Abstract
The ketogenic diet (KD) is an effective therapy primarily used in pediatric patients whom are refractory to current anti-seizure medications. The mechanism of the KD is not completely understood, but is thought to involve anti-inflammatory and anti-oxidant processes. The nutritionally-regulated transcription factor peroxisome proliferator activated receptor gamma, PPARγ, regulates genes involved in anti-inflammatory and anti-oxidant pathways. Moreover, endogenous ligands of PPARγ include fatty acids suggesting a potential role in the effects of the KD. Here, we tested the hypothesis that PPARγ contributes to the anti-seizure efficacy of the KD. We found that the KD increased nuclear protein content of the PPARγ2 splice variant by 2–4 fold (p < 0.05) in brain homogenates from wild-type (WT) and epileptic Kv1.1 knockout (KO) mice, while not affecting PPARγ1. The KD reduced the frequency of seizures in Kv1.1KO mice by ~70% (p < 0.01). GW9662, a PPARγ antagonist, prevented KD-mediated changes in PPARγ2 expression and prevented the anti-seizure efficacy of the KD in Kv1.1KO mice. Further supporting the association of PPARγ2 in mediating KD actions, the KD significantly prolonged the latency to flurothyl-induced seizure in WT mice by ~20–35% (p < 0.01), but was ineffective in PPARγ2KO mice and neuron-specific PPARγKO mice. Finally, administering the PPARγ agonist pioglitazone increased PPARγ2 expression by 2-fold (p < 0.01) and reduced seizures in Kv1.1KO mice by ~80% (p < 0.01). Our findings implicate brain PPARγ2 among the mechanisms by which the KD reduces seizures and strongly support the development of PPARγ2 as a therapeutic target for severe, refractory epilepsy.
Keywords: Epilepsy, peroxisome proliferator activated receptor, ketogenic diet, nutrition, seizure, Kv1.1, Kcna1, PPARgamma2
INTRODUCTION
Approximately 30% of people with epilepsy do not achieve adequate seizure control with current anti-seizure drugs. The high fat, low carbohydrate ketogenic diet (KD) is an highly effective therapeutic option for this non-responsive population completely abolishing seizures in 7–10% of patients and reducing seizure frequency by >50% in two-thirds of patients (Freeman et al., 2006). Due to the strict regimen of the KD, it is primarily prescribed to pediatric patients, but is effective in adult epilepsies (Stafstrom and Rho, 2012). Despite compliance issues in adults, the KD is currently under investigation as treatment for other neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and Amyotrophic Lateral Sclerosis (Stafstrom and Rho, 2012). Although the mechanisms of KD anti-seizure efficacy are not completely understood, the KD has been demonstrated to involve disease-modifying pathways including central anti-inflammatory and anti-oxidant pathways and those that improve mitochondrial function (Masino and Rho, 2012).
In the periphery, the KD is thought to engage peroxisome proliferator activated receptors (PPAR), type II nuclear transcription factors that regulate lipid and energy metabolism (Masino and Rho, 2012; Cullingford, 2004). Interestingly, one PPAR isoform, PPARγ, is not only a master regulator of adipogenesis, lipid metabolism and insulin sensitivity (Medina-Gomez et al., 2007; Medina-Gomez and Vidal-Puig, 2007), but also regulates anti-inflammatory, anti-oxidant and mitochondrial genes similar to the KD (Mandrekar-Colucci et al., 2013, Fong et al., 2010; Bernardo and Minghetti, 2006). Moreover, there is significant interest in the potential therapeutic applications of PPARγ agonist-mediated neuroprotection in diverse neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and Amyotrophic Lateral Sclerosis (Heneka and Landreth, 2007). Interest in PPARγ in the epilepsy community has had a late and slow start with the first research study appeared in 2006 and the total count to date is a little over a dozen (Okada et al., 2006; Luna-Medina et al., 2007; Maurois et al., 2008; Yu et al., 2008; Hong et al., 2008, 2011, 2013; Abdallah, 2010; Han et al., 2011; Jeong et al., 2011; Adabi Mohazab et al., 2012; Chuang et al., 2012; Hughes et al., 2014; Boes et al., 2015). These studies span acute seizure models, post-status epilepticus (SE) models, kindling and, for the most part, consistently support beneficial neuroprotective and anti-seizure effects of PPARγ agonists in epilepsy.
PPARγ activation is initiated by ligand binding. The PPARγ ligand binding pocket is large (1300 angstroms3) which allows structural promiscuity for a wide variety of endogenous or natural agonists (i.e. unsaturated fatty acids, eicosanoids, oxidized lipids, nitroalkenes), synthetic agonists (e.g. thiazolidinediones which are clinically useful in treatment of Type II Diabetes Mellitus) and synthetic anatagonists (currently there are no known endogenous antagonists) (Itoh et al., 2008; Fong et al., 2010; Kroker and Bruning, 2015; Sauer, 2015). The nature of the large ligand binding pocket makes PPARγ an effective sensor and transducer of environmental nutritional and inflammatory states. There are two PPARγ isoforms, PPARγ1 and PPARγ2, which result from alternative splicing and differential promoter use (Tonotonoz et al., 1994). The isoforms are identical except that PPARγ2 contains an additional 30 amino acids at its N-terminus that convey a 5–10 fold more effective ligand-independent transactivation and increased ligand binding affinity to the ligand-binding domain relative to PPARγ1 (Werman et al., 1997; Shao et al., 1998; Castillo et al., 1999; Bugge et al., 2009). The expression of PPARγ1 appears ubiquitous, whereas PPARγ2 is restricted to adipose tissue; however, high fat diets can induce the expression of PPARγ2 (Vidal-Puig et al., 1996, 1997).
A recent report suggests that KD-treatment of normal mice increases PPARγ in brain (Jeong et al., 2011); however, PPARγ isoforms were not distinguished and, more importantly, whether this change in PPARγ expression held importance for KD effects in an animal model of epilepsy was not determined. Here, using multiple genetic and pharmacologic tools we sought to determine whether (i) the KD regulates PPARγ isoforms in epileptic brain, (ii) such regulation contributes to KD anti-seizure efficacy, and (iii) activation of PPARγ alone attenuates spontaneous recurrent seizures.
2. MATERIALS AND METHODS
2.1. Animals
All mice were housed in the Animal Resource Facilities at Creighton University School of Medicine in a temperature (25°C)- and humidity (50–60%)-controlled and pathogen-free environment. Mice were given food and water ad libitum and kept on a 12-hour light/dark cycle. Heterozygous Kcna1-null mice on a C3HeB/FeJ congenic background were purchased from Jackson Laboratories (Bar Harbor, Maine) and bred to obtain Kv1.1 wild-type (WT) and Kv1.1 knockout (KO) littermates. Heterozygous Pparγ2-null mice on a mixed 129sv-C57Bl/6 background were provided by Gema Medina-Gomez (Universidad Rey Carlos, Madrid, Spain) (Medina-Gomez et al., 2005, 2007) and bred to obtain PPARγ2WT, PPARγ2Het and PPARγ2KO littermates. Tail clips were taken at postnatal day (P)10–P15 and sent to Transnetyx Inc. for genotyping (Cordova, TN, U.S.A.). Homozygous floxed Pparγfl/fl and homozygous neuronal-specific synapsin I-Cre+Pparγfl/lf knockout (Pparγfl/fl-NKO; Cre expression driven by synapsin I) mice were provided by J.M. Olefsky (University of California-San Diego) and M.W. Schwartz (University of Washington) and bred to provide control Pparγfl/fl mice and Pparγfl/fl-NKO mice (Lu et al., 2011). All procedures involving animals were in accordance with National Institutes of Health guidelines, the EU Directive 2010/63/EU and were approved by the Institutional Animal Care and Use Committees at Creighton University School of Medicine.
2.2. Dietary and pharmacological treatments
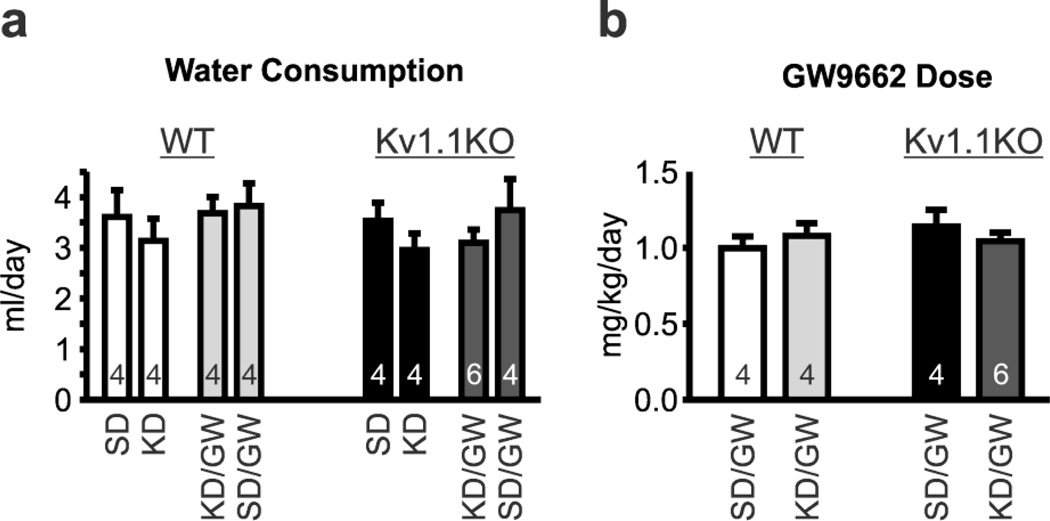
On P21, mice were randomly weaned onto either a standard diet (SD) or a KD (6.3:1, fat to carbohydrates plus proteins; Bio-Serv F3666, Frenchtown, NJ, U.S.A.) for 10–14 days. For dietary experiments involving singly housed Kv1.1WT and Kv1.1KO mice, drinking water contained either 0.0323% DMSO vehicle or the PPARγ antagonist GW9662 (2.68 mg/ml) to obtain an average dosage of 1.062±0.038 mg/kg/day (n=18) (Iwanami et al., 2010; Min et al., 2012). There was no difference in the average daily water consumption or resulting GW9662 dosage between experimental groups (Fig. 1). For experiments involving pioglitazone, on P32–33 Kv1.1KO mice were intraperitoneal-injected with saline vehicle for two days, followed by five days of daily pioglitazone injections (10 mg/kg/day, i.p., at 9:00AM) (Abdallah, 2010).
Figure 1.
(a) Water consumption during dietary treatments and administration of either vehicle or the PPARγ antagonist GW9662 in drinking water. (b) Calculated daily dosage of GW9662. Neither genotype nor treatment had a significant effect on water consumption (not shown) or resulting GW9662 dosage (n = 4–6 mice). Wild-type, WT; knockout, KO; ketogenic diet, KD; standard diet, SD.
2.3. Blood β -hydroxybutyrate and glucose measurements
β-hydroxybutyrate and glucose levels were measured every three days from blood samples collected from the tail vein of each mouse using a test strip system and reader (Precision Xtra Advance Diabetes Management System with Precision Xtra blood ketone test strips and blood glucose test strips; Abbott Diabetes Care Inc., Alameda, CA, U.S.A.).
