (as opposed to mixed populations of residents of nursing homes, seniors’ apartments, and group homes), and d) the high event rates of hip fractures in such populations, with a clinical need to identify effective fracture prevention strategies.
2. Methods
2.1. Inclusion and exclusion criteria for studies
We examined randomized controlled trials that assessed the efficacy of a policy of provision of free hip protectors in preventing hip fracture in elderly nursing home residents of either gender. The inclusion criteria were similar to that used in a previous systematic review [10], except we restricted this updated systematic review to individually or cluster randomized trials composed exclusively of nursing home residents. Moreover, unlike in our previous review, we did not exclude CRTs in which no adjustment for cluster randomization was performed. A median or mean follow-up period of at least 6 months was required. Trials in which participants who dropped out or died were replaced with eligible participants from a waiting list were excluded.
2.2. Search strategy
An electronic search of the following databases was conducted without language restrictions in February 2005 and updated in February 2006: Ovid Medline (1966–2006), Medline in Process, Cochrane Database for Systematic Reviews, American College of Physicians Journal Club Online (1991–2006), Cochrane Clinical Trials Registry, Database of Abstracts and Reviews, EMBASE (1980–2006), CINAHL (1982–2006), and AARP Ageline (1978–August 2004). The MeSH headings for “orthotic devices” or “protective devices” or “protective clothing” or the root of the text-word “hip protector” were combined with the MeSH heading of “fractures” or the root of the text-word “fracture” as well as the MeSH heading “hip” or the root of the text-word “hip.” We cross-referenced included studies and contacted experts in the field for additional references. We also reviewed the references included in a recently updated Cochrane systematic review by Parker et al. [12,13].
2.3. Selection of trials for inclusion and data abstraction
Two investigators independently reviewed all of the retrieved abstracts and titles and any studies deemed potentially relevant were subject to full-text review by both reviewers. Agreement was reached by both reviewers on which papers should be included in the review. Two reviewers independently assessed methodologic quality characteristics and abstracted data from all included studies. Consensus was reached by both reviewers with respect to abstracted data. In the case of any questions regarding trial methodology or event data, we attempted to contact one or more authors of the study in question by e-mail. If primary authors could not be contacted by e-mail or if the primary authors chose not to respond, we abstracted the data as presented in the primary publication (with consensus among reviewers on interpretation of the data). If primary authors provided new unpublished information, the new information was incorporated in the systematic review.
The population studied was that of nursing home residents since data from individually and cluster randomized trials of hip protectors have been published for this population. A relatively homogenous clinical population of nursing home residents was chosen for study, as opposed to mixed populations including individuals residing in seniors’ apartments or group homes, in order to minimize clinical heterogeneity. Furthermore, application of hip protectors in nursing homes by staff may be considered an important cointervention, not available in other group residence settings; this issue could be another contributor to clinical heterogeneity of pooled analyses of mixed populations. The nursing home population was of greatest clinical interest, as this population is known to be at extremely high risk for osteoporosis [14–16], falls [17], cognitive impairment (leading to falls and hip fracture) [16], and hip fracture [18–20].
2.4. Statistical analyses
A Bayesian random effects model was chosen for pooling of binary outcome data of CRTs and IRTs because it allows for formal incorporation of both prior and likelihood information on all unknown parameters including ICC, in the analysis [21]. The outcome measure of interest was the odds of a participant in the treatment group sustaining one (or more) hip fractures, compared to a participant in the control group. WinBUGS version 1.4.1 (MRC Biostatistics Unit, Cambridge, UK) was used for all Bayesian statistical analyses (random number seed 314159 for all analyses) [22]. In order to transform the prior and likelihood function data to a posterior inference (final result), simulations were performed using Markov chain Monte Carlo Methods. For each outcome, we performed 41,000 simulations, with Gibbs sampling of results for posterior distributions started at 1,000 (sampling every 5th value); three chains were run simultaneously for each model. For each CRT, the VIF was considered to be equal to the value of [1 + (m − 1)(ICC)], where m was the average number of individuals per cluster [3]. In the case of CRTs in which the average cluster size was not reported or could not be calculated based on data in the paper, we assumed a normal distribution of mean cluster sizes and divided the sum of the maximum cluster size and minimum cluster size by two (rounded to the nearest person). The WinBUGs code for the model used in the primary analysis is shown in the Appendix. Assumptions used in the primary model and sensitivity analyses are described below.
