Abstract
There is a growing body of evidence that the nondegradable fluorophores that accumulate as the lipofuscin of retinal pigment epithelium (RPE) are involved in mechanisms leading to the degeneration of RPE in macular degeneration. Most of the constituents of RPE lipofuscin are inadvertent products of the retinoid visual cycle, the enzymatic pathway by which the 11-cis-retinal chromophore of rhodopsin is generated. Indeed, a major constituent of RPE lipofuscin, the pyridinium bisretinoid A2E, is a diretinal conjugate that forms in photoreceptor cells and is deposited in RPE cells as a consequence of the phagocytosis of the outer segment membrane by RPE cells. Given the adverse effects of A2E, there is considerable interest in combating its deposition so as to protect against vision loss. These efforts, however, necessitate an understanding of factors that modulate its formation. Here we show that an amino acid variant in murine Rpe65, a visual-cycle protein required for the regeneration of 11-cis-retinal, is associated with reduced A2E accumulation.
The visual cycle employs a panel of proteins whose roles in enzymatic processing and trafficking enable regeneration of the 11-cis chromophore of rhodopsin (1) (Fig. 1). One of these proteins, retinal pigment epithelium (RPE) 65 (2), has been known for some time to be essential for the regeneration of rhodopsin. Recent studies have shown that RPE65 serves as a binding protein for retinyl esters (3–5), thereby delivering these hydrophobic compounds to the isomerohydrolase, which converts them to 11-cis-retinol (6). Mutations in RPE65, including missense- or nonsense-point mutations, insertions, deletions, and splice site defects, lead to severe, childhood-onset retinal degeneration (7–9), including ≈5–10% of cases of Lebers congenital amaurosis (10). A null mutation of Rpe65 in mice results in a complete absence of 11-cis-retinaldehyde with the visual cycle arrest producing a severely depressed electroretinographic response (11). Retinal dystrophy with functional deficits similar to that observed in Rpe65–/– mice is also present in the Swedish Briard dog, a strain carrying a 4-bp deletion in RPE65. Transfer of the RPE65 gene has restored vision in both the Briard dog (12, 13) and Rpe65–/– mice (14).
Fig. 1.
Lipofuscin fluorophores as by-products of the visual cycle. (A) The retinoid cycle and formation of the lipofuscin fluorophores A2E, iso-A2E, and all-trans-retinal (ATR) dimer. Upon the photoisomerization of 11-cis-retinal, all-trans-retinal is released from rhodopsin and reduced to all-trans-retinol by all-trans-retinol dehydrogenase (atrDH). Lecithin retinol acyltransferase (LRAT) generates all-trans-retinyl esters from all-trans-retinol, and RPE65 presents retinyl esters to isomerohydrolase (IMH) for processing to 11-cis-retinol. 11-cis-retinol is then oxidized by 11-cis-retinol dehydrogenase (11-cRDH) to regenerate 11-cis-retinal. All-trans-retinal that evades reduction reacts with phosphatidylethanolamine (PE) (2:1) to generate the precursor A2-PE, from which A2E and iso-A2E form, or ATR dimer. (B) Structures of A2E and iso-A2E. These pigments interconvert under the influence of light.
Work in the Rpe65-null mutant mouse has also shown that nearly all of the lipofuscin that accumulates in RPE cells with age is derived as a byproduct of the visual cycle. This conclusion is based on the observation that in Rpe65–/– mice, wherein the 11-cis and all-trans-retinal chromophores are absent, RPE lipofuscin is decreased >90% (15). Thus, it is not surprising that all of the RPE lipofuscin fluorophores isolated to date, including A2E, the photoisomer iso-A2E, minor cis-isomers of A2E, and ATR dimer (5, 16–19), are generated by reactions between phosphatidylethanolamine and first one and then a second molecule of all-trans-retinal (Fig. 1). The all-trans-retinal that enters the A2E biosynthetic pathway is generated upon photoisomerization of 11-cis-retinal. Although most of the all-trans-retinal is reduced to all-trans-retinol by a dehydrogenase in the photoreceptor cell, all-trans-retinal that eludes reduction is available to form these lipofuscin fluorophores.