2.4. Intracranial electroencephalography (iEEG) electrode implantation and seizure analysis
On ~P27, mice underwent surgical implantation of electrodes. Mice were maintained under isoflurane anesthesia and normothermic conditions. One ground (1.5mm anterior lamba, 1.5mm lateral) and two subdural (1mm posterior Bregma, 1.5mm bilateral) iEEG electrodes were implanted, secured and attached to a headmount. Following a 5–6 day recovery, seizures were monitored for forty-eight hours using a continuous infrared video surveillance system time-synced to an EEG recording system (Pinnacle Technology, Inc., Lawrence, KS, U.S.A) as we have described (Roundtree et al., 2016; Simeone et al., 2014a; Fenoglio-Simeone et al., 2009a,b). EEG recordings were acquired with a 250 Hz sampling rate and band-pass filtered between 0.5 and 40 Hz. During seizure monitoring, mice were single-housed in hexagon cages. EEG recordings were imported into Spike2 v6–7 software (Cambridge Electronic Design, Cambridge, England. U.K.) for initial seizure identification using time-frequency analysis. Subsequently, EEG seizures were confirmed using Sirenia software (Pinnacle Technology, Inc.) that time-synced EEG and video recordings and behavioral manifestations were manually verified by 2 blinded investigators. Incidence and severity of each seizure was scored for the first 15 min of each hour during the time period of highest seizure occurrence for Kv1.1KO mice (00:00–08:00) (Simeone et al., 2014a; Fenoglio-Simeone et al., 2009b). Seizure severity was scored using a modified Racine scale: 0-normal; 1-myoclonic jerk; 2-side-to-side head movement; 3-forelimb/hindlimb clonus, tail extension, a single rearing event; 4-continuous rearing and falling; 5-severe tonic-clonic seizures. To weigh the incidence and severity of seizures, seizure burden index (SBI) scores were calculated using the equation: SBI = [Σ(σiγi)]/ε, where σ indicates the severity; i indicates each stage of seizure (1–5); γ is the frequency; and ε indicates the total number of epochs scored as we have described (Simeone et al., 2014a; Roundtree et al., 2016; Simeone et al., 2016).
2.5. Immunofluorescent western blot
PPARγ is a type II nuclear receptor. As such, it is primarily located in the nucleus regardless of the presence or absence of ligand. Therefore, we determined relative PPARγ protein levels in nuclear extracts. Nuclei were isolated from whole brain homogenate using a nuclear extraction kit according to manufacturer instructions (AY2002; Affymetrix, Inc., Santa Clara, CA, U.S.A.). Nuclear extracts were mixed with Laemmli’s loading buffer (Bio-Rad, Hercules, CA, U.S.A.) containing beta-mercaptoethanol (Sigma-Aldrich, St. Louis MO, U.S.A.), heated to 99°C and run through precast PAGE gels (Bio-Rad). Proteins were transferred to Immobilon-FL PVDF membranes (EMD Millipore, Billerica, MA, U.S.A.), which were then blocked in Odyssey blocking buffer (OBB; Li-Cor Biosciences, Lincoln, NE, U.S.A.) and phosphate buffered saline (PBS) at a 1:1 ratio for one hour at room temperature (RT). Membranes were incubated with the following primaries overnight at 4°C: rabbit anti-PPARγ (1:400, 07–466, EMD Millipore, was used for Fig. 3 and Fig. 6; 1:1000, ABN1445, EMD Millipore, was used for Fig. 5; both antibodies recognize PPARγ1 at 52 kDa and PPARγ2 at 56 kDa) and mouse anti-β-actin (1:8,000; 926–42212, Li-Cor Biosciences). After PBS-T washes membranes were incubated in secondary antibodies for one hour at RT: goat anti-rabbit (1:5,000; 926–32221, Li-Cor Biosciences) and goat anti-mouse (1:20,000–1:40,000; 926–32210, Li-Cor Biosciences). Membranes were washed in PBS-T and rinsed in distilled water. Samples were run in duplicate on each gel. Densitometric analysis was conducted using images captured on an Odyssey FC (Licor, Lincoln, NE) and PPARγ protein signal was normalized to within well β-actin values. Averages of the duplicates were determined and values normalized to the WT-SD values within each gel.
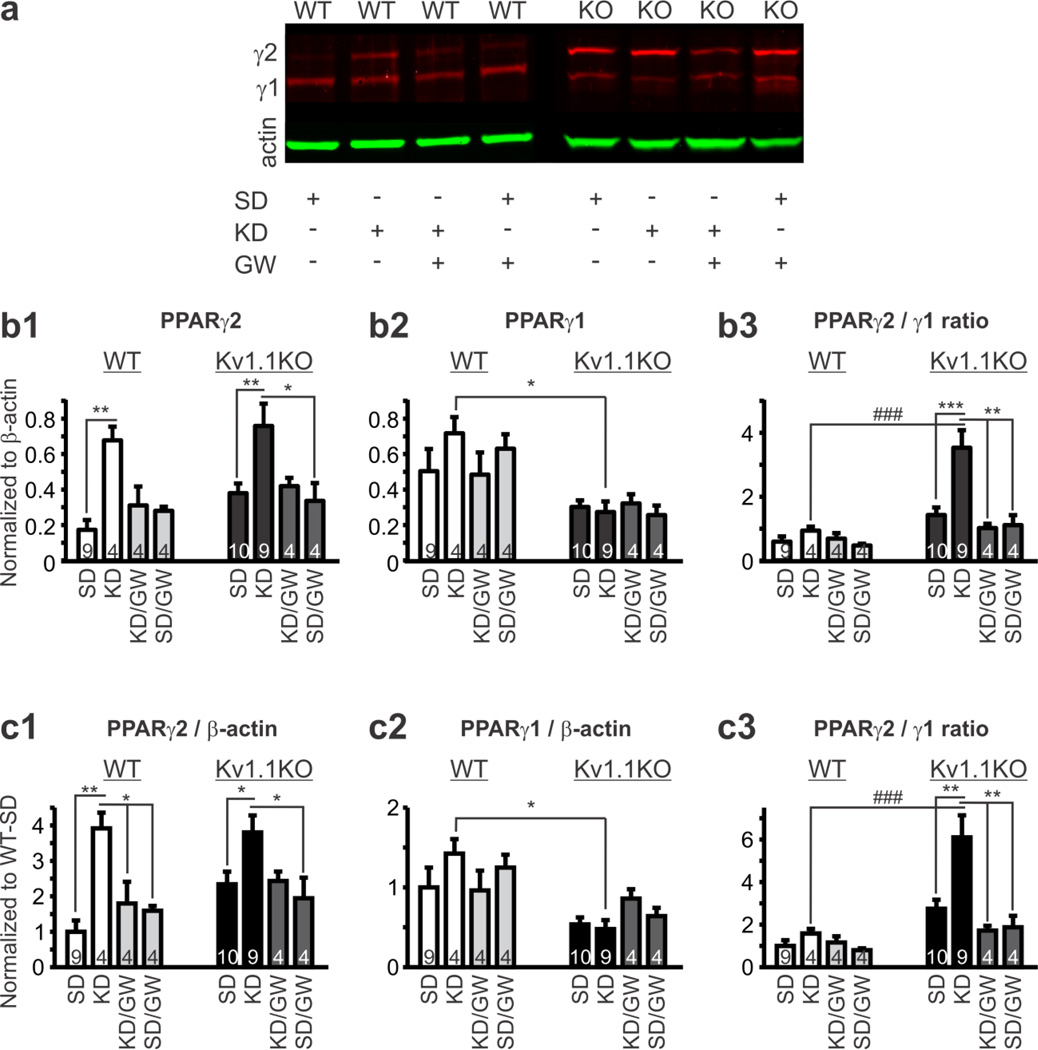
Figure 3.
Ketogenic Diet (KD) treatment increases nuclear content of PPARγ2 in brain tissue. Wild-type (WT) and Kv1.1KO mice were weaned onto both a standard diet (SD) or KD for 10-4 days and the drinking water contained either vehicle or the PPARγ antagonist GW9662. The mice were euthanized and brains were processed for western blot analysis. (a) Example western blots of the genotypes and treatment groups. (b) PPARγ1 and γ2 bands normalized to β-actin. (b1) PPARγ2/β-actin ratio (n = 4–10 mice; two-way ANOVA, interaction: F(3,40) = 0.224, P = 0.8792, treatment: F(3,40) = 8.555, P = 0.0002, genotype: F(1,40) = 2.627, P = 0.1129; Tukey’s multiple comparisons post-hoc test, *P < 0.05, **P < 0.001 as compared to mice fed an SD within genotype). (b2) PPARγ1/β-actin ratio (n = 4–10 mice; two-way ANOVA, interaction: F(3,40) = 0.9445, P = 0.4282, treatment: F(3,40) = 0.3968, P = 0.756, genotype: F(1,40) = 18.03, P = 0.0001; Tukey’s multiple comparisons post-hoc test, *P < 0.05 between mice fed a KD in each genotype). (b3) PPARγ2/ PPARγ1 ratio (n = 4–10 mice; two-way ANOVA, interaction: F(3,40) = 3.434, P = 0.0258, treatment: F(3,40) = 6.14, P = 0.0016, genotype: F(1,40) = 15.96, P = 0.0003; Tukey’s multiple comparisons post-hoc test, **P < 0.05, ***P < 0.001 as compared to mice fed an SD within genotype, ###P < 0.001 between mice fed a KD in each genotype). (c) PPARγ/β-actin ratios normalized to wild-type mice fed a SD. (c1) PPARγ2 (n = 4–10 mice; two-way ANOVA, interaction: F(3,40) = 0.7943, P = 0.5043, treatment: F(3,40) = 8.976, P = 0.0001, genotype: F(1,40) = 2.619, P = 0.1135; Tukey’s multiple comparisons post-hoc test, *P < 0.05, **P < 0.01 as compared to mice fed an SD within genotype). (c2) PPARγ1 (n = 4–10 mice; two-way ANOVA, interaction: F(3,40) = 1.582, P = 0.2088, treatment: F(3,40) = 0.3805, P = 0.7676, genotype: F(1,40) = 14.55, P = 0.0005; Tukey’s multiple comparisons post-hoc test, *P < 0.05 between mice fed a KD in each genotype). (c3) PPARγ2/ PPARγ1 ratio (n = 4–10 mice; two-way ANOVA, interaction: F(3,40) = 3.003, P = 0.0416, treatment: F(3,40) = 5.278, P = 0.0037, genotype: F(1,40) = 15.05, P = 0.0004; Tukey’s multiple comparisons post-hoc test, **P < 0.01, ***P < 0.001 as compared to mice fed an SD within genotype, ###P < 0.001 between mice fed a KD in each genotype). Numbers in each bar graph indicate the number of animals in each group.
