Abstract
Study Design Retrospective database review.
Objective To identify trends of the recombinant human bone morphogenetic protein-2 (rhBMP-2) use in the treatment of lumbar degenerative spondylolisthesis (LDS).
Methods PearlDiver Patient Record Database was used to identify patients who underwent lumbar fusion for LDS between 2005 and 2011. The distribution of bone morphogenetic protein use rate (BR) in various surgical procedures was recorded. Patient numbers, reoperation numbers, BR, and per year BR (PYBR) were stratified by geographic region, gender, and age.
Results There were 11,335 fusion surgeries, with 3,461 cases using rhBMP-2. Even though PYRB increased between 2005 and 2008, there was a significant decrease in 2010 for each procedure: 404 (34.5%) for posterior interbody fusion, 1,282 (34.3%) for posterolateral plus posterior interbody fusion (PLPIF), 1,477 (29.2%) for posterolateral fusion, and 335 (22.4%) for anterior lumbar interbody fusion. In patients using rhBMP-2, the reoperation rate was significantly lower than in patients not using rhBMP-2 (0.69% versus 1.07%, p < 0.0001). Male patients had higher PYBR compared with female patients in 2008 and 2009 (p < 0.05). The West region and PLPIF had the highest BR and PYBR.
Conclusions Our data shows that the revision rates were significantly lower in patients treated with rhBMP-2 compared with patients not treated with rhBMP-2. Furthermore, rhBMP-2 use in LDS varied by year, region, gender, and type of fusion technique. In the West region, the posterior approach and patients 65 to 69 years of age had the highest rate of rhBMP-2 use.
Keywords: fusion, rhBMP-2, ALIF, posterolateral, demographics, PearlDiver patient record database, lumbar degenerative spondylolisthesis
Introduction
Lumbar degenerative spondylolisthesis (LDS) is one of the most common lumbar spinal diseases associated with low back pain, radiculopathy, and neurogenic intermittent claudication.1 The pathogenesis of lumbar spondylolisthesis begins with the slipping of one vertebral body over the other, narrowing the spinal canal and leading to a wide range of clinical presentations.2 Currently, patients with LDS have treatment options that range from conservative (nonsteroidal anti-inflammatory drugs, physical therapy, epidural steroid injection and bracing) to surgical (decompressive laminectomy with or without fusion and instrumentation).3 4 When conservative treatments fail to provide relief for severe lumbar spondylolisthesis, decompression and fusion can be considered.5 There are several surgical approaches for segmental fusion, including anterior lumbar interbody fusion (ALIF), posterolateral fusion (PL), posterolateral plus posterior interbody fusion (PLPIF), and posterior interbody fusion (PIF).6 7 8 To promote the fusion rates, pedicle screw instrumentation, bone graft material, and osteoinductive adjuvants are used.9 10
Human recombinant bone morphogenetic protein (rhBMP)-2 and rhBMP-7 have been used as osteoinductive adjuvants for spinal fusion.11 12 Commercially available rhBMP-2 (INFUSE Bone Graft, Medtronic, Memphis, Tennessee, United States) has been approved for use in ALIF with a threaded interbody cage. Due to the rhBMP-2 osteogenic potential, off-label use has been extended to PL, PIF, and PLPIF.9 13 14
Although use of rhBMP-2 leads to high fusion rates, complications including excessive and ectopic bone formation, carcinogenicity, bone resorption, neuritis, seroma formation, retrograde ejaculation, and dural ossification leading to neural impingement have been reported.13 15 16 17
Due to the large variations in spine procedures across the United States, it is essential to understand the use of rhBMP-2 in the surgical management of LDS and how its use is affected by different fusion procedures and patient demographics. It is also unclear whether the presence of rhBMP-2 could decrease the reoperation rate. To gain a better understanding, we examined retrospective data on patients treated for LDS with rhBMP-2 in the United States Medicare population from 2005 to 2011.