In inbred strains of mice, an Rpe65 polymorphism exists whereby the amino acid at residue 450 is either leucine or methionine. The fact that this amino acid variant produces changes in Rpe65 activity is indicated by studies showing that recovery of the electroretinographic response following a photobleach is retarded in C57BL/6J-c2J mice that carry methionine at codon 450 as compared with BALB/cByJ mice that have a leucine at that position; C57BL/6J-c2J mice also demonstrate resistance to light damage (20–22). Biochemical studies have demonstrated that the slower recovery of vision in the C57BL/6J-c2J mice reflects more laggard rhodopsin regeneration and, correspondingly, reduced capacity for photon catch (23).
Because the source of all-trans-retinal for A2E formation is the photoisomerization of 11-cis-retinal, and given that the Leu450Met substitution in Rpe65 slows the kinetics of 11-cis-retinal production, the question remains as to whether this amino acid variant may result in reduced A2E accumulation (15, 24). By analogy, isotretinoin (13-cis-retinoic acid), the acne medication that induces night blindness and protection against light damage by a retarding of 11-cis-retinal regeneration, can reduce A2E deposition in the RPE of Abcr–/– mice (25, 26). The latter null mutant mice have been shown to accumulate this fluorophore in abundance (27). Here we report the results of our exploration of A2E levels in mice bearing methionine vs. leucine at residue 450 of Rpe65.
Materials and Methods
Mice. Albino BALB/cByJ and albino C57BL/6J-c2J mice were purchased from The Jackson Laboratory as retired breeders (8–9 months of age). Abcr null mutant mice (129/SV × C57BL/6J) were generated in collaboration with Bristol-Myers Squibb and Lexicon Genetics (The Woodlands, TX) by using a strategy described in ref. 27. Abcr–/– and Abcr+/+ mice were raised under 12-h on–off cyclic lighting with an in-cage illuminance of 30–80 lux. Experiments were performed with pigmented Abcr-null mutant and WT mice aged 7–15 months, as indicated. In Abcr–/– and Abcr+/+ mice, Rpe65 was sequenced by the PCR restriction fragment length polymorphism method. DNA derived from mouse tails by standard procedures was PCR-amplified with the forward 5′-ACCAGAAATTTGGAGGGAAAC-3′ and reverse 5′-CCCTTCCATTCAGAGCTTCA-3′ primers. The resulting 545-bp product was digested to completion with 50-fold excess of the MwoI restriction enzyme (New England Biolabs) and analyzed on 2% agarose gels. The sequence variant corresponding to Met-450 yielded discrete fragments of 180 and 365 bp because of the presence of the MwoI restriction site. Because the Leu-450 codon eliminates the MwoI site, undigested or partially digested products were interpreted as being homozygous or heterozygous for Leu-450, respectively. Initially, the results of restriction analysis were confirmed by direct sequencing to confirm the reliability of the PCR restriction fragment length polymorphism method. All procedures were approved by the Institutional Animal Care and Use Committee and complied with guidelines set forth by The Association for Research in Vision and Ophthalmology.
Tissue Extraction and HPLC Analysis. Posterior eye cups were pooled and homogenized in PBS with a tissue grinder. An equal volume of a mixture of chloroform/methanol (2:1) was added, and the sample was extracted three times. To remove insoluble material, extracts were filtered through cotton and passed through a reversed-phase (C18 Sep-Pak, Millipore) cartridge with 0.1% trifluoroacetic acid in methanol. After removing solvent by evaporation under gas, the extract was dissolved in methanol containing 0.1% trifluoroacetic acid, for HPLC analysis. For quantification of A2E, a Waters 600E HPLC was used with a C18 column (4 × 150 mm) and the following gradient of acetonitrile in water (containing 0.1% trifluoroacetic acid): 90–100% (0–10 min), 100% acetonitrile (10–20 min), and a flow rate of 0.8 ml/min with monitoring at 430 nm. The injection volume was 10 μl. Extraction and injection for HPLC were performed under dim red light. Levels of A2E and iso-A2E were determined by reference to an external standard of HPLC-purified A2E/iso-A2E. Because A2E and iso-A2E reach photoequilibrium in vivo (28), use of the term A2E will refer to both isomers, unless stated otherwise.