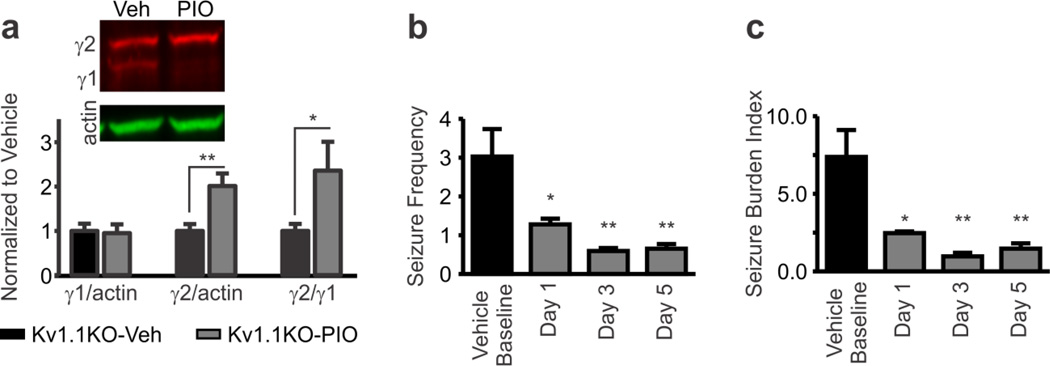
Figure 6.
PPARγ agonism increases brain PPARγ2 and decreases seizures. (a) Treatment with a PPARγ agonist pioglitazone increases nuclear content of PPARγ2 in Kv1.1KO brain tissue, but has no effect on PPARγ1; thus, increasing the PPARγ2/γ1 ratio. Mice (P35) were administered pioglitazone (PIO; 10 mg/kg/day, i.p., at 9:00AM) daily for six days. Three hours after the last injection brain cell nuclear extracts were analyzed for PPARγ by Western blot. *P < 0.05, **P < 0.01 (n = 10 control and 6 PIO mice; unpaired t-test). (b, c) Pioglitazone reduced the frequency of seizures and seizure burden index (a measure of seizure frequency and modified Racine scale severity; see Methods). On P32–33, mice were i.p.-injected with saline vehicle for two days, followed by five days of daily pioglitazone injections (10 mg/kg/day, i.p., at 9:00AM). *P < 0.05, **P < 0.01 as compared to vehicle (n = 4 mice; repeated measures one-way ANOVA with Tukey’s multiple comparisons post-hoc test).
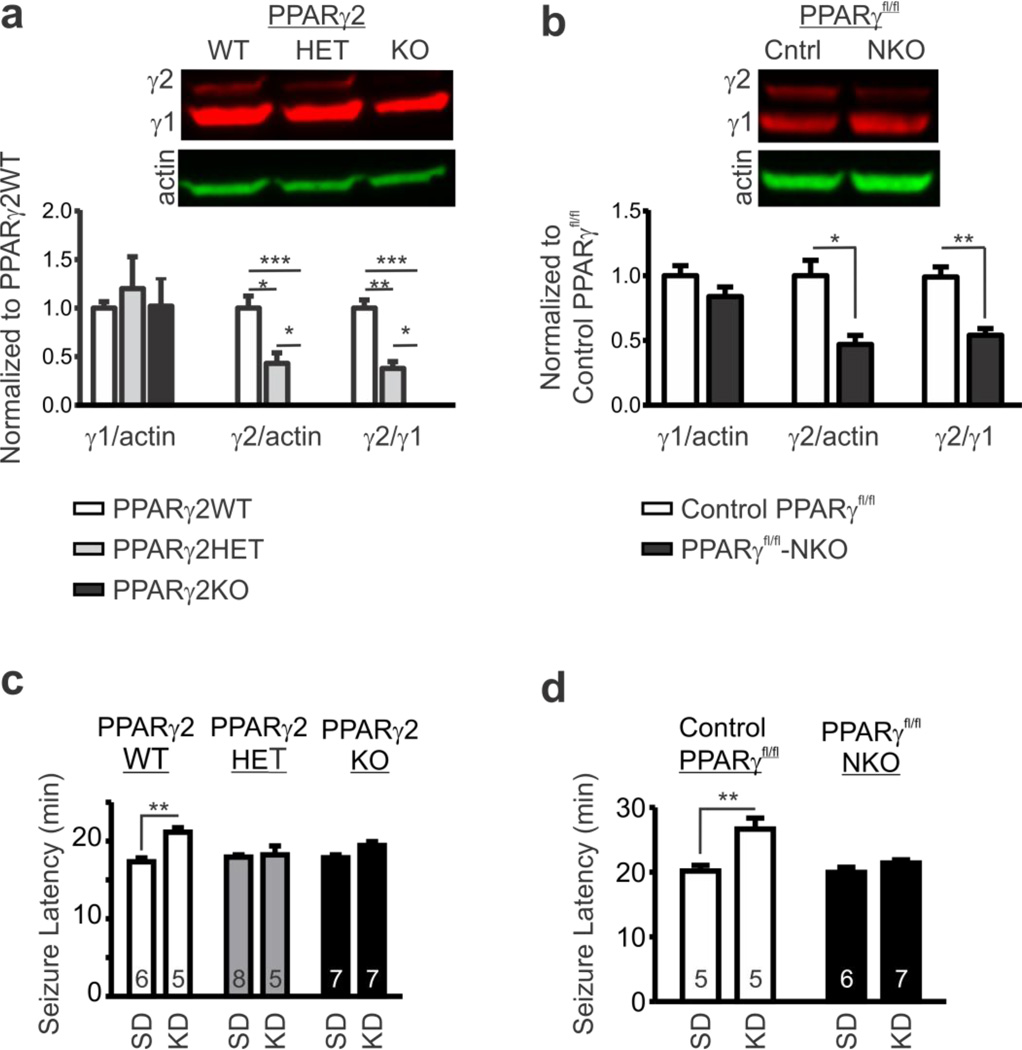
Figure 5.
Genetic knockout of PPARγ eliminates KD-mediated seizure protection. (a) Example western blot of nuclear extracts from brain homogenates of PPARγ2 wild-type (WT), heterozygous (HET) and knockout (KO) littermates and quantification of PPARγ1 and PPARγ2 bands normalized to β-actin and further normalized to PPARγ2WT (n = 3; one-way ANOVA with Tukey’s multiple comparisons post-hoc test, *P < 0.05, **P < 0.01, ***P < 0.001). (b) Example western blots of nuclear extracts from brain homogenates of floxed (fl/fl) control PPARγfl/fl mice and neuron-specific Synapsin I-Cre+ PPARγfl/fl knockout (NKO) and quantification of PPARγ1 and PPARγ2 bands normalized to β-actin and further normalized to control PPARγfl/fl (n = 3; unpaired t-test, *P < 0.05, **P < 0.01). (c) Latency to flurothyl-induced generalized tonic-clonic seizures of PPARγ2WT, HET and KO littermates fed a SD or KD (n = 5–8 mice; two-way ANOVA, interaction: F(2,32) = 4.728, P = 0.0159, treatment: F(1,32) = 16.97, P = 0.0003, genotype: F(1,32) = 1.997, P = 0.1523; Tukey’s multiple comparisons posthoc test, **P < 0.01 as compared to SD). (d) Latency to flurothyl-induced generalized tonicclonic seizures of control PPARγfl/fl mice and PPARγfl/fl-NKO mice fed a SD or KD (n = 5–7; two-way ANOVA, interaction: F(1,19) = 6.588, P = 0.0189, treatment: F(1,19) = 16.43, P = 0.0007, genotype: F(1,19) = 7.805, P = 0.0116; Tukey’s multiple comparisons post-hoc test, **P < 0.01 as compared to SD).
2.6. Immunoflourescent histochemistry
Mice were quickly anesthetized with isoflurane, decapitated, brains were removed and frozen in methyl-butane on dry ice. Sections affixed to slides were fixed in 4% paraformaldehyde in 0.1 M PB (pH 7.4), washed with 0.01 M PBS/0.3% Triton X-100, pH 7.4 (PBS-T) and blocked with 10% normal goat serum/PBS-T. Sections were incubated with rabbit anti-PPARγ (1:400, 07–466, EMD Millipore) overnight at room temperature followed by AF-594 conjugated goat anti-rabbit IgG (1:600, Invitrogen, Grand Island, NY, U.S.A.) for 3 h at room temperature after which sections were immediately cover-slipped with mounting media containing DAPI. Sections were imaged using an EVOS fluorescent imaging system outfitted with Texas Red, GFP and DAPI light cubes (Thermofischer Scientific, Waltham, MA, U.S.A.).
2.7. Flurothyl-induced Seizures
All experiments were performed in a fume hood. Mice (P32–36) were acclimated 1 h before testing. Mice were individually placed in a 2.7 L airtight glass chamber. A 10% solution (in 95% ethanol) of flurothyl (bis-2,2,2-trifluoroethyl ether; Sigma-Aldrich, St. Louis, MO, U.S.A.) was delivered by a syringe pump (KD Scientific, Holliston, MA, U.S.A.) at a constant rate of 0.05 ml/min and allowed to drip onto a Whatman grade 1 filter paper until the mouse reached a generalized tonic-clonic seizure with loss of posture (Araki et al., 2002). Seizure latency was measured from the first drop of flurothyl onto the filter paper to the onset of the generalized tonic-clonic seizure.
2.8. Reagents and Statistics
Unless otherwise specified, all reagents were purchased from Sigma-Aldrich. Statistical significance was determined with Prism6 software (Graphpad Software Inc., La Jolla, CA, U.S.A.) using unpaired t-test, one-way ANOVA or two-way ANOVA for genotype and treatment with an appropriate post hoc test where appropriate.
3. RESULTS
3.1. KD increases PPARγ2 in the brains of normal WT mice and epileptic Kv1.1KO mice
The Kv1.1KO mouse is a model of temporal lobe epilepsy that exhibits severe and frequent spontaneous recurrent seizures (SRS) (Simeone et al., 2013, 2014a,b; Fenoglio-Simeone et al., 2009a,b Kim et al., 2015; Roundtree et al., 2016). We have previously demonstrated that the KD is highly efficacious in Kv1.1KO mice attenuating seizures by 70–80% (Fenoglio-Simeone et al., 2009a; Kim et al., 2015). Here, we sought to determine whether PPARγ expression is changed in KD-treated control WT mice and epileptic Kv1.1KO mice.
Similar to previous studies (Lu et al., 2011; Moreno et al., 2004; Sarruf et al., 2009; Gahring et al., 2005), we found that PPARγ appears to be primarily located in the nucleus of cells in the principal layers of the hippocampus (Fig. 2). Because we were interested in whether there were differences in the amount of PPARγ protein readily available to exert transcriptional effects, we examined whether nuclear content of the two splice variants of PPARγ (γ1, γ2) in homogenates of mouse brain tissue differed between WT and Kv1.1KO littermates. The two PPARγ splice variants were differentially regulated between genotypes. In western blot experiments, we found that PPARγ1 predominated in WT brain (0.5 ± 0.13 WT v. 0.3 ± 0.04 KO, n = 9–10, p = 0.126, unpaired t-test), whereas PPARγ2 predominated in Kv1.1KO brain (0.17 ± 0.06 WT v. 0.38 ± 0.05 KO, p < 0.05, unpaired t-test) resulting in a significantly larger PPARγ2/γ1 ratio in the epileptic tissue (0.59 ± 0.16 WT v. 1.4 ± 0.24 KO, p < 0.05, unpaired ttest) (Fig. 3a).
