Abstract
How do cells sense and control their cholesterol levels? Whereas most of the cell cholesterol is located in the plasma membrane, the effectors of its abundance are regulated by a small pool of cholesterol in the endoplasmic reticulum (ER). The size of the ER compartment responds rapidly and dramatically to small changes in plasma membrane cholesterol around the normal level. Consequently, increasing plasma membrane cholesterol in vivo from just below to just above the basal level evoked an acute (<2 h) and profound (≈20-fold) decrease in ER 3-hydroxy-3-methylglutaryl-CoA reductase activity in vitro. We tested the hypothesis that the sharply inflected ER response to cholesterol is governed by the thermodynamic activity (fugacity) of plasma membrane cholesterol. The following two independent measures of plasma membrane cholesterol activity in human red cells and fibroblasts were used: susceptibility to cholesterol oxidase and cholesterol transfer to cyclodextrin. Both indicators revealed a threshold at the physiologic set point of plasma membrane cholesterol. Incrementing the phospholipid compartment in the plasma membrane with lysophosphatidylcholine, previously shown to decrease cholesterol oxidase susceptibility, reduced the transfer of plasma membrane cholesterol to cyclodextrin and to the ER. Conversely, the membrane intercalator, n-octanol, increased cholesterol oxidation, transfer, and ER pool size, perhaps by displacing cholesterol from plasma membrane phospholipids. We conclude that the activity of the fraction of cholesterol in excess of other plasma membrane lipids sets the cholesterol level in the ER. Cholesterol-sensitive elements therein respond by nulling the active plasma membrane pool, thereby keeping the cholesterol matched to the other plasma membrane lipids.
Sterols such as cholesterol serve essential functions in eukaryotic plasma membranes and endomembrane compartments (1–3). Of the unesterified cholesterol in human fibroblasts, ≈85% is in the plasma membrane (4, 5) and perhaps another 10% is in the endocytic pathway with which it is in dynamic continuity (6, 7). One measure of the endoplasmic reticulum (ER) cholesterol pool suggests that it contains ≈0.5% of the total (8, 9). The level of the ER pool is set by a brisk circulation of cholesterol to and from the plasma membrane (10, 11). Small changes in plasma membrane cholesterol near the physiologic set point evoke large responses in ER cholesterol within minutes (9). Sterol-sensitive proteins in the ER then increase its esterification (12), decrease its biosynthesis (13), and sequester transcription factor precursors that activate genes promoting sterol accretion (2, 14). These responses complete a feedback loop that restores the plasma membrane pool to its physiological level.
There is evidence that the primary signal of the abundance of plasma membrane cholesterol is its excess over the other lipids, predominantly phospholipids. First, the molar ratio of cholesterol to phospholipids in plasma membranes is usually maintained just below unity (4). Second, cholesterol accretion is coordinated with the levels of phospholipids and sphingolipids (3). Third, studies of model systems suggest that membrane sterols in excess of the phospholipids have a high chemical potential. In one formulation, most of the membrane cholesterol exists in stoichiometric complexes with polar lipids, whereas cholesterol exceeding this equivalence point is free as active monomers (15, 16). In another view, the excess cholesterol forms pure domains (3, 17). The outcome in either case could be that the chemical potential of super-threshold cholesterol causes its redistribution to the homeostatic ER pool (16).
Here, we report a test of this hypothesis in cells by relating the effects of plasma membrane cholesterol level on the ER cholesterol pool to the following two independent measures of plasma membrane cholesterol activity: its susceptibility to cholesterol oxidase and its transfer from the membrane to an acceptor, cyclodextrin.