Results
To probe for an effect of the leucine to methionine variant of Rpe65 on A2E levels, we measured A2E and the related photoisomer iso-A2E in the eyes of pigmented Abcr+/+ and Abcr–/– mice homozygous for either the Leu-450 or Met-450 allele. The representative chromatograms presented in Fig. 2 demonstrate the detection of A2E and the slightly less polar pigment iso-A2E, the Z-isomer of A2E at the C13-C14 double bond, in Abcr+/+ mice (age 7 months). A2E and iso-A2E interconvert in vivo (28), and both pigments were detectable in extracts from all eye cups. HPLC quantitation (Fig. 2C) revealed that in mice homozygous for methionine at position 450, A2E and iso-A2E were present in amounts that were ≈28% and ≈13%, respectively, of the levels present in the Leu-450 mice.
Fig. 2.
Analysis of hydrophobic extracts of eye cups excised from Abcr+/+ mice (age 7 months) with the Leu-450 or Met-450 variant of Rpe65. (A and B) Typical chromatograms obtained by reverse-phase HPLC with monitoring at 430 nm illustrate the detection of A2E and iso-A2E. (C) Quantitation of A2E and iso-A2E in eye cups of Abcr+/+ mice with either the Leu-450 or Met-450 polymorphism. Levels were determined as integrated peak areas normalized to an external standard of A2E. Values are expressed as picomoles per eye and for each group are based on measures obtained after pooling eight eyes into a single sample.
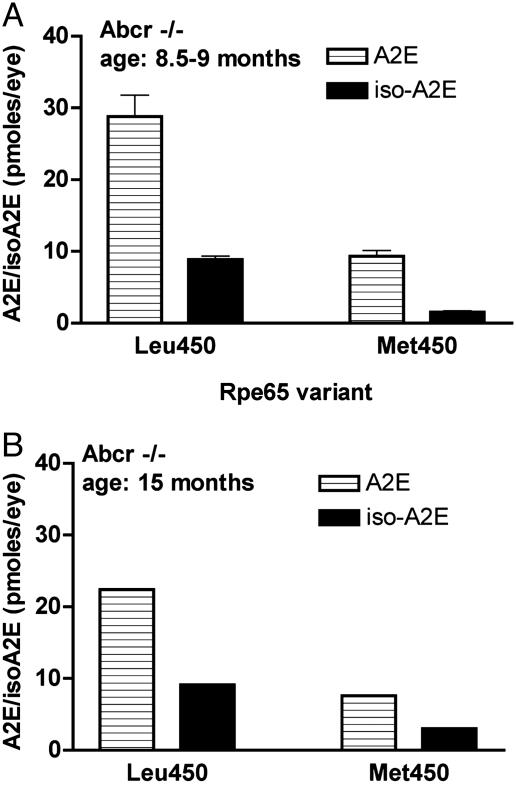
As anticipated from the work of Travis and colleagues (5, 27, 29), levels of A2E were appreciably greater in mice carrying a null mutation in Abcr as compared with the WT (Abcr+/+) mice (Figs. 2 and 3). Nevertheless, the suppression in A2E accumulation associated with the methionine variant in Rpe65 was readily detectable when 8.5- to 9-month-old Abcr–/– mice homozygous for the leucine or methionine variant were compared: methionine at amino acid 450 was associated with levels of A2E that were 32% of that measured in mice with the leucine variant, whereas iso-A2E was reduced to 16% of that with Leu-450 present (Fig. 3A). In 15-month-old Abcr–/– mice, similar differences in A2E accumulation were conferred by the leucine to methionine variant (Fig. 3B).