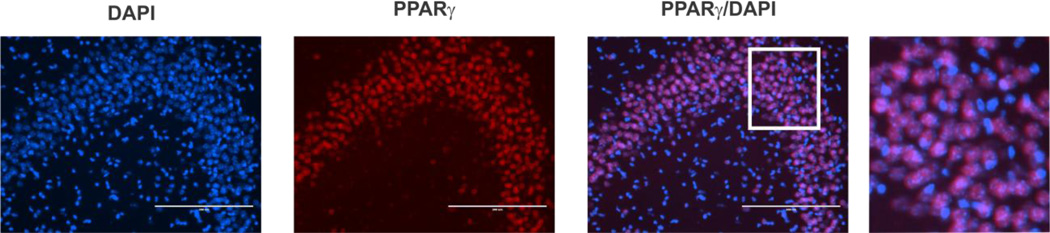
Figure 2.
PPARγ co-localizes with the nuclear stain DAPI in many cells of the hippocampal CA3 region. Scale bar = 200 µm. Right most picture is a digital magnification of the area within the white box.
Next, we determined whether KD-treatment affected nuclear PPARγ protein levels. A two week KD treatment selectively increased PPARγ2 2–4 fold in both genotypes, whereas PPARγ1 was unaffected (Fig. 3b1,b2,c1,c2). This effect of the KD was greater in WT where the 4-fold increase brought levels of PPARγ2 equal to those of Kv1.1KO mice treated with KD and resulted in a near doubling of the PPARγ2/γ1 ratio in both genotypes (Fig. 3b3). Further normalization to SD-treated WT values, revealed that even though KD-treatment increased PPARγ2 four-fold in both genotypes, the resulting PPARγ2/γ1 ratio was increased only ~58% in WT whereas it was increased to ~510% in Kv1.1KO (Fig. 3c3).
Previous studies have shown that, in a positive feed forward loop, PPARγ increases expression of itself and its coactivators such as PGC-1α (Fong et al., 2010). To determine whether the KD engages PPARγ itself to increase PPARγ2 we co-administrated the blood-brain-barrier permeable PPARγ antagonist GW9662 in the drinking water (~1 mg/kg/day; Fig. 1; Iwanami et al., 2010; Min et al., 2012). GW9662 prevented the KD-induced increase of PPARγ2 in both genotypes (Fig. 3b,c) suggesting that the KD increases PPARγ2 in a PPARγ-dependent manner.
3.2. Inhibition of PPARγ by GW9662 prevents KD attenuation of SRS in epileptic Kv1.1KO mice
We next determined whether PPARγ contributed to the anti-seizure efficacy of the KD using pharmacologic techniques. Mice were weaned onto either a standard diet (SD) or KD. On ~P27, mice underwent surgical implantation of subdural recording electrodes. On ~P33, continuous video-EEG recordings were obtained for forty-eight hours. Seizure frequency was quantified and further weighted for Racine scale severity to calculate a seizure burden index score for each mouse. A seizure burden index score provides an important consolidated metric to convey the effect of a given treatment on both the frequency and severity of seizures (Simeone et al 2014a; Barker-Haliski et al., 2015; Roundtree et al., 2016). Similar to our previous findings (Fenoglio-Simeone et al., 2009a,b Kim et al., 2015), KD treatment decreased seizure frequency and burden of Kv1.1KO mice by ~70% (Fig.4). GW9662 co-treatment prevented the KD from significantly reducing Kv1.1KO seizures (Fig. 4). These data suggest that PPARγ may be involved in the anti-seizure mechanisms of the KD.
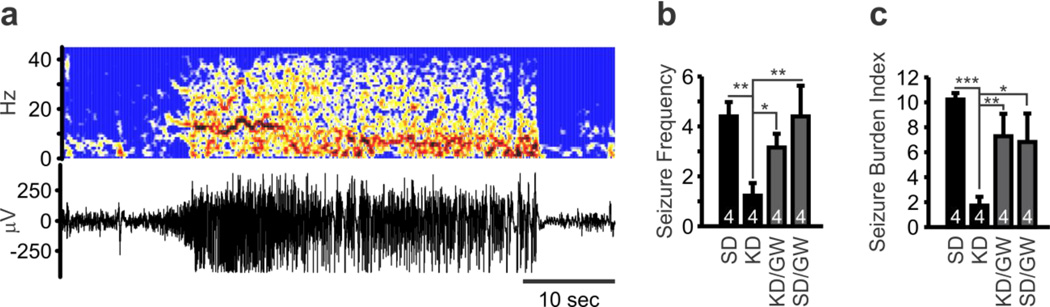
Figure 4.
PPARγ antagonism attenuates ketogenic diet (KD) reduction of seizures. Wild-type (WT) and Kv1.1KO mice were weaned onto both a standard diet (SD) or KD for 10-4 days and the drinking water contained either vehicle or the PPARγ antagonist GW9662. On P27, mice were outfitted with subdural electrodes. After 5–6 days recovery, video-EEG recordings were obtained for 48 consecutive hours. (a) Representative EEG recording and corresponding time-frequency map constructed with a short-time Fourier transform of a generalized tonic-clonic seizure (modified Racine Scale 5) in a Kv1.1KO mouse fed a SD. (b) KD-treatment reduced the frequency of seizures in Kv1.1KO mice. This effect was blocked by GW9662. No seizures were detected in WT mice regardless of treatment (not shown). (n = 4; two-way ANOVA, interaction: F(3,23) = 3.753, P = 0.025, treatment: F(3,23) = 3.753, P =0.025, genotype: F(1,23) = 71.45, P < 0.0001; Tukey’s multiple comparisons post-hoc test, **P < 0.01, *P < 0.001 as compared to Kv1.1KO mouse fed a SD). (c) Seizure burden index is a weighted measure of the frequency and Racine scale severity of seizures. KD-treatment reduced the seizure burden index in Kv1.1KO mice. This effect was blocked by GW9662. No seizures were detected in WT mice regardless of treatment (not shown). (n = 4; two-way ANOVA, interaction: F(3,23) = 4.986, P = 0.0083, treatment: F(3,23) = 4.986, P = 0.0083, genotype: F(1,23) = 68.96, P < 0.0001; Tukey’s multiple comparisons post-hoc test, **P < 0.01, *P < 0.001 as compared to Kv1.1KO mouse fed a SD).
3.3. Genetic loss of either PPARγ2 or neuronal PPARγ prevents KD-mediated increases of seizure threshold in non-epileptic mice
We further determined the contribution of PPARγ in the effects of KD on raising seizure thresholds using two genetic strains of mice: PPARγ2KO mice and neuron-specific PPARγ KO mice. Previous studies have demonstrated complete loss and partial loss of PPARγ2 in KO and heterozygous mice, respectively (Medina-Gomez et al., 2005), whereas neuron-specific Synapsin I-Cre+ PPARγfl/fl knockout (PPARγfl/fl-NKO) mice had up to 90% reduction of PPARγ mRNA in the hippocampus and other brain regions compared to PPARγfl/fl control mice (Lu et al., 2011). We found that PPARγ2 protein is absent and ~60% reduced in nuclear extracts of brain homogenates from our PPARγ2KO and PPARγ2HET mice, respectively, and found that PPARγ1 protein levels where similar to PPARγ2WT (Fig. 5a). Interestingly, PPARγ2 protein was also reduced by ~50% in nuclear extracts of brain homogenates from our Pparγfl/fl-NKO mice relative to PPARγfl/fl control mice, whereas the PPARγ1 protein was slightly, but non-significantly, decreased by ~16% (p = 0.21) (Fig. 5b). This was unexpected because Cre-mediated deletion of exons 1 and 2 in neurons of PPARγfl/fl-NKO mice is predicted to result in loss of PPARγ1 and a nonfunctional, N-terminal, 43 amino acid translational product of PPARγ2 that lacks part of the activation function 1, AF1, domain and the first zinc finger of the DNA binding domain (He et al., 2003). The greater reduction of PPARγ2 that we have observed may indicate that PPARγ2 has a more prominent role in neurons relative to PPARγ1.
To determine whether genetic loss of PPARγ would affect the KD, knockout mice and their control littermates were fed either a SD or KD for two weeks, at the end of which the latency to flurothyl-induced generalized tonic-clonic (GTC) seizures was measured. KD-treatment increased seizure latency of PPARγ2WT mice, but failed to protect heterozygous and PPARγ2KO littermates (Fig. 5c). KD-treatment was ineffective in Pparγfl/fl-NKO mice, whereas seizure latencies of PPARγfl/fl control mice fed a KD were significantly increased (Fig. 5d). These data suggest that PPARγ2 and neuronal PPARγ may play an important role for KD-mediated seizure protection.
Our data also indicates that inhibition of or loss of PPARγ does not unmask a convulsant mechanism. GW9662 treatment alone did not worsen Kv1.1KO seizures (Fig. 3b,c), nor did treatment induce spontaneous seizures in WT mice (n = 4, not shown). We also found that two weeks of GW9662 treatment did not influence seizure thresholds of WT mice exposed to the volatile convulsant flurothyl (n = 6, not shown). Furthermore, we did not observe spontaneous seizures in PPARγ2KO mice and Pparγfl/fl-NKO mice, and these mutant mice did not differ in flurothyl seizure threshold compared to control mice (Fig. 5c,d).
3.4. The PPARγ agonist pioglitazone increases brain PPARγ2 and attenuates SRS in epileptic Kv1.1KO mice
To determine whether activating PPARγ alone reduced seizure frequency and/or burden (to levels resembling the KD), Kv1.1KO mice were treated with pioglitazone, a PPARγ agonist and Type II Diabetes Mellitus therapeutic thiazolidinedione compound. Kv1.1KO mice were treated with saline vehicle for two days followed by five days of pioglitazone treatment (10 mg/kg/day, i.p.). Pioglitazone crosses the blood-brain-barrier and the 10 mg/kg/day dose has been used frequently in previous studies of neuroprotection (Abdallah, 2010, Adabi Mohazab et al., 2012, Grommes et al., 2013). Similar to the KD, pioglitazone increased PPARγ2 in Kv1.1KO brain homogenates by 2-fold relative to vehicle (Fig. 6a) and decreased seizure frequency and burden by ~80% (Fig. 6b,c). These results support that PPARγ activation is sufficient to reduce seizures in an animal model of severe epilepsy.
3.5. Loss of PPARγ does not alter KD effects on β-hydroxybutyrate, glucose or weight
Peripheral increases in blood ketone bodies (e.g., β-hydroxybutyrate) and/or reductions in blood glucose have been implicated in the mechanism of action of the KD (Masino and Rho, 2012). Therefore, we determined whether our pharmacologic or genetic manipulations of PPARγ attenuated the KD effects on peripheral ketone bodies and glucose. We measured blood concentrations of β-hydroxybutyrate and glucose in mice from all experimental groups. We found that antagonizing PPARγ with GW9662 did not affect KD modulation of concentrations of blood β-hydroxybutyrate, blood glucose, or body weight of Kv1.1KO or WT mice (Fig. 7a). Similarly, these parameters were not influenced by genetic loss of PPARγ2 (Fig. 7b) or neuronal PPARγ (Fig. 7c). Pharmacological treatment with the PPARγ agonist pioglitazone did not change blood β-hydroxybutyrate, glucose or body weight in either Kv1.1KO or WT mice on SD (Fig. 7d). These data suggest two conclusions. First, peripheral and central PPARγ are not involved in regulating blood ketone bodies or glucose in normal and epileptic mice fed an SD or KD. This finding is in agreement with studies demonstrating differential effects of pioglitazone on blood glucose of normal and diabetic rats, and no effect on ketone bodies (Ikeda et al., 1990; Larsen et al., 2003). Second, central PPARγ is an important, inducible anti-seizure mechanism in these mouse models.