Materials and Methods
Materials. We obtained 1α, 2α-[3H]cholesterol from Amersham Biosciences. We obtained 2-hydroxypropyl-β-cyclodextrin (used in all fibroblast experiments) and methyl-β-cyclodextrin (used in all red cell experiments), egg yolk lysophosphatidylcholine (LPC; mostly palmitic and stearic acid derivatives; nominal molecular mass, 525 Da), and cholesterol oxidase (Streptomyces sp.) from Sigma. Cholesterol was obtained from Steraloids (Newport, RI) (our preparations of cyclodextrin cholesterol were free of oxysterols detectable by HPLC). The Amplex Red kit was obtained from Molecular Probes. Blood was donated by a healthy volunteer. The cells were separated from the plasma and buffy coat and washed twice with five volumes of PBS. In experiments in which the effect of LPC on red cell transfer was measured, the cells were first washed with PBS containing 0.5 mg/ml BSA and then washed twice with PBS.
Cell Culture. Human skin fibroblasts were maintained in DMEM containing 100 μg/ml penicillin, 100 μg/ml streptomycin, and 10% FCS (8). Fibroblast and red cell plasma membrane cholesterol were decreased and increased, respectively, with cyclodextrin and complexes of cholesterol with cyclodextrin, as described in the figure legends (9, 18).
Analyses. Cholesterol was determined by HPLC (10) or by using Amplex Red. For protein assay, we used the BCA kit (Pierce). We assayed 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoAR) as described (19) except that 50 mM NaF and 10 mM DTT were added to the reaction mixtures, and the reactions proceeded for 20 min at 37°C.
Results and Discussion
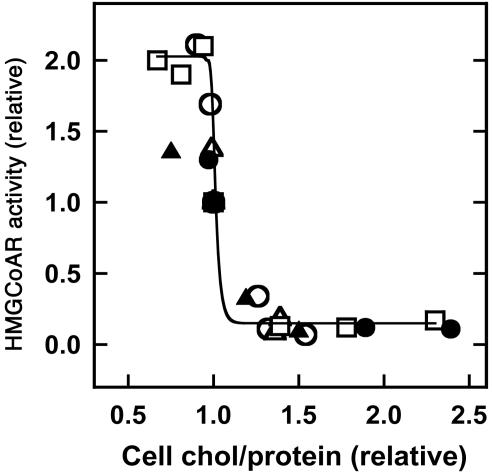
HMGCoAR, the rate-limiting enzyme in cholesterol biosynthesis, is regulated at several levels. In particular, the enzyme is degraded within a few hours when cells are treated with large amounts of 25-hydroxycholesterol (13, 20, 21). One action of this regulatory oxysterol is to rapidly increase the level of ER cholesterol (9), thereby priming HMGCoAR for homeostatic down-regulation (13). We reasoned that the inactivation of HMGCoAR could serve as a useful indicator of the dose–response relationship between plasma membrane and ER cholesterol. Fig. 1 demonstrates such a relationship. Increasing plasma membrane cholesterol from 25% below its resting level to 25% above its resting level caused the activity of HMGCoAR to fall by ≈20-fold. The half-time for this response was ≈30 min (data not shown). As found earlier for ER cholesterol (9), the sharp threshold shown in Fig. 1 is centered on the physiological set point for plasma membrane cholesterol. Whereas HMGCoAR activity is also rapidly inhibited by the AMP-dependent kinase (22), a role for phosphorylation in the observed response to plasma membrane cholesterol remains to be evaluated. In any case, Fig. 1 is consistent with the premise (9) that super-threshold plasma membrane cholesterol rapidly regulates homeostatic ER elements by acutely modulating the cholesterol level in that compartment. According to this point of view, the action of 25-hydroxycholesterol in rapidly raising ER cholesterol could reflect its ability to increase the activity of plasma membrane cholesterol, perhaps by displacing the sterol from association with phospholipids.
Fig. 1.