Fig. 3.
Quantitation of A2E and iso-A2E in eye cups of Abcr–/– mice expressing either the Leu-450 or Met-450 variant of Rpe65. Levels were determined as integrated peak areas normalized to an external standard of A2E. (A) A2E and iso-A2E in eyes of Abcr–/– mice (age 8.5–9 months). Values (mean ± SD) are expressed as picomoles per eye and are based on two to five samples and two eye cups per sample. (B) Eye cups obtained from Abcr–/– mice (age 15 months). Values are based on single samples with two eyes per sample.
In testing for the effect of the leucine-to-methionine variant on A2E levels, we also measured A2E and iso-A2E in the eyecups of albino BALB/cByJ (n = 26) and C57BL/6J-c2J (n = 24) mice obtained at age 8–9 months. The use of albinotic mice in the case of both strains eliminated effects of pigmentation on quantal catch (21). In eyes from the BALB/cByJ mice, a strain that expresses leucine at position 450 of Rpe65, levels of A2E and iso-A2E were comparable with Abcr+/+/Leu-450 mice of a similar age range (Figs. 2 and 4). However, in C57BL/6J-c2J mice the quantity of A2E was 26% of that in the BALB/cByJ mice (P < 0.01) (Fig. 4). The relative level of iso-A2E was 12% (P < 0.01). Note that a more pronounced reduction in iso-A2E was consistently observed (Figs. 2, 3, 4), even across extractions and analyses carried out by two investigators (S.K. and N.F.). We had observed that when solutions of A2E or iso-A2E in methanol are exposed to room light, isomerization occurs such that the resulting mixtures contain an ≈4:1 ratio of A2E/iso-A2E (28). In the current experiments, we observed considerably less iso-A2E than would be expected under conditions of photoequilibrium. The explanation for this difference is unclear at this time.
Fig. 4.
Quantitation of A2E and iso-A2E in eye cups of BALB/cByJ and C57BL/6J-c2J mice obtained as retired breeders. Levels were determined as integrated peak areas normalized to an external standard of A2E. Values (mean ± SEM) are expressed as picomoles per eye; four experiments with 8–23 eyes per group in each experiment. *, P < 0.01, unpaired Student's t test.
Discussion
We have shown that the levels of the RPE lipofuscin fluorophores A2E and iso-A2E are lower in C57BL/6J-c2J mice that have the methionine variant at position 450 of Rpe65 as compared with BALB/cByJ mice in which the amino acid residue is leucine. The same leucine-to-methionine substitution also confers a diminished tendency toward A2E/iso-A2E formation in Abcr–/– and Abcr+/+ mice. Whether the comparison was made between C57BL/6J-c2J and BALB/cByJ mice or between Leu-450 and Met-450 in the Abcr–/– and Abcr+/+ mice, the levels of A2E in the presence of the methionine variant were consistently observed to be 25–30% of that occurring with the leucine variant. The difference in the quantity of A2E occurred in association with the Rpe65 Leu450Met polymorphism in pigmented and albino mice and in mice of varying ages. Interestingly, isotretinoin (13-cis-retinoic acid) reduces A2E deposition in RPE cells of Abcr–/– mice by a similar magnitude (26). Although isotretinoin was suggested to act, at least partially, by inhibiting 11-cis-retinol dehydrogenase (25, 26, 30), slowing of the visual cycle at the stage involving RPE65 can clearly provide protection against A2E formation.