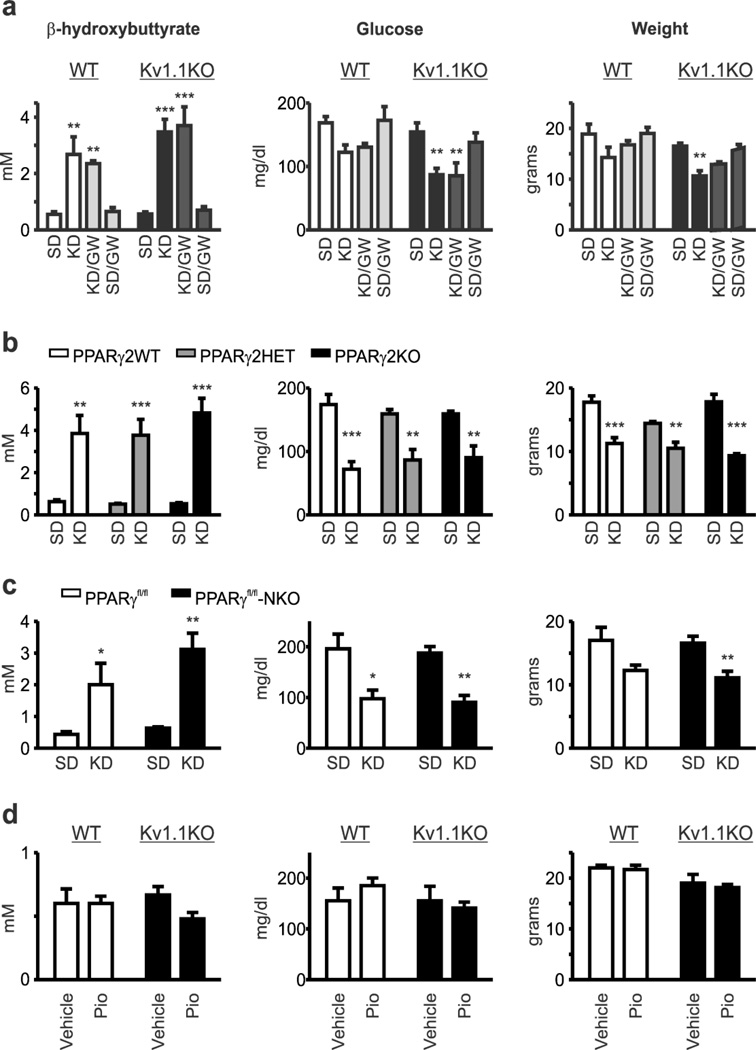
Figure 7.
Ketone bodies, glucose and weight are unaffected by PPARγ modulation regardless of genotype or dietary treatment. (a) Ketogenic diet (KD) treatment increases blood β-hydroxybutrate, decreases blood glucose and decreases weight of Kv1.1 wild-type (WT) and knockout (KO) mice to a similar degree. PPARγ antagonism with GW9662 did not change KD effects on these parameters. Reported values are from P35 mice that were weaned onto either a SD or KD with vehicle or GW9662 in the drinking water on P21 (n = 4–6). Blood β-hydroxybutrate (n = 4–6; two-way ANOVA, interaction: F(3,27) = 0.5033, P = 0.6832, treatment: F(3,27) = 20.82, P < 0.0001, genotype: F(1,27) = 1.759, P = 0.1959; Tukey’s multiple comparisons post-hoc test, *P < 0.05, **P < 0.01, ***P < 0.001 as compared to SD), blood glucose (n = 4–6; two-way ANOVA, interaction: F(3,27) = 0.3481, treatment: F(3,27) = 9.046, P = 0.0003, genotype: F(1,27) = 9.923, P = 0.004; Tukey’s multiple comparisons post-hoc test, **P < 0.01 as compared to SD) and body weight (n = 4–6; two-way ANOVA, interaction: F(3,27) = 0.1806, P = 0.9087, treatment: F(3,27) = 8.211, P = 0.0005, genotype: F(1,27) = 15.83, P = 0.0005; Tukey’s multiple comparisons post-hoc test, **P < 0.01 as compared to SD). (b) Genetic loss of PPARγ2 did not alter KD effects on blood β-hydroxybutrate, blood glucose or weight. Reported values are from P30 mice that were weaned onto either a SD or KD with vehicle or GW9662 in the drinking water on P21 (n = 4–9 mice). Blood β-hydroxybutrate (n = 4–9 mice; two-way ANOVA, interaction: F(2,28) = 0.7771, P = 0.4694, treatment: F(1,28) = 79.01, P < 0.0001, genotype: F(2,28) = 0.7421, P = 0.4853; Tukey’s multiple comparisons post-hoc test, **P < 0.01, ***P < 0.001 as compared to SD), blood glucose (n = 4–9 mice; two-way ANOVA, interaction: F(2,30) = 0.8746, P = 0.4274, treatment: F(1,30) = 57.43, P < 0.0001, genotype: F(2,30) = 0.01317, P = 0.9869; Tukey’s multiple comparisons post-hoc test, **P < 0.01, ***P < 0.001 as compared to SD) and weight (n = 4–9 mice; two-way ANOVA, interaction: F(2,29) = 4.813, P = 0.0157, treatment: F(1,29) = 90.6, P < 0.001, genotype: F(2,29) = 3.13, P = 0.0588; Tukey’s multiple comparisons post-hoc test, **P < 0.01, ***P < 0.001 as compared to SD). (c) Neuron-specific loss of PPARγ did not alter KD effects on blood β-hydroxybutrate, blood glucose or weight. Reported values are from P30 mice that were weaned onto either a SD or KD with vehicle or GW9662 in the drinking water on P21 (n = 3–11 mice). Blood β-hydroxybutrate (n = 3–9 mice; two-way ANOVA, interaction: F(1,20) = 0.3642, P = 0.553, treatment: F(1,20) = 20.91, P = 0.0002, genotype: F(1,20) = 1.043, P = 0.3193; Holm-Sidak’s multiple comparisons post-hoc test, *P < 0.05, ***P < 0.001 as compared to SD), blood glucose (n = 3–11 mice; two-way ANOVA, interaction: F(1,22) = 0.1269, P = 0.7251, treatment: F(1,22) = 27.96, P < 0.0001, genotype: F(1,22) = 0.0038, P = 0.9512; Tukey’s multiple comparisons post-hoc test, *P < 0.05, **P < 0.01 as compared to SD) and weight (n = 3–11 mice; two-way ANOVA, interaction: F(1,22) = 0.00181, P = 0.9665, treatment: F(1,22) = 12.21, P = 0.0021, genotype: F(1,22) = 0.109, P = 0.7444; Tukey’s multiple comparisons post-hoc test, **P < 0.01). (d) The PPARγ agonist pioglitazone (10 mg/kg/day, i.p., for five days) did not affect blood β-hydroxybutrate concentrations, blood glucose concentrations or weight of wild-type (WT) or Kv1.1 knockout (Kv1.1KO) mice as compared to vehicle injected mice (P40; n = 3–8).
4. DISCUSSION
In the present study, we used Western blot, immunohistochemistry and video-EEG in conjunction with in vivo pharmacologic and genetic manipulations to determine the contribution of the nutrient-sensitive transcription factor PPARγ to the anti-seizure efficacy of KD therapy. (i) We found that in the hippocampus PPARγ was primarily located in the nucleus of cells in pyramidal layers. (ii) Interestingly, the KD preferentially increases nuclear expression of the PPARγ2 splice variant in the brain of control and epileptic mice. This is reflected in an increase in the PPARγ2/γ1 splice variant ratio which is much more prominent in epileptic mice due to a concomitant decrease in PPARγ1. (iii) We found that KD treatment significantly reduces spontaneous recurrent seizures (SRS) of epileptic mice. This effect is abolished by co-administration of a PPARγ antagonist. (iv) Further, PPARγ antagonism prevents KD-mediated increases of nuclear PPARγ2 in the brain of both control and epileptic mice. (v) KD treatment increases the threshold of flurothyl-induced seizures in control mice. This effect is not present in mutant mice lacking PPARγ2 and mutant mice lacking neuronal PPARγ. (vi) Finally, administration of PPARγ agonist increases nuclear PPARγ2 in the brain of epileptic mice and significantly reduces SRS. (vii) Pharmacologic and genetic manipulation of PPARγ does not affect KD-mediated changes in weight, blood glucose or blood β-hydroxybutyrate suggesting that PPARγ does not achieve its effects by altering these biochemical consequences of the KD. This is the first study to systematically determine a role for PPARγ in the effects of the KD and provide evidence for the anti-seizure potential of PPARγ agonists in an animal model of chronic epilepsy. Collectively, our data implicate brain PPARγ2 among the mechanisms by which the KD reduces seizures.
4.1. Seizures increase brain PPARγ
Similar to findings in chronic neurodegenerative disorders and acute stroke models (Kitamura et al., 1999; Diab et al., 2002; Victor et al., 2006; Fong et al., 2010; Wang et al., 2012), the expression of brain PPARγ increases subsequent to SE induced by lithium-pilocarpine, unilateral intrahippocampal kainic acid, intraperitoneal injection of kainic acid and electrically-induced self-sustaining SE (Yu et al., 2008; Hong et al., 2008; Jeong et al., 2011; Chuang et al., 2012; Boes et al., 2015). Concomitantly, the products of the PPARγ-regulated genes Pgc-1alpha and Ucp2 are increased after lithium-pilocarpine and intrahippocampal kainic acid (Han et al., 2011; Chuang et al., 2012). In the current study, we examined the expression of both PPARγ splice variants, γ1 and γ2, in the brains of wild-type mice and epileptic Kv1.1KO. Similar to a previous report (Gahring et al 2005), we found that PPARγ1 predominated in wild-type brain. In contrast, in epileptic Kv1.1KO brain we found that PPARγ2 was the dominant form resulting in a PPARγ2/γ1 ratio that was increased three-fold in Kv1.1KO brains relative to WT.