Acute effect of plasma membrane cholesterol concentration on HMGCoAR activity. To induce the expression of the enzyme, human fibroblasts were grown overnight in replicate flasks containing medium plus lipoprotein-deficient serum. Plasma membrane cholesterol was then reduced or increased by incubating the monolayers of cells (containing about 15 μgof cholesterol and 0.4 mg of protein) for 12 min at 37°C with 1 ml of hydroxypropyl-β-cyclodextrin (1.5–5.0 mg/ml) or complexes of hydroxypropyl-β-cyclodextrin (0.2–5 mg/ml) plus cholesterol (0–32 μg/ml). The modified cells were then incubated for 2 h (two experiments, filled symbols) or 4 h (three experiments, open symbols) and disrupted, and enzyme activity, protein, and cholesterol were assayed in duplicate. HMGCoAR activities, which are scaled to untreated controls (≈25 pmol of product per min per mg of protein), are plotted against cell cholesterol relative to untreated controls and fit to a sigmoid function.
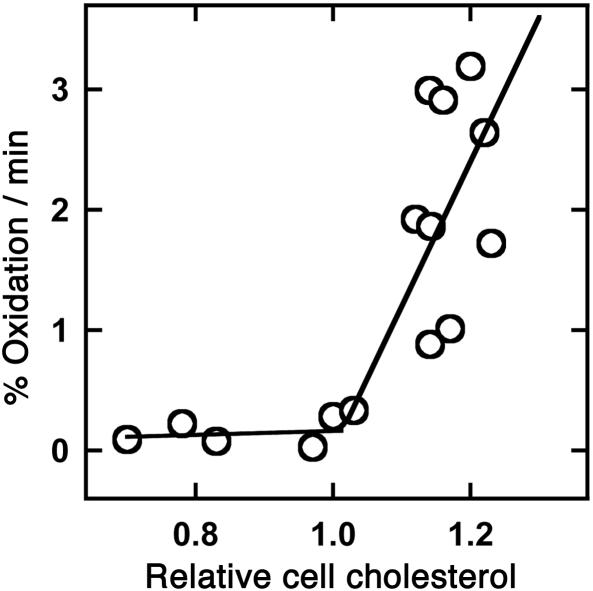
The action of cholesterol oxidase on membrane cholesterol depends highly on the environment of the substrate (23, 24). Red cell cholesterol is normally a very poor substrate for this enzyme; however, increasing the sterol level even slightly above the physiological level increases oxidation dramatically (25, 26). Here, we show that the transition in the cholesterol oxidase susceptibility of human fibroblasts is also sharp and occurs at the physiological set point (Fig. 2). In particular, a rise of 20% in fibroblast plasma membrane cholesterol increased the rate of cholesterol oxidase action by ≈8-fold. It might be asked why a small enrichment leads to the oxidation of the entire cholesterol pool (25). We have found that the sterol oxidation product, Δ4-cholestenone, remains in the membrane and promotes the attack of the enzyme at least as well as the parent sterol.
Fig. 2.
Dependence on plasma membrane cholesterol concentration of cholesterol susceptibility to cholesterol oxidase. Fibroblasts were grown overnight in 96-well plates, and plasma membrane cholesterol was then adjusted as described for Fig. 1. The cells were washed and treated with 0.9 units of cholesterol oxidase in PBS for 10 min at 37°C. The H2O2 reaction product in aliquots of the overlying medium was determined with Amplex Red. Cholesterol remaining in the cells was determined by adding 0.2% Triton X-100 to the wells and allowing the oxidation reaction to run to completion before assay with Amplex Red. Values for the initial rate of cell cholesterol oxidation are averages of six replicate wells in each of eight experiments; they were plotted against relative cell cholesterol, and the curve was fit by eye to two straight lines.
Crystallographic studies suggest that cholesterol oxidase accesses substrate sterol molecules as they transiently project from the bilayer surface (27). The high activity of plasma membrane cholesterol above the threshold may reflect the frequency of such projections (18, 26). Much of the great variation in the action of cholesterol oxidase on membrane cholesterol that is reported in the literature might then reflect the strong dependence of the chemical activity (projection rate) of the substrate on numerous experimental variables.