In this study we relied, in part, on the use of two strains of mice (BALB/cByJ and C57BL/6J-c2J) that were used previously to analyze genotype in relation to susceptibility to prolonged light exposure (20). By using progeny of a backcross between BALB/cByJ and C57BL/6J-c2J, it was found that a trait that accounted for ≈50% of the protective effect against light-induced retinal degeneration cosegregated with the Rpe65 gene on chromosome 3. Gene sequencing disclosed that C57BL/6J-c2J mice had an ATG (methionine) at codon 450 and BALB/cByJ mice had a CTG (leucine). Subsequent biochemical studies aimed at comparing the two strains of mice revealed that methionine at position 450 conferred a slowing of the rate of rhodopsin regeneration, whereas electroretinographic recording demonstrated a lower intrinsic gain within rod photoreceptors (21, 23). The difference in A2E levels measured in mice with the Leu-450 vs. Met-450 variant in the present work reflects a methionine-associated reduction of 70–75%. It is notable that a study of BALB/cByJ vs. C57BL/6J-c2J mice reported that the rate of rhodopsin regeneration in the presence of Met-450 was decreased by a similar magnitude (75%) (23). Within the setting of this earlier work implicating RPE65 as having a rate-determining role in the visual cycle, it is likely that the reduced flux of all-trans-retinal that accompanies slowing of the visual cycle in the presence of the methionine variant in RPE65 is responsible for the decreased formation in A2E that we observed in the C57BL/6J-c2J mice. This notion is supported by our studies with Abcr–/– and Abcr+/+ mice of a mixed background (129/Sv × C57BL/6), wherein the RPE65-leucine-450 variant is derived from the 129/Sv embryonic stem cell donor and RPE65-methionine-450 is derived from the C57BL/6 host. In both Abcr–/– and Abcr+/+ mice homozygous for the RPE65-methionine-450 allele, A2E levels were depressed.
Of the mouse strains studied, only C57BL/6 mice or mouse lines derived from this strain (23) have been reported to carry the methionine variant. Although RPE65 protein levels are reported to be higher in some strains of rats than others, these differences did not correlate with sequence variations in RPE65 (31). Exactly how the leucine-to-methionine variant affects the activity of murine Rpe65 is not known. Rpe65 mRNA expression is not different in the C57BL/6J-c2J mice as compared with the BALB/cByJ mice, although the protein is present at higher levels in BALB/cByJ than in C57BL/6J-c2J (6, 23). Perhaps, therefore, with methionine as amino acid 450, protein stability is reduced. Alternatively, methionine at this position may alter the affinity with which retinyl esters bind to Rpe65. It has been recently demonstrated that palmitoylation of sRPE65 (soluble RPE65) generates mRPE65 (membrane-associated RPE65), the form that binds and mobilizes retinyl esters (32). Thus, perhaps the methionine residue at position 450 interferes with palmitoylation, thereby slowing the flow of retinyl esters to the IMH that converts them to 11-cis-retinol.
A2E and its isomers are the best characterized of the lipofuscin fluorophores, although a condensation product of two molecules of all-trans-retinal, all-trans-retinal dimer (ATR dimer) (18), has been described and others are suspected (33). Because of its unusual pyridinium bisretinoid structure (34), A2E cannot be enzymatically degraded and thus accumulates. The amassing of A2E by RPE cells is particularly substantial in mice with null mutations in one or both alleles of Abcr (Abca4) (5, 27, 29), the gene responsible for Stargardt disease in humans (35). At sufficient concentrations, A2E perturbs cell membranes (36–39), confers a susceptibility to blue light-induced apoptosis (40–43), and alters lysosomal function (44, 45). In light of this adverse behavior, there is considerable interest in retarding A2E formation as a means to prevent vision loss in Stargardt disease and, perhaps, age-related macular degeneration (24). It is thus significant that studies have shown that RPE lipofuscin is substantially reduced when the 11-cis and all-trans-retinal chromophores are absent because of either dietary deficiency or gene knockout (15, 46). Light exposure, an obvious determinant of the rate of flux of all-trans-retinal through the visual cycle, can also moderate the rate of A2E synthesis (5, 18, 47). Additionally, Radu et al. (26) reported that isotretinoin (13-cis-retinoic acid), an acne medication (Accutane, Roche Laboratories, Nutley, NJ) known previously to delay dark adaptation (25), dampens the deposition of A2E in RPE cells. Our data demonstrate that controlling for other genetic variants in the Abcr–/– and Abcr+/+ mice, such as the Rpe65 Leu450Met polymorphism, is needed to accurately estimate the therapeutic effect. Although 13-cis-retinoic acid itself has severe side effects, an alternate therapeutic strategy could involve the use of synthetic analogs. Compounds that compete with retinyl esters for binding to RPE65 would have particular therapeutic efficacy.