Further studies are needed to determine the cause of this flip in isoform, but could involve isoform-specific altered nuclear translocation or gene expression. Potential causes that preferentially enhance nuclear translocation of PPARγ2 over PPARγ1 may be generation of isoform-specific ligands, altered expression of isoform-specific co-factors or post-translational modifications. Alternatively, transcription, translation or splicing of PPARγ2 could be increased in epilepsy. Recently, it was found that at the initial stage of adipogenesis another nuclear receptor, glucocorticoid receptor (GR), is transiently recruited along with the transcription factor C/EBPβ to a complex consisting of PBP/MED1/TRAP220 and p300 to enhancer regions of the Pparg2 isoform. In response to glucocortocoids, this results in a transient increase in H3K9 acetylation and enhances the induction of PPARγ2, which becomes the principal driver of adipogenesis (Steger et al., 2010). Intriguingly both C/EBPβ and glucocorticoids increase in the brain with seizures (Lu et al., 2013; Engel et al., 2013; Maguire and Salpekar, 2013). PGC-1α activation of PPARγ also enhances interactions with p300/CBP (Puigserver et al., 1999). Whether PPARγ2 regulates distinct gene sets is unclear, but it has been shown to upregulate catalase expression to a greater degree than PPARγ1 and is important in providing protection against lipotoxicity (Medina-Gomez et al., 2007a, b; Yakunin et al., 2014).
Assuming that the seizure/injury-induced changes in PPARγ expression are part of an endogenous neuroprotective mechanism that limits damage similar to that proposed for other neurodegenerative disorders (Kitamura et al., 1999; Diab et al., 2002; Victor et al., 2006; Fong et al., 2010; Wang et al., 2012), then loss of PPARγ should exacerbate markers of injury and possibly seizures. Chuang et al. (2012) found that pretreatment with bilateral focal injections of the PPARγ antagonist GW9662 reduces UCP2 and exacerbates intrahippocampal kainic acid SE-induced increases in ROS, oxidized proteins, mitochondrial Bax, cytosolic cytochrome C, DNA fragmentation and decreases in mitochondrial respiratory complex I (MRCI) activity. Unfortunately, the authors failed to report the details of SE, so it is not known whether PPARγ antagonism worsened the SE (Chuang et al., 2012). In contrast, in a similar study GW9662 did not worsen cell loss due to intrahippocampal kainic acid, but it did abrogate the protective effects of the PPARγ agonist NP031112 (Luna-Medina et al., 2007). Similarly, in the current study we administered GW9662 to epileptic Kv1.KO mice and WT littermates, however, contrary to expectations, inhibiting PPARγ did not increase SRS in Kv1.KO mice nor did it induce seizures or lower seizure threshold in WT mice. Furthermore, we also found that PPARγ2KO mice and neuron specific-PPARγ KO mice did not have spontaneous seizures. In fact their seizure thresholds were no different than control littermates. From these experimental results we can draw the tentative conclusion that the presumed seizure-induced increase in PPARγ may afford some neuroprotection against injury, but may not raise the seizure threshold per se.
4.2. PPARγ2 contributes to the anti-seizure effects of the KD
Although the possibility of a role for PPARγ in the mechanism of the ketogenic diet has been briefly speculated in review articles for the past several years (Kobow et al., 2012; Masino and Rho, 2012; Gano et al., 2014), until the current study there has only been one study published which experimentally explored this possibility (Jeong et al., 2011). Jeong et al. (2011) fed WT mice a KD for four weeks and found a three-fold increase of PPARγ protein in cell lysate from hippocampal tissue compared to SD-fed mice, but did not determine the importance of splice variants or whether PPARγ played an active role in the mechanism of the KD. In the current study, we aimed to determine whether the KD changes PPARγ expression in the brains of epileptic and normal mice; and, if so, whether it contributes to the anti-seizure effects of the KD. Treating epileptic Kv1.1KO mice for two weeks with a KD reduced seizures by ~70% as we have reported previously (Fenoglio-Simeone et al., 2009b; Kim et al. 2015; Simeone et al., 2016). KD-treatment increased PPARγ2 in both genotypes resulting in PPARγ2/γ1 ratios that were 2-fold and 6-fold higher for WT and Kv1.1KO brain compared to SD-fed WT mice. Co-administration of GW9662 prevented the increase in nuclear PPARγ2 and prevented KD-mediated seizure reduction in Kv1.1KO mice. Combined with the findings that GW9662 did not worsen seizures or lower seizure threshold, these results support our previous interpretation that PPARγ does not play a role in setting the endogenous seizure threshold, and that the effects of GW9662 on KD anti-seizure efficacy are not merely a result of unmasking a seizuregenic mechanism of inhibiting PPARγ. Rather, antagonism of PPARγ directly interferes with the anti-seizure mechanism of the KD. To further test the importance of PPARγ in the KD mechanism, we obtained PPARγ2KO mice and conditional neuron-specific PPARγ KO mice. We found that KD-treatment was unable to raise the seizure threshold of PPARγ2KO mice and conditional neuron-specific PPARγ KO mice.
Stereotypical biochemical consequences of KD treatment include ketone body production and lower glucose. Elegant studies have convincingly demonstrated that both ketone bodies and restricted glucose have anti-seizure effects. Ketone bodies may achieve seizure control via entering the TCA cycle and increasing ATP production, directly providing anti-oxidant capacity and reducing damaging ROS, inhibiting cyclophilin D induction of mitochondrial membrane permeability transition which preserves the calcium buffering capacity of mitochondria and prevents cell death, and by opening of KATP channels (Maalouf et al., 2007; Kim et al., 2007, 2015; Masino and Rho, 2012; Haces et al., 2008). Reduced glucose may increase seizure thresholds or dampen hyperexcitability by inducing ATP release which is dephosphorylated to adenosine activating adenosineA1 receptors and hyperpolarizing neuronal membranes by opening KATP channels (Kawamura 2010, 2014). In the current study, pharmacologic and genetic reduction of PPARγ expression/function did not affect the stereotypic KD increase of blood β-hydroxybutyrate or decrease of glucose indicating that (i) PPARγ does not play a role in these effects of the KD, (ii) the attenuation of KD anti-seizure efficacy is not an indirect effect of PPARγ on ketones or glucose and (iii) central actions of PPARγ, more specifically PPARγ2, contribute to the anti-seizure mechanism of the KD.
4.3. What does the KD provide that could be activating PPARγ?
The KD provides plenty of fat, and unsaturated fatty acids, such as omega-3 and omega-6 long chain polyunsaturated fatty acids, are notably increased in blood serum of patients (Fraser et al., 2003). Importantly, long chain polyunsaturated fatty acids and their metabolites, eicosanoids, oxidized lipids and nitroalkenes are all natural ligands for PPARγ (Yamamoto et al., 2005; Fong et al., 2010). Also, it was recently determined that the saturated fatty acid decanoic acid (a.k.a. capric acid), a primary constituent of the medium chain triglyceride ketogenic diet, is a ligand for PPARγ at physiologically relevant concentrations (Malapaka et al., 2012). In vivo treatment suggests that decanoic acid is a selective PPARγ modulator (i.e., partial agonist) as it improves glucose sensitivity and lipid profiles without weight gain in diabetic mice (Malapaka et al., 2012). In vitro experiments in hippocampal slices found that decanoic acid decreases PTZ-induced epileptiform activity in a concentration-dependent manner (Chang et al., 2013). Furthermore, decanoic acid-induced increases in citrate synthase, catalase and MRCI activity in SH-SY5Y neuronal cultures were inhibited by a PPARγ antagonist (Hughes et al., 2014). Alternatively, Jeong et al. (2011) found that treatment of cultured HT22 cells (a hippocampal neuronal cell line) with the ketone body acetoacetate (5 mM) increases PPARγ over a 12 hour period; however, thus far we have been unable to replicate this finding in primary hippocampal neuronal cultures (Simeone et al., unpublished observations). Therefore, at the moment it seems that the unsaturated and saturated fatty acids provided by a ketogenic diet may be the ligands for PPARγ, and that PPARγ may be involved in the anti-seizure mechanism of the various formulations of the ketogenic diet regardless of the type of fat content.
The selective increase of nuclear PPARγ2 over PPARγ1 may be due to the additional 30 amino acids in the PPARγ2 N-terminal transactivation domain that convey a 5–10 fold more effective ligand-independent transactivation and increased ligand binding affinity to the LBD relative to PPARγ1 (Werman et al., 1997; Shao et al., 1998; Castillo et al., 1999; Bugge et al., 2009). PPARγ2 is the only PPARγ isoform regulated at the transcriptional level by nutrition (Medina-Gomez et al., 2007a). The PPARγ2 expanded ligand-independent transactivation domain also confers differential interaction with transcriptional co-factors and post-translational modifications that would most likely result in tissue-specific differences in the regulation of gene sets by the PPARγ splice variants. This is evident in the periphery where PPARγ2, but not PPARγ1, is induced during high fat diets and initiates adipogenesis, increases lipid-buffering and reduces lipotoxicity (Medina-Gomez et al., 2007b). Therefore, the most likely mechanisms for this isoform-specificity of the KD probably involve either regulation of signaling cascades responsible for post-translational modifications that contribute to selective nuclear translocation or transcription of PPARγ2 and/or providing a PPARγ ligand, possibly selective for PPARγ2.
5. CONCLUSIONS
The results of this study clearly demonstrate that PPARγ plays an important role in the anti-seizure mechanism of the KD, one of the only non-surgical treatments for refractory epilepsy, and strongly support pursuing central PPARγ2 as a novel therapeutic target for refractory epilepsy. We further demonstrate that the PPARγ agonist and Type II Diabetes Mellitus drug pioglitazone increases PPARγ2 and effectively attenuates SRS of chronically epileptic mice. PPARγ agonists and the KD regulate similar anti-inflammatory, anti-oxidant and pro-mitochondrial pathways. These include, but are not limited to, upregulation of IκB, inhibition of NFκB, reduction of cytokines such as IL-1β, IL-6 and TNF-α, upregulation of genes encoding mitochondrial enzymes involved in oxidative phosphorylation (e.g., multiple subunits of complexes I, II, IV and V), induction of mitochondrial biogenesis and upregulation of UCP2, catalase and glutathione (Masino and Rho, 2012; Mandrekar-Colucci et al., 2013; Fong et al., 2010; Bernardo et al., 2006; Heneka and Landreth, 2007; Chuang et al., 2012; Hong et al., 2008, 2012, 2013; Abdallah, 2010; Adabi Mohazab et al., 2012; Bough et al., 2006; Miglio et al., 2009; Sullivan et al., 2004; Yang and Cheng, 2010; Yu et al., 2008). All of these have been suggested as possible disease modifying targets for epilepsy. Further studies are needed to identify the downstream mechanisms by which PPARγ attenuates seizures and determine how PPARγ contributes to the many other hypotheses of the KD (Masino and Rho, 2012). Our current findings in an epilepsy model, and the continuing investigations of KD and PPARγ agonist use in stroke, Alzheimer’s disease, Parkinson’s disease and ALS, suggest that PPARγ may also contribute to the KD effects in these neurodegenerative diseases.
Highlights.
The ketogenic diet increases PPARγ2 in the brains of epileptic mice.
Pharmacologic inhibition of PPARγ2 prevents ketogenic diet anti-seizure effects.
Genetic loss of PPARγ2 prevents ketogenic diet anti-seizure effects.
PPARγ agonism increases PPARγ2 and attenuates spontaneous recurrent seizures in epileptic mice.
Acknowledgments
Acknowledgement Statement
The authors thank G. Medina-Gomez (Universidad Rey Juan Carlos) for the kind gift of PPARγ2 heterozygous mice. We thank J.M. Olefsky (University of California-San Diego) and M.W. Schwartz (University of Washington) for the kind gifts of control PPARγfl/fl mice and Synapsin I-Cre+ PPARγfl/fl knockout mice. This work was supported by a Nebraska State LB692 grant (TAS), Epilepsy Foundation of America grant (TAS), Citizens United for Research in Epilepsy Foundation grant (TAS), NIH NS072179 (KAS) and NIH NS085389 (TAS). The project described was also supported by the National Center for Research Resources grant G20RR024001. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Please note, the last author has published under the names K. Dorenbos, K.A. Fenoglio, K.A. Fenoglio-Simeone and K.A. Simeone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adabi Mohazab R, Javadi-Paydar M, Delfan B, Dehpour AR. Possible involvement of PPAR-gamma receptor and nitric oxide pathway in the anticonvulsant effect of acute pioglitazone on pentylenetetrazole-induced seizures in mice. Epilepsy Res. 2012;101:28–35. doi: 10.1016/j.eplepsyres.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Abdallah DM. Anticonvulsant potential of the peroxisome proliferator-activated receptor γ agonist pioglitazone in pentylenetetrazole-induced acute seizures and kindling in mice. Brain Res. 2010;1351:246–253. doi: 10.1016/j.brainres.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Araki H, Kobayashi Y, Hashimoto Y, Futagami K, Kawasaki H, Gomita Y. Characteristics of flurothyl-induced seizures and the effect of antiepileptic drugs on flurothyl-induced seizures in Mongolian gerbils. Pharmacol. Biochem. Behav. 2002;74(1):141–147. doi: 10.1016/s0091-3057(02)00965-6. [DOI] [PubMed] [Google Scholar]
- Barker-Haliski ML, Dahle EJ, Heck TD, Pruess TH, Vanegas F, Wilcox KS, White HS. Evaluating an etiologically relevant platform for therapy development for temporal lobe epilepsy: effects of carbamazepine and valproic acid on acute seizures and chronic behavioral comorbidities in the Theiler's murine encephalomyelitis virus mouse model. J. Pharmacol. Exp. Ther. 2015;353(2):318–329. doi: 10.1124/jpet.114.222513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo A, Minghetti L. PPAR-gamma agonists as regulators of microglial activation and brain inflammation. Curr Pharm Des. 2006;12:93–109. doi: 10.2174/138161206780574579. [DOI] [PubMed] [Google Scholar]
- Boes K, Russmann V, Ongerth T, Licko T, Salvamoser JD, Siegl C, Potschka H. Expression regulation and targeting of the peroxisome proliferator-activated receptor γ following electrically-induced status epilepticus. Neurosci. Lett. 2015;604:151–156. doi: 10.1016/j.neulet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- Bugge A, Grontved L, Aagaard MM, Borup R, Mandrup S. The PPARgamma2 A/B-domain plays a gene-specific role in transactivation and cofactor recruitment. Mol. Endocrinol. 2009;23:794–808. doi: 10.1210/me.2008-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo G, Brun RP, Rosenfield JK, Hauser S, Park CW, Troy AE, Wright ME, Spiegelman BM. An adipogenic cofactor bound by the differentiation domain of PPARgamma. EMBO J. 1999;18:3676–3687. doi: 10.1093/emboj/18.13.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P, Terbach N, Plant N, Chen PE, Walker MC, Williams RS. Seizure control by ketogenic diet-associated medium chain fatty acids. Neuropharmacology. 2013;69:105–114. doi: 10.1016/j.neuropharm.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YC, Lin TK, Huang HY, Chang WN, Liou CW, Chen SD, Chang AYW, Chan SHH. Peroxisome proliferator-activated receptors gamma/mitochondrial uncoupling protein 2 signaling protects against seizure-induced neuronal cell death in the hippocampus following experimental status epilepticus. J Neuroinflammation. 2012;9:184. doi: 10.1186/1742-2094-9-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullingford TE. The ketogenic diet; fatty acids, fatty acid-activated receptors and neurological disorders. Prostaglandins. Leukot Essent Fatty Acids. 2004;70:253–264. doi: 10.1016/j.plefa.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, Drew PD, Racke MK. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J. Immunol. 2002;168:2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- Engel T, Sanz-Rodgriguez A, Jimenez-Mateos EM, Concannon CG, Jimenez-Pacheco A, Moran C, Mesuret G, Petit E, Delanty N, Farrell MA, O'Brien DF, Prehn JH, Lucas JJ, Henshall DC. CHOP regulates the p53-MDM2 axis and is required for neuronal survival after seizures. Brain. 2013;136:577–592. doi: 10.1093/brain/aws337. [DOI] [PubMed] [Google Scholar]
- Fenoglio-Simeone K, Mazarati A, Sefidvash-Hockley S, Shin D, Wilke J, Milligan H, Sankar R, Rho JM, Maganti R. Anticonvulsant effects of the selective melatonin receptor agonist ramelteon. Epilepsy Behav. 2009a;16:52–57. doi: 10.1016/j.yebeh.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Fenoglio-Simeone KA, Wilke JC, Milligan HL, Allen CN, Rho JM, Maganti RK. Ketogenic diet treatment abolishes seizure periodicity and improves diurnal rhythmicity in epileptic Kcna1-null mice. Epilepsia. 2009b;50:2027–2034. doi: 10.1111/j.1528-1167.2009.02163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong WH, Tsai HD, Chen YC, Wu JS, Lin TN. Anti-apoptotic actions of PPAR-gamma against ischemic stroke. Mol Neurobiol. 2010;41:180–186. doi: 10.1007/s12035-010-8103-y. [DOI] [PubMed] [Google Scholar]
- Fraser DD, Whiting S, Andrew RD, Macdonald EA, Musa-Veloso K, Cunnane SC. Elevated polyunsaturated fatty acids in blood serum obtained from children on the ketogenic diet. Neurology. 2003;60:1026–1029. doi: 10.1212/01.wnl.0000049974.74242.c6. [DOI] [PubMed] [Google Scholar]
- Freeman J, Veggiotti P, Lanzi G, Tagliabue A, Perucca E. The ketogenic diet: From molecular mechanisms to clinical effects. Epilepsy Res. 2006;68:145–180. doi: 10.1016/j.eplepsyres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Gahring LC, Persiyanov K, Days EL, Rogers SW. Age-related loss of neuronal nicotinic receptor expression in the aging mouse hippocampus corresponds with cyclooxygenase-2 and PPAR gamma expression and is altered by long-term NS398 administration. J. Neurobiol. 2005;62:453–468. doi: 10.1002/neu.20106. [DOI] [PubMed] [Google Scholar]
- Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res. 2014;55:2211–2228. doi: 10.1194/jlr.R048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grommes C, Karlo JC, Caprariello A, Blankenship D, Dechant A, Landreth GE. The PPARγ agonist pioglitazone crosses the blood-brain barrier and reduces tumor growth in a human xenograft model. Cancer Chemother. Pharmacol. 2013;71(4):929–936. doi: 10.1007/s00280-013-2084-2. [DOI] [PubMed] [Google Scholar]
- Haces ML, Hernández-Fonseca K, Medina-Campos ON, Montiel T, Pedraza-Chaverri J, Massieu L. Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp. Neurol. 2008;211(1):85–96. doi: 10.1016/j.expneurol.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Han Y, Xie N, Cao L, Zhao X, Liu X, Jiang H, Chi Z. Adenosine monophosphate-activated protein kinase and peroxisome proliferator-activated receptor gamma coactivator 1α signaling provides neuroprotection in status epilepticus in rats. Neurosci. Lett. 2011;500:133–138. doi: 10.1016/j.neulet.2011.06.020. [DOI] [PubMed] [Google Scholar]
- He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Landreth GE. PPARs in the brain. Biochim Biophys Acta. 2007;1771:1031–1045. doi: 10.1016/j.bbalip.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Hong S, Xin Y, HaiQin W, GuiLian Z, Ru Z, ShuQin Z, HuQing W, Li Y, Yun D. The PPARγ agonist rosiglitazone prevents cognitive impairment by inhibiting astrocyte activation and oxidative stress following pilocarpine-induced status epilepticus. Neurol Sci. 2012;33:559–566. doi: 10.1007/s10072-011-0774-2. [DOI] [PubMed] [Google Scholar]
- Hong S, Huang Y, Yu X, Li Y, Yang J, Li R, Deng Y, Zhao G. Peroxisome proliferator-activated receptor gamma agonist, rosiglitazone, suppresses CD40 expression and attenuates inflammatory responses after lithium pilocarpine-induced status epilepticus in rats. Int J Dev Neurosci. 2008;26:505–515. doi: 10.1016/j.ijdevneu.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Hong S, Xin Y, HaiQin W, GuiLian Z, Ru Z, ShuQin Z, HuQing W, Li Y, Ning B, YongNan L. The PPARγ agonist rosiglitazone prevents neuronal loss and attenuates development of spontaneous recurrent seizures through BDNF/TrkB signaling following pilocarpine-induced status epilepticus. Neurochem Int. 2013;63:405–412. doi: 10.1016/j.neuint.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Hughes SD, Kanabus M, Anderson G, Hargreaves IP, Rutherford T, O'Donnell M, Cross JH, Rahman S, Eaton S, Heales SJ. The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J. Neurochem. 2014;129:426–433. doi: 10.1111/jnc.12646. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Taketomi S, Sugiyama Y, Shimura Y, Sohda T, Meguro K, Fujita T. Effects of pioglitazone on glucose and lipid metabolism in normal and insulin resistant animals. Arzneimittelforschung. 1990;40(2 Pt 1):156–162. [PubMed] [Google Scholar]
- Itoh T, Fairall L, Amin K, Inaba Y, Szanto A, Balint BL, Nagy L, Yamamoto K, Schwabe JW. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat. Struct. Mol. Biol. 2008;15:924–931. doi: 10.1038/nsmb.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanami J, Mogi M, Tsukuda K, Min LJ, Sakata A, Jing F, Iwai M, Horiuchi M. Low dose of telmisartan prevents ischemic brain damage with peroxisome proliferator-activated receptor-gamma activation in diabetic mice. J. Hypertens. 2010;28(8):1730–1737. doi: 10.1097/HJH.0b013e32833a551a. [DOI] [PubMed] [Google Scholar]
- Jeong EA, Jeon BT, Shin HJ, Kim N, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Ketogenic diet-induced peroxisome proliferator-activated receptor-gamma activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp Neurol. 2011;232:195–202. doi: 10.1016/j.expneurol.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Jr, Ruskin DN, Geiger JD, Boison D, Masino SA. Ketogenic diet sensitizes glucose control of hippocampal excitability. J Lipid Res. 2014;55(11):2254–2260. doi: 10.1194/jlr.M046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Jr, Ruskin DN, Masino SA. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J Neurosci. 2011;30(11):3886–3895. doi: 10.1523/JNEUROSCI.0055-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Simeone KA, Simeone TA, Pandya JD, Wilke JC, Ahn Y, Geddes JW, Sullivan PG, Rho JM. Ketone Bodies Mediate Anti-Seizure Effects Through Mitochondrial Permeability Transition. Ann. Neurol. 2015;78:77–87. doi: 10.1002/ana.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim doY, Davis LM, Sullivan PG, Maalouf M, Simeone TA, van Brederode J, Rho JM. Ketone bodies are protective against oxidative stress in neocortical neurons. J Neurochem. 2007;101(5):1316–1326. doi: 10.1111/j.1471-4159.2007.04483.x. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Shimohama S, Koike H, Kakimura Ji, Matsuoka Y, Nomura Y, Gebicke-Haerter PJ, Taniguchi T. Increased expression of cyclooxygenases and peroxisome proliferator-activated receptor-gamma in Alzheimer's disease brains. Biochem. Biophys. Res. Commun. 1999;254:582–586. doi: 10.1006/bbrc.1998.9981. [DOI] [PubMed] [Google Scholar]