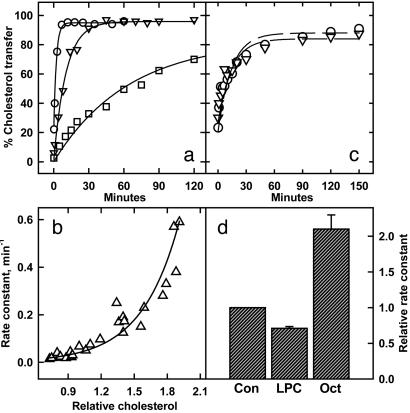
The passive transfer of sterols from membranes to acceptors is similarly limited by the frequency of their projection from the bilayer (18). The finding that the rate of transfer from lipid monolayers to cyclodextrin increased markedly as cholesterol was titrated beyond molar equivalence with phospholipids has been taken as evidence of the high chemical activity of the excess cholesterol (16). Such a concentration dependence is seen also with red cells (Fig. 3a). In this system, an increase in membrane cholesterol of ≈75% across the physiological range accelerated its transfer 7-fold (Fig. 3b). Such concentration dependence has been observed also in fibroblasts (28). (Note that the rate constants per millimolar cyclodextrin observed here are close to those cited for lipid monolayers in ref. 16.) The reverse process, transfer from cyclodextrin to membranes, does not vary with cholesterol concentration (Fig. 3c). The high activity of the excess sterol, therefore, seems to have a thermodynamic rather than a kinetic basis.
Fig. 3.
Transfer of cholesterol between red cells and cyclodextrin-cholesterol. Erythrocytes (100 μl of packed cells containing ≈80 μg of cholesterol) were allowed to achieve cholesterol mass equilibrium with 9.9 ml of PBS containing methyl-β-cyclodextrin (5 mg/ml) bearing 40–90 μg/ml cholesterol (18). Cells and cyclodextrin were separated by centrifugation. Either the cell or the cyclodextrin compartment was labeled with [3H]cholesterol and then recombined with the other compartment for analysis of transfer kinetics. The transfer reaction at 10°C used 60 μl of packed red cells and 5.94 ml of medium with which it was in mass equilibrium. (a) Transfer of [3H]cholesterol to cyclodextrin complexes from labeled red cells bearing 1.7 (○), 1.1 (▿), or 0.73 (□) times the control cholesterol level. (b) Dependence on red cell cholesterol of the rate of transfer of [3H]cholesterol to cyclodextrin complexes. First-order rate constants were obtained in 15 experiments such as those shown in a.(c) Transfer of [3H]cholesterol from labeled cyclodextrin to red cells. Cyclodextrin-[3H]cholesterol complexes were prepared by equilibrating cyclodextrin-cholesterol with labeled red cells and separating it by centrifugation. The labeled donor preparations were then mixed at 10°C with unlabeled red cells that had been mass equilibrated in parallel with unlabeled cyclodextrin-cholesterol complexes, and the time course of transfer was then determined. (○) Unenriched red cells; (▿) red cells enriched 1.7-fold in cholesterol. (d) Effect of membrane intercalators on red cell [3H]cholesterol efflux. Labeled red cells were added to mass-equilibrated cyclodextrin-cholesterol mixtures to which had been added LPC (10.8 μM; i.e., 0.5 μmol/μmol cell cholesterol) or n-octanol (Oct, 2.1 mM). A high level of LPC was used to compensate for its binding to the cyclodextrin. As widely observed, mild red cell spiculation occurred; however, the low level of free LPC did not extract cholesterol or lyse the cells. n-Octanol did not cause cholesterol extraction, cell lysis, or shape change. The amount of octanol in the membranes could not be calculated because its potential binding to cyclodextrin is unknown. Transfer kinetics in three or four experiments are plotted relative to controls (Con).