A posterior distribution of the odds ratio (OR) of the treatment effect of hip protector allocation in decreasing the risk of hip fracture was modeled. We checked for non-convergence of posteriors of the respective models by examining the percentage of the Monte Carlo error divided by the standard deviation, the dynamic trace, the time series plot, and the quantile trace. The results were expressed as the posterior mean with corresponding 95% credibility interval (CRI). Using the traditional definition of 95% CRI, there is a 95% probability that the true treatment effect, in this case, the OR, lies within this interval [23]. The posterior density function of the OR was provided.
2.5. Assumptions and priors used in the statistical analyses
Distribution assumptions of unknown quantities in the analyses were prespecified. A Normal distribution with an unknown mean and precision (precision is defined as the reciprocal of the variance) was assumed for the natural logarithm of the OR of an individual sustaining one or more hip fractures. A Normal distribution (0,1.0 E-6), representing weak prior information, was assumed for the mean. We also assumed a Normal (rhom, rhotau) distribution for the ICC estimates from the respective CRTs. A Beta (1,1) prior distribution was assumed for the mean, rhom; this is equivalent to assuming a flat Uniform (0,1) prior. A flat Uniform distribution for priors of ICCs has been previously suggested in the analysis of individual CRTs with binary data;[24] this is because ICCs must range from 0–1 and be nonnegative by definition [24]. We used the Uniform and Half-Normal priors recommended by Gelman for the variance components of the model [25].
For CRTs in which the ICC or VIF was not reported (or could not be estimated based on the data reported in the trial or was not available from the primary author), ICC values were imputed and multiple sensitivity analyses were performed. It has been previously noted that ICCs for process variables (nonoutcome variables, ICCs in the range of 0.05–0.15) are generally an order of magnitude higher than those for outcome variables (ICCs generally lower than 0.05) (in implementation trials in primary care) [4]. We chose to explore the impact of a wide range of plausible imputed ICC ranging from 0.01 to 0.5 for trials not reporting an ICC or VIF. In the primary analysis, the mean of the ICC of the CRTs for which this information was missing, was assumed to be the same as the mean of the known ICCs.
3. Results
3.1. Studies included in the systematic review
Two reviewers independently examined 338 unique titles or abstracts and 84 potentially relevant references in full-text form; four papers were included in the review (Fig. 1). Of the four trials included in this systematic review, one was considered individually randomized—Jantti et al., 1998 [37] and three were considered cluster randomized—Ekman et al., 1997 [38], Harada et al., 2001 [39], Meyer et al., 2003 [40]. In the case of the Harada et al. trial, information on cluster randomization was obtained from the author, as details of randomization were not explicitly reported in the primary paper. Two trials of mixed populations (nursing home and other populations) were excluded, as they were not comprised exclusively of nursing home residents [28,35].
Fig. 1.
Process of exclusion of studies from the systematic review [26–36].
3.2. Assessment of methodologic features of the included studies
An assessment of the methodologic characteristics of the four included trials is shown in Table 1. Of note, central computerized randomization was explicitly reported in one trial. As sham hip protectors were not used in any of the studies, strict blinding of participants, caregivers, and investigators was not achieved in any of the trials. Losses to follow-up in treatment or control groups ranged from 0% to 47% or were not documented. Moreover, adherence with the intervention was quite poor in all of the studies, ranging from 34% to 70% (using the definition of compliance or adherence suggested by the primary authors of each study).
Table 1.