Materials and Methods
For our retrospective study, we used the PearlDiver Patient Record Database (available at www.pearldiverinc.com; PearlDiver Inc., Fort Wayne, Indiana, United States). Our Institutional Review Board deemed this study exempt from review, as all patient information was deidentified. In our study, we used orthopedic billing records from the standard analytical files from Medicare, containing more than 40 million patients per year. The database was queried for patients undergoing surgical fusion for LDS from 2005 through 2011. Patients were identified by entry of International Classification of Diseases, ninth edition (ICD-9) diagnosis code 738.4 for LS and 84.52 for bone morphogenetic protein. The number of patients having a record of Current Procedural Terminology (CPT) codes associated with ALIF/lateral lumbar interbody fusion (LLIF), PL, PIF, PLPIF, or reoperation lumbar fusion were recorded, including CPT-22558 (the ALIF code is also used for commonly performed LLIF, also known as eXtreme Lateral Interbody Fusion or Direct Lateral Interbody Fusion), CPT-22585, CPT-22586, CPT-22845, CPT-22614, CPT-22634, CPT-22632, and CPT-22849 (Table 1). The search results yielded the number of patients divided by year, gender, age group (less than 65, 65 to 69, 70 to 74, 75 to 79, 80 to 84, and greater than 84), and geographic region of the United States (Northeast, South, Midwest, West). Bone morphogenetic protein use rate (BR) and per year bone morphogenetic protein use rate (PYBR) were calculated for each group. Patients were followed for up to 3 years to permit assessment of long-term outcome.
Table 1. ICD-9 Procedure codes and CPT codes searched in the PearlDiver database.
| Code | Diagnosis/procedure |
|---|---|
| ICD-9 | |
| 738.4 | Acquired spondylolisthesis (degenerative spondylolisthesis; spondylolisthesis acquired excludes congenital [756.12]) |
| 84.52 | BMP |
| CPT | |
| 22558 | Anterior approach for lumbar fusion or lumbar lateral interbody |
| 22585 | Each additional interspace (use with 22558) |
| 22586 | Arthrodesis presacral interbody technique |
| 22845 | Anterior instrumentation; 2–3 vertebral segments |
| 22614 | Posterior or posterolateral technique for fusion/arthrodesis |
| 22634 | A combined posterior or posterolateral technique with posterior interbody arthrodesis at an additional level |
| 22632 | Arthrodesis an additional level fusion |
| 22849 | Reoperation lumbar fusion (reinsertion of spine) |
Abbreviations: BMP, bone morphogenetic protein; CPT, Current Procedural Terminology; ICD-9, International Classification of Diseases, ninth edition.
For statistical analysis, we used SPSS 13.0 software (IBM, Armonk, New York, United States). The chi-square test was used to determine statistical significance with regard to procedure, gender, age, and region. Linear regression was performed to test the significance of trends over time. The level of significance was p < 0.05.
Results
Between 2005 and 2011, there were 11,335 fusion surgeries in patients with LDS, of which 3,461 cases were performed with the use of rhBMP-2. Within the rhBMP-2 cases, there were 2,410 female and 1,082 male patients. Nine hundred fifty-three surgeries with rhBMP-2 were done in the Midwest, 271 in the Northeast, 1,314 in the South, and 975 in the West. The overall distribution of rhBMP-2 use by age was 559 cases in patients less than 65 years old, 1,035 cases in patients 65 to 69 years old, 930 cases in patients 70 to 74 years old, 655 cases in patients 75 to 79 years old, 241 cases in patients 80 to 84 years old, and 78 cases in patients greater than 84 years old.
Per Year Bone Morphogenetic Protein Use Rate
By Year
Overall, the average PYBR was 30.5% between 2005 and 2011. Although there was a significant decrease in 2010 (24.9%, p < 0.0001), in 2011 PYBR increased to 30.5%, coming close to the 7-year average (30.5%, Table 2).
Table 2. The number of patients with or without BMP and PYBR from 2005 to 2011.
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | Total | |
|---|---|---|---|---|---|---|---|---|
| BMP casesa | 388 | 411 | 491 | 588 | 671 | 395 | 568 | 3,461 |
| Total casesb | 1,338 | 1,354 | 1,546 | 1,784 | 2,106 | 1,587 | 1,863 | 11,335 |
| PYRB (%)c | 29.00 | 30.35 | 31.76 | 32.96 | 31.86 | 24.89 | 30.49 | 30.53 |
Abbreviations: BMP, bone morphogenetic protein; PYBR, per year bone morphogenetic protein use rate.
Number of patients with lumbar degenerative spondylolisthesis using BMP in the operation.
Total number of patients with lumbar degenerative spondylolisthesis treated by operation.
BMP cases in 1 year/total cases in 1 year × 100 (%).
By Surgical Procedure
The mean PYBR of each procedure was 22.4% for ALIF, 29.2% for PL, 34.3% for PLPIF, and 34.5% for PIF from 2005 to 2011. The trend of PYBR for each procedure increased from 2005 to 2008 and was lowest in 2010 (Fig. 1a). The yearly distribution of surgical procedures using rhBMP-2 was the greatest in PL, followed by PLPIF, PIF, and ALIF (Fig. 1b).
Fig. 1.
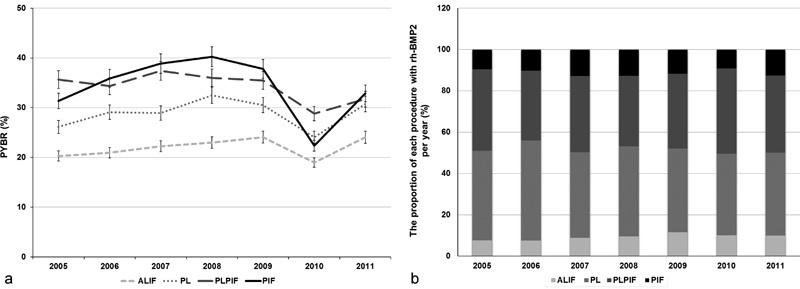
Bone morphogenetic protein use in different surgical procedures. (a) PYBR in for each produce from 2005 and 2011 with PIF (34.53%) having the highest mean PYBR of the four procedures; (b) the proportion of each procedure cases by year from 2005 to 2011. Abbreviations: ALIF, anterior lumbar interbody fusion; PIF, posterior interbody fusion; PL, posterolateral fusion; PLIF, posterolateral plus posterior interbody fusion; PYBR, per year bone morphogenetic protein use rate; rh-BMP2, recombinant human bone morphogenetic protein-2.
Bone Morphogenetic Protein Use Rate
By Age
The overall rate of rhBMP-2 use changed with age, with the lowest rate (27.1%) observed in patient 80 to 84 years old, followed by patients less than 65 years (29.1%), 75 to 79 years old (30.9%), greater than 84 years old (31.1%), 70 to 74 years old (31.2%), and 65 to 69 years old (31.3%, Table 3). Looking at the age distribution by fusion type, the highest rate of rhBMP-2 use for ALIF, PLPIF, and PIF was in patients > 84 years of age (31.4, 39.7, and 46.2%, p < 0.001, respectively). The greatest rhBMP-2 use for PL was found in patients 65 to 69 years old (30.6%, p < 0.001, Table 3).
Table 3. The number of patients using or without BMP of different surgical techniques by age.
| ALIF | PL | PLPIF | PIF | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | N | B | R | N | B | R | N | B | R | N | B | R | N | B | R |
| <65 | 303 | 81 | 21.09 | 493 | 204 | 29.27 | 455 | 207 | 31.27 | 114 | 67 | 37.02 | 1,365 | 559 | 29.05 |
| 65–69 | 353 | 92 | 20.67 | 927 | 409 | 30.61 | 763 | 404 | 34.62 | 234 | 130 | 35.71 | 2,277 | 1,035 | 31.25 |
| 70–74 | 250 | 82 | 24.7 | 931 | 403 | 30.21 | 646 | 338 | 34.35 | 221 | 107 | 32.62 | 2,048 | 930 | 31.23 |
| 75–79 | 166 | 54 | 24.55 | 746 | 303 | 28.88 | 413 | 226 | 35.37 | 138 | 72 | 34.29 | 1,463 | 655 | 30.93 |
| 80–84 | 68 | 15 | 18.07 | 384 | 122 | 24.11 | 144 | 82 | 36.28 | 52 | 22 | 29.73 | 648 | 241 | 27.11 |
| >84 | 24 | 11 | 31.43 | 104 | 36 | 25.71 | 38 | 25 | 39.68 | 7 | 6 | 46.15 | 173 | 78 | 31.08 |
| Total | 1,164 | 335 | 22.35 | 3,585 | 1,477 | 29.18 | 2,459 | 1,282 | 34.27 | 766 | 404 | 34.53 | |||
Abbreviations: ALIF, anterior lumbar interbody fusion; B, number of patients using BMP; BMP, bone morphogenetic protein; PIF, posterior interbody fusion; PL, posterolateral fusion; PLIF, posterolateral plus posterior interbody fusion; N, number of patients not using BMP; R, ratio of BMP use = B/(N + B) × 100 (%) of each procedure for every age.
By Demographic Region
The BR was the highest in the West (37.3%) followed by the Midwest (32.8%), the South (28.3%), and the Northeast (19.7%, p < 0.001; Fig. 2a).When examining the regional BR per procedure, we observed an uneven distribution (Fig. 2b). The lowest BR for ALIF was found in the South (18.4%), and the Northeast region had the lowest BR for PL (16.2%), PLPIF (26.3%), and PIF (23.8%).
Fig. 2.
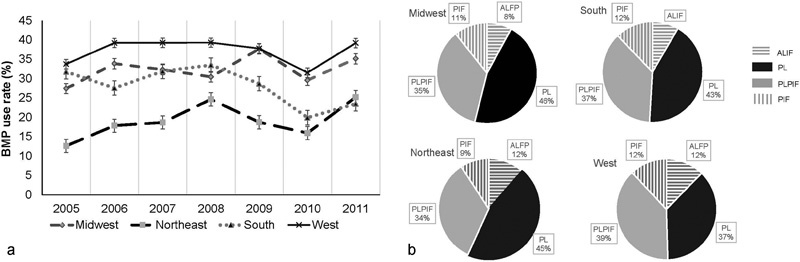
The BMP use rate by demographic region. (a) The PYBR of the four regions. West region had the highest PYBR among four regions with the mean PYBR of 37.33%. (b) The distribution of BMP use by the four procedures in each region. Abbreviations: ALIF, anterior lumbar interbody fusion; BMP, bone morphogenetic protein; PIF, posterior interbody fusion; PL, posterolateral fusion; PLIF, posterolateral plus posterior interbody fusion; PYBR, per year bone morphogenetic protein use rate.
By Gender
We found that the BR was higher in female patients in all years except 2008 and 2009. In those 2 years, male patients were more likely to receive rhBMP2 compared with the female patients (2008: male 35.2%, female 32.1%; 2009: male 33.3%, female 31.2%; p < 0.0001, Table 4).
Table 4. The number of patients using or not using BMP by sex from 2005 to 2011.
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | |
|---|---|---|---|---|---|---|---|
| Female | |||||||
| N | 649 | 647 | 707 | 779 | 980 | 800 | 856 |
| B | 283 | 301 | 348 | 368 | 444 | 272 | 394 |
| R (%) | 30.36 | 31.75 | 32.99 | 32.08 | 31.18 | 25.37 | 31.52 |
| Male | |||||||
| N | 301 | 296 | 348 | 369 | 455 | 392 | 439 |
| B | 105 | 110 | 143 | 200 | 227 | 123 | 174 |
| R (%) | 25.86 | 27.09 | 29.12 | 35.15 | 33.28 | 23.88 | 28.38 |
Abbreviations: B, number of patients using BMP; BMP, bone morphogenetic protein; N, number of patients not using BMP; R, ratio of BMP use = B/(N + B) × 100 (%) of each procedure for every age.
Reoperation Rate of Patients with or without Bone Morphogenetic Protein
From 2005 to 2011, the overall reoperation rate of patients who used rhBMP-2 was significantly lower than the rate in patients who did not use rhBMP-2 (0.7 versus 1.1%, p < 0.0001, Fig. 3). Furthermore, the same pattern was seen looking at the reoperation rates in different procedures with and without rhBMP-2: ALIF (0 versus 1%, p < 0.0001), PL (0.8 versus 1%, p < 0.001), PLPIF (0.9 versus 1.2%, p < 0.01), and PIF (0.3 versus 0.9%, p < 0.0001).
Fig. 3.
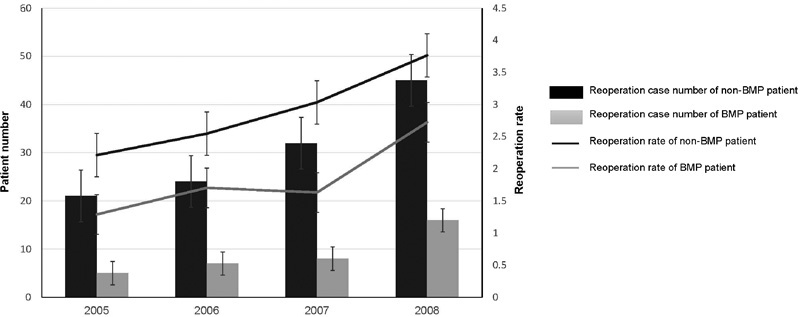
The reoperation rate by 3-year follow-up. From 2005 to 2008, patients using bone morphogenetic protein (BMP) had a lower reoperation rate in each year compared with patients not using BMP (1.29 and 2.21%, respectively, in 2005, p < 0.0001; 1.70 and 2.55%, respectively, in 2006, p < 0.0001; 1.63 and 3.03%, respectively, in 2007, p < 0.0001; 2.72 and 3.76%, respectively, in 2008, p < 0.001).
Discussion
LDS affects between 0.5 and 4.5% of the adult population, especially patients over the age of 60.3 7 18 In general, nerve decompression and fusion is the most effective surgical intervention. Even though isolated posterior and anterior approaches are the most common procedures, combined anterior-posterior or posterior interbody techniques may provide an optimal reconstruction for patients with severe pathology.18 19 20 21 22
rhBMP-2 and its osteogenic potential were first reported by Urist in 1965.23 Studies focusing on various approaches and carriers have demonstrated the regenerative potential of rhBMP-2 in enhancing segmental fusion.24 25 26 Although a large body of literature favors the use of rhBMP-2, some studies indicate potential adverse effects.16 17 27
In the present study, patients using rhBMP-2 had a significantly lower reoperation rate within a 7-year period compared with patients who did not use rhBMP-2. Even though the effect of rhBMP-2 was positive, we found that the PYBR changed in the 7 years, with an upward trend from 2005 to 2008 and a decrease in 2010, which may be due to the Food and Drug Administration notification and published reports on complications on bone morphogenetic protein use.
In all patients with LDS, rhBMP-2 was used mainly for posterior and posterior-lateral procedures, compared with the on-label indication (ALIF). This result could be potentially explained with the increased use of the posterior approach for various spinal degenerative conditions, as well as high fusion rates, good patient outcomes, and restoration of sagittal balance in patients with spondylolisthesis.28 29
Previous studies found that the differences in LDS treatment with patient age were a result of various general conditions, tolerance to trauma after the surgery, bone density, and degree of degeneration.13 14 30 Similarly, in our study, the use of rhBMP-2 in LDS treatment showed no linear correlation with age. It is unclear why certain age groups had high rhBMP-2 rates and if the patient profiles within the database had influence.
In our study, we observed PYBR changes associated with gender, with female patients having more rhBMP-2 procedures than male patients. Earlier studies on idiopathic scoliosis demonstrated that use of rhBMP-2 was more common in the West region.31 Similarly, we found the West region to have the highest rate of rhBMP-2 use for the LDS treatments. However, the distribution of the four fusion procedures in each region was not the same. The possible explanation may be regional variations in surgeon preference and training, economic factors, and patient expectations.
Although this study provides a timely and critical update on the trends of rhBMP-2 use, there are several limitations to this study. The database has been reported to carry a potential risk for inaccuracy in capturing the correct diagnoses and procedures. The variations in coding and data input may introduce additional inaccuracies in patient selections from the database. To address these limitations, we first utilized the procedural codes and then the diagnostic codes to improve the specificity of our search. In addition, the total number of cases over the 7-year period did not match the sum of number of cases per year, which happens if patients get older, if they relocate, or if they had more than one operation between 2005 and 2011; in those instances, they will be counted in the database for several years. To address these limitations, we calculated the total number of each parameter by the output of the PearlDiver database without double-count. Furthermore, we were unable to comment on the effect of bone morphogenetic protein dose with various procedures due to the inexistence of dose-specific ICD-9 codes. Finally, in our study, the follow-up period was 3 years, but it is possible that these results would change over a longer period. Despite these limitations, we believe that our data represents important and relevant information on the trends of rhBMP-2 use for the treatment of LDS.
Conclusion
Our study shows that the utilization of rhBMP-2 for the LDS treatment varied by year, region, gender, and type of fusion technique. Patients age 60 to 65 years old and patients in the West had more procedures with rhBMP-2. The reoperation rate of patients using rhBMP-2 was significantly lower than in patients not using rhBMP-2. Further studies on lumbar spondylolisthesis looking at the geographic variability, a longer follow-up, and the cost profile of rhBMP-2 utilization are needed.
Acknowledgment
This work was funded by AOSpine and departmental funds. AOSpine is a clinical division of the AO Foundation—an independent medically guided nonprofit organization. The AOSpine Knowledge Forums are pathology-focused working groups acting on behalf of AOSpine in their domain of scientific expertise. Each forum consists of a steering committee of up to 10 international spine experts who meet on a regular basis to discuss research, assess the best evidence for current practices, and formulate clinical trials to advance spine care worldwide. Study support is provided directly through AOSpine's Research department.
Disclosures Qingqiang Yao: none Jeremiah R. Cohen: Personal fees (AO Foundation) Zorica Buser: Consultancy (Xenco Medical) Jong-Beom Park: none Darrel S. Brodke: Consultancy (Amedica); Royalties (Amedica, DePuy Synthes, Medtronic); Stock/stock options (Amedica); Travel expenses (DePuy Synthes) Hans-Joerg Meisel: Consultancy (Zyga, DiFusion); Royalties (Medtronic, Fehling); Stock/stock options (DiFusion); Grant (BG Clinic Bergmannstrost); Speakers' bureau (BG Clinic Bergmannstrost) Jim A. Youssef: Consultancy (NuVasive, Integra, Amedica); Royalties (NuVasive, Integra, Amedica, Osprey Biomedical); Stock/stock options (Amedica, Vertiflex, Benvenue, Paradigm Spine, Promethean Surgical, ISD, Spinicity, Spinal Ventures, Providence Medical); Research support (NuVasive, Globus Medical, Vertiflex, Integra); Board of directors (Spinicity) Jeffrey C. Wang: Royalties (Stryker, Osprey, Aesculap, Biomet, Amedica, Seaspine, Synthes); Stock ownership (Fziomed, Alphatech); Private investments (Promethean Spine, Paradigm Spine, Benevenue, NexGen, Amedica, Vertiflex, electroCore, Surgitech, VG Innovations, CoreSpine, Expanding Orthopaedics, Osprey, Bone Biologics, Curative Biosciences, PearlDiver); Board of directors (North American Spine Society [nonfinancial, reimbursement for travel for board meetings, courses, etc.], North American Spine Foundation [nonfinancial], Cervical Spine Research Society [nonfinancial, reimbursement for travel for board meetings], AOSpine/AO Foundation [both, 86597 combined for honorariums for board and educational activities], Collaborative Spine Research Foundation [nonfinancial]); Fellowship support: AO Foundation [spine fellowship funding paid directly to institution/employer]) S. Tim Yoon: Grant (Biomet Spine, AOSpine, NIH); Personal fees (Biomet Spine, Stryker Spine, Meditech Spine); Stock ownership (Phygen, Alphatec, Medyssey); Society officer (ISSLS)
Qingqiang Yao and Jeremiah R. Cohen contributed equally.
References
- 1.Martin C R, Gruszczynski A T, Braunsfurth H A, Fallatah S M, O'Neil J, Wai E K. The surgical management of degenerative lumbar spondylolisthesis: a systematic review. Spine (Phila Pa 1976) 2007;32(16):1791–1798. doi: 10.1097/BRS.0b013e3180bc219e. [DOI] [PubMed] [Google Scholar]
- 2.Mannion A F Pittet V Steiger F Vader J P Becker H J Porchet F; Zürich Appropriateness of Spine Surgery (ZASS) Group. Development of appropriateness criteria for the surgical treatment of symptomatic lumbar degenerative spondylolisthesis (LDS) Eur Spine J 20142391903–1917. [DOI] [PubMed] [Google Scholar]
- 3.Vibert B T, Sliva C D, Herkowitz H N. Treatment of instability and spondylolisthesis: surgical versus nonsurgical treatment. Clin Orthop Relat Res. 2006;443(443):222–227. doi: 10.1097/01.blo.0000200233.99436.ea. [DOI] [PubMed] [Google Scholar]
- 4.Boswell M V, Trescot A M, Datta S. et al. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10(1):7–111. [PubMed] [Google Scholar]
- 5.Watters W C III, Bono C M, Gilbert T J. et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J. 2009;9(7):609–614. doi: 10.1016/j.spinee.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Bednar D A. Surgical management of lumbar degenerative spinal stenosis with spondylolisthesis via posterior reduction with minimal laminectomy. J Spinal Disord Tech. 2002;15(2):105–109. doi: 10.1097/00024720-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Altaf F, Jalgaonkar A, Raman S A. Prospective study of posterior lumbar interbody fusion and posterolateral fusion for degenerative spondylolisthesis. Spine J. 2011;11(10):112S–113S. [Google Scholar]
- 8.Asazuma T, Yamugishi M, Sato M, Ichimura S, Fujikawa K, Crock H V. Posterior spinal fusion for lumbar degenerative diseases using the Crock-Yamagishi (C-Y) spinal fixation system. J Spinal Disord Tech. 2004;17(3):174–177. doi: 10.1097/00024720-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Tannoury C A, An H S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014;14(3):552–559. doi: 10.1016/j.spinee.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 10.Behrbalk E, Uri O, Parks R M, Musson R, Soh R C, Boszczyk B M. Fusion and subsidence rate of stand alone anterior lumbar interbody fusion using PEEK cage with recombinant human bone morphogenetic protein-2. Eur Spine J. 2013;22(12):2869–2875. doi: 10.1007/s00586-013-2948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaccaro A R, Patel T, Fischgrund J. et al. A pilot study evaluating the safety and efficacy of OP-1 Putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine (Phila Pa 1976) 2004;29(17):1885–1892. doi: 10.1097/01.brs.0000137062.79201.98. [DOI] [PubMed] [Google Scholar]
- 12.Boden S D, Zdeblick T A, Sandhu H S, Heim S E. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine (Phila Pa 1976) 2000;25(3):376–381. doi: 10.1097/00007632-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 13.Crandall D G, Revella J, Patterson J, Huish E, Chang M, McLemore R. Transforaminal lumbar interbody fusion with rhBMP-2 in spinal deformity, spondylolisthesis, and degenerative disease—part 1: large series diagnosis related outcomes and complications with 2- to 9-year follow-up. Spine (Phila Pa 1976) 2013;38(13):1128–1136. doi: 10.1097/BRS.0b013e31828864e6. [DOI] [PubMed] [Google Scholar]
- 14.Burkus J K, Heim S E, Gornet M F, Zdeblick T A. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech. 2003;16(2):113–122. doi: 10.1097/00024720-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Crandall D G, Revella J, Patterson J, Huish E, Chang M, McLemore R. Transforaminal lumbar interbody fusion with rhBMP-2 in spinal deformity, spondylolisthesis, and degenerative disease—part 2: BMP dosage-related complications and long-term outcomes in 509 patients. Spine (Phila Pa 1976) 2013;38(13):1137–1145. doi: 10.1097/BRS.0b013e3182880298. [DOI] [PubMed] [Google Scholar]
- 16.Carragee E J, Mitsunaga K A, Hurwitz E L, Scuderi G J. Retrograde ejaculation after anterior lumbar interbody fusion using rhBMP-2: a cohort controlled study. Spine J. 2011;11(6):511–516. doi: 10.1016/j.spinee.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Epstein N E. Complications due to the use of BMP/INFUSE in spine surgery: the evidence continues to mount. Surg Neurol Int. 2013;4 05:S343–S352. doi: 10.4103/2152-7806.114813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaccaro A R, Garfin S R. Degenerative lumbar spondylolisthesis with spinal stenosis, a prospective study comparing decompression with decompression and intertransverse process arthrodesis: a critical analysis. Spine (Phila Pa 1976) 1997;22(4):368–369. doi: 10.1097/00007632-199702150-00002. [DOI] [PubMed] [Google Scholar]
- 19.Ghogawala Z, Benzel E C, Amin-Hanjani S. et al. Prospective outcomes evaluation after decompression with or without instrumented fusion for lumbar stenosis and degenerative grade I spondylolisthesis. J Neurosurg Spine. 2004;1(3):267–272. doi: 10.3171/spi.2004.1.3.0267. [DOI] [PubMed] [Google Scholar]
- 20.Kornblum M B Fischgrund J S Herkowitz H N Abraham D A Berkower D L Ditkoff J S Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis Spine (Phila Pa 1976) 2004297726–733., discussion 733–734 [DOI] [PubMed] [Google Scholar]
- 21.Hackenberg L, Halm H, Bullmann V, Vieth V, Schneider M, Liljenqvist U. Transforaminal lumbar interbody fusion: a safe technique with satisfactory three to five year results. Eur Spine J. 2005;14(6):551–558. doi: 10.1007/s00586-004-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anand N, Hamilton J F, Perri B, Miraliakbar H, Goldstein T. Cantilever TLIF with structural allograft and RhBMP2 for correction and maintenance of segmental sagittal lordosis: long-term clinical, radiographic, and functional outcome. Spine (Phila Pa 1976) 2006;31(20):E748–E753. doi: 10.1097/01.brs.0000240211.23617.ae. [DOI] [PubMed] [Google Scholar]
- 23.Urist M R. Bone: formation by autoinduction. Science. 1965;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 24.Robin B N, Chaput C D, Zeitouni S, Rahm M D, Zerris V A, Sampson H W. Cytokine-mediated inflammatory reaction following posterior cervical decompression and fusion associated with recombinant human bone morphogenetic protein-2: a case study. Spine (Phila Pa 1976) 2010;35(23):E1350–E1354. doi: 10.1097/BRS.0b013e3181e85756. [DOI] [PubMed] [Google Scholar]
- 25.Patel V V Zhao L Wong P et al. An in vitro and in vivo analysis of fibrin glue use to control bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth Spine J 200664397–403., discussion 404 [DOI] [PubMed] [Google Scholar]
- 26.Glassman S D, Dimar J R, Carreon L Y, Campbell M J, Puno R M, Johnson J R. Initial fusion rates with recombinant human bone morphogenetic protein-2/compression resistant matrix and a hydroxyapatite and tricalcium phosphate/collagen carrier in posterolateral spinal fusion. Spine (Phila Pa 1976) 2005;30(15):1694–1698. doi: 10.1097/01.brs.0000172157.39513.80. [DOI] [PubMed] [Google Scholar]
- 27.Smucker J D, Rhee J M, Singh K, Yoon S T, Heller J G. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine (Phila Pa 1976) 2006;31(24):2813–2819. doi: 10.1097/01.brs.0000245863.52371.c2. [DOI] [PubMed] [Google Scholar]
- 28.Sears W. Posterior lumbar interbody fusion for degenerative spondylolisthesis: restoration of sagittal balance using insert-and-rotate interbody spacers. Spine J. 2005;5(2):170–179. doi: 10.1016/j.spinee.2004.05.257. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs W C, Vreeling A, De Kleuver M. Fusion for low-grade adult isthmic spondylolisthesis: a systematic review of the literature. Eur Spine J. 2006;15(4):391–402. doi: 10.1007/s00586-005-1021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steiger F, Becker H J, Standaert C J. et al. Surgery in lumbar degenerative spondylolisthesis: indications, outcomes and complications. A systematic review. Eur Spine J. 2014;23(5):945–973. doi: 10.1007/s00586-013-3144-3. [DOI] [PubMed] [Google Scholar]
- 31.Daffner S D, Beimesch C F, Wang J C. Geographic and demographic variability of cost and surgical treatment of idiopathic scoliosis. Spine (Phila Pa 1976) 2010;35(11):1165–1169. doi: 10.1097/BRS.0b013e3181d88e78. [DOI] [PubMed] [Google Scholar]