If the observed threshold in the behavior of plasma membrane cholesterol signifies the high activity of the fraction in excess of phospholipids, then increasing the phospholipids should reduce cholesterol activity. Although it is not easy to introduce diacylphospholipids into plasma membranes, lysophosphatides readily partition into cell surfaces where they form stoichiometric complexes with sterols (29). We have observed (26) that adding LPC to red cells reduced the susceptibility of their cholesterol to cholesterol oxidase. We now show that LPC also inhibits the transfer of red cell membrane cholesterol to cyclodextrin (Fig. 3d). Although LPC complexed with the cyclodextrin, the observed inhibition of cholesterol transfer was not caused by a reduction of the binding capacity of cyclodextrin for cholesterol because equilibrium (plateau) distributions between donor and acceptor were not altered by LPC (data not shown). [An earlier study reported that the kinetics of transfer of cholesterol between artificial phosphatidylcholine vesicles was unaffected by LPC (30). This finding agrees with our results because the cholesterol content of those membranes was so low that an active cholesterol pool would not be expected.]
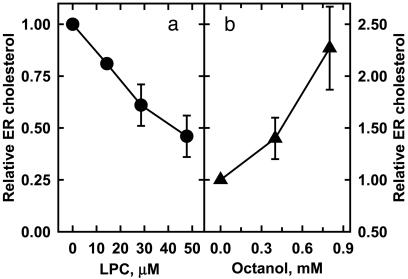
In addition to its inhibitory effect on cholesterol oxidase susceptibility and cholesterol transfer, LPC was shown to rapidly reduce the level of cholesterol in the fibroblast ER (Fig. 4a). Although we cannot be certain of the mechanism of this action because of the possible signaling effects of LPC (31), the data are consistent with our hypothesis.
Fig. 4.
Effect of membrane intercalators on ER cholesterol. (a) The plasma membrane cholesterol in flasks of human fibroblasts was varied as in Fig. 1. DMEM (4 ml) containing 10% serum plus lysophosphatidylcholine up to 0.2 μmol was added. This LPC amounted to 1.8 μM per μM of cell cholesterol, but much of the LPC was presumably taken up by serum proteins in the medium. The LPC was shown not to alter the cell cholesterol level. After 2 h at 37°C, the cells were homogenized, and ER cholesterol was determined (8). (b) The same as described for a except that n-octanol was used. By using a partition coefficient of 7657 (35), we estimated that, at its highest concentration, there should be ≤0.1 mol n-octanol per mol phospholipid in the plasma membrane. This dosage is within the range used in model bilayer and natural membrane studies (32, 33, 35); in addition, octanol uptake by lipoproteins in the medium might reduce membrane loading still further. Error bars indicate SD for three experiments.
Millimolar amounts of n-octanol are known to enhance the susceptibility of red cell cholesterol to cholesterol oxidase (26). We reasoned that this action might reflect its activation of membrane cholesterol because small amounts of this intercalator alter the molecular organization of artificial and natural membranes without disrupting them. In particular, n-octanol increases the hydration of the polar-head region of bilayers (32). It also seems to associate with and increase the order of phospholipid fatty acyl chains (33–35). Cholesterol weakens this effect, perhaps by competing with the alkanol for phospholipid sites (35). Octanol might, therefore, create active cholesterol by displacing it from the phospholipids.
Here, we report that n-octanol both accelerates the transfer of red cell cholesterol to cyclodextrin (Fig. 3d) and increases the level of ER cholesterol in fibroblasts (Fig. 4b). This agent also prevents the lysis of red cells by LPC (data not shown), consistent with the possibility that the actions of these two intercalators on membrane-cholesterol activity are opposed.
Concluding Comments
These results and those previously published demonstrate a concordance among three putative gauges of plasma membrane-cholesterol activity: susceptibility to cholesterol oxidase, transfer to cyclodextrin, and the level of ER cholesterol. Furthermore, earlier work showed that these three parameters respond to the abundance of plasma membrane sphingomyelin in accordance with our hypothesis. That is, treatment with sphingomyelinase C or genetic manipulation to reduce plasma membrane sphingomyelin increased the susceptibility of plasma membrane cholesterol to cholesterol oxidase, its efflux to cyclodextrin, the level of fibroblast ER cholesterol, and the rate of cholesterol esterification, which is a gauge of the ER pool (3, 8, 36).