- Kobow K, Auvin S, Jensen F, Löscher W, Mody I, Potschka H, Prince D, Sierra A, Simonato M, Pitkänen A, Nehlig A, Rho JM. Finding a better drug for epilepsy: antiepileptogenesis targets. Epilepsia. 2012;53:1868–1876. doi: 10.1111/j.1528-1167.2012.03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroker AJ, Bruning JB. Review of the Structural and Dynamic Mechanisms of PPARγ Partial Agonism. PPAR Res. 2015 doi: 10.1155/2015/816856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Jensen PB, Sørensen RV, Larsen LK, Vrang N, Wulff EM, Wassermann K. Differential influences of peroxisome proliferator-activated receptors gamma and -alpha on food intake and energy homeostasis. Diabetes. 2003;52(9):2249–2259. doi: 10.2337/diabetes.52.9.2249. [DOI] [PubMed] [Google Scholar]
- Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, Bandyopadhyay G, Nalbandian S, Fan W, Gayen JR, Mahata SK, Webster NJ, Schwartz MW, Olefsky JM. Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat. Med. 2011;17:618–622. doi: 10.1038/nm.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wu DM, Zheng YL, Hu B, Cheng W, Zhang ZF, Li MQ. Troxerutin counteracts domoic acid-induced memory deficits in mice by inhibiting CCAAT/enhancer binding protein beta-mediated inflammatory response and oxidative stress. J. Immunol. 2013;190:3466–3479. doi: 10.4049/jimmunol.1202862. [DOI] [PubMed] [Google Scholar]
- Luna-Medina R, Cortes-Canteli M, Sanchez-Galiano S, Morales-Garcia JA, Martinez A, Santos A, Perez-Castillo A. NP031112, a thiadiazolidinone compound, prevents inflammation and neurodegeneration under excitotoxic conditions: potential therapeutic role in brain disorders. J. Neurosci. 2007;27:5766–5776. doi: 10.1523/JNEUROSCI.1004-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145(1):256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Salpekar JA. Stress, seizures, and hypothalamic-pituitary-adrenal axis targets for the treatment of epilepsy. Epilepsy Behav. 2013;26:352–362. doi: 10.1016/j.yebeh.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malapaka RR, Khoo S, Zhang J, Choi JH, Zhou XE, Xu Y, Gong Y, Li J, Yong EL, Chalmers MJ, Chang L, Resau JH, Griffin PR, Chen YE, Xu HE. Identification and mechanism of 10-carbon fatty acid as modulating ligand of peroxisome proliferator-activated receptors. J. Biol. Chem. 2012;287:183–195. doi: 10.1074/jbc.M111.294785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar-Colucci S, Sauerbeck A, Popovich PG, McTigue DM. PPAR agonists as therapeutics for CNS trauma and neurological diseases. ASN Neuro. 2013;5:e00129. doi: 10.1042/AN20130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Rho JM. Mechanisms of Ketogenic Diet Action. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 4th. Bethesda, MD: National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- Maurois P, Rocchi S, Pages N, Bac P, Stables JP, Gressens P, Vamecq J. The PPARgamma agonist FMOC-L-leucine protects both mature and immature brain. Biomed. Pharmacother. 2008;62:259–263. doi: 10.1016/j.biopha.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Medina-Gomez G, Virtue S, Lelliott C, Boiani R, Campbell M, Christodoulides C, Perrin C, Jimenez-Linan M, Blount M, Dixon J, Zahn D, Thresher RR, Aparicio S, Carlton M, Colledge WH, Kettunen MI, Seppänen-Laakso T, Sethi JK, O'Rahilly S, Brindle K, Cinti S, Oresic M, Burceli R, Vidal-Puig A. The link between nutritional status and insulin sensitivity is dependent on the adipocyte-specific peroxisome proliferator-activated receptor-gamma2 isoform. Diabetes. 2005;54(6):1706–1716. doi: 10.2337/diabetes.54.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Gomez G, Gray SL, Yetukuri L, Shimomura K, Virtue S, Campbell M, Curtis RK, Jimenez-Linan M, Blount M, Yeo GS, Lopez M, Seppänen-Laakso T, Ashcroft FM, Oresic M, Vidal-Puig A. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3:e64. doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Gomez G, Gray S, Vidal-Puig A. Adipogenesis and lipotoxicity: role of peroxisome proliferator-activated receptor gamma (PPARgamma) and PPARgammacoactivator-1 (PGC1) Public Health Nutr. 2007;10:1132–1137. doi: 10.1017/S1368980007000614. [DOI] [PubMed] [Google Scholar]
- Miglio G, Rosa AC, Rattazzi L, Collino M, Lombardi G, Fantozzi R. PPARgamma stimulation promotes mitochondrial biogenesis and prevents glucose deprivation-induced neuronal cell loss. Neurochem Int. 2009;55:496–504. doi: 10.1016/j.neuint.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Min LJ, Mogi M, Shudou M, Jing F, Tsukuda K, Ohshima K, Iwanami J, Horiuchi M. Peroxisome Proliferator-Activated Receptor-γ Activation With Angiotensin II Type 1 Receptor Blockade Is Pivotal for the Prevention of Blood-Brain Barrier Impairment and Cognitive Decline in Type 2 Diabetic Mice. Hypertension. 2012 doi: 10.1161/HYPERTENSIONAHA.112.192401. [DOI] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Cerù MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Okada K, Yamashita U, Tsuji S. Ameliorative effect of pioglitazone on seizure responses in genetically epilepsy-susceptible EL mice. Brain Res. 2006;1102:175–178. doi: 10.1016/j.brainres.2006.04.108. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O’Malley B, Spiegelman B. Activation of PPARgamma coactivator-1 through transcription factor docking. Science. 1999;286:1368–1371. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- Roundtree HM, Simeone TA, Johnson C, Matthews SA, Samson KK, Simeone KA. Orexin Receptor Antagonism Improves Sleep and Reduces Seizures in Kcna1-null Mice. Sleep. 2016;39(2):57–68. doi: 10.5665/sleep.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD, Schwartz MW. Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology. 2009;150:707–712. doi: 10.1210/en.2008-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer S. Ligands for the Nuclear Peroxisome Proliferator-Activated Receptor Gamma. Trends Pharmacol. Sci. 2015;36:688–704. doi: 10.1016/j.tips.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Shao D, Rangwala SM, Bailey ST, Krakow SL, Reginato MJ, Lazar MA. Interdomain communication regulating ligand binding by PPARgamma. Nature. 1998;396:377–380. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- Simeone KA, Matthews SA, Rho JM, Simeone TA. Ketogenic diet treatment increases longevity in Kcna1-null mice, a model of sudden unexpected death in epilepsy. Epilepsia. 2016 doi: 10.1111/epi.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone KA, Matthews SA, Samson KK, Simeone TA. Targeting deficiencies in mitochondrial respiratory complex I and functional uncoupling exerts anti-seizure effects in a genetic model of temporal lobe epilepsy and in a model of acute temporal lobe seizures. Exp. Neurol. 2014a;251:84–90. doi: 10.1016/j.expneurol.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone TA, Samson KK, Matthews SA, Simeone KA. In vivo ketogenic diet treatment attenuates pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices from epileptic Kv1.1α knockout mice. Epilepsia. 2014b;55:e44–e49. doi: 10.1111/epi.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone TA, Simeone KA, Samson KK, Kim, do Y, Rho JM. Loss of the Kv1.1 potassium channel promotes pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices. Neurobiol. Dis. 2013;54:68–81. doi: 10.1016/j.nbd.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:e59. doi: 10.3389/fphar.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger DJ, Grant GR, Schupp M, Tomaru T, Lefterova MI, Schug J, Manduchi E, Stoeckert CJ, Jr, Lazar MA. Propagation of adipogenic signals through an epigenomic transition state. Genes Dev. 2010;24:1035–1044. doi: 10.1101/gad.1907110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann. Neurol. 2004;55:576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Yang X, Cheng B. Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. J. Mol. Neurosci. 2010;42:145–153. doi: 10.1007/s12031-010-9336-y. [DOI] [PubMed] [Google Scholar]
- Yu X, Shao XG, Sun H, Li YN, Yang J, Deng YC, Huang YG. Activation of cerebral peroxisome proliferator-activated receptors gamma exerts neuroprotection by inhibiting oxidative stress following pilocarpine-induced status epilepticus. Brain Res. 2008;1200:146–158. doi: 10.1016/j.brainres.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Victor NA, Wanderi EW, Gamboa J, Zhao X, Aronowski J, Deininger K, Lust WD, Landreth GE, Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur. J. Neurosci. 2006;24:1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- Vidal-Puig A, Jimenez-Liñan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J. Clin. Invest. 1996;97:2553–2561. doi: 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Puig AJ, Considine RV, Jimenez-Liñan M, Werman A, Pories WJ, Caro JF, Flier JS. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J. Clin. Invest. 1997;99:2416–2422. doi: 10.1172/JCI119424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Jiang R, He Q, Zhang Y, Zhang Y, Li Y, Zhuang R, Luo Y, Li Y, Wan J, Tang Y, Yu H, Jiang Q, Yang J. Expression Pattern of Peroxisome Proliferator-Activated Receptors in Rat Hippocampus following Cerebral Ischemia and Reperfusion Injury. PPAR Res. 2012 doi: 10.1155/2012/596394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werman A, Hollenberg A, Solanes G, Bjorbaek C, Vidal-Puig AJ, Flier JS. Ligand-independent activation domain in the N terminus of peroxisome proliferator-activated receptor gamma (PPARgamma). Differential activity of PPARgamma1 and-2 isoforms and influence of insulin. J. Biol. Chem. 1997;272:20230–20235. doi: 10.1074/jbc.272.32.20230. [DOI] [PubMed] [Google Scholar]
- Yakunin E, Kisos H, Kulik W, Grigoletto J, Wanders RJ, Sharon R. The regulation of catalase activity by PPAR γ is affected by α-synuclein. Ann. Clin. Transl. Neurol. 2014;1:145–159. doi: 10.1002/acn3.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Itoh T, Abe D, Shimizu M, Kanda T, Koyama T, Nishikawa M, Tamai T, Ooizumi H, Yamada S. Identification of putative metabolites of docosahexaenoic acid as potent PPARgamma agonists and antidiabetic agents. Bioorg Med. Chem. Lett. 2005;15:517–522. doi: 10.1016/j.bmcl.2004.11.053. [DOI] [PubMed] [Google Scholar]