Abstract
Study Design Pilot study using the rabbit model.
Objective Low back pain is often associated with disk degeneration. Cell therapy for degenerating disks may promote tissue regeneration and repair. Human dermal fibroblasts, obtained from the patient's skin tissue or donated tissue, may be a promising cell therapy option for degenerating disks. The objective of these studies is to determine the effects of intradiscal transplantation of neonatal human dermal fibroblasts (nHDFs) on intervertebral disk (IVD) degeneration by measuring disk height, magnetic resonance imaging (MRI) signal intensity, gene expression, and collagen immunostaining.
Methods New Zealand white rabbits (n = 16) received an annular puncture to induce disk degeneration and were treated with nHDFs or saline 4 weeks later. At 2 and 8 weeks post-treatment, X-ray and MRI images were obtained. IVDs were isolated and examined for changes in collagen staining and gene expression.
Results In the nHDF-treated group, there was a 10% increase in the disk height index after 8 weeks of treatment (p ≤ 0.05), and there was no significant difference in the saline-treated group. When compared with the saline-treated disks, disks treated with nHDFs showed reduced expression of inflammatory markers, a higher ratio of collagen type II over collagen type I gene expression, and more intense immunohistochemical staining for both collagen types I and II.
Conclusions Human dermal fibroblast introduction into the disk reduced inflammation and promoted tissue rich in both type I and type II collagens. The results of this study suggest that nHDFs would be a feasible cell therapy option for disk degeneration.
Keywords: cell therapy, human dermal fibroblast, disk degeneration, rabbit model
Introduction
Spine disorders present a major burden on the medical, social, and economic structures of all developed countries. Treatments that strategize to biologically repair or regenerate the intervertebral disk (IVD) tissues hold great promise for discogenic low back pain. One strategy is to introduce viable cells into the degenerating IVD that would promote matrix repair, restore physiologic function, and decrease inflammation and pain. Animal studies using sand rats, dogs, and rabbits have shown that cells injected into the disk can survive.1 2 3 4 A variety of inflammatory and injury models have shown that cell therapy can decrease the production of inflammatory cytokines.5 6 7 A previous study from our group showed that chondrocyte cell therapy could reduce the expression of an inflammatory cytokine, interleukin-8 (IL-8), in the rabbit degenerative intervertebral disk.8 Several pilot clinical studies showed that injection of cells into the IVDs of patients led to symptom relief.9 10 11
Cell therapy with neonatal human dermal fibroblasts (nHDFs) may be an effective option for treating disk degeneration. Dermal fibroblasts can easily and noninvasively be obtained from patients or donors. Human dermal fibroblasts have the potential to transdifferentiate into fat-, cartilage-, bone-,12 and chondrocyte-like cells.13 Preclinical and clinical studies have shown that nHDFs embedded in human collagen-based extracellular matrix can integrate well into the host and help heal surgical wounds.14 15 The purpose of this research was to conduct a pilot study to determine if transplantation of nHDFs could reduce disk degeneration and inflammation using the rabbit disk degeneration model.
Materials and Methods
Cell Culture
The nHDFs isolated from human foreskin were purchased from Invitrogen Life Technologies (Carlsbad, California, United States). The cells were cultured in Dulbecco's modified Eagle medium (high glucose) supplemented with 1% penicillin–streptomycin, 25 mM HEPES (all from Invitrogen Life Technologies) and 10% fetal bovine serum (Omega Scientific, Tarzana, California, United States). On the day of injection, nHDFs were labeled with infrared dye for cell tracking purposes. Monolayer cells were detached with trypsin, labeled with CellVue NIR815 Fluorescent dye (LI-COR Biosciences, Lincoln, Nebraska, United States) as recommended by the manufacturer, and suspended in serum free media at a concentration of 1.0 × 107 cells/mL.
Rabbit Disk Degeneration and Cell Treatment
New Zealand white rabbits (Myrtle's Rabbitry, Thompson Station, Tennessee, United States) weighing ∼2.5 to 3 kg were used in this study with the approval of the Institutional Animal Care and Use Committee (Fig. 1). Under general anesthesia and using aseptic conditions, a left abdominal incision was made and the ventral surfaces of four consecutive lumbar IVDs (L2–L3, L3–L4, L4–L5, and L5–L6) were exposed. Using an 18-gauge needle, the annulus fibrosus (AF) was punctured to a depth of 5 mm in the ventral aspect into the nucleus pulposus (NP). The needle was rotated 360 degrees. Suction was applied by withdrawing through a 5-mL syringe for 10 seconds to denucleate the IVD. A vascular staple was placed on the psoas muscle at the L4–L5 level as a marker. The surgical wound was repaired in layers. Meloxicam (1.5 mg) was also given orally (1 day before surgery and 2 to 3 days after the operation). An analgesic (buprenorphine HCl 0.01 to 0.03 mg/kg) was given up to twice daily for 2 to 3 days, when needed, in consultation with the veterinary staff. After recovery from anesthesia, the rabbits were returned to their cages and mobilized ad lib.
Fig. 1.
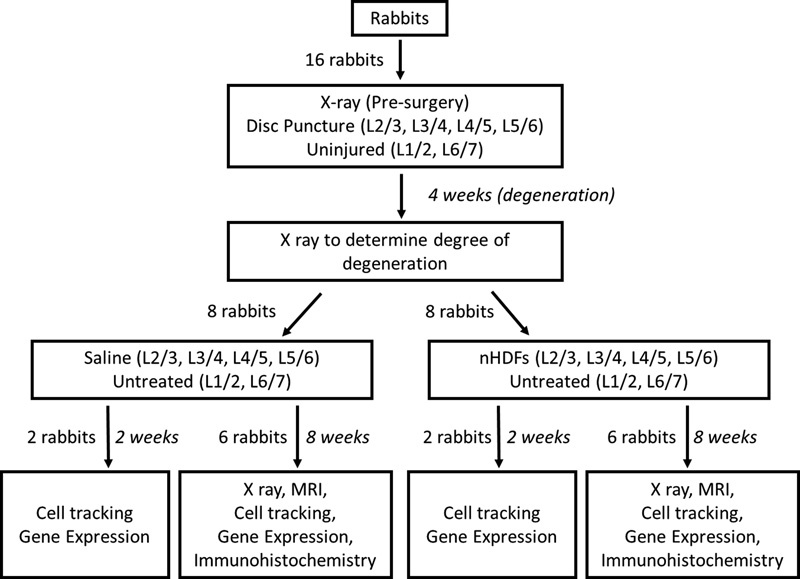
Study design. New Zealand white rabbits (n = 16) underwent surgery to induce disk degeneration by annular puncture on four disks (L2–L3, L3–L4, L4–L5, and L5–L6). Four weeks later, rabbits underwent a second surgery and were randomized to receive either neonatal human dermal fibroblasts (nHDFs) or saline treatment in the injured disks (L2–L3, L3–L4, L4–L5, and L5–L6). L1–L2 and L6–L7 intervertebral disks (IVDs) from each rabbit were left uninjured and untreated. Rabbits were euthanized at 2 weeks (n = 4, two per group) or 8 weeks (n = 12, six per group) post-treatment. X-ray images were obtained before the first surgery, 4 weeks after disk injury, and 8 weeks post-treatment. After euthanasia, rabbit spines underwent magnetic resonance imaging (MRI) and infrared imaging. Individual IVDs were further isolated to study changes in gene expression and collagen staining.
Four weeks postinjury, an X-ray was taken to confirm degeneration of the disks. Then, a right abdominal incision was made. The ventral surfaces of the L2–L3, L3–L4, L4–L5, and L5–L6 IVDs were exposed. Treatments with nHDFs or saline were randomized between rabbits. A volume of 8 µL (the maximum volume that we could easily inject into the rabbit lumbar disks ex vivo) of either nHDF suspension or saline was injected into each degenerated rabbit IVD with an Exmire syringe (Ito Corporation, Fuji, Japan) and 27-gauge needle. The postoperative procedures are described above.
Radiographic Analysis
X-ray images of sedated rabbits were obtained before the surgery, 4 weeks after disk injury, and 8 weeks post-treatment. Disk height indexes (DHIs; n = 12) were calculated by three orthopedic researchers who were blinded to the treatment groups using the method of Lü et al with a slight modification (DHI = IVD height/adjacent IVD body height).16 The average IVD height was calculated by averaging the measurements obtained from the anterior, middle, and posterior portions of the IVD and dividing that by the average of adjacent vertebral body heights. Changes in the DHI of injected disks were expressed as %DHI and normalized to the measured preoperative DHI (%DHI = postoperative DHI/preoperative DHI × 100) as previously described.17
Magnetic Resonance Imaging Analysis
Magnetic resonance imaging (MRI) was performed on the rabbit spines removed en bloc using a 1.5-T MRI machine (Siemens, Malvern, Pennsylvania, United States) with a hand coil. Transverse relaxation time (T2)-weighted sections in the sagittal plane were obtained in the following settings: fast spin echo sequence with time to repetition, 4,000 milliseconds; time to echo, 97 milliseconds; slice thickness, 3.5 mm; field of view, 250, and matrix, 256 × 320. MRIs (n = 12) were graded by five blinded independent readers according to the modified Thompson scale as described previously.18
MRI indexes (n = 12) were calculated as described by Sobajima et al.19 20 MRIs were transformed into picture files using Image J software (National Institutes of Health, Bethesda, Maryland, United States). NPs were outlined to define the regions of interest. High signal intensity area, highest signal intensity, and average signal intensity of this region of interest were calculated. The MRI index was defined as the product of the high signal intensity area and average signal intensity. MRI indexes were normalized to the uninjured intact disks in the same animal.
Cell Tracking
The spine segments and individual disks were scanned with the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, Nebraska, United States) to detect signals at the 700- and 800-nm wavelengths. Infrared fluorescence intensity counts per cubic millimeter of the individual disks were determined using the Odyssey imaging software (LI-COR Biosciences) and exported to Microsoft Excel (Redmond, Washington, United States). The infrared fluorescence intensities between the 2- and 8-week points were compared using a nonparametric t test.
Gene Expression Assays
Total RNA was processed for each IVD. Real-time PCR analysis was performed as described in previous studies.8 Taqman gene expression assays were used for analysis or normalization for the following genes: rabbit COL1A2, COL2A1, IL-8, HPRT1 and 18 S rRNA (Oc03396113_m1, Oc03396134_m1, Oc03397860_m1, Oc03399461_m1, Hs99999901_s1; Applied Biosystems, Foster City, California, United States). A PrimeTime Mini qPCR Assays were designed for rabbit chemokine (C-C motif) ligand 2 (CCL2) and rabbit COL3A1 (CCL2 primers: 5′-TCT TGT CCA GGTTGG CAATG-3′ and 5′-CCC AAA GAA GCT GTG ATC TTC A-3′; CCL2 probe: 5′-/56-FAM/CCA AGC AGA /ZEN/AGT GGGTCC AGG ATG /3IABkFQ/-3′; COL3A1 primers: 5′-ACA GCCTTG CGT GTT CTATAT T-3′ and 5′-GGC AGA AGG AAA CAG CAA ATT C-3′; COL3A1 probe: 5′-/56-FAM/TAC ACA GTT /ZEN/CTG GAG GAT GGCTGC /3IABkFQ/-3′ [Integrated DNA Technologies, Coralville, Iowa, United States]). DataAssist Software (Applied Biosystems) was used to calculate the relative gene expression using the comparative CT (ΔΔCT) method. Intact controls were used as reference samples.
Immunostaining for Collagen Types I and II
Intervertebral disk segments (n = 12) were fixed with 10% formaldehyde, embedded in paraffin, and sectioned (5 μm). Sections were deparaffinized, treated with Proteinase K, incubated with 0.5% hydrogen peroxide, and blocked with 5% horse serum before incubating overnight with mouse anti-collagen type I antibody (1:1,000; C2456, Sigma, St. Louis, Missouri, United States) or mouse anti-collagen type II antibody biotinylated (1:2,000; no. 7006, Chondrox, Redmond, Washington, United States). Because the anti-collagen type II antibody was already biotinylated, a secondary antibody incubation was not necessary for these sections. For collagen type I staining, the sections underwent secondary antibody incubation with biotinylated horse anti-mouse immunoglobulin G (1:400). All the antibodies were diluted in PBS with 3% horse serum. Vectastain ABC kit (Vector Labs, Burlingame, California, United States) and 3,3′-diaminobenzidine (DAB) were used to develop the immunostaining. The sections were counterstained with hematoxylin solution (VWR, Radnor, Pennsylvania, United States). Negative controls were performed by omitting the primary antibodies.
The degree of collagen types I and II immunopositive staining was assessed by scoring the staining intensity and the number of stained areas. Representative images were taken at 200× magnification on a Nikon Eclipse 50i microscope using NIS-Elements Imaging Software (Nikon, Tokyo, Japan).
Statistical Methods
The Kruskal-Wallis test and Mann-Whitney test were used to analyze nonparametric data (MRI grading) for the effect of treatment. The changes in disk height indexes were assessed by the Spearman rank correlation test. The MRI indexes were compared using the Student t test. The infrared fluorescence intensities were compared using a nonparametric t test. The differences were considered significant when the p value was equal to or below 0.05.
Results
Tracking of nHDFs Injected into Rabbit Degenerating IVDs In Vivo
To determine if the cells transplanted into the degenerating IVD remained in the IVD, the nHDFs were labeled with infrared dye and tracked at the end of treatment. The infrared dye-labeled nHDFs were transplanted into the degenerating rabbit IVDs at a concentration of 107 cells/mL. After euthanasia, isolated spines and individual IVDs were scanned with an infrared scanner 2 and 8 weeks after transplantation. As seen in Fig. 2, the rabbit spine and disk contours were detected in the 700-nm wavelength channel (represented in red). Injected cells were detected in the 800-nm wavelength channel and represented in green or yellow (yellow represents overlapping signals of the 800- and 700-nm wavelengths). At both 2 weeks (Fig. 2, A' and A”) and 8 weeks (Fig. 2, B' and B”) after transplantation, infrared dye-labeled cells were detected in the spines and individual IVDs. The average intensity of the IVDs was 226,555 counts at 2 weeks post-treatment (n = 2 rabbits) and 75,239 counts at 8 weeks post-treatment (n = 3 rabbits). Although there was a threefold decrease in signal intensity from 2 to 8 weeks, this data suggests that some of the cells injected into the IVD remained in the IVD for up to 8 weeks.
Fig. 2.
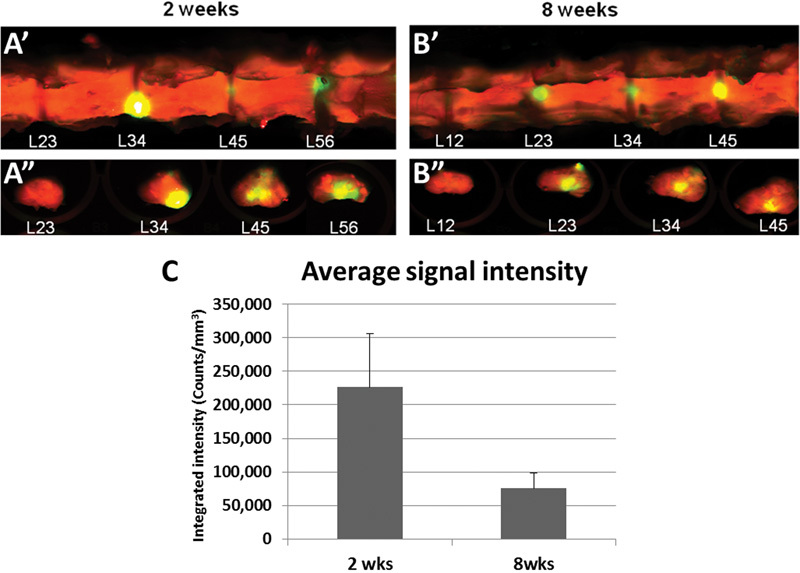
Neonatal human dermal fibroblasts were tracked after injection into the degenerating rabbit intervertebral disk in vivo. Infrared dye-labeled neonatal human dermal fibroblasts were injected into the degenerating rabbit disks and imaged at 2 and 8 weeks post-treatment. The rabbit spine and disk contours were detected in the 700-nm wavelength channel represented in red. The injected cells were detected in the 800-nm wavelength channel and represented in green or yellow (yellow represents overlapping signals of the 800- and 700-nm wavelengths). Coronal images of the spine and transverse images of the individual disks were taken at (A' and A”) 2 weeks post-treatment and at (B' and B”) 8 weeks post-treatment. (C) Average integrated signal intensity of the infrared fluorescence in the 800-nm wavelength of the intervertebral disks. Error bars represent the standard error of the mean.
Radiographic Assessment 8 Weeks Post-Treatment
DHIs were calculated by three orthopedic researchers who were blinded to the treatment groups. Four weeks after disk injury, a reduction of disk height occurred due to injury-induced degeneration. Representative lateral radiographs are shown in Fig. 3 (left panel). DHI at 4 weeks after injury had decreased by ∼30% compared with the initial DHI before surgery. Eight weeks after saline treatment, there was a small increase in the DHI but this increase was not significant. In the disks that were treated with nHDFs, there was a 10% restoration in the DHI. This difference was significant in comparison with the DHI before treatment (p ≤ .05; Fig. 3, right panel).
Fig. 3.
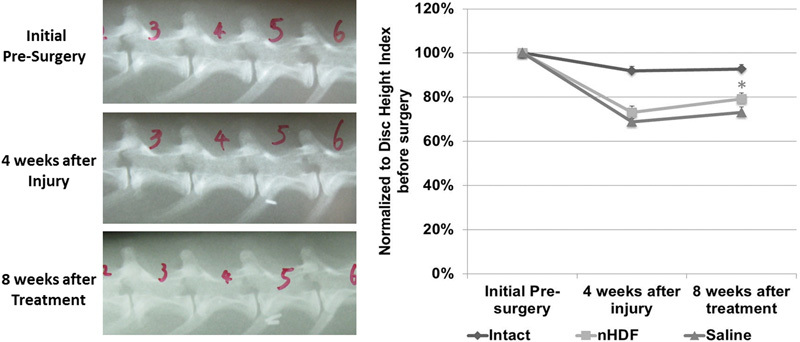
Lateral radiographs and changes in disk height index. (Left) Representative serial lateral radiographs of a lumbar spine of a rabbit obtained initially presurgery, 4 weeks after injury, and 8 weeks after treatment. (Right) Average disk height indexes were calculated presurgery, 4 weeks after injury, and 8 weeks after treatment in disks that were intact, injured, and treated with neonatal human dermal fibroblasts (nHDFs) and injured and treated with saline. The average disk height index was normalized to the presurgical disk height index. *Significant difference between the two time points: 4 weeks after injury and 8 weeks after nHDF treatment (p < 0.05). Error bars represent the standard error of the mean.
Magnetic Resonance Imaging Assessment 8 Weeks Post-Treatment
At 8 weeks post-treatment, rabbit spines underwent MRI. Representative MRIs are shown in Fig. 4A. Four levels (L2–L3, L3–L4, L4–L5, and L5–L6) were injured and treated and the other levels (L1–L2 and L6–L7) were left uninjured and intact. MRI grading was calculated using the Modified Thompson Grading Scale by five independent orthopedic researchers who were blinded to the treatment groups. The NPs in the intact uninjured disks showed lower average MRI grades and higher MRI signal intensities than those in the injured nHDF-treated or saline-treated disks (Fig. 4B). The average MRI grade for the nHDF-treated disks was 3.5 and the average for the saline-treated disks was 2.8. The nHDF-treated disks had higher MRI grades and lower signal intensities than the saline-treated IVDs.
Fig. 4.
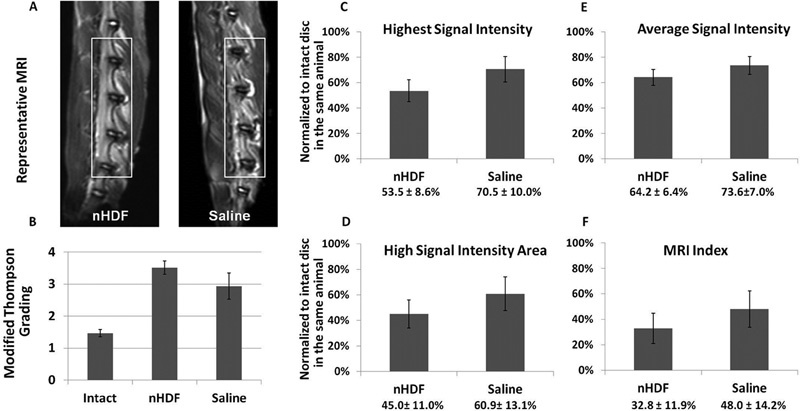
Magnetic resonance imaging (MRI) analysis 8 weeks after neonatal human dermal fibroblast (nHDF) or saline treatment. (A) Representative MRI scans of the lumbar spine of rabbits at 8 weeks post-treatment. (B) Average MRI grading using the Modified Thompson Grading Scale. (C to F) Highest signal intensity, average signal intensity, and high signal intensity areas were calculated to determine the MRI index (nucleus pulposus [NP] average signal intensity × NP area). Error bars represent the standard error of the mean.
Due to the subjectivity of MRI grading, MRI indexes were also included in our analysis. The highest signal intensity, average signal intensity, and high signal intensity area were calculated to determine the MRI index (Fig. 4C to 4F). The MRI index (defined as the NP area × NP average signal intensity) of each treated disk was then normalized to the intact disk in the same animal. On average, the MRI indexes showed that the saline-treated IVDs had higher signal intensities than the nHDF-treated IVDs, although this difference was not significant.
Collagen Gene Expression Analyses
Collagen types I and II are markers that are usually found in the NP and AF tissues, and collagen type III is a marker for fibrotic tissue. At 2 and 8 weeks after treatment, expression of COL1A2, COL2A1, and COL3A1 genes from rabbit IVDs treated with nHDFs and saline increased in comparison to uninjured controls (Fig. 5A to 5C). The expression of COL3A1 was highly upregulated in the nHDF-treated disks at 2 weeks, which later decreased to the same level as those treated with saline at 8 weeks. Cartilage and NP tissues usually contain a higher ratio of collagen type II over type I. The ratios of COL2A1 to COL1A2 gene expression were calculated and normalized to that of the saline-treated samples. At 2 weeks post-treatment, the ratio of COL2A1 to COL1A2 gene expression was similar between the nHDF (0.84) and the saline (1.00) treatment groups (Fig. 5C). At 8 weeks post-treatment, the ratio was higher in the IVDs treated with nHDFs (2.71) than in those treated with saline (1.00).
Fig. 5.
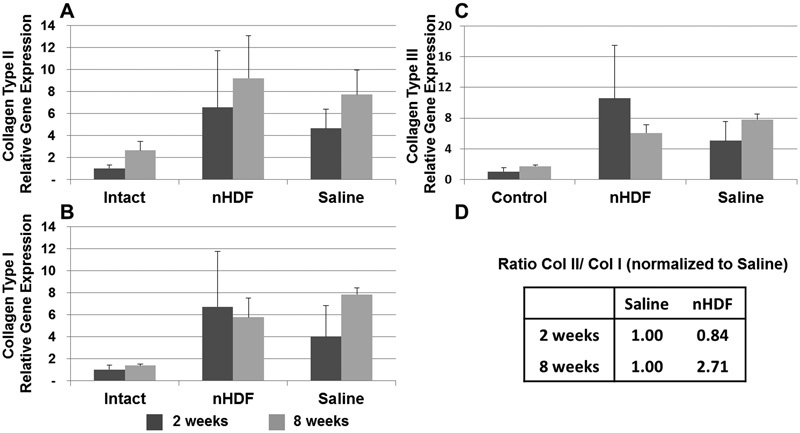
Expression analysis of collagen genes using real-time polymerase chain reaction (PCR). RNA from uninjured untreated control disks and injured disks treated with neonatal human dermal fibroblasts (nHDFs) or saline was isolated for real-time PCR analysis. (A–C) At 2 and 8 weeks after treatment, expression levels of collagen types II, I, and III genes were analyzed. (D) The ratio of collagen II (Col II) to collagen I (Col I) gene expression was calculated and normalized to that of the saline treated disks. Error bars represent the standard error of the mean.
Collagen Types I and II Immunostaining in the IVD
Histologic analysis showed evidence of fibrocartilage formation in the injured and treated disks (data not shown). To determine if treatment led to a difference in fibrocartilage formation, sections were immunostained for both collagen types I and II and analyzed. Collagen type II was detected in the NP areas and collagen type I staining was found in the AF areas. Areas of new fibrocartilage formation had staining for both types of collagens. The disks treated with nHDF had a higher staining intensity and a larger number of areas positive for both collagen types I and II than those treated with saline. Positive immunostaining of the collagens was not distinct at low magnification (10×), therefore representative images in Fig. 6 are of high magnification (200×).
Fig. 6.
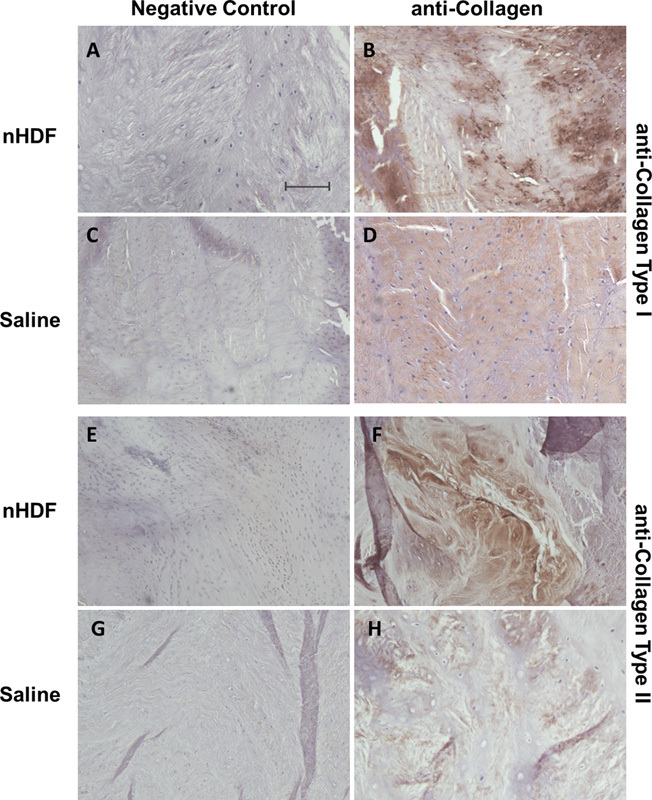
Representative immunohistochemical staining of collagen types I and II. Collagen type I staining of the annulus fibrosus regions in disks treated with (B) neonatal human dermal fibroblasts (nHDFs) or (D) saline and (A, C) their respective negative controls. Collagen type II staining of nucleus pulposus regions of disks treated with (F) nHDFs or (H) saline and (E, G) their respective negative controls. Negative controls had primary antibodies omitted. Note: Staining of both collagen types I and II is more intense in nHDF-treated disks compared with those treated with saline. Scale bar = 50 μm.
Cytokine and Chemokine Gene Expression Analyses
For both nHDF- and saline-treated disks, the expression levels of inflammatory cytokine IL-8 and chemokine CCL2 genes were elevated compared with uninjured intact controls at 2 weeks (Fig. 7). Although the saline-treated disks showed a 4.5-fold increase in IL-8 and 15.4-fold increase in CCL2 gene expression, the nHDF-treated disks had only a 1.5-fold increase in IL-8 and 5-fold increase in CCL2 gene expression. Treatment with nHDFs reduced the expression of IL-8 and CCL2 genes by two-thirds.
Fig. 7.
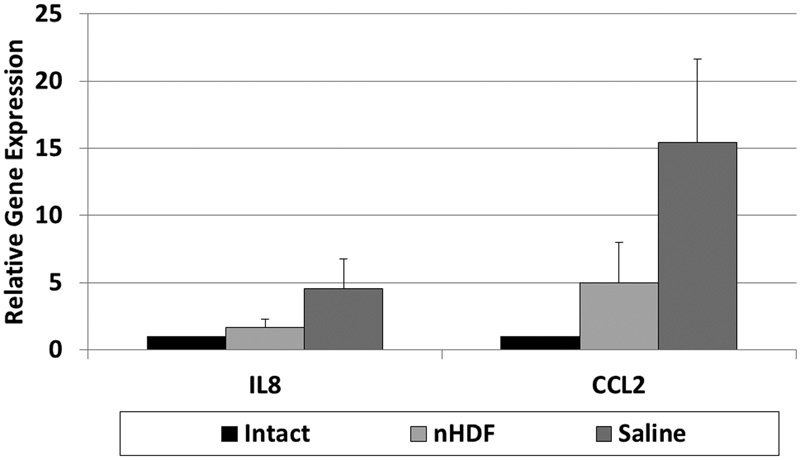
Expression analysis of inflammatory cytokine and chemokine genes using real-time polymerase chain reaction (PCR). RNA from uninjured untreated control disks and injured disks treated with neonatal human dermal fibroblasts (nHDFs) or saline was isolated for real-time PCR analysis. At 2 weeks after treatment, expression levels of interleukin-8 (IL8) and chemokine (C-C motif) ligand 2 (CCL2) genes were analyzed. Error bars represent the standard error of the mean.
Discussion
Cell therapy is a promising approach to help regenerate the intervertebral disk. In this pilot study, we tested nHDF as a cell therapy option for disk degeneration using the rabbit model. Disk height in the nHDF-treated IVDs increased significantly 8 weeks after treatment (Fig. 3), and the height of the saline-treated IVDs did not change significantly. Because the average MRI signal intensity of the nHDF-treated IVDs was lower than that of the saline-treated IVDs, the increase in disk height may be due to the formation of fibrocartilage. Fibrocartilage contains less water than hyaline cartilage, resulting in a lower MRI signal intensity.21 The NP tissue has a higher ratio of proteoglycan to collagen, more water content, and higher MRI signal than both hyaline cartilage and AF tissue.22 Increasing the IVD height alone may reduce pressure on sensitive tissues, which could in turn decrease some pain. The upregulation of collagen type III in the nHDF-treated disks at 2 weeks may indicate that fibrocartilage may have formed early after transplantation. At 8 weeks, collagen type III expression is decreased and collagen type II is increased in the nHDF-treated disks (Fig. 5A and 5C). Future studies will be needed to determine how the presence of fibrocartilage affects IVD biomechanical function.
Injury to the disk caused an upregulation of collagen type II and collagen type I gene expression (Fig. 5A and B). The ratio of collagen II to collagen I gene expression in the nHDF-treated IVDs was close to the ratio seen in the saline-treated IVDs at 2 weeks (Fig. 5D). At the later 8-week point, the ratio of collagen II to collagen I was higher in nHDF-treated samples than in saline-treated samples. The shift toward higher collagen II expression and lesser collagen I and collagen III may indicate that the new collagen expression may be more cartilaginous and less fibrotic. Immunohistochemical studies showed that the staining for both collagen types I and II was more pronounced in the nHDF-treated disks than in the saline-treated disks. The source of collagen production may be from the transplanted nHDFs or resident disk cells. NP cells in the degenerated disk may increase expression of collagen type II when exposed to transplanted cells or during the normal disk repair process. In some cases, dermal fibroblasts may be induced to express collagen type II when exposed to different growth factors, scaffold proteins, and fluctuating pressures.13 23 24 Studies have shown that human dermal fibroblasts have the potential to transdifferentiate into different cell types.13 One reason may be that skin dermal fibroblasts express mesenchymal stem cell surface markers: CD44, CD73, and CD105.25
Both IL-8 and CCL2 have been associated with inflammation and recruitment of immune cells to injured or inflamed tissues. IL-8 has been shown to be upregulated in the rabbit disk injury model and in cultured intervertebral disk cells isolated from surgical patients or after exposure to IL-1.26 27 28 CCL2 has been shown to be upregulated in the rabbit disk hernia model and in cultured intervertebral disks from surgical patients.27 29 As in other cell therapy studies of inflammatory and injury models,5 6 7 our study showed that transplantation of nHDFs can decrease the early signs of disk inflammation. After 2 weeks of treatment with nHDFs, the expression of IL-8 and CCL2 genes decreased by two-thirds compared with the saline-treated disks. A reduction in cytokine and chemokine expression in the disk may reduce recruitment of immune cells and inflammation. In an earlier study, the disks treated with rabbit articular chondrocytes also showed a reduction in IL-8 gene expression compared with saline-injected ones. The IL-8 gene expression levels were similar to those treated with nHDF (both were 1.6-fold of the intact). Our studies show that injured disks can induce the expression of chemokines that may recruit immune cells into the disk, although it has been thought to be a relatively immune-privileged site. It is unclear if these cells would initiate an immune response that would later lead to rejection and if immunosuppression would be necessary when treating with allogenic cells. Studies with murine embryonic stem cells in rabbit degenerative disk model suggest there is no immune rejection after 8 weeks.30
In conclusion, this pilot study has shown that nHDFs injected into the rabbit degenerating disk can mildly increase disk height, increase the ratio of collagen type II to collagen type I gene expression, and reduce the expression of inflammatory markers. Human dermal fibroblasts can easily be obtained from skin biopsies of patients themselves or human foreskin donors.31 In 2011, the Food and Drug Administration approved the use of autologous dermal fibroblasts to reduce fine wrinkles or nasolabial folds around the nose and mouth.32 Future studies will be needed to determine the minimum effective dosage of human dermal fibroblasts and biomechanical outcome measures after human dermal fibroblast therapy. Also, studies that compare the effectiveness of different cell types (e.g., mesenchymal stem cells versus dermal fibroblasts) or the immunogenicity of different cell sources (allogenic versus autologous) will give us a better picture as to which cell therapy option would be most promising for the disk. Because disk loading and disk nutrient and oxygen supplies differ in small animal and humans, clinical studies would ultimately be required to determine if cell therapy is beneficial in reducing back pain. In conclusion, the data in these studies will help to lay the groundwork to test human dermal fibroblast therapy for restoring biological function and reducing symptoms of low back pain associated with degenerative disk disease.
Acknowledgments
SpinalCyte, LLC provided the research funds to perform this study. The authors do not own stocks, royalties, or have any other financial relationship with SpinalCyte, LLC.
The authors gratefully acknowledge Dr. Martin Heyworth for critically editing the manuscript and Kerry Kraushaar and the personnel at the Midwest Orthopaedics at Rush Advanced Imaging department.
Footnotes
Disclosures Ana Chee: Research support (SpinalCyte, Yuhan Corporation, NASS, AO Foundation, RTI Surgical, Baxter) Peng Shi: Research support (SpinalCyte) Thomas Cha: Research support (SpinalCyte, North American Spine Society, Gordon and Betty Moore Foundation); Consultant (Bio2, GE Healthcare) Ting-Hsien Kao: Research support (SpinalCyte) Shu-Hua Yang: Research support (SpinalCyte) Jun Zhu: none Ding Chen: none Yejia Zhang: Research support (SpinalCyte) Howard S. An: Consultant (Bioventus, RTI Surgical, Life Spine, Halozyme); Royalties (U&I, Zimmer Spine); Research support (SpinalCyte, Yuhan Corporation, RTI Surgical, Baxter, NASS, AO Foundation); Institutional support (Endowment); Stock/options (U&I, Spinal Kinetics, Advanced Biologics, Medyssey); Advisory board or board of directors (Articular Engineering, International Society for the Study of the Lumbar Spine, Spinal Kinetics, Advanced Biologics, Medyssey); Fellowship support (OREF, Omega)
References
- 1.Gruber H E, Johnson T L, Leslie K. et al. Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine (Phila Pa 1976) 2002;27(15):1626–1633. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 2.Ganey T, Libera J, Moos V. et al. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine (Phila Pa 1976) 2003;28(23):2609–2620. doi: 10.1097/01.BRS.0000097891.63063.78. [DOI] [PubMed] [Google Scholar]
- 3.Sakai D, Mochida J, Iwashina T. et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27(3):335–345. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 4.Sakai D, Mochida J, Iwashina T. et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976) 2005;30(21):2379–2387. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 5.Oh J Y, Lee R H, Yu J M. et al. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther. 2012;20(11):2143–2152. doi: 10.1038/mt.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mert T, Kurt A H, Arslan M, Çelik A, Tugtag B, Akkurt A. Anti-inflammatory and anti-nociceptive actions of systemically or locally treated adipose-derived mesenchymal stem cells in experimental inflammatory model. Inflammation. 2015;38(3):1302–1310. doi: 10.1007/s10753-014-0101-1. [DOI] [PubMed] [Google Scholar]
- 7.Urdzíková L M, Růžička J, LaBagnara M. et al. Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat. Int J Mol Sci. 2014;15(7):11275–11293. doi: 10.3390/ijms150711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Chee A, Shi P. et al. Allogeneic articular chondrocyte transplantation downregulates interleukin 8 gene expression in the degenerating rabbit intervertebral disk in vivo. Am J Phys Med Rehabil. 2015;94(7):530–538. doi: 10.1097/PHM.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meisel H J, Ganey T, Hutton W C, Libera J, Minkus Y, Alasevic O. Clinical experience in cell-based therapeutics: intervention and outcome. Eur Spine J. 2006;15 03:S397–S405. doi: 10.1007/s00586-006-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meisel H J, Siodla V, Ganey T, Minkus Y, Hutton W C, Alasevic O J. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24(1):5–21. doi: 10.1016/j.bioeng.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine (Phila Pa 1976) 2010;35(11):E475–E480. doi: 10.1097/BRS.0b013e3181cd2cf4. [DOI] [PubMed] [Google Scholar]
- 12.Junker J P, Sommar P, Skog M, Johnson H, Kratz G. Adipogenic, chondrogenic and osteogenic differentiation of clonally derived human dermal fibroblasts. Cells Tissues Organs. 2010;191(2):105–118. doi: 10.1159/000232157. [DOI] [PubMed] [Google Scholar]
- 13.Singh M, Pierpoint M, Mikos A G, Kasper F K. Chondrogenic differentiation of neonatal human dermal fibroblasts encapsulated in alginate beads with hydrostatic compression under hypoxic conditions in the presence of bone morphogenetic protein-2. J Biomed Mater Res A. 2011;98(3):412–424. doi: 10.1002/jbm.a.33129. [DOI] [PubMed] [Google Scholar]
- 14.Boyd M, Flasza M, Johnson P A, Roberts J S, Kemp P. Integration and persistence of an investigational human living skin equivalent (ICX-SKN) in human surgical wounds. Regen Med. 2007;2(4):363–370. doi: 10.2217/17460751.2.4.363. [DOI] [PubMed] [Google Scholar]
- 15.Flasza M, Kemp P, Shering D. et al. Development and manufacture of an investigational human living dermal equivalent (ICX-SKN) Regen Med. 2007;2(6):903–918. doi: 10.2217/17460751.2.6.903. [DOI] [PubMed] [Google Scholar]
- 16.Lü D S Shono Y Oda I Abumi K Kaneda K Effects of chondroitinase ABC and chymopapain on spinal motion segment biomechanics. An in vivo biomechanical, radiologic, and histologic canine study Spine (Phila Pa 1976) 199722161828–1834., discussion 1834–1835 [DOI] [PubMed] [Google Scholar]
- 17.An H S Takegami K Kamada H et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits Spine (Phila Pa 1976) 200530125–31., discussion 31–32 [DOI] [PubMed] [Google Scholar]
- 18.Masuda K, Aota Y, Muehleman C. et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976) 2005;30(1):5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 19.Sobajima S, Shimer A L, Chadderdon R C. et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5(1):14–23. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- 20.Sobajima S, Kompel J F, Kim J S. et al. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine (Phila Pa 1976) 2005;30(1):15–24. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]
- 21.Berquist T H. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2013. MRI of the Musculoskeletal System. 6th ed; p. 1173. [Google Scholar]
- 22.Mwale F Roughley P Antoniou J Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc Eur Cell Mater 2004858–63., discussion 63–64 [DOI] [PubMed] [Google Scholar]
- 23.French M M, Rose S, Canseco J, Athanasiou K A. Chondrogenic differentiation of adult dermal fibroblasts. Ann Biomed Eng. 2004;32(1):50–56. doi: 10.1023/b:abme.0000007790.65773.e0. [DOI] [PubMed] [Google Scholar]
- 24.Zhao G, Yin S, Liu G. et al. In vitro engineering of fibrocartilage using CDMP1 induced dermal fibroblasts and polyglycolide. Biomaterials. 2009;30(19):3241–3250. doi: 10.1016/j.biomaterials.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 25.Alt E, Yan Y, Gehmert S. et al. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell. 2011;103(4):197–208. doi: 10.1042/BC20100117. [DOI] [PubMed] [Google Scholar]
- 26.Kuelling F A, Foley K T, Liu J J. et al. The anabolic effect of plasma-mediated ablation on the intervertebral disc: stimulation of proteoglycan and interleukin-8 production. Spine J. 2014;14(10):2479–2487. doi: 10.1016/j.spinee.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Burke J G, Watson R W, McCormack D, Dowling F E, Walsh M G, Fitzpatrick J M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84(2):196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y Chee A Shi P et al. Intervertebral disc cells produce interleukins found elevated in patients with back pain Am J Phys Med Rehabil 2015; October 22 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida M, Nakamura T, Sei A, Kikuchi T, Takagi K, Matsukawa A. Intervertebral disc cells produce tumor necrosis factor alpha, interleukin-1beta, and monocyte chemoattractant protein-1 immediately after herniation: an experimental study using a new hernia model. Spine (Phila Pa 1976) 2005;30(1):55–61. doi: 10.1097/01.brs.0000149194.17891.bf. [DOI] [PubMed] [Google Scholar]
- 30.Sheikh H, Zakharian K, De La Torre R P. et al. In vivo intervertebral disc regeneration using stem cell-derived chondroprogenitors. J Neurosurg Spine. 2009;10(3):265–272. doi: 10.3171/2008.12.SPINE0835. [DOI] [PubMed] [Google Scholar]
- 31.Villegas J, McPhaul M. Establishment and culture of human skin fibroblasts. Current Protocols in Molecular Biology. 2005;71(28.3):28.3.1–28.3.9. doi: 10.1002/0471142727.mb2803s71. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt C. FDA approves first cell therapy for wrinkle-free visage. Nat Biotechnol. 2011;29(8):674–675. doi: 10.1038/nbt0811-674. [DOI] [PubMed] [Google Scholar]