Assessment of the methodologic features of included studies
| Study (Individual or cluster randomization, unit of cluster if applicable) | Computerized central randomization reported in primary publication | Use of sham hip protectors | Intention to treat data extractable | Loss to follow-up (%) | Hip protector adherence in treatment groupa (Trial duration) | Adjustment for clustering in calculations of a) sample size and b) final results | ICC or VIF reportedb |
|---|---|---|---|---|---|---|---|
| Harada et al., 2001 [39] (Cluster—Room)c | Nod | No | Yes | T, 7%; C, 11% | 70%e (12 months) | a) No, b) No | No |
| Jantti et al., 1998 [37] (Individual) | Nof | No | Yes | T, 31%; C, 47% | 68% (12 months) | Not applicable | Not applicable |
| Ekman et al., 1997 [38] (Cluster—Nursing Home) | Nod | No | Yes | Unclear | 44% (11 months) | a) No, b) No | No |
| Meyer et al., 2003 [40] (Cluster—Nursing Home) | Yes | No | Yes | T, 2%; C, 5% | 34% (15 months) | a) Yes, b) Yes | Nog |
Abbreviations: T, Treatment group; C, Control group.
Daytime use of hip protectors at the end of the trial was assumed, unless otherwise indicated.
ICC, Intra-cluster correlation coefficient; VIF, variance inflation factor.
The unit of randomization was not clearly described in the primary publication. Through e-mail communication with the primary author, we learned the trial was cluster randomized, with the cluster unit being a room (of 4 or fewer patients, with average cluster size not known).
Method of randomization not clearly reported in the primary publication.
24-hour wear of hip protectors.
Randomization by closed envelope method.
Based on data presented in the primary paper, we were able to estimate the ICC and the value of ICC was confirmed to be 0.0247 through e-mail correspondence with the author.
For the CRTs, the unit of randomization was reported to be a nursing home in the Ekman et al. [38] and Meyer et al. [40] trials. Dr. Harada informed us that the unit of randomization in his study was by room number and that rooms contained up to four residents (average cluster size unknown) [39]. The ICC or VIF was not reported in the primary publication of any of the CRTs. An adjustment for cluster randomization in the analysis of final results was performed only in the Meyer et al. trial [40]. Based on the data pertaining to the adjusted analysis in the Meyer et al. trial, we were able to calculate an approximate estimate for the ICC and verified it to be 0.0247 through e-mail correspondence with the primary authors.
3.3. Characteristics of the included trials
In the four trials included in the review, a total of 1,037 participants were allocated to the treatment arms and 885 participants allocated to the control arms [37–40]. There was no gender restriction in the trials, with the exception of the Harada et al. trial [39], which included only women. For the three CRTs, we estimated the respective mean cluster sizes to be 19 in the Meyer et al. trial, 186 in the Ekman et al. trial, and 3 in the Harada et al. trial (minimum plus maximum divided by two for the latter trial). The duration of trials ranged from 11 to 15 months (Table 1). The Safe-hip protector (Tytex) brand of hip protector was used in the Harada et al. [12,39], and Meyer et al. [40] trials (Table 2); three protectors were provided per participant. The JOFA AB hip protector was used in the Ekman et al. trial [38] whereas a hip protector designed by the primary authors was used in the Jantti et al. trial [37], with the number of hip protectors provided per participant being unclear in each of these studies. Educational cointerventions or measures to encourage adherence (such as employment of staff to educate participants or caregivers or encourage adherence) were used in the Meyer et al. [40] trial. Of note, in the Meyer et al. study, individuals in the control group were not prohibited from wearing hip protectors and 40/483 (8%) of the control participants used a hip protector during the study [40].
Table 2.
Hip fracture rates in the individual studies
| Study | Brand of hip protector studied | Hip fracture rate in the control groupa | Hip fracture rate in the treatment groupa |
|---|---|---|---|
| Harada et al., 2001 [39] | Safehip | 8/76 (10.5%) | 1/88 (1.1%) |
| Jantti et al., 1998 [37] | Designed by authors | 7/36 (19.4%) | 1/36 (2.8%) |
| Ekman et al., 1997 [38] | JOFA AB | 17/442 (3.8%) | 4/302 (1.3%) |
| Meyer et al., 2003 [40] | Safehip | 39/483 (8.1%) | 21/459 (4.6%) |
Number of individuals who sustained one or more hip fractures.
3.4. Participant hip fracture rates
The number of individuals who sustained one or more hip fractures in the treatment and control groups, were as follows: Harada et al.—treatment 1/88 (1.1%), control 8/76 (10.5%) [39], Jantti et al.—treatment 1/36 (2.8%), control 7/36 (19.4%) [37], Ekman et al.—treatment 4/302 (1.3%), control 17/442 (3.8%) [38], and Meyer et al.—treatment 21/459 (4.6%), control 39/483 (8.1%) [40] (Table 2). Multiple hip fractures sustained by the same individual were reported only in the Meyer et al. trial with 42 hip fractures sustained by 39 individuals in the control group of that trial [40].
3.5. Pooled analyses
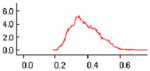
In the primary pooled analysis, when a mean ICC of 0.0247 was assumed for all CRTs (equivalent to the known ICC in the Meyer et al. trial), the mean odds of an elderly individual allocated to hip protector use sustaining one or more hip fractures was 0.40 (95% CRI 0.25, 0.61) (Tables 3 and 4). The results were robust in multiple sensitivity analyses in which the unknown values of the unreported ICCs, varying from 0.01 to 0.5, were imputed (Tables 3 and 4). In all analyses, we found no evidence of nonconvergence. A graphical assessment for nonconvergence is shown for each analysis in Table 4. The percentage Monte Carlo error divided by the standard deviation was 5.7% for the primary analysis and varied from 5.4% to 5.8% for the respective sensitivity analyses.
Table 3.
Results of sensitivity analyses exploring various imputed values for the unknown ICCs in estimating the odds of an individual sustaining a hip fracture with hip protector allocation
| Assumption for the unknown ICCs | OR | 95% credibility interval | Posterior density |
|---|---|---|---|
| Mean imputed ICC = 0.0247 (Equal to the known ICC) | 0.40 | 0.25, 0.61 |

|
| Imputed ICCs = 0.01 | 0.39 | 0.23, 0.60 |

|
| Imputed ICCs = 0.05 | 0.39 | 0.24, 0.60 |

|
| Imputed ICCs = 0.1 | 0.38 | 0.25, 0.57 |
|
| Imputed ICCs = 0.15 | 0.40 | 0.25, 0.61 |

|
| Imputed ICCs = 0.5 | 0.38 | 0.25, 0.57 |

|
Abbreviation: ICC, Intra-cluster correlation coefficient.
Table 4.
Assessment for non-convergence for posterior densities of the odds ratio of hip fracture with hip protector allocation
Abbreviation: ICC, Intra-cluster correlation coefficient.
4. Discussion
We have proposed a Bayesian random effects data for pooling data from cluster and individually randomized trials, incorporating an adjustment for clustering. Using this model, we determined that there is evidence that hip protectors significantly decrease the risk of hip fracture in elderly nursing home residents. However, this conclusion is limited by methodologic and reporting deficiencies of the included studies. In particular, lack of reporting of the VIF or ICC in many of the included studies was noted. We have tried to adjust for this limitation by imputing various values of potential ICC’s for the studies not reporting this variable and performing multiple sensitivity analyses. Our findings were found to be robust in the sensitivity analyses tested. Of particular note, we have observed a positive treatment effect of the hip protector intervention in a specialized population of nursing home residents, whereas pooled analyses of mixed populations including residents of group homes, hostels, or seniors’ apartments were generally negative [10,12,13]. As an explanation of these findings, we hypothesize that the application of these devices by staff in nursing homes may represent a clinically important cointervention, which would generally not be available in less supervised settings. It is also possible that a herd effect may contribute to the positive effect of the intervention seen in the CRTs of hip protectors in nursing homes [41]; for example, the staff may have been particularly motivated to apply the devices on residents in institutions adopting such a strategy [41].
Established guidelines for pooling of data from CRTs do not currently exist, leading to variable quality of published meta-analyses. In a recent systematic review, Laopaiboon noted that in 52% (13/25) of meta-analyses of CRTs, inappropriate methods that ignored clustering effect, were used for pooling of results [9]. Laopaiboon has suggested that lack of provision of software for analysis of CRTs by the Cochrane Collaboration may have contributed to inappropriate meta-analyses of CRTs [9]. At present, the freely available Cochrane collaboration software (Review Manager 4.2.8) cannot accommodate adjustments for cluster-randomized designs [42]. However, WinBUGs software, which was used in the model that we proposed, is currently freely available through the MRC Biostatistics Unit web site [22]. The code that we have provided can be used with the latest WinBUGs software for analysis of data from CRTs of other interventions. Thus, we have proposed an option for pooling of data from CRTs, which is currently accessible and free of charge. Furthermore, the posterior for the treatment effect of hip protectors in elderly nursing home residents generated in our model may be used as an informative prior in future Bayesian trials or meta-analyses of this intervention.
Our study is subject to several limitations, other than the imputation of unreported ICC’s as described above. First, the average cluster size had to be estimated in one of the studies, potentially resulting in some estimation error. Second, the quality of any systematic review is limited by the quality of the studies that are included. In the studies we identified, losses to follow-up and noncompliance rates were relatively high. Moreover, strict blinding of participants, caregivers, and adjudicators of outcomes could not have been achieved in any of the trials studied as sham hip protectors were not used. Third, selection bias may have also occurred in the case of refusal of participants in clusters to accept the intervention or attrition [43]. Finally, we chose to use noninformative priors in our meta-analytic model, and that approach may be criticized by advocates of the use of informative priors in Bayesian models. The reasons for choosing a noninformative prior was to limit criticism of potential subjectivity in prior estimation, and to generate posteriors analogous to those that would be generated using a frequentist approach.
We hope that after publication of the extended CONSORT guidelines for reporting of CRTs [6], important information such as ICCs and cluster sizes will be explicitly reported in future CRTs, making imputation of these values less necessary in future meta-analyses. We also hope that guidelines for systematically reviewing and pooling data from CRTs will be established. A Bayesian approach may be a feasible option for pooling such complex data. Although we have proposed a Bayesian model for meta-analysis binary outcomes of cluster with (or without) IRTs, an analogous model has yet to be developed for pooling of continuous data. Furthermore, more complicated models will need to be developed to accommodate meta-analysis of matched pair, crossover, or factorial designs, should those be used in future CRTs. Indeed, as the design of randomized controlled trials has become more complex, methods used for pooling of data from such trials will need to become more complex in the future. The flexibility of the Bayesian approach offers a particularly attractive option for incorporating complex issues and assumptions in pooling data from specialized randomized controlled trials. Finally, we have used this approach to highlight the effectiveness of hip protectors in decreasing the risk of hip fractures in a vulnerable, often ignored population with a significant burden of disease.
Acknowledgments
No funding was received for this project. We would like to thank the Reviewer for valuable comments that led to improvements in the proposed analytical model and the manuscript. We would also like to thank Drs. David Spiegelhalter and Andrew Gelman for their advice on Bayesian modeling. We would like to thank Dr. Sharon Straus for reviewing the manuscript and providing us with extremely helpful clinical and methodological insights. Furthermore, we would like Dr. Anthony Hodsman for his thoughtful review of the PhD thesis of Anna Sawka.
Appendix. The WinBUGs code for the model used
| model |
| { |
| for(i in 1: Num1) { |
| rc[i] ~ dbin(pc[i], nc[i]) |
| rt[i] ~ dbin(pt[i], nt[i]) |
| logit(pc[i]) <- mu[i] |
| logit(pt[i]) <- mu[i] + delta[i] |
| mu[i] ~ dnorm(0.0,1.0E-5) |
| delta[i] ~ dnorm(d, tau) |
| } |
| for(i in (Num1 + 1): Num2) { |
| rc[i] ~ dbin(pc[i], nc[i]) |
| rt[i] ~ dbin(pt[i], nt[i]) |
| logit(pc[i]) <- mu[i] |
| logit(pt[i]) <- mu[i] + delta[i] |
| mu[i] ~ dnorm(0.0,1.0E-5) |
| delta[i] ~ dnorm(d, tauAdj) |
| #Assuming a Uniform distribution for rho estimates |
| rho[i]~dnorm(rhom,rhotau) |
| VIF[i]<-(1+(m[i]-1)*rho[i]) |
| } |
| #Takes the average of the VIFs from cluster RCTs |
| VIFM<-(VIF[2]+VIF[3]+VIF[4])/3 |
| #Same VIF for all cluster RCTs |
| tauAdj<-tau/VIFM |
| #Prior Distribution for d |
| d~dnorm(0,1.0E-6) |
| #Beta Prior Distribution for rhom |
| rhom~dbeta(1,1) |
| #Prior Distributions for rhotau and sigmatau |
| rhotau<-1/(sigmarho*sigmarho) |
| tau<-1/(sigmatau*sigmatau) |
| sigmarho~dunif(0,1) |
| sigmatau~dnorm(0,10000)I(0,) |
| OR<-exp(d) |
| } |
| # Data |
| list(rt = c(1,1,21,4), nt = c(36,88,459,302), rc = c(7,8,39,17), nc = c(36,76,483,442), rho = c(0,Xi1,0.0247,Xi2), m = c(0,3,19,186), Num1 = 1, Num2 = 4) |
| #Initial Values |
| #CHAIN 1 |
| list(d = 0, mu = c(0,0,0,0), delta = c(0,0,0,0), rhom = 0.01, sigmarho = 0.03, sigmatau = 1) |
| #CHAIN 2 |
| list(d = 0, mu = c(0,0,0,0), delta = c(0,0,0,0), rhom = 0.02, sigmarho = 0.05, sigmatau = 1) |
| #CHAIN 3 |
| list(d = 0, mu = c(0,0,0,0), delta = c(0,0,0,0), rhom = 0.05, sigmarho = 0.1, sigmatau = 1) |
Xi1 and Xi2 represent imputed values for the ICC for the trials not reporting the ICC. The mean of these values is equal to 0.0247 (the known ICC) in the primary analysis.
References
- 1.Elbourne DR, Campbell MK. Extending the CONSORT statement to cluster randomized trials: for discussion. Stat Med. 2001;20:489–96. doi: 10.1002/1097-0258(20010215)20:3<489::aid-sim806>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Altman DG. Statistics in medical journals: some recent trends. Stat Med. 2000;19:3275–89. doi: 10.1002/1097-0258(20001215)19:23<3275::aid-sim626>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Klar N, Donner A. Current and future challenges in the design and analysis of cluster randomization trials. Stat Med. 2001;20:3729–40. doi: 10.1002/sim.1115. [DOI] [PubMed] [Google Scholar]
- 4.Campbell MK, Mollison J, Steen N, Grimshaw JM, Eccles M. Analysis of cluster randomized trials in primary care: a practical approach. Fam Pract. 2000;17:192–6. doi: 10.1093/fampra/17.2.192. [DOI] [PubMed] [Google Scholar]
- 5.Cornfield J. Randomization by group: a formal analysis. Am J Epidemiol. 1978;108:100–2. doi: 10.1093/oxfordjournals.aje.a112592. [DOI] [PubMed] [Google Scholar]
- 6.Campbell MK, Elbourne DR, Altman DG CONSORT group. CONSORT statement: extension to cluster randomised trials. BMJ. 2004;328:702–8. doi: 10.1136/bmj.328.7441.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Stat Med. 2002;21:2971–80. doi: 10.1002/sim.1301. [DOI] [PubMed] [Google Scholar]
- 8.Donner A, Piaggio G, Villar J. Statistical methods for the meta-analysis of cluster randomization trials. Stat Methods Med Res. 2001;10:325–38. doi: 10.1177/096228020101000502. [DOI] [PubMed] [Google Scholar]
- 9.Laopaiboon M. Meta-analyses involving cluster randomization trials: a review of published literature in health care. Stat Methods Med Res. 2003;12:515–30. doi: 10.1191/0962280203sm347oa. [DOI] [PubMed] [Google Scholar]
- 10.Sawka AM, Boulos P, Beattie K, Thabane L, Papaioannou A, Gafni A, et al. Do hip protectors decrease the risk of hip fracture in institutional and community dwelling elderly? Osteoporos Int. 2005;16:1461–74. doi: 10.1007/s00198-005-1932-2. [DOI] [PubMed] [Google Scholar]
- 11.Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR. Methods in health service research. An introduction to Bayesian methods in health technology assessment. BMJ. 1999;319:508–12. doi: 10.1136/bmj.319.7208.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker MJ, Gillespie LD, Gillespie WJ. Hip protectors for preventing hip fractures in the elderly. Cochrane Database Syst Rev. 2005:1. doi: 10.1002/14651858.CD001255. [DOI] [PubMed] [Google Scholar]
- 13.Parker MJ, Gillespie LD, Gillespie WJ. Hip protectors for preventing hip fractures in the elderly. BMJ. 2006;332:571–4. doi: 10.1136/bmj.38753.375324.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandler JM, Zimmerman SI, Girman CJ, Martin AR, Hawkes W, Hebel JR, et al. Low bone mineral density and risk of fracture in white female nursing home residents. JAMA. 2000;284:972–7. doi: 10.1001/jama.284.8.972. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerman SI, Girman CJ, Buie VC, Chandler J, Hawkes W, Martin A, et al. The prevalence of osteoporosis in nursing home residents. Osteoporos Int. 1999;9:151–7. doi: 10.1007/s001980050129. [DOI] [PubMed] [Google Scholar]
- 16.Weller I, Schatzker J. Hip fractures and Alzheimer’s disease in elderly institutionalized Canadians. Ann Epidemiol. 2004;14:319–24. doi: 10.1016/j.annepidem.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Vu MQ, Weintraub N, Rubenstein LZ. Falls in the nursing home: are they preventable? J Am Med Dir Assoc. 2004;5:401–6. doi: 10.1097/01.JAM.0000144553.45330.AD. [DOI] [PubMed] [Google Scholar]
- 18.Butler M, Norton R, Lee-Joe T, Cheng A, Campbell AJ. The risks of hip fracture in older people from private homes and institutions. Age Ageing. 1996;25:381–5. doi: 10.1093/ageing/25.5.381. [DOI] [PubMed] [Google Scholar]
- 19.Ooms ME, Vlasman P, Lips P, Nauta J, Bouter LM, Valkenburg HA. The incidence of hip fractures in independent and institutionalized elderly people. Osteoporos Int. 1994;4:6–10. doi: 10.1007/BF02352254. [DOI] [PubMed] [Google Scholar]
- 20.Brennan nee Saunders J, Johansen A, Butler J, Stone M, Richmond P, Jones S, et al. Place of residence and risk of fracture in older people: a population-based study of over 65-year-olds in Cardiff. Osteoporos Int. 2003;14:515–9. doi: 10.1007/s00198-003-1404-5. [DOI] [PubMed] [Google Scholar]
- 21.Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR. Bayesian methods in health technology assessment: a review. Health Technol Assess. 2000;4(38):1–130. [PubMed] [Google Scholar]
- 22.MRC Biostatistics Unit (Cambridge University) [Accessed March 4, 2005];The BUGS Project—Bayesian inference using Gibbs sampling. Available at http://www.mrc-bsu.cam.ac.uk/bugs/welcome.shtml2004.
- 23.Spiegelhalter DJ, Abrams KR, Myles JP. Section 3.8 point estimation, interval estimation, and interval hypotheses. In: Spiegelhalter DJ, Abrams KR, Myles JP, editors. Bayesian approaches to clinical trials and healthcare evaluation. John Wiley and Sons; 2004. pp. 64–7. [Google Scholar]
- 24.Turner RM, Omar RZ, Thompson SG. Bayesian methods of analysis for cluster randomized trials with binary outcome data. Stat Med. 2001;20:453–72. doi: 10.1002/1097-0258(20010215)20:3<453::aid-sim803>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 25.Gelman A. Prior distributions for variance parameters in hierarchical models. Bayesian Analysis. 2005;1(2):1–19. [Google Scholar]
- 26.Birks YF, Hildreth R, Campbell P, Sharpe C, Torgerson DJ, Watt I. Randomised controlled trial of hip protectors for the prevention of second hip fractures. Age Ageing. 2003;32:442–4. doi: 10.1093/ageing/32.4.442. [DOI] [PubMed] [Google Scholar]
- 27.Birks YF, Porthouse J, Addie C, Loughney K, Saxon L, Baverstock M, et al. Randomized controlled trial of hip protectors among women living in the community. Osteoporos Int. 2004;15:701–6. doi: 10.1007/s00198-004-1599-0. [DOI] [PubMed] [Google Scholar]
- 28.Cameron ID, Venman J, Kurrle SE, Lockwood K, Birks C, Cumming RG, et al. Hip protectors in aged-care facilities: A randomized trial of use by individual higher-risk residents. Age Ageing. 2001;30:477–81. doi: 10.1093/ageing/30.6.477. [DOI] [PubMed] [Google Scholar]
- 29.Cameron ID, Cumming RG, Kurrle SE, Quine S, Lockwood K, Salkeld G, et al. A randomised trial of hip protector use by frail older women living in their own homes. Inj Prev. 2003;9:138–41. doi: 10.1136/ip.9.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Schoor NM, Smit JH, Twisk JW, Bouter LM, Lips P. Prevention of hip fractures by external hip protectors: a randomized controlled trial. JAMA. 2003;289:1957–62. doi: 10.1001/jama.289.15.1957. [DOI] [PubMed] [Google Scholar]
- 31.Chan DK, Hillier G, Coore M, Cooke R, Monk R, Mills J, et al. Effectiveness and acceptability of a newly designed hip protector: a pilot study. Arch Gerontol Geriatr. 2000;30:25–34. doi: 10.1016/s0167-4943(99)00048-5. [DOI] [PubMed] [Google Scholar]
- 32.Hubacher M, Wettstein A. Acceptance of hip protectors for hip fracture prevention in nursing homes. Osteoporos Int. 2001;12:794–9. doi: 10.1007/s001980170057. [DOI] [PubMed] [Google Scholar]
- 33.Kannus P, Parkkari J, Niemi S, Pasanen M, Palvanen M, Jarvinen M, et al. Prevention of hip fracture in elderly people with use of a hip protector. N Engl J Med. 2000;343:1506–13. doi: 10.1056/NEJM200011233432101. [DOI] [PubMed] [Google Scholar]
- 34.Lauritzen JB, Petersen MM, Lund B. Effect of external hip protectors on hip fractures. Lancet. 1993;341:11–3. doi: 10.1016/0140-6736(93)92480-h. [DOI] [PubMed] [Google Scholar]
- 35.O’Halloran PD, Cran GW, Beringer TR, Kernohan G, O’Neill C, Orr J, et al. A cluster randomised controlled trial to evaluate a policy of making hip protectors available to residents of nursing homes. Age Ageing. 2004;33:582–8. doi: 10.1093/ageing/afh200. [DOI] [PubMed] [Google Scholar]
- 36.Villar M, Hill P, Inskip H, Thompson P, Cooper C. Will elderly rest home residents wear hip protectors? Age Ageing. 1998;27:195–8. doi: 10.1093/ageing/27.2.195. [DOI] [PubMed] [Google Scholar]
- 37.Jantti PO, Aho HJ, Maki-Jokela PL, Heikinheimo RJ. Hip protectors and hip fractures. Age Ageing. 1998;27:758–9. doi: 10.1093/ageing/27.6.758. [DOI] [PubMed] [Google Scholar]
- 38.Ekman A, Mallmin H, Michaelsson K, Ljunghall S. External hip protectors to prevent osteoporotic hip fractures. Lancet. 1997;350:563–4. doi: 10.1016/S0140-6736(05)63140-6. [DOI] [PubMed] [Google Scholar]
- 39.Harada A, Mizuno M, Takemura M, Tokuda H, Okuizumi H, Niino N. Hip fracture prevention trial using hip protectors in Japanese nursing homes. Osteoporos Int. 2001;12:215–21. doi: 10.1007/s001980170132. [DOI] [PubMed] [Google Scholar]
- 40.Meyer G, Warnke A, Bender R, Muhlhauser I. Effect on hip fractures of increased use of hip protectors in nursing homes: cluster randomised controlled trial. BMJ. 2003;326:76. doi: 10.1136/bmj.326.7380.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hahn S, Puffer S, Torgerson DJ, Watson J. Methodological bias in cluster randomised trials. BMC Med Res Methodol. 2005;5:10. doi: 10.1186/1471-2288-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The Cochrane Collaboration. [Accessed March 4, 2005];Cochrane reviewers’ handbook (version 4.2.2) Available at http://www.cochrane.org/resources/handbook/2004.
- 43.Puffer S, Torgerson D, Watson J. Evidence for risk of bias in cluster randomised trials: review of recent trials published in three general medical journals. BMJ. 2003;327:785–9. doi: 10.1136/bmj.327.7418.785. [DOI] [PMC free article] [PubMed] [Google Scholar]