Acknowledgments
We thank Illar Pata for expert technical assistance. This work was supported by National Institutes of Health Grants EY12951 (to J.R.S.), GM34509 (to K.N.), and EY13435 (to R.A.), the American Health Assistance Foundation (J.R.S.), the Foundation Fighting Blindness (R.A.), National Institutes of Health Vision Training Grant EY139933 (to N.F.), and unrestricted funds from Research to Prevent Blindness to the Department of Ophthalmology.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: RPE, retinal pigment epithelium.
∥Fishkin, N. E., Pescitelli, G., Itagaki, Y., Berova, N., Allikmets, R., Nakanishi, K. & Sparrow, J. R. (2004) Invest. Ophthalmol. Visual Sci. 45, E-abstract 1803.
References
- 1.Rando, R. R. (2001) Chem. Rev. (Washington, D.C.) 101, 1881–1896. [DOI] [PubMed] [Google Scholar]
- 2.Hamel, C. P., Tsilou, E., Pfeffer, B. A., Hooks, J. J., Detrick, B. & Redmond, T. M. (1993) J. Biol. Chem. 268, 15751–15757. [PubMed] [Google Scholar]
- 3.Gollapalli, D. R., Maiti, P. & Rando, R. R. (2003) Biochemistry 42, 11824–11830. [DOI] [PubMed] [Google Scholar]
- 4.Jahng, W. J., David, C., Nesnas, N., Nakanishi, K. & Rando, R. R. (2003) Biochemistry 42, 6159–6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mata, N. L., Weng, J. & Travis, G. H. (2000) Proc. Natl. Acad. Sci. USA 97, 7154–7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moiseyev, G., Crouch, R. K., Goletz, P., Oatis, J., Redmond, T. M. & Ma, J.-X. (2003) Biochemistry 42, 2229–2238. [DOI] [PubMed] [Google Scholar]
- 7.Gu, S. M., Thompson, D. A., Srikumari, C. R., Lorenz, B., Finckh, U., Nicoletti, A., Murthy, K. R., Rathmann, M., Kumaramanickavel, G., Denton, M. J. & Gal, A. (1997) Nat. Genet. 17, 194–197. [DOI] [PubMed] [Google Scholar]
- 8.Marlhens, F., Bareil, C., Griffoin, J. M., Zrenner, E., Amalric, P., Eliaou, C., Liu, S. Y., Harris, E., Redmond, T. M., Arnaud, B., et al. (1997) Nat. Genet. 17, 139–141. [DOI] [PubMed] [Google Scholar]
- 9.Thompson, D. A. & Gal, A. (2003) Prog. Retin. Eye Res. 22, 683–703. [DOI] [PubMed] [Google Scholar]
- 10.Cremers, F. P., Van Den Hurk, J. A. & Den Hollander, A. I. (2002) Hum. Mol. Genet. 11, 1169–1176. [DOI] [PubMed] [Google Scholar]
- 11.Redmond, T. M., Yu, S., Lee, E., Bok, D., Hamasaki, D., Chen, N., Goletz, P., Ma, J.-X., Crouch, R. K. & Pfeifer, K. (1998) Nat. Genet. 20, 344–351. [DOI] [PubMed] [Google Scholar]
- 12.Narfstrom, K., Katz, M. L., Bragadottir, R., Seeliger, M., Boulanger, A., Redmond, T. M., Caro, L., Lai, C. M. & Rakoczy, P. E. (2003) Invest. Ophthalmol. Visual Sci. 44, 1663–1672. [DOI] [PubMed] [Google Scholar]
- 13.Acland, G. M., Aguirre, G. D., Ray, J., Zhang, Q., Aleman, T. S., Cideciyan, A. V., Pearce-Kelling, S. E., Anand, V., Zeng, Y., Maguire, A. M., et al. (2001) Nat. Genet. 28, 92–95. [DOI] [PubMed] [Google Scholar]