From these findings, we infer that nearly all of the cholesterol in the unperturbed plasma membrane has a relatively low chemical potential. Membrane cholesterol activity would seem to rise fairly linearly with its concentration above the physiological set point. The fact that this threshold lies just below the molar equivalence of plasma membrane cholesterol with the phospholipids suggests that most of the bilayer lipids contribute to its definition. The low activity and high activity populations of cholesterol would seem to be in rapid equilibrium in that all of the sterol in the enriched membranes behaves as a single pool on the time scale of several seconds. The physical state of the excess sterol is not known; it could be a bilayer solution of monomers (15, 16) or self-associated (3, 17). It is also uncertain how the low and high activity forms of cholesterol might relate to rafts (cholesterol-rich bilayer domains; refs. 2, 3, and 37). In this regard, it has been proposed that the excess (active) cholesterol fraction is simply that in the liquid-disordered phase of the plasma membrane bilayer in equilibrium with its liquid-ordered domains (2). It is possible that some of the many physiological processes reported to be acutely modulated by plasma membrane cholesterol could, in fact, reflect their dependence on active cholesterol rather than on the formation of phaseseparated domains.
The finding that the cholesterol content of the plasma membrane homes to the threshold observed in these experiments suggests that a primary homeostatic mechanism operates upstream of the ER. That is, the magnitude of the active cholesterol fraction in the plasma membrane sets the level of ER cholesterol to which its battery of regulatory proteins then responds so as to return plasma membrane cholesterol to the equivalence point (16).
It is not known how cholesterol is conveyed between the plasma membrane and ER. Simple aqueous diffusion of cholesterol down its activity gradient seems unlikely (2, 3, 38). One reason is that the sterol moves between the plasma membrane and ER in minutes (8, 38, 39), whereas its unmediated transfer between membranes typically takes hours in vitro (18, 40). The physiologic transport process could be regulated, as the size of the ER cholesterol pool is altered by agents acting on protein kinases C (41). Metabolic energy could be used to keep the membrane pools from reaching thermodynamic equilibrium, given that the level of cholesterol in the plasma membrane may exceed that in the ER by more than two orders of magnitude (1, 4, 5, 8, 9), whereas the cholesterol affinities of the relevant phospholipids differ in the extreme by <20-fold (37, 42). However, our functional definition of the ER cholesterol pool as that residing in membranes bearing acyl-CoA:cholesterol acyl transferase (8, 9) might underestimate its true size. Furthermore, the concentration of sterols in these nonideal membrane bilayers may not accurately represent their thermodynamic activity. In any case, one can envision a cytoplasmic transport pathway responsive both to cholesterol activity (e.g., the frequency of projection of cholesterol from the cytoplasmic surface of the plasma membrane bilayer) and to regulatory signals.
The high chemical activity of excess cholesterol could drive other regulatory processes at both the endofacial and exofacial surfaces of the plasma membrane (2, 3). In particular, the cholesterol transferred from plasma membranes to plasma lipoproteins could be drawn primarily from the active pool. ABCA1 and SR-BI, integral plasma membrane proteins that stimulate the delivery of plasma membrane cholesterol to high-density (apo)lipoproteins, also promote its susceptibility to cholesterol oxidase and modulate the ER cholesterol pool (39, 43). Perhaps these proteins increase the activity of plasma membrane cholesterol or the presentation of active sterol to the acceptors. Confining cholesterol export to the active excess would create a second feedback loop that nulls plasma membrane cholesterol to the same level as does the set of regulatory proteins that are responsive to the ER pool.
Acknowledgments
We thank Fredric Cohen and Godfrey Getz for helpful comments on the manuscript. This work was supported in part by National Institutes of Health Grant HL 28448 (to Y.L.).
Abbreviations: LPC, lysophosphatidylcholine; ER, endoplasmic reticulum; HMGCoAR, 3-hydroxy-3-methylglutaryl-CoA reductase.
References
- 1.Yeagle, P. L. (1985) Biochim. Biophys. Acta 822, 267–287. [DOI] [PubMed] [Google Scholar]
- 2.Simons, K. & Ikonen, E. (2000) Science 290, 1721–1726. [DOI] [PubMed] [Google Scholar]
- 3.Ohvo-Rekila, H., Ramstedt, B., Leppimaki, P. & Slotte, J. P. (2002) Prog. Lipid Res. 41, 66–97. [DOI] [PubMed] [Google Scholar]
- 4.Lange, Y., Swaisgood, M. H., Ramos, B. V. & Steck, T. L. (1989) J. Biol. Chem. 264, 3786–3793. [PubMed] [Google Scholar]
- 5.Lange, Y., Ye, J., Rigney, M. & Steck, T. (2000) J. Biol. Chem. 275, 17468–17475. [DOI] [PubMed] [Google Scholar]
- 6.Lange, Y. (1991) J. Lipid Res. 32, 329–339. [PubMed] [Google Scholar]
- 7.Lange, Y., Ye, J. & Steck, T. L. (1998) J. Biol. Chem. 273, 18915–18922. [DOI] [PubMed] [Google Scholar]
- 8.Lange, Y. & Steck, T. L. (1997) J. Biol. Chem. 272, 13103–13108. [DOI] [PubMed] [Google Scholar]
- 9.Lange, Y., Ye, J., Rigney, M. & Steck, T. L. (1999) J. Lipid Res. 40, 2264–2270. [PubMed] [Google Scholar]
- 10.Lange, Y., Echevarria, F. & Steck, T. L. (1991) J. Biol. Chem. 266, 21439–21443. [PubMed] [Google Scholar]
- 11.Lange, Y. & Steck, T. L. (1996) Trends Cell Biol. 6, 205–208. [DOI] [PubMed] [Google Scholar]
- 12.Chang, T. Y., Chang, C. C. & Cheng, D. (1997) Annu. Rev. Biochem. 66, 613–638. [DOI] [PubMed] [Google Scholar]
- 13.Sever, N., Song, B. L., Yabe, D., Goldstein, J. L., Brown, M. S. & DeBose-Boyd, R. A. (2003) J. Biol. Chem. 278, 52479–52490. [DOI] [PubMed] [Google Scholar]
- 14.Brown, A. J., Sun, L., Feramisco, J. D., Brown, M. S. & Goldstein, J. L. (2002) Mol. Cell 10, 237–245. [DOI] [PubMed] [Google Scholar]
- 15.McConnell, H. M. & Radhakrishnan, A. (2003) Biochim. Biophys. Acta 1610, 159–173. [DOI] [PubMed] [Google Scholar]
- 16.Radhakrishnan, A. & McConnell, H. M. (2000) Biochemistry 39, 8119–8124. [DOI] [PubMed] [Google Scholar]
- 17.Huang, J. & Feigenson, G. W. (1999) Biophys. J. 76, 2142–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steck, T. L., Ye, J. & Lange, Y. (2002) Biophys. J. 83, 2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown, M. S., Dana, S. E. & Goldstein, J. L. (1973) Proc. Natl. Acad. Sci. USA 70, 2162–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berkhout, T. A., Simon, H. M., Patel, D. D., Bentzen, C., Niesor, E., Jackson, B. & Suckling, K. E. (1996) J. Biol. Chem. 271, 14376–14382. [DOI] [PubMed] [Google Scholar]
- 21.McGee, T. P., Cheng, H. H., Kumagai, H., Omura, S. & Simoni, R. D. (1996) J. Biol. Chem. 271, 25630–25638. [DOI] [PubMed] [Google Scholar]
- 22.Hardie, D. G. (1992) Biochim. Biophys. Acta 1123, 231–238. [DOI] [PubMed] [Google Scholar]
- 23.McMullen, T. P. W. & McElhaney, R. N. (1996) Curr. Opin. Colloid Interface Sci. 1, 83–90. [Google Scholar]
- 24.Wang, M. M., Olsher, M., Sugar, I. P. & Chong, P. L. (2004) Biochemistry 43, 2159–2166. [DOI] [PubMed] [Google Scholar]
- 25.Lange, Y., Cutler, H. B. & Steck, T. L. (1980) J. Biol. Chem. 255, 9331–9337. [PubMed] [Google Scholar]
- 26.Lange, Y., Matthies, H. & Steck, T. L. (1984) Biochim. Biophys. Acta 769, 551–562. [DOI] [PubMed] [Google Scholar]
- 27.Chen, X., Wolfgang, D. E. & Sampson, N. S. (2000) Biochemistry 39, 13383–13389. [DOI] [PubMed] [Google Scholar]
- 28.Haynes, M. P., Phillips, M. C. & Rothblat, G. H. (2000) Biochemistry 39, 4508–4517. [DOI] [PubMed] [Google Scholar]
- 29.Ramsammy, L. S. & Brockerhoff, H. (1982) J. Biol. Chem. 257, 3570–3574. [PubMed] [Google Scholar]
- 30.McLean, L. R. & Phillips, M. C. (1984) Biochemistry 23, 4624–4630. [DOI] [PubMed] [Google Scholar]
- 31.Xu, Y. (2002) Biochim. Biophys. Acta 1582, 81–88. [DOI] [PubMed] [Google Scholar]
- 32.Ho, C. & Stubbs, C. D. (1997) Biochemistry 36, 10630–10637. [DOI] [PubMed] [Google Scholar]
- 33.Westerman, P. W., Pope, J. M., Phonphok, N., Doane, J. W. & Dubro, D. W. (1988) Biochim. Biophys. Acta 939, 64–78. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell, D. C., Lawrence, J. T. & Litman, B. J. (1996) J. Biol. Chem. 271, 19033–19036. [DOI] [PubMed] [Google Scholar]
- 35.Rowe, E. S., Zhang, F., Leung, T. W., Parr, J. S. & Guy, P. T. (1998) Biochemistry 37, 2430–2440. [DOI] [PubMed] [Google Scholar]
- 36.Fukasawa, M., Nishijima, M., Itabe, H., Takano, T. & Hanada, K. (2000) J. Biol. Chem. 275, 34028–34034. [DOI] [PubMed] [Google Scholar]
- 37.Silvius, J. R. (2003) Biochim. Biophys. Acta 1610, 174–183. [DOI] [PubMed] [Google Scholar]
- 38.Prinz, W. (2002) Semin. Cell Dev. Biol. 13, 197–203. [DOI] [PubMed] [Google Scholar]
- 39.Lange, Y., Strebel, F. & Steck, T. L. (1993) J. Biol. Chem. 268, 13838–13843. [PubMed] [Google Scholar]
- 40.Yancey, P. G., Bortnick, A. E., Kellner-Weibel, G., de la Llera-Moyo, M., Phillips, M. C. & Rothblatt, G. H. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 712–719. [DOI] [PubMed] [Google Scholar]
- 41.Lange, Y., Ye, J. & Steck, T. L. (2002) Biochem. Biophys. Res. Commun. 290, 488–493. [DOI] [PubMed] [Google Scholar]
- 42.Niu, S. L. & Litman, B. J. (2002) Biophys. J. 83, 3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaughan, A. M. & Oram, J. F. (2003) J. Lipid Res. 44, 1373–1380. [DOI] [PubMed] [Google Scholar]