- 14.Dejneka, N. S., Surace, E. M., Aleman, T. S., Cideciyan, A. V., Lyubarsky, A., Savchenko, A., Redmond, T. M., Tang, W., Wei, Z., Rex, T. S., et al. (2004) Mol. Ther. 9, 182–188. [DOI] [PubMed] [Google Scholar]
- 15.Katz, M. L. & Redmond, T. M. (2001) Invest. Ophthalmol. Visual Sci. 42, 3023–3030. [PubMed] [Google Scholar]
- 16.Eldred, G. E. & Lasky, M. R. (1993) Nature 361, 724–726. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Shabat, S., Itagaki, Y., Jockusch, S., Sparrow, J. R., Turro, N. J. & Nakanishi, K. (2002) Angew. Chem. Int. Ed. 41, 814–817. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Shabat, S., Parish, C. A., Vollmer, H. R., Itagaki, Y., Fishkin, N., Nakanishi, K. & Sparrow, J. R. (2002) J. Biol. Chem. 277, 7183–7190. [DOI] [PubMed] [Google Scholar]
- 19.Liu, J., Itagaki, Y., Ben-Shabat, S., Nakanishi, K. & Sparrow, J. R. (2000) J. Biol. Chem. 275, 29354–29360. [DOI] [PubMed] [Google Scholar]
- 20.Danciger, M., Matthes, M. T., Yasamura, D., Akhmedov, N. B., Rickabaugh, T., Gentleman, S., Redmond, T. M., La Vail, M. M. & Farber, D. B. (2000) Mamm. Genome 11, 422–427. [DOI] [PubMed] [Google Scholar]
- 21.Nusinowitz, S., Nguyen, L., Radu, R. A., Kashani, Z., Farber, D. B. & Danciger, M. (2003) Exp. Eye Res. 77, 627–638. [DOI] [PubMed] [Google Scholar]
- 22.Wenzel, A., Grimm, C., Samardzija, M. & Reme, C. E. (2003) Invest. Ophthalmol. Visual Sci. 44, 2798–2802. [DOI] [PubMed] [Google Scholar]
- 23.Wenzel, A., Reme, C. E., Williams, T. P., Hafezi, F. & Grimm, C. (2001) J. Neurosci. 21, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sparrow, J. R. (2003) Proc. Natl. Acad. Sci. USA 100, 4353–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sieving, P. A., Chaudhry, P., Kondo, M., Provenzano, M., Wu, D., Carlson, T. J., Bush, R. A. & Thompson, D. A. (2001) Proc. Natl. Acad. Sci. USA 98, 1835–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radu, R. A., Mata, N. L., Nusinowitz, S., Liu, X., Sieving, P. A. & Travis, G. H. (2003) Proc. Natl. Acad. Sci. USA 100, 4742–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weng, J., Mata, N. L., Azarian, S. M., Tzekov, R. T., Birch, D. G. & Travis, G. H. (1999) Cell 98, 13–23. [DOI] [PubMed] [Google Scholar]
- 28.Parish, C. A., Hashimoto, M., Nakanishi, K., Dillon, J. & Sparrow, J. R. (1998) Proc. Natl. Acad. Sci. USA 95, 14609–14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mata, N. L., Tzekov, R. T., Liu, X., Weng, J., Birch, D. G. & Travis, G. H. (2001) Invest. Ophthalmol. Visual Sci. 42, 1685–1690. [PubMed] [Google Scholar]
- 30.Gamble, M. V., Mata, N. L., Tsin, A. T., Mertz, J. R. & Blaner, W. S. (2000) Biochim. Biophys. Acta 1476, 3–8. [DOI] [PubMed] [Google Scholar]
- 31.Iseli, H.-P., Wenzel, A., Hafezi, F., Reme, C. E. & Grimm, C. (2002) Exp. Eye Res. 75, 407–413. [PubMed] [Google Scholar]
- 32.Xue, L., Gollapalli, D. R., Maiti, P., Jahng, W. J. & Rando, R. R. (2004) Cell 117, 761–771. [DOI] [PubMed] [Google Scholar]
- 33.Fishkin, N., Jang, Y. P., Itagaki, Y., Sparrow, J. R. & Nakanishi, K. (2003) Org. Biomol. Chem. 1, 1101–1105. [DOI] [PubMed] [Google Scholar]
- 34.Sakai, N., Decatur, J., Nakanishi, K. & Eldred, G. E. (1996) J. Am. Chem. Soc. 118, 1559–1560. [Google Scholar]
- 35.Allikmets, R., Shroyer, N. F., Singh, N., Seddon, J. M., Lewis, R. A., Bernstein, P. S., Peiffer, A., Zabriskie, N. A., Li, Y., Hutchinson, A., et al. (1997) Science 277, 1805–1807. [DOI] [PubMed] [Google Scholar]
- 36.Sparrow, J. R., Fishkin, N., Zhou, J., Cai, B., Jang, Y. P., Krane, S., Itagaki, Y. & Nakanishi, K. (2003) Vision Res. 43, 2983–2990. [DOI] [PubMed] [Google Scholar]
- 37.Sparrow, J. R., Parish, C. A., Hashimoto, M. & Nakanishi, K. (1999) Invest. Ophthalmol. Visual Sci. 40, 2988–2995. [PubMed] [Google Scholar]
- 38.De, S. & Sakmar, T. P. (2002) J. Gen. Physiol. 120, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suter, M., Reme, C. E., Grimm, C., Wenzel, A., Jaattela, M., Esser, P., Kociok, N., Leist, M. & Richter, C. (2000) J. Biol. Chem. 275, 39625–39630. [DOI] [PubMed] [Google Scholar]
- 40.Sparrow, J. R., Nakanishi, K. & Parish, C. A. (2000) Invest. Ophthalmol. Visual Sci. 41, 1981–1989. [PubMed] [Google Scholar]
- 41.Schutt, F., Davies, S., Kopitz, J., Holz, F. G. & Boulton, M. E. (2000) Invest. Ophthalmol. Visual Sci. 41, 2303–2308. [PubMed] [Google Scholar]
- 42.Sparrow, J. R. & Cai, B. (2001) Invest. Ophthalmol. Visual Sci. 42, 1356–1362. [PubMed] [Google Scholar]
- 43.Sparrow, J. R., Cai, B., Fishkin, N., Jang, Y. P., Krane, S., Vollmer, H. R., Zhou, J. & Nakanishi, K. (2003) in Retinal Degenerations: Mechanisms and Experimental Therapy, eds. LaVail, M. M., Hollyfield, J. G. & Anderson, R. E. (Kluwer Academic/Plenum, New York), pp. 205–211.
- 44.Holz, F. G., Schutt, F., Kopitz, J., Eldred, G. E., Kruse, F. E., Volcker, H. E. & Cantz, M. (1999) Invest. Ophthalmol. Visual Sci. 40, 737–743. [PubMed] [Google Scholar]
- 45.Finneman, S. C., Leung, L. W. & Rodriguez-Boulan, E. (2002) Proc. Natl. Acad. Sci. USA 99, 3842–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katz, M. L., Norberg, M. & Stientjes, H. J. (1992) Invest. Ophthalmol. Visual Sci. 33, 2612–2618. [PubMed] [Google Scholar]
- 47.Radu, R. A., Mata, N. L., Bagla, A. & Travis, G. H. (2004) Proc. Natl. Acad. Sci. USA 101, 5928–5933. [DOI] [PMC free article] [PubMed] [Google Scholar